Do Elderly Lung Cancer Patients Aged ≥75 Years Benefit from Immune Checkpoint Inhibitors?
Abstract
1. Introduction
2. Standard Anticancer Drug Therapies for Elderly Patients with Advanced NSCLC
3. Standard Second-Line Treatment for Elderly Patients with NSCLC
4. Initial Treatment for Extensive-Stage Small-Cell Lung Cancer
5. Efficacy of ICIs in Elderly NSCLC Patients (≥75 years) in Phase 3 Studies
6. Efficacy of ICIs for Elderly NSCLC Patients in Non-Randomized Studies
7. Comparison of Adverse Events Between Elderly and Non-Elderly Patients Treated with ICI Monotherapy
8. Conclusions and Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Gridelli, C.; Langer, C.; Maione, P.; Rossi, A.; Schild, S.E. Lung Cancer in the Elderly. J. Clin. Oncol. 2007, 25, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Presley, C.J.; Reynolds, C.H.; Langer, C.J. Caring for the Older Population with Advanced Lung Cancer. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Cancer Stat Facts: Lung and Bronchus Cancer. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 19 June 2020).
- Khozin, S.; Abernethy, A.P.; Nussbaum, N.C.; Zhi, J.; Curtis, M.D.; Tucker, M.; Lee, S.E.; Light, D.E.; Gossai, A.; Sorg, R.A.; et al. Characteristics of Real-World Metastatic Non-Small Cell Lung Cancer Patients Treated With Nivolumab and Pembrolizumab During the Year Following Approval. Oncologist 2018, 23, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, H.; Ninomiya, K.; Kenmotsu, H.; Morise, M.; Daga, H.; Goto, Y.; Kozuki, T.; Miura, S.; Sasaki, T.; Tamiya, A.; et al. The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV. Int. J. Clin. Oncol. 2019, 24, 731–770. [Google Scholar] [CrossRef] [PubMed]
- Statistical Data about Cancer. Available online: http://ganjoho.jp/reg_stat/statistics/dl/index.html (accessed on 19 June 2020).
- Ferrara, R.; Mezquita, L.; Auclin, E.; Chaput, N.; Besse, B. Immunosenescence and immunecheckpoint inhibitors in non-small cell lung cancer patients: Does age really matter? Cancer Treat. Rev. 2017, 60, 60–68. [Google Scholar] [CrossRef]
