Systemic Treatment Selection for Patients with Advanced Pancreatic Neuroendocrine Tumours (PanNETs)
Abstract
1. Introduction
2. Evidence Regarding the Use of Available Systemic Treatment Option
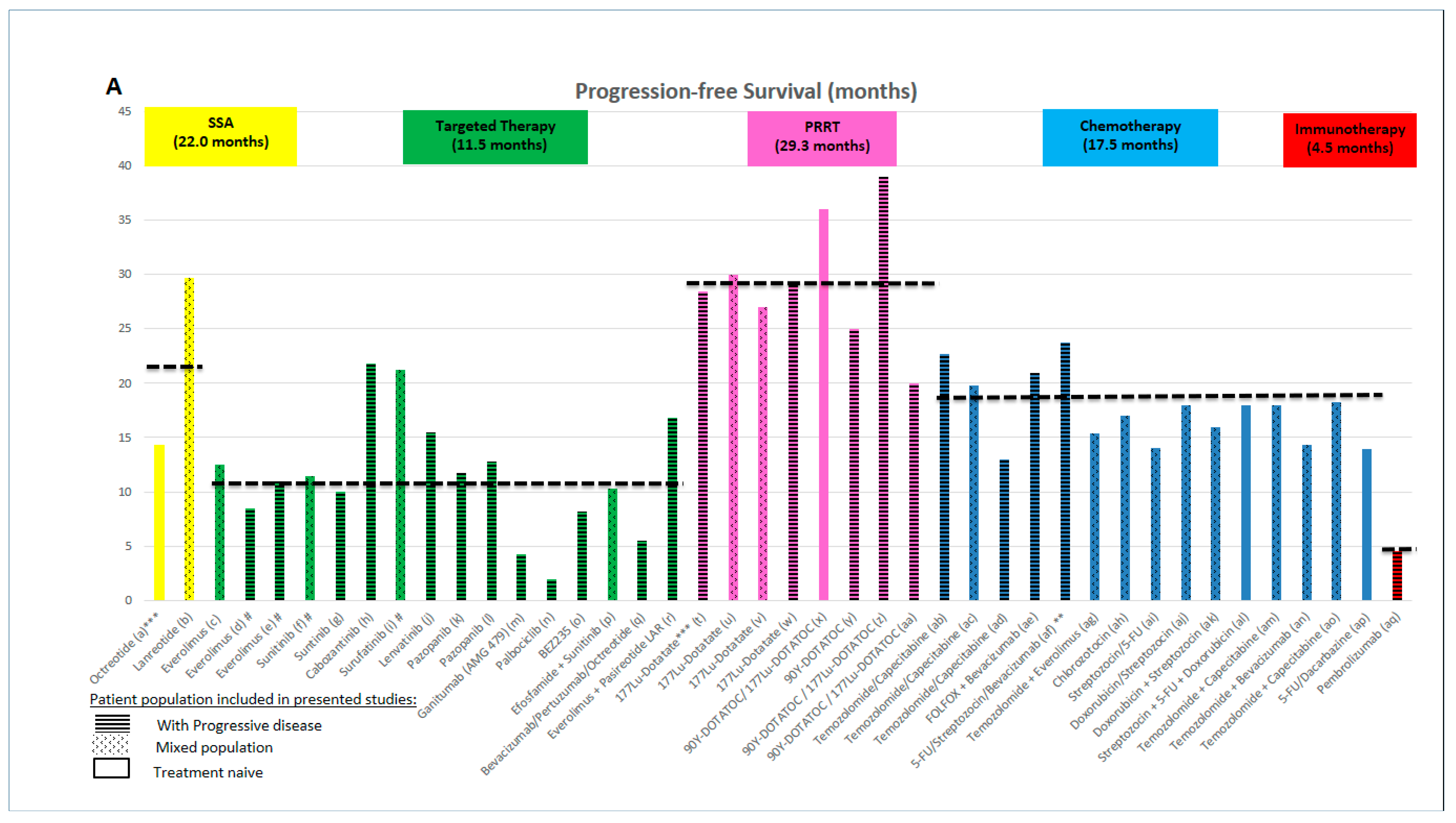
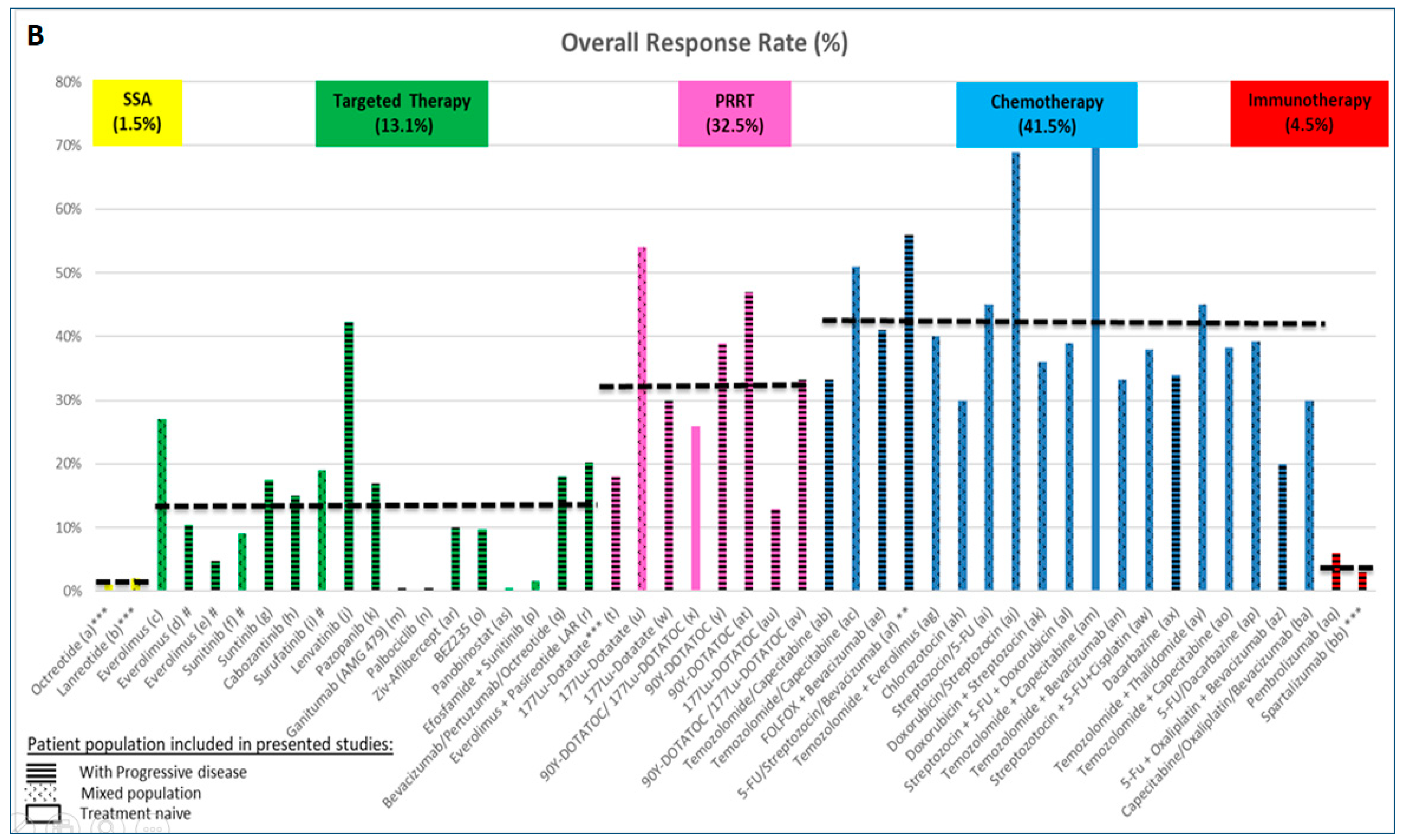
2.1. Evidence Supporting the Use of Somatostatin Analogues (SSAs)
2.2. Evidence Supporting the Use of Targeted Therapies
2.3. Evidence Supporting the Use of Peptide Receptor Radionuclide Therapy (PRRT)
2.4. Evidence Supporting the Use of Chemotherapy
2.5. Evidence Supporting the Use of Immunotherapy
3. Considerations for Treatment Selection
3.1. Building an Individualized Plan
- (1).
