Circulating Exosomes Inhibit B Cell Proliferation and Activity
Abstract
1. Introduction
2. Results
2.1. Clinicopathological Characteristics of HNSCC Patients
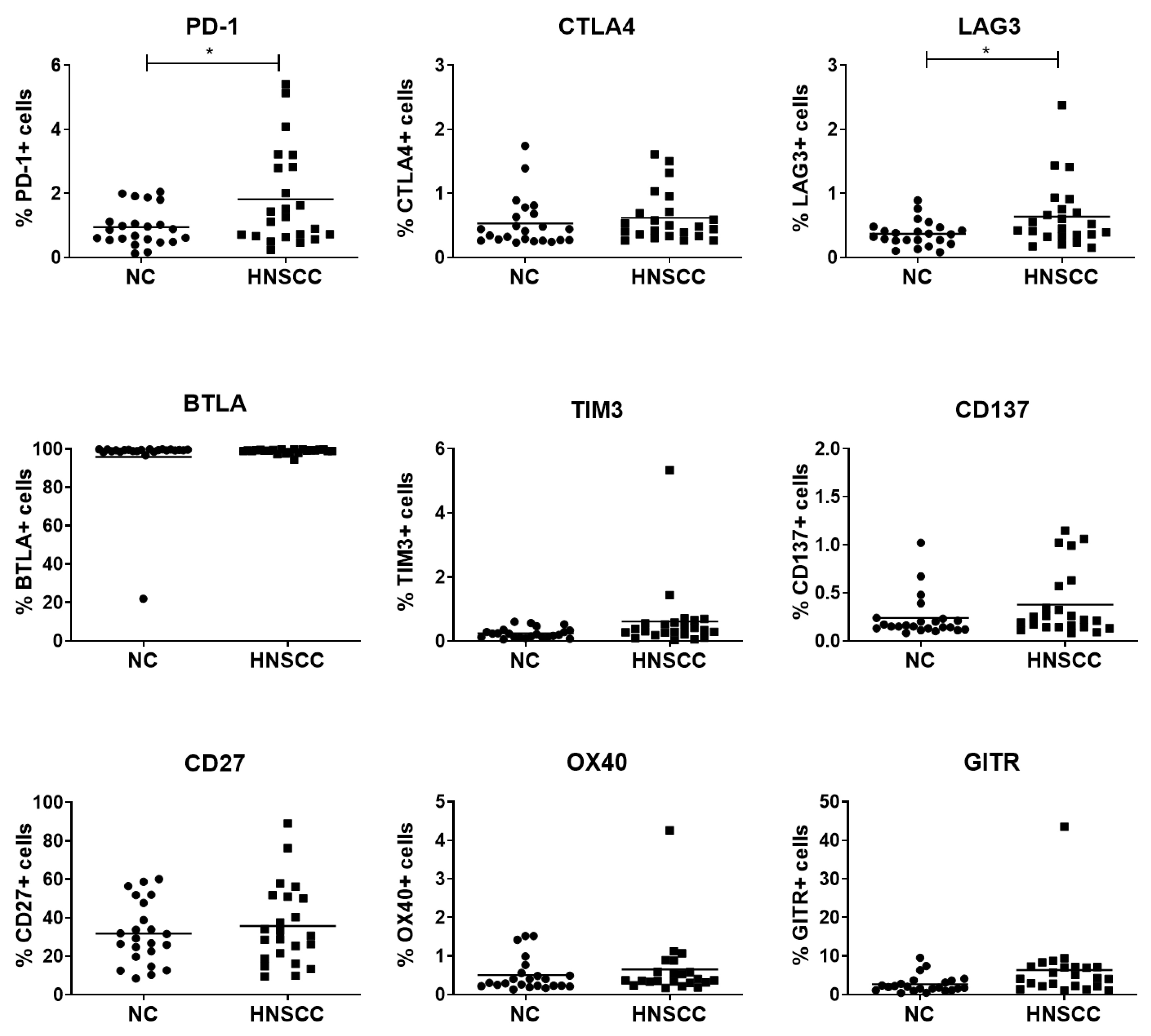
2.2. Expression of Checkpoint Receptors on B Cells
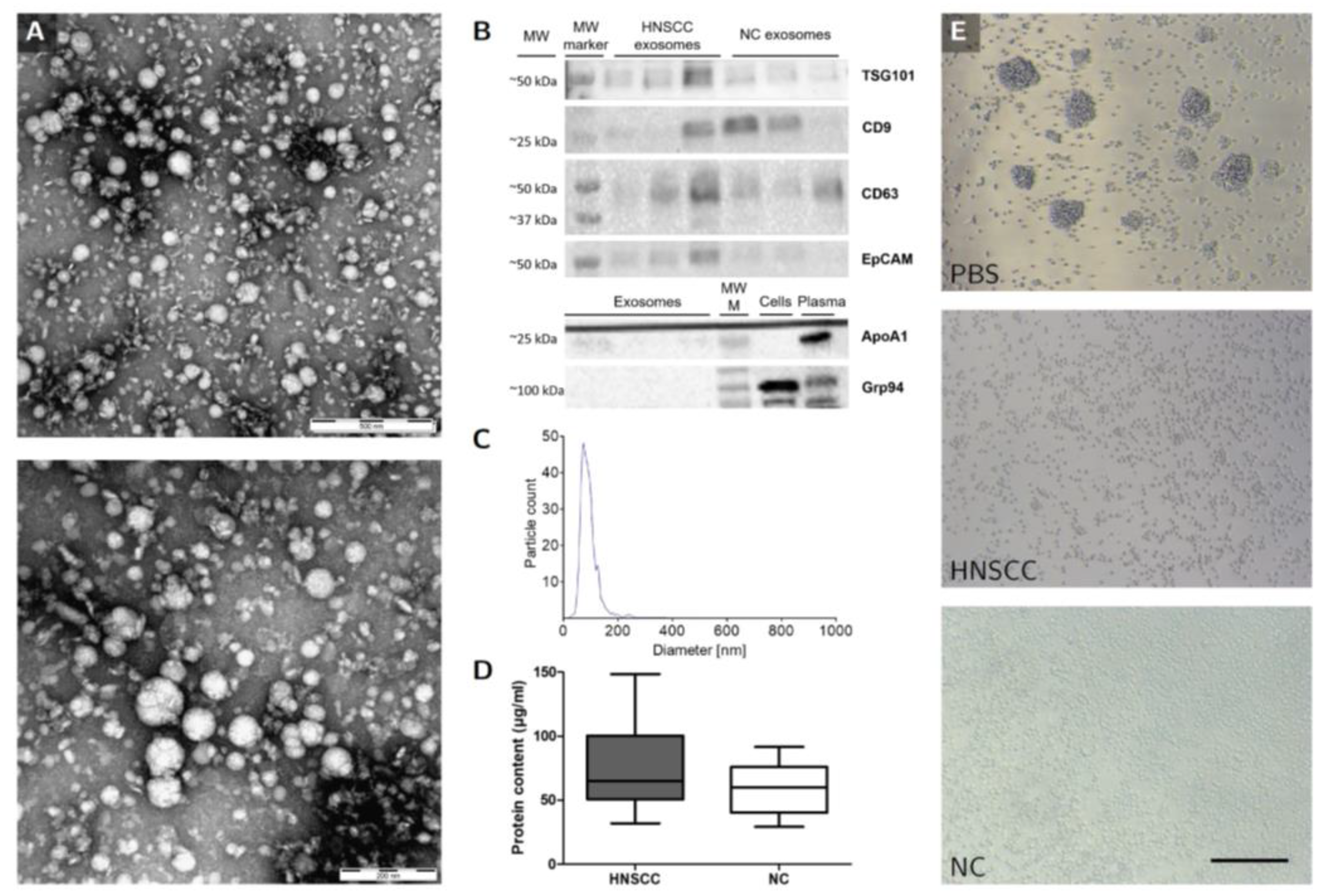
2.3. Characterization of Plasma-Derived Exosomes
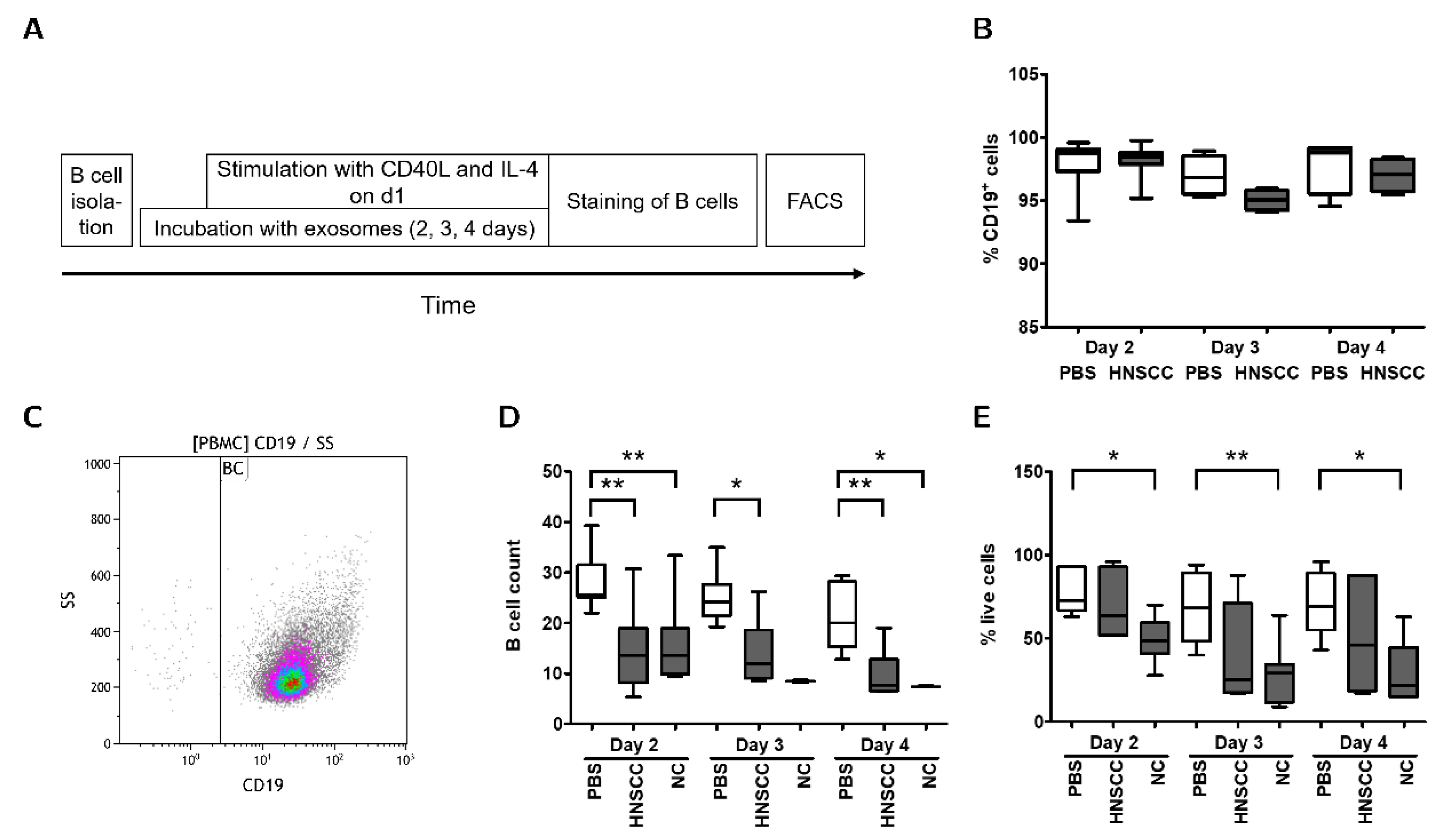
2.4. Exosomes Inhibit Proliferation and Survival of B Cells In Vitro
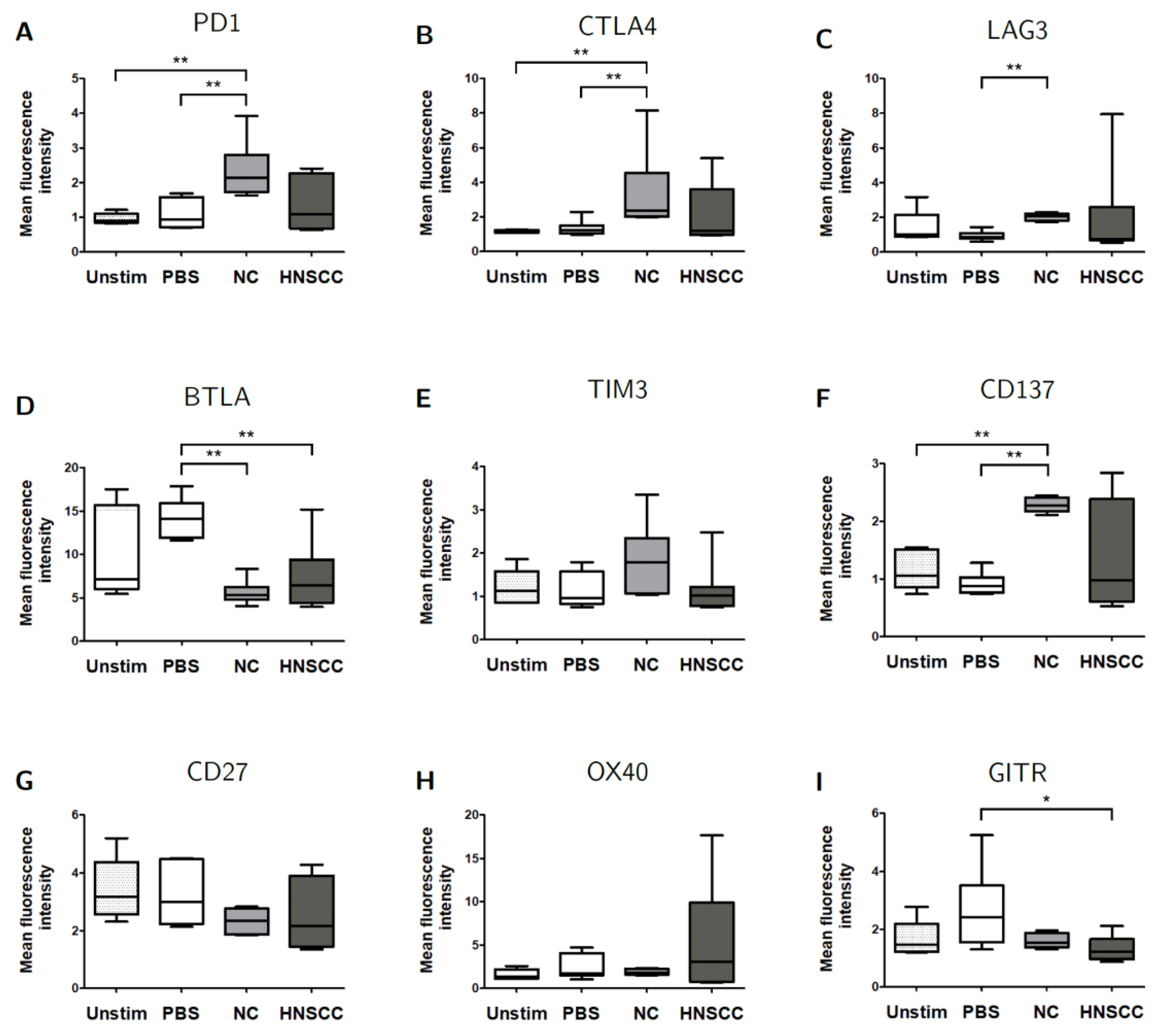
2.5. Exosomes Modulate the Expression of Checkpoint Receptors on B Cells
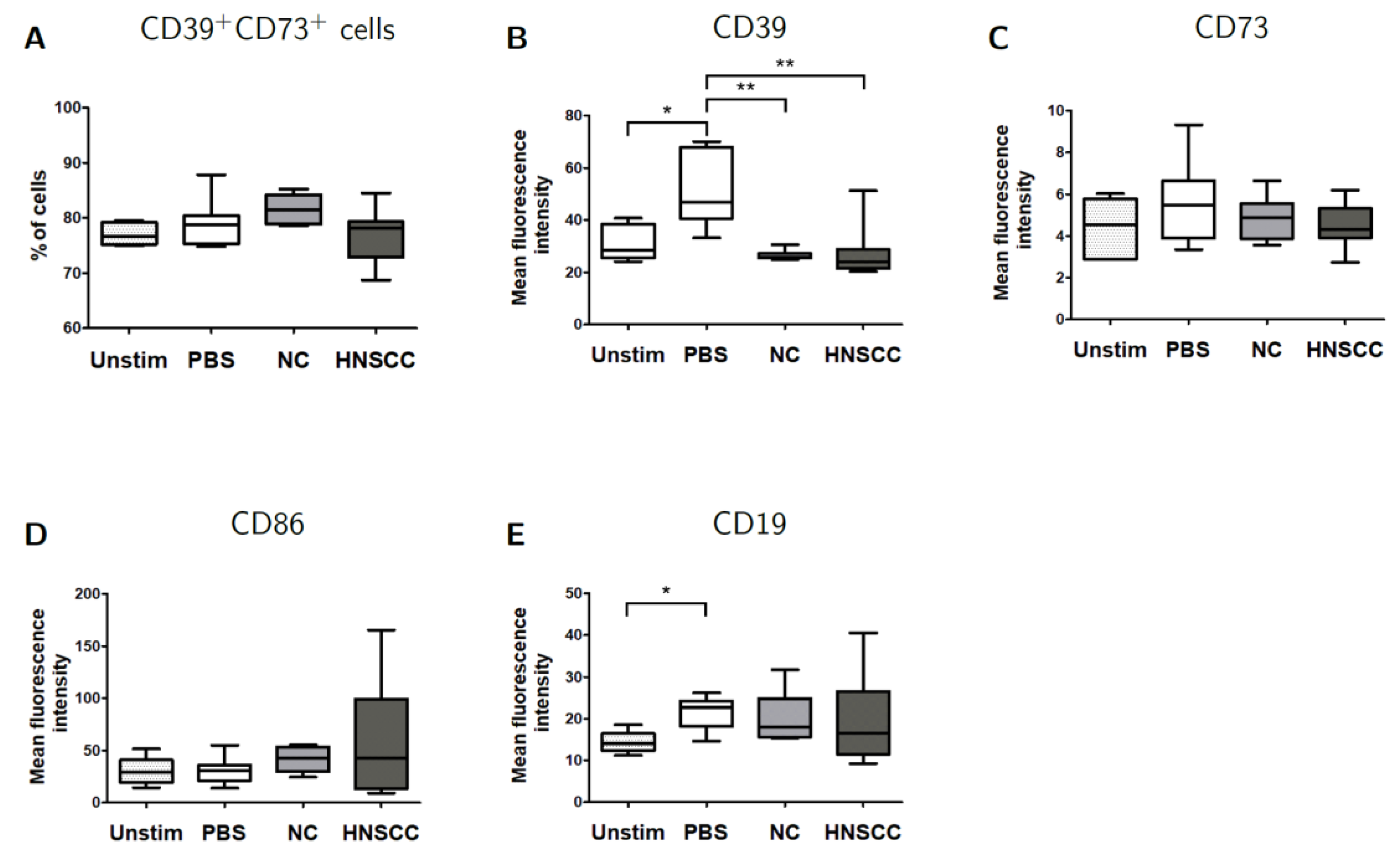
2.6. Effects of Exosomes on CD39/CD73 Expression
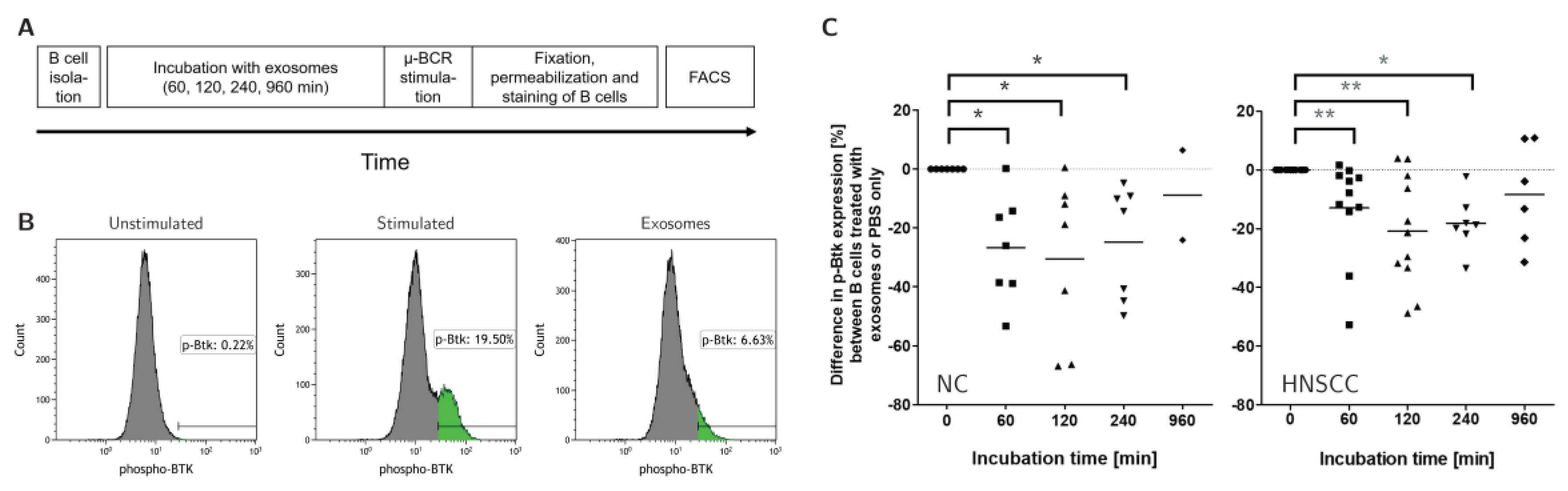
2.7. Exosomes Reduce the Activity of the B-Cell Receptor Pathway
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Exosome Isolation by Mini Size-Exclusion Chromatography (Minisec)
4.3. Bicinchoninic Acid (BCA) Protein Assay
4.4. Transmission Electron Microscopy (TEM)
4.5. SDS-PAGE and Western Blot
4.6. B Cell Culture
4.7. Flow Cytometry
4.8. Intracellular p-BTK Staining
4.9. ATP Hydrolysis Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.S.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Head and Neck Carcinoma Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2017, 24, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. 2015, 33, 3293–3304. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Haskó, G. Immunity, inflammation and cancer: A leading role for adenosine. Nat. Rev. Cancer 2013, 13, 842–857. [Google Scholar] [CrossRef]