- Moreira, A.; Gross, S.; Kirchberger, M.C.; Erdmann, M.; Schuler, G.; Heinzerling, L. Senescence markers: Predictive for response to checkpoint inhibitors. Int. J. Cancer 2019, 144, 1147–1150. [Google Scholar] [CrossRef]
- Carmichael, J.A.; Wing-San Mak, D.; O’Brien, M. A Review of Recent Advances in the Treatment of Elderly and Poor Performance NSCLC. Cancers 2018, 10, 236. [Google Scholar] [CrossRef]
- Okamoto, I.; Nokihara, H.; Nomura, S.; Niho, S.; Sugawara, S.; Horinouchi, H.; Azuma, K.; Yoneshima, Y.; Murakami, H.; Hosomi, Y.; et al. Comparison of Carboplatin Plus Pemetrexed Followed by Maintenance Pemetrexed with Docetaxel Monotherapy in Elderly Patients with Advanced Nonsquamous Non-Small Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e196828. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab Versus Chemotherapy for Previously Untreated, PD-L1-expressing, Locally Advanced or Metastatic Non-Small-Cell Lung Cancer (KEYNOTE-042): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet 2019, 393, 1819–1830. [Google Scholar]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Gajra, A.; Jatoi, A. Non–small-cell lung cancer in elderly patients: A discussion of treatment options. J. Clin. Oncol. 2014, 32, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Bunn, P.A., Jr.; Dimou, A. Systemic Therapy for Elderly Patients with Advanced Non-Small-Cell Lung Cancers. J. Clin. Oncol. 2018, 36, 2571–2574. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, C.; Khozin, S.; Suzman, D.; Zhang, L.; Tang, S.; Wahby, S.; Goldberg, K.B.; Kim, G.; Pazdur, R.U.S. Food and Drug Administration Approval Summary: Atezolizumab for Metastatic Non-Small Cell Lung Cancer. Clin. Cancer Res. 2017, 23, 4534–4539. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Rudin, C.M.; Awad, M.M.; Navarro, A.; Gottfried, M.; Peters, S.; Csőszi, T.; Cheema, P.K.; Rodriguez-Abreu, D.; Wollner, M.; Yang, J.C.; et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Nosaki, K.; Saka, H.; Hosomi, Y.; Baas, P.; de Castro, G., Jr.; Reck, M.; Wu, Y.L.; Brahmer, J.R.; Felip, E.; Sawada, T.; et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer 2019, 135, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Ciuleanu, T.E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Marur, S.; Singh, H.; Mishra-Kalyani, P.; Larkins, E.; Keegan, P.; Sridhara, R.; Blumenthal, G.M.; Pazdur, R. FDA Analyses of Survival in Older Adults With Metastatic Non-Small Cell Lung Cancer in Controlled Trials of PD-1/PD-L1 Blocking Antibodies. Semin. Oncol. 2018, 45, 220–225. [Google Scholar] [CrossRef]
- Jotte, R.; Cappuzzo, F.; Vynnychenko, I.; Stroyakovskiy, D.; Rodríguez-Abreu, D.; Hussein, M.; Soo, R.; Conter, H.J.; Kozuki, T.; Huang, K.C.; et al. Atezolizumab in Combination with Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results from a Randomized Phase III Trial. J. Thorac Oncol. 2020. [Google Scholar] [CrossRef]
- Reck, M.; Mok, T.S.K.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir. Med. 2019, 7, 387–401. [Google Scholar] [CrossRef]
- Spigel, D.R.; McCleod, M.; Jotte, R.M.; Einhorn, L.; Horn, L.; Waterhouse, D.M.; Creelan, B.; Babu, S.; Leighl, N.B.; Chandler, J.C.; et al. Safety, Efficacy, and Patient-Reported Health-Related Quality of Life and Symptom Burden with Nivolumab in Patients with Advanced Non-Small Cell Lung Cancer, Including Patients Aged 70 Years or Older or with Poor Performance Status (CheckMate 153). J. Thorac. Oncol. 2019, 14, 1628–1639. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Ardizzoni, A.; Ciuleanu, T.; Cobo, M.; Laktionov, K.; Szilasi, M.; Califano, R.; Carcereny, E.; Griffiths, R.; Paz-Ares, L.; et al. CheckMate 171: A phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations. Eur. J. Cancer 2020, 127, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Grossi, F.; Crinò, L.; Logroscino, A.; Canova, S.; Delmonte, A.; Melotti, B.; Proto, C.; Gelibter, A.; Cappuzzo, F.; Turci, D.; et al. Use of nivolumab in elderly patients with advanced squamous non-small-cell lung cancer: Results from the Italian cohort of an expanded access programme. Eur. J. Cancer 2018, 100, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Grossi, F.; Genova, C.; Crinò, L.; Delmonte, A.; Turci, D.; Signorelli, D.; Passaro, A.; Soto