- Pathology: tumours with low Ki-67 or low-grade are usually associated with higher expression of SSTR, a more indolent disease course and poorer responses to chemotherapy, in comparison with tumours with high Ki-67 or higher-grade [117]. Low Ki-67 seems to be an independent predictor of better response to PRRT [118], although the observed benefit was present in both G1 (HR 0.24 (95%-CI 0.13–0.44)) and G2 (HR 0.15 (95%-CI 0.07–0.34)) patients with midgut NETs [36]. Similarly, the benefit from lanreotide was comparable in both groups of patients [G1 (HR 0.43 (95%-CI 0.25–0.74)) vs. G2 (Ki-67 up to 10%) (HR 0.45 (95%-CI 0.22–0.91)]. Patients with PanNETs with a Ki-67 >10% were not included in the CLARINET study [19]; thus, evidence to support the use of SSAs in this patient group is scarce [119]. The phase III study exploring the role of sunitinib in the treatment of patients with PanNETs suggested an impact on PFS [120], more marked in the group of patients with Ki-67 < 5% (HR 0.38 (95%-CI 0.16–0.92)) [vs Ki-67 > 5% (HR 0.63 (95%-CI 0.24–1.71))] [34]; however, it has to be mentioned that the limited number of patients with Ki-67 data available in this study (36 patients out of the 86 patients in each arm), limited the power of this subgroup analysis. Everolimus showed similar PFS benefit in both groups: G1 (HR 0.41 (95% CI 0.31–0.53), G2 (HR 0.21 (95% CI 0.11–0.42)) [23].
- (2).
- Functional vs. Non-functional disease: functionality is associated with well differentiated tumours, lower grade, low Ki-67, and higher expression of SSTRs. For symptomatic control, SSAs or PRRT are considered. However, the PFS impact reported in the CLARINET study in patients with non-functioning PanNETs supports the use of lanreotide, regardless of functioning status [19,60]. No subgroup analysis by functional status was performed in the NETTER-1 study [36]. The non-functional population may benefit more (HR 0.26 (0.13–0.54)) from sunitinib therapy compared to the functional cohort (HR 0.75 (0.30–1.84)); acknowledging the limited number of patients (and therefore limited power) in this latest group (86 vs. 46 patients, respectively) [26]. Such information is not available for everolimus [23].
- (3).
- Tumour load, liver involvement: large tumour burden as well as large liver tumour load and presence of extrahepatic metastases are negative prognostic features in patients with PanNETs [10]. However, benefits from SSAs and PRRT were seen in both the CLARINET and NETTER trials, with improvements in PFS, irrespective of tumour load or the presence of extrahepatic disease [19,36]. In addition, the benefit from sunitinib in the landmark trial was seen regardless of number of sites of disease (≤2 or ≥3) [121]. Despite this similar effect being reported in clinical trials, for patients with higher tumour load, the aim of therapy may be in favour of reducing tumour burden, for which strategies with higher objective response rates may be considered. These could be in the form of chemotherapy or PRRT, while SSAs and targeted therapies (reported to achieve lower response rate) may be reserved for patients with lower tumour burden. One of the exceptions may be lenvatinib, which leads to high response rates [72] and may change the management approach, if the results are confirmed in phase III studies. The type of distant metastases also represents the aggressiveness of the disease and it is also illustrated in a recently published analysis of SEER data from 2010 to 2014 [122]. Involvement of liver and bone is connected with worse prognosis followed by brain metastases (not very common in PanNETs). Scoring systems to assign groups with different survivals may also be useful in calculating the individual risk in different NENs [122]. Tumour growth rate (TGR) is another factor that is able to predict PFS and response to treatment [123].