- Hoffmann, T.; Dworacki, G.; Tsukihiro, T.; Meidenbauer, N.; Gooding, W.; Johnson, J.T.; Whiteside, T.L. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin. Cancer Res. 2002, 8, 2553–2562. [Google Scholar] [PubMed]
- Reichert, T.E.; Strauss, L.; Wagner, E.M.; Gooding, W.; Whiteside, T.L. Signaling abnormalities, apoptosis, and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin. Cancer Res. 2002, 8, 3137–3145. [Google Scholar]
- Whiteside, T.L. Induced regulatory T cells in inhibitory microenvironments created by cancer. Expert Opin. Biol. Ther. 2014, 14, 1411–1425. [Google Scholar] [CrossRef]
- Ruivo, C.; Adem, B.; Silva, M.; Melo, S.A. The Biology of Cancer Exosomes: Insights and New Perspectives. Cancer Res. 2017, 77, 6480–6488. [Google Scholar] [CrossRef]
- Whiteside, T.L. Exosomes carrying immunoinhibitory proteins and their role in cancer. Clin. Exp. Immunol. 2017, 189, 259–267. [Google Scholar] [CrossRef]
- Brinton, L.T.; Sloane, H.S.; Kester, M.; Kelly, K.A. Formation and role of exosomes in cancer. Cell. Mol. Life Sci. 2014, 72, 659–671. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Exosomes and tumor-mediated immune suppression. J. Clin. Investig. 2016, 126, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.; Floros, T.; Theodoraki, M.-N.; Hong, C.-S.; Jackson, E.K.; Lang, S.; Whiteside, T.L. Suppression of Lymphocyte Functions by Plasma Exosomes Correlates with Disease Activity in Patients with Head and Neck Cancer. Clin. Cancer Res. 2017, 23, 4843–4854. [Google Scholar] [CrossRef]
- Theodoraki, M.-N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical Significance of PD-L1+ Exosomes in Plasma of Head and Neck Cancer Patients. Clin. Cancer Res. 2017, 24, 896–905. [Google Scholar] [CrossRef]