Parra, H.; Catino, A.; Landi, L.; et al. Real-life results from the overall population and key subgroups within the Italian cohort of nivolumab expanded access program in non-squamous non-small cell lung cancer. Eur. J. Cancer 2019, 123, 72–80. [Google Scholar] [CrossRef]
- Galli, G.; De Toma, A.; Pagani, F.; Randon, G.; Trevisan, B.; Prelaj, A.; Ferrara, R.; Proto, C.; Signorelli, D.; Ganzinelli, M.; et al. Efficacy and safety of immunotherapy in elderly patients with non-small cell lung cancer. Lung Cancer 2019, 137, 38–42. [Google Scholar] [CrossRef]
- Youn, B.; Trikalinos, N.A.; Mor, V.; Wilson, I.B.; Dahabreh, I.J. Real-world use and survival outcomes of immune checkpoint inhibitors in older adults with non-small cell lung cancer. Cancer 2020, 126, 978–985. [Google Scholar] [CrossRef]
- Smit, H.J.M.; Aerts, J.; van den Heuvel, M.; Hiltermann, T.J.N.; Bahce, I.; Smit, E.F.; Dingemans, A.C.; Hendriks, L.E.; Stigt, J.A.; Schramel, F.M.N.H.; et al. Effects of checkpoint inhibitors in advanced non-small cell lung cancer at population level from the National Immunotherapy Registry. Lung Cancer 2020, 140, 107–112. [Google Scholar] [CrossRef]
- Yamaguchi, O.; Imai, H.; Minemura, H.; Suzuki, K.; Wasamoto, S.; Umeda, Y.; Osaki, T.; Kasahara, N.; Uchino, J.; Sugiyama, T.; et al. Efficacy and safety of immune checkpoint inhibitor monotherapy in pretreated elderly patients with non-small cell lung cancer. Cancer Chemother. Pharmacol. 2020, 85, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Mazzaschi, G.; Barbieri, F.; Passiglia, F.; Mazzoni, F.; Berardi, R.; Proto, C.; Cecere, F.L.; Pilotto, S.; Scotti, V.; et al. First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. Eur. J. Cancer 2020, 130, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Califano, R.; Gomes, F.; Ackermann, C.J.; Rafee, S.; Tsakonas, G.; Ekman, S. Immune checkpoint blockade for non-small cell lung cancer: What is the role in the special populations? Eur. J. Cancer 2020, 125, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.J.; Eguchi, M.; Perraillon, M.C. Factors associated with utilization of high cost agents for the treatment of metastatic non-small cell lung cancer. J. Natl. Cancer Inst. 2019. [Google Scholar] [CrossRef] [PubMed]