- (4).
- Other individual factors may be considered when selecting therapy. A watch-and-wait approach could be a suitable strategy in selected patients with grade 1 PanNETs when comorbidities or other patient-related factors may play a significant role. In addition, selection of a specific targeted therapy (i.e., sunitinib vs. everolimus) may be supported, depending on specific comorbidities: favouring everolimus for patients with hypertension or in the scenario of an insulinoma and favouring sunitinib for patients with diabetes or past medical history of lung comorbidities. The presence of MGMT deficiency has been suggested as a predictive marker for temozolomide, even though its role as a predictive factor in patients with PanNETs is still to be clarified, and testing should not be employed in routine clinical practice [94,124]. Similarly, the predictive role of PD-L1 as a biomarker for immunotherapy treatment has been suggested in other disease groups [125,126,127]. However, despite 42% of patients with PanNETs being expected to express PD-L1 [128], the lack of responses in studies using immunotherapy [48,49,50] indicates that this biomarker may not be relevant in NENs.
- (5).
- Prior therapies administered may impact future therapy options; both sunitinib and everolimus showed efficacy in the setting of treatment-naïve and pre-treated (including SSAs or chemotherapy) patients [22,24,27,65]. In contrast, the CLARINET study only included treatment-naïve patients and therefore the use of SSAs for patients who had already progressed on previous treatments is unclear [19]. Unfortunately, use of PRRT frontline or as a subsequent line of therapy could not be definitely settle down based on the study by Brabander and colleagues, where no baseline characteristics for the studied PanNETs population was provided [80]. Chemotherapy has been used in first/subsequent lines of therapies in PanNET patients and benefit has been shown, regardless of prior treatment. This could be illustrated in the E2211 trial, which included patients with advanced PanNET who were randomized to receive Temozolomide or TemCap combination. The fact that almost half of patients in the E2211 trial received prior targeted therapies and that 53% of patients randomised to TemCap received concomitant SSAs supports the role of chemotherapy, regardless of the line of therapy [31].
3.2. Challenges in the Sequencing of Therapy
4. Ongoing Challenges
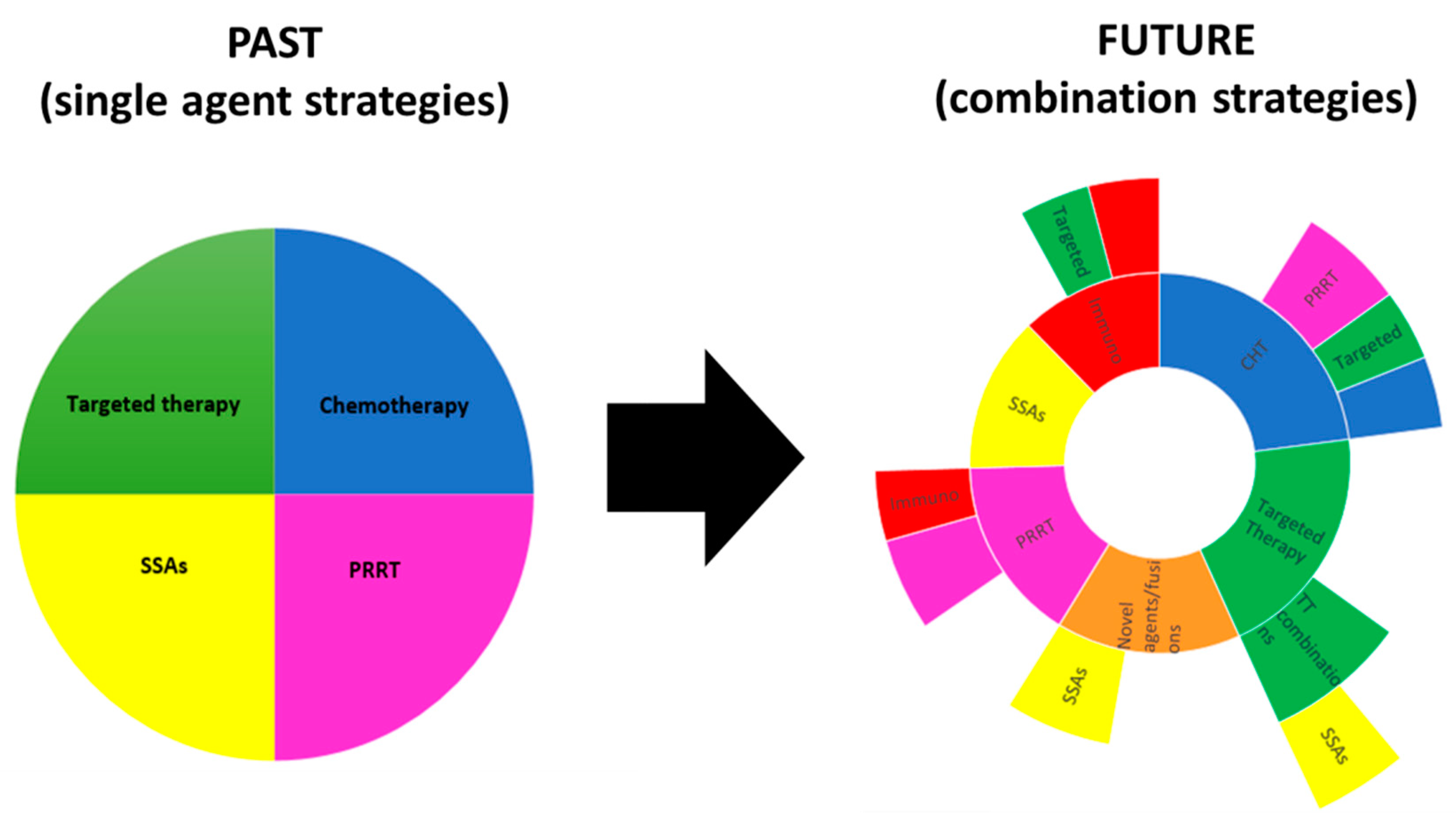