- Sarvaria, A.; Madrigal, J.; Saudemont, A. B cell regulation in cancer and anti-tumor immunity. Cell. Mol. Immunol. 2017, 14, 662–674. [Google Scholar] [CrossRef]
- Xiao, X.; Lao, X.-M.; Chen, M.-M.; Liu, R.-X.; Wei, Y.; Ouyang, F.-Z.; Chen, D.-P.; Zhao, X.-Y.; Zhao, Q.; Li, X.-F.; et al. PD-1hi Identifies a Novel Regulatory B-cell Population in Human Hepatoma that Promotes Disease Progression. Cancer Discov. 2016, 6, 546–559. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, Y.; Dong, L.; Zhang, Q.; Wang, C.; Zhang, Y.; Li, X.; Fu, Z. Circulating exosomes from esophageal squamous cell carcinoma mediate the generation of B10 and PD-1high Breg cells. Cancer Sci. 2019, 110, 2700–2710. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, Q.; Cheng, Y.; Chen, X.; Wang, G.; Shi, M.; Zhang, T.; Cao, Y.; Pan, H.; Zhang, L.; et al. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1+ regulatory B cell expansion. J. Immunother. Cancer 2018, 6, 145. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, T.; Li, M.; Yin, L.; Xue, J. Immunosuppressive B cells expressing PD-1/PD-L1 in solid tumors: A mini review. Qjm Int. J. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, G.; Wang, Z.; Liu, B.; Han, N.; Li, J.; Lu, C.; Liu, X.; Zhang, Q.; Yang, Q.; et al. PD-1-expressing B cells suppress CD4+ and CD8+ T cells via PD-1/PD-L1-dependent pathway. Mol. Immunol. 2019, 109, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.-N.; Hoffmann, T.K.; Whiteside, T.L. Separation of plasma-derived exosomes into CD3(+) and CD3(−) fractions allows for association of immune cell and tumour cell markers with disease activity in HNSCC patients. Clin. Exp. Immunol. 2018, 192, 271–283. [Google Scholar] [CrossRef]