- Dranitsaris, G.; Zhu, X.; Adunlin, G.; Vincent, M.D. Cost effectiveness vs. affordability in the age of immuno-oncology cancer drugs. Expert Rev. Pharm. Outcomes Res. 2018, 18, 351–357. [Google Scholar] [CrossRef] [PubMed]
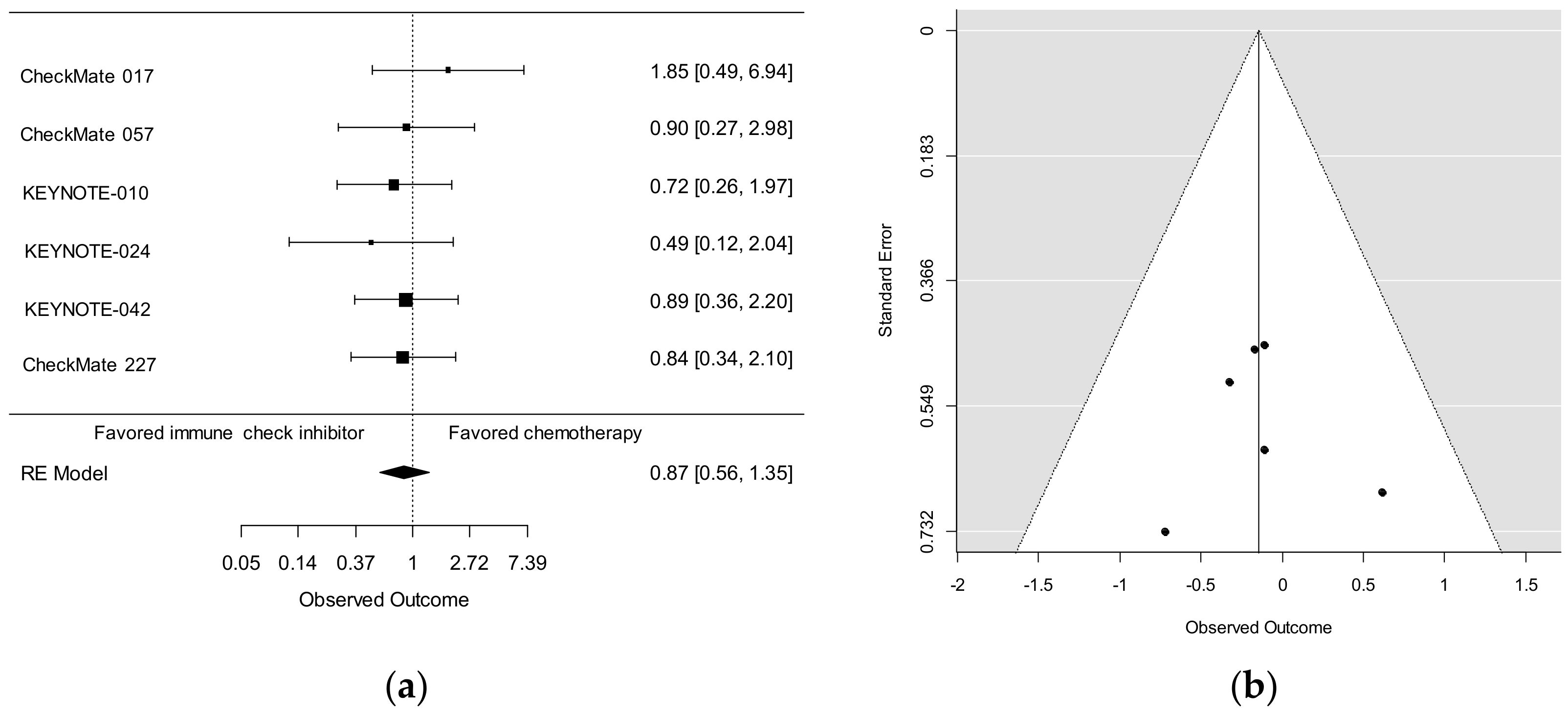
| 1st Author | Study | Line | Histology | Therapy | Median Age | Subgroup | Patients | Hazard Ratio |
|---|---|---|---|---|---|---|---|---|
| Year | Name | (PD-L1 status) | (Range) | Age | Number | (95% CI) | ||
| Brahmer | CM017 | 2nd | Sq | Nivolumab | 62 (39–85) | <65 | 152 | 0.52 (0.35–0.75) |
| 2015 | Docetaxel | 65 (42–84) | ≥65, <75 | 91 | 0.56 (0.34–0.91) | |||
| ≥75 | 29 | 1.85 (0.76–4.51) | ||||||
| Borghaei | CM057 | ≥2nd | Non-Sq | Nivolumab | 61 (37–84) | <65 | 339 | 0.81 (0.62–1.04) |
| 2015 | Docetaxel | 64 (21–85) | ≥65, <75 | 200 | 0.63 (0.45–0.89) | |||
| ≥75 | 43 | 0.90 (0.43–1.87) | ||||||
| Nosaki | KN010 | ≥2nd | NSCLC | Pembrolizumab | 63 (56–69)* | <75 | 943 | 0.64 (0.55–0.75) |
| 2019 | (PD-L1 ≥1%) | Docetaxel | 62 (56–69)* | ≥75 | 90 | 0.72 (0.43–1.21) | ||
| Nosaki | KN024 | 1st | NSCLC | Pembrolizumab | 64.5 (33–90) | <75 | 260 | 0.64 (0.42–0.97) |
| 2019 | (PD-L1 ≥50%) | Chemotherapy | 66.0 (38–85) | ≥75 | 45 | 0.49 (0.17–1.39) | ||
| Nosaki | KN042 | 1st | NSCLC | Pembrolizumab | 63 (57–69)* | <75 | 1145 | 0.79 (0.68–0.92) |
| 2019 | (PD-L1 ≥1%) | Chemotherapy | 63 (57–69)* | ≥75 | 129 | 0.89 (0.59–1.35) | ||
| Hellmann | CM227 | 1st | NSCLC | Nivolumab + Ipi | 64 (26–87) | <65 | 611 | 0.70 (0.58–0.85) |
| 2019 | Chemotherapy | 64 (29–87) | ≥65, <75 | 442 | 0.76 (0.61–0.95) | |||
| ≥75 | 113 | 0.84 (0.55–1.29) | ||||||
| Reck | IM150 | 1st | Non-Sq | ABCP | 63 (31–89) | <65 | 441 | 0.78 (0.60–1.00) |
| 2019 | BCP | 63 (31–90) | ≥65, <75 | 281 | 0.69 (0.49–0.96) | |||
| ≥75, <85 | 72 | 0.78 (0.50–1.76) | ||||||
| ≥85 | 6 | NR | ||||||
| Jotte | IM131 | 1st | Sq | A + CnP | 66 (23–83) | <65 | 326 | 0.89 (0.68–1.15) |