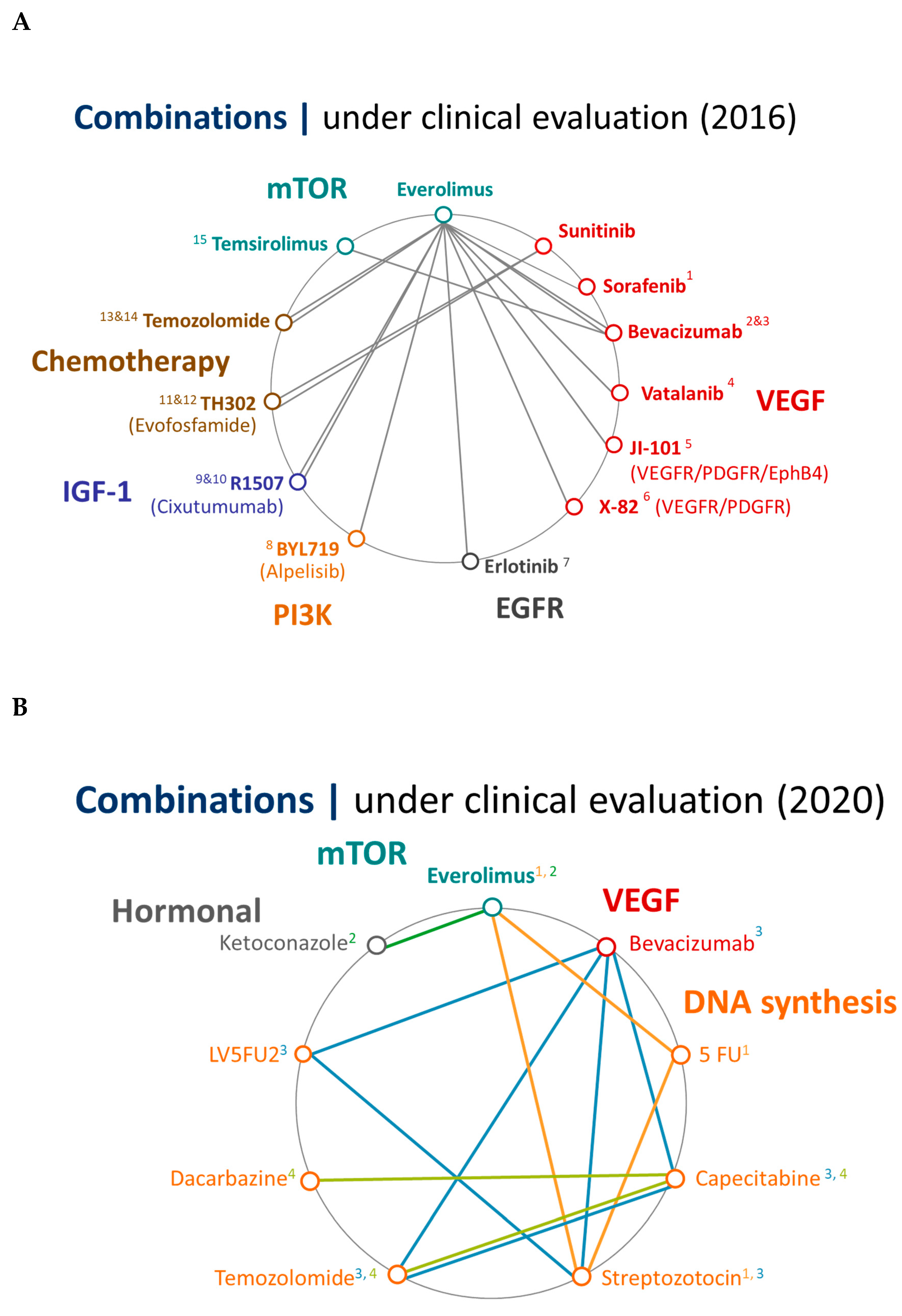
5. Future Perspectives
6. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Lawrence, B.; Gustafsson, B.I.; Chan, A.; Svejda, B.; Kidd, M.; Modlin, I.M. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol. Metab. Clin. N. Am. 2011, 40, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonero, R.; Capdevila, J.; Crespo-Herrero, G.; Díaz-Pérez, J.A.; Martínez Del Prado, M.P.; Alonso Orduña, V.; Sevilla-García, I.; Villabona-Artero, C.; Beguiristain-Gómez, A.; Llanos-Muñoz, M.; et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): Results from the National Cancer Registry of Spain (RGETNE). Ann. Oncol. 2010, 21, 1794–1803. [Google Scholar] [CrossRef]
- Kuiper, P.; Verspaget, H.W.; Van, H.S.; Overbeek, L.; Biemond, I.; Lamers, C.B. Pathological incidence of duodenopancreatic neuroendocrine tumors in the Netherlands: A Pathologisch Anatomisch Landelijk Geautomatiseerd Archief study. Pancreas 2010, 39, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Highlights in GEP-NETs from the 2016 NANETS Symposium—Hematology & Oncology. Available online: https://www.hematologyandoncology.net/supplements/highlights-in-gep-nets-from-the-2016-nanets-symposium/ (accessed on 18 July 2020).