- Theodoraki, M.-N.; Yerneni, S.; Gooding, W.E.; Ohr, J.; Clump, D.A.; Bauman, J.E.; Ferris, R.L.; Whiteside, T.L. Circulating exosomes measure responses to therapy in head and neck cancer patients treated with cetuximab, ipilimumab, and IMRT. OncoImmunology 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.-N.; Matsumoto, A.; Beccard, I.; Hoffmann, T.K.; Whiteside, T.L. CD44v3 protein-carrying tumor-derived exosomes in HNSCC patients’ plasma as potential noninvasive biomarkers of disease activity. OncoImmunology 2020, 9, 1747732. [Google Scholar] [CrossRef]
- Theodoraki, M.-N.; Hoffmann, T.K.; Jackson, E.K.; Whiteside, T.L. Exosomes in HNSCC plasma as surrogate markers of tumour progression and immune competence. Clin. Exp. Immunol. 2018, 194, 67–78. [Google Scholar] [CrossRef]
- Anel, A.; Gallego-Lleyda, A.; De Miguel, D.; Naval, J.; Martínez-Lostao, L. Role of Exosomes in the Regulation of T-cell Mediated Immune Responses and in Autoimmune Disease. Cells 2019, 8, 154. [Google Scholar] [CrossRef]
- Östman, S.; Taube, M.; Telemo, E. Tolerosome-induced oral tolerance is MHC dependent. Immunology 2005, 116, 464–476. [Google Scholar] [CrossRef]
- Robbins, P.D.; Dorronsoro, A.; Booker, C.N. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J. Clin. Investig. 2016, 126, 1173–1180. [Google Scholar] [CrossRef]
- Gercel-Taylor, C.; Shah, S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods 2015, 87, 3–10. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Goularte, O.D.; Rufner, K.; Wilkinson, B.; Kaye, J. An Inhibitory Ig Superfamily Protein Expressed by Lymphocytes and APCs Is Also an Early Marker of Thymocyte Positive Selection. J. Immunol. 2004, 172, 5931–5939. [Google Scholar] [CrossRef] [PubMed]