| 2020 | CnP | 65 (38–86) | ≥65, <75 | 279 | 0.84 (0.63–1.13) | |||
| ≥75, <85 | 77 | 0.74 (0.45–1.23) | ||||||
| ≥85 | 1 | NR |
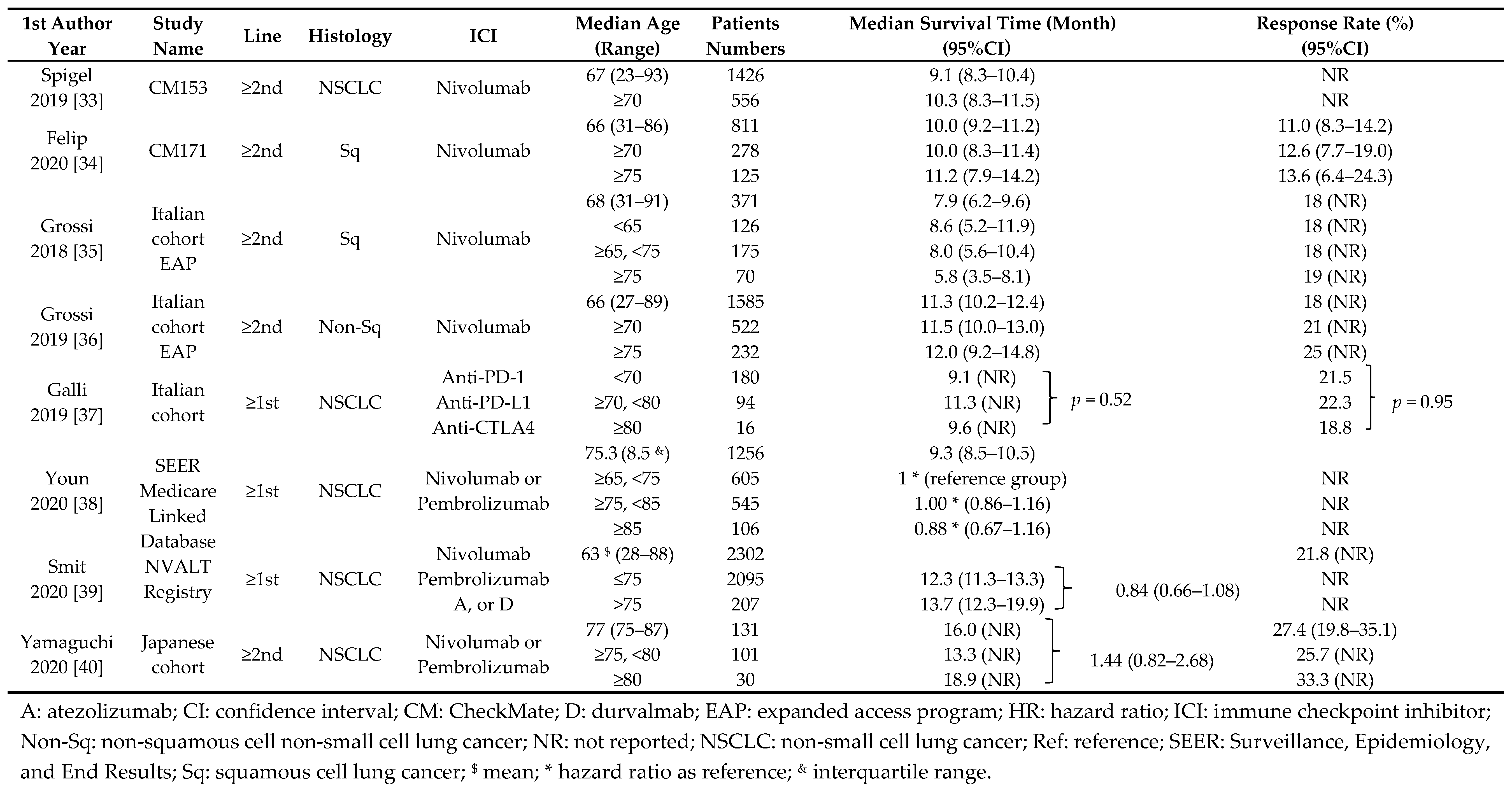
| 1st Author | Median Age | Patients | Treatment-Related Adverse Events (%) | |
|---|---|---|---|---|
| Year | (Range) | Numbers | Any Grade | Grade 3/4 |
| Grossi | 68 (31–91) | 371 | 29 | 6 |
| 2018 | <65 | 126 | 32 | 3 |
| ≥65, <75 | 175 | 28 | 9 | |
| 75 | 70 | 29 | 3 | |
| Grossi | 66 (27–89) | 1585 | 33 | 6 |
| 2019 | ≥70 | 522 | 33 | 7 |
| ≥75 | 232 | 34 | 7 | |
| Felip | 66 (31–86) | 811 | 58 | 14 |
| 2020 | ≥70 | 278 | 63 | 16 |
| ≥75 | 125 | 69 | 18 | |
| Spigel | 67 (23–93) | 1426 | 62 | 12 |
| 2019 | ≥70 | 556 | 64 | 14 |
| Yamaguchi | 77 (75–87) | 131 | 38* | NR |
| 2020 | ≥75, <80 | 101 | 37 * | NR |
| ≥80 | 30 | 41 * | NR | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takigawa, N.; Ochi, N.; Nakagawa, N.; Nagasaki, Y.; Taoka, M.; Ichiyama, N.; Mimura, A.; Nakanishi, H.; Kohara, H.; Yamane, H. Do Elderly Lung Cancer Patients Aged ≥75 Years Benefit from Immune Checkpoint Inhibitors? Cancers 2020, 12, 1995. https://doi.org/10.3390/cancers12071995
Takigawa N, Ochi N, Nakagawa N, Nagasaki Y, Taoka M, Ichiyama N, Mimura A, Nakanishi H, Kohara H, Yamane H. Do Elderly Lung Cancer Patients Aged ≥75 Years Benefit from Immune Checkpoint Inhibitors? Cancers. 2020; 12(7):1995. https://doi.org/10.3390/cancers12071995
Chicago/Turabian StyleTakigawa, Nagio, Nobuaki Ochi, Nozomu Nakagawa, Yasunari Nagasaki, Masataka Taoka, Naruhiko Ichiyama, Ayaka Mimura, Hidekazu Nakanishi, Hiroyuki Kohara, and Hiromichi Yamane. 2020. "Do Elderly Lung Cancer Patients Aged ≥75 Years Benefit from Immune Checkpoint Inhibitors?" Cancers 12, no. 7: 1995. https://doi.org/10.3390/cancers12071995
APA StyleTakigawa, N., Ochi, N., Nakagawa, N., Nagasaki, Y., Taoka, M., Ichiyama, N., Mimura, A., Nakanishi, H., Kohara, H., & Yamane, H. (2020). Do Elderly Lung Cancer Patients Aged ≥75 Years Benefit from Immune Checkpoint Inhibitors? Cancers, 12(7), 1995. https://doi.org/10.3390/cancers12071995