- Thibult, M.-L.; Mamessier, E.; Gertner-Dardenne, J.; Pastor, S.; Just-Landi, S.; Xerri, L.; Chetaille, B.; Olive, D. PD-1 is a novel regulator of human B-cell activation. Int. Immunol. 2012, 25, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, H.M.; Brouwer, M.; Linsley, P.S.; van Lier, R.A. Activated T cells can induce high levels of CTLA-4 expression on B cells. J. Immunol. 1995, 155, 1776–1783. [Google Scholar]
- Quandt, D.; Hoff, H.; Rudolph, M.; Fillatreau, S.; Brunner, M. A new role of CTLA-4 on B cells in thymus-dependent immune responses in vivo. J. Immunol. 2007, 179, 7316–7324. [Google Scholar] [CrossRef]
- Do, P.; Beckwith, K.A.; Cheney, C.; Tran, M.; Beaver, L.; Griffin, B.G.; Mo, X.; Liu, Y.; Lapalombella, R.; Hertlein, E.; et al. Leukemic B Cell CTLA-4 Suppresses Costimulation of T Cells. J. Immunol. 2019, 202, 2806–2816. [Google Scholar] [CrossRef]
- Herrmann, A.; Lahtz, C.; Nagao, T.; Song, J.Y.; Chan, W.C.; Lee, H.; Yue, C.; Look, T.; Mülfarth, R.; Li, W.; et al. CTLA4 Promotes Tyk2-STAT3-Dependent B-cell Oncogenicity. Cancer Res. 2017, 77, 5118–5128. [Google Scholar] [CrossRef]
- Zhang, X.; Voskens, C.J.; Sallin, M.; Maniar, A.; Montes, C.L.; Zhang, Y.; Lin, W.; Li, G.; Burch, E.; Tan, M.; et al. CD137 Promotes Proliferation and Survival of Human B Cells. J. Immunol. 2009, 184, 787–795. [Google Scholar] [CrossRef]
- Kisielow, M.; Kisielow, J.; Karjalainen, K.; Capoferri-Sollami, G. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur. J. Immunol. 2005, 35, 2081–2088. [Google Scholar] [CrossRef]
- Nakaima, Y.; Watanabe, K.; Koyama, T.; Miura, O.; Fukuda, T. CD137 Is Induced by the CD40 Signal on Chronic Lymphocytic Leukemia B Cells and Transduces the Survival Signal via NF-κB Activation. PLoS ONE 2013, 8, e64425. [Google Scholar] [CrossRef]
- Vendel, A.C.; Calemine-Fenaux, J.; Izrael-Tomasevic, A.; Chauhan, V.; Arnott, D.; Eaton, D.L. B and T lymphocyte attenuator regulates B cell receptor signaling by targeting Syk and BLNK. J. Immunol. 2009, 182, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Lin, H.-W.; Chien, C.-L.; Lai, Y.-L.; Sun, W.-Z.; Chen, C.-A.; Cheng, W.-F. BTLA blockade enhances Cancer therapy by inhibiting IL-6/IL-10-induced CD19high B lymphocytes. J. Immunother. Cancer 2019, 7, 313. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, J.; Yamazaki, S.; Takahashi, T.; Ishida, Y.; Sakaguchi, S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 2002, 3, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Teodorovic, L.S.; Riccardi, C.; Torres, R.M.; Pelanda, R. Murine B Cell Development and Antibody Responses to Model Antigens are Not Impaired in the Absence of the TNF Receptor GITR. PLoS ONE 2012, 7, e31632. [Google Scholar] [CrossRef]
- Ludwig, S.; Sharma, P.; Theodoraki, M.-N.; Pietrowska, M.; Yerneni, S.S.; Lang, S.; Ferrone, S.; Whiteside, T.L. Molecular and Functional Profiles of Exosomes From HPV(+) and HPV(−) Head and Neck Cancer Cell Lines. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef]
- Muller, L.; Mitsuhashi, M.; Simms, P.; Gooding, W.E.; Whiteside, T.L. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci. Rep. 2016, 6, 20254. [Google Scholar] [CrossRef]
- Wang, K.; Wei, G.; Liu, D. CD19: A biomarker for B cell development, lymphoma diagnosis and therapy. Exp. Hematol. Oncol. 2012, 1, 36. [Google Scholar] [CrossRef]
- Saze, Z.; Schuler, P.J.; Hong, C.-S.; Cheng, N.; Jackson, E.K.; Whiteside, T.L. Adenosine production by human B cells and B cell–mediated suppression of activated T cells. Blood 2013, 122, 9–18. [Google Scholar] [CrossRef]
- Jeske, S.S.; Brand, M.; Ziebart, A.; Laban, S.; Doescher, J.; Greve, J.; Jackson, E.K.; Hoffmann, T.K.; Brunner, C.; Schuler, P. Adenosine-producing regulatory B cells in head and neck cancer. Cancer Immunol. Immunother. 2020, 69, 1205–1216. [Google Scholar] [CrossRef]
- Dalporto, J. B cell antigen receptor signaling 101. Mol. Immunol. 2004, 41, 599–613. [Google Scholar] [CrossRef]
- Hong, C.-S.; Funk, S.; Muller, L.; Boyiadzis, M.; Whiteside, T.L. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J. Extracell. Vesicles 2016, 5, 364. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
| Parameter | HNSCC Patients (Exosome Isolation) n = 21 | Control Group (Exosome Isolation) n = 10 | p-Value * | HNSCC Patients (B Cell Isolation) n = 23 | Control Group (B Cell Isolation) n = 23 | p-Value * |
|---|---|---|---|---|---|---|
| Mean age (range) | 64.1 (49–79) | 29.2 (20–58) | 0.0001 | 59 (37–74) | 56 (27–84) | 0.58 |
| Sex | ||||||
| Female | 3 (14.3%) | 6 (60%) | 9 (39.1%) | 13 (56.5%) | ||
| Male | 18 (85.7%) | 4 (40%) | 0.0001 | 14 (60.9%) | 10 (43.5%) | 0.24 |
| T classification | ||||||
| T1 | 1 (4.8%) | 4 (17.4%) | ||||
| T2 | 5 (23.8%) | 8 (34.8%) | ||||
| T3 | 6 (28.6%) | 5 (21.7%) | ||||
| T4 | 9 (42.9%) | 6 (26.1%) | ||||
| N classification | ||||||
| N0 | 0 | 7 (30.4%) | ||||
| N1 | 3 (14.3%) | 5 (21.7%) | ||||
| N2 | 13 (61.9%) | 7 (30.4%) | ||||
| N3 | 5 (23.8%) | 4 (17.4%) | ||||
| M classification | ||||||
| M0 | 21 (100%) | 23 (100%) | ||||
| M1 | 0 | 0 | ||||
| HPV-status | ||||||
| Positive (p16) | 3 (14.3%) | 7 | ||||
| Localization | ||||||
| Pharynx | 12 (57.1%) | 14 (60.9%) | ||||
| Larynx | 5 (23.8%) | 4 (17.4%) | ||||
| Mouth | 4 (19.0%) | 5 (21.7%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schroeder, J.C.; Puntigam, L.; Hofmann, L.; Jeske, S.S.; Beccard, I.J.; Doescher, J.; Laban, S.; Hoffmann, T.K.; Brunner, C.; Theodoraki, M.-N.; et al. Circulating Exosomes Inhibit B Cell Proliferation and Activity. Cancers 2020, 12, 2110. https://doi.org/10.3390/cancers12082110
Schroeder JC, Puntigam L, Hofmann L, Jeske SS, Beccard IJ, Doescher J, Laban S, Hoffmann TK, Brunner C, Theodoraki M-N, et al. Circulating Exosomes Inhibit B Cell Proliferation and Activity. Cancers. 2020; 12(8):2110. https://doi.org/10.3390/cancers12082110
Chicago/Turabian StyleSchroeder, Jan C., Lisa Puntigam, Linda Hofmann, Sandra S. Jeske, Inga J. Beccard, Johannes Doescher, Simon Laban, Thomas K. Hoffmann, Cornelia Brunner, Marie-Nicole Theodoraki, and et al. 2020. "Circulating Exosomes Inhibit B Cell Proliferation and Activity" Cancers 12, no. 8: 2110. https://doi.org/10.3390/cancers12082110
APA StyleSchroeder, J. C., Puntigam, L., Hofmann, L., Jeske, S. S., Beccard, I. J., Doescher, J., Laban, S., Hoffmann, T. K., Brunner, C., Theodoraki, M.-N., & Schuler, P. J. (2020). Circulating Exosomes Inhibit B Cell Proliferation and Activity. Cancers, 12(8), 2110. https://doi.org/10.3390/cancers12082110