- Hauso, O.; Gustafsson, B.I.; Kidd, M.; Waldum, H.L.; Drozdov, I.; Chan, A.K.C.; Modlin, I.M. Neuroendocrine tumor epidemiology: Contrasting Norway and North America. Cancer 2008, 113, 2655–2664. [Google Scholar] [CrossRef]
- Caldarella, A.; Crocetti, E.; Paci, E. Distribution, incidence, and prognosis in neuroendocrine tumors: A population based study from a cancer registry. Pathol. Oncol. Res. 2011, 17, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Lepage, C.; Bouvier, A.M.; Phelip, J.M.; Hatem, C.; Vernet, C.; Faivre, J. Incidence and management of malignant digestive endocrine tumours in a well defined French population. Gut 2004, 53, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Lye, K.D.; Kidd, M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003, 97, 934–959. [Google Scholar] [CrossRef]
- Panzuto, F.; Merola, E.; Rinzivillo, M.; Partelli, S.; Campana, D.; Iannicelli, E.; Pilozzi, E.; Mercantini, P.; Rossi, M.; Capurso, G.; et al. Advanced digestive neuroendocrine tumors: Metastatic pattern is an independent factor affecting clinical outcome. Pancreas 2014, 43, 212–218. [Google Scholar] [CrossRef]
- Sackstein, P.E.; O’Neil, D.S.; Neugut, A.I.; Chabot, J.; Fojo, T. Epidemiologic trends in neuroendocrine tumors: An examination of incidence rates and survival of specific patient subgroups over the past 20 years. Semin. Oncol. 2018, 45, 249–258. [Google Scholar] [CrossRef]
- Ito, T.; Lee, L.; Jensen, R.T. Treatment of symptomatic neuroendocrine tumor syndromes: Recent advances and controversies. Exp. Opin. Pharmacother. 2016, 17, 2191–2205. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Hong, S.-M.; Ro, J.Y. Recent updates on grading and classification of neuroendocrine tumors. Ann. Diagn. Pathol. 2017, 29, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.; Kim, K.W.; Kim, H.J.; Kim, D.W.; Kim, K.P.; Hong, S.-M.; Ryu, J.-S.; Tirumani, S.H.; Krajewski, K.; Ramaiya, N. What Is New in the 2017 World Health Organization Classification and 8th American Joint Committee on Cancer Staging System for Pancreatic Neuroendocrine Neoplasms? Korean J. Radiol. 2019, 20, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.-F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef]
- Caplin, M.E.; Phan, A.T.; Cadiot, G.; Wall, L.; Martinez, S.; Blumberg, J.; Ruszniewski, P. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: The CLARINET open-label extension study. Endocr. Relat. Cancer 2016, 23, 191–199. [Google Scholar] [CrossRef]
- Phan, A.T.; Yao, J.C.; Fogelman, D.R.; Hess, K.R.; Ng, C.S.; Bullock, S.A.; Malinowski, P.; Regan, E.; Kulke, M. A prospective, multi-institutional phase II study of GW786034 (pazopanib) and depot octreotide (sandostatin LAR) in advanced low-grade neuroendocrine carcinoma (LGNEC). J. Clin. Oncol. 2010, 28, 4001. [Google Scholar] [CrossRef]
- Wolin, E.M.; Pavel, M.; Cwikla, J.B.; Phan, A.T.; Raderer, M.; Sedlackova, E.; Cadiot, G.; Capdevila, J.; Wall, L.; Rindi, G.; et al. Final progression-free survival (PFS) analyses for lanreotide autogel/depot 120 mg in metastatic enteropancreatic neuroendocrine tumors (NETs): The CLARINET extension study. J. Clin. Oncol. 2017, 35, 4089. [Google Scholar] [CrossRef]
- Rinke, A.; Müller, H.-H.; Schade-Brittinger, C.; Klose, K.-J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.-F.; Bläker, M.; et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Müller, H.-H.; Arnold, R.; PROMID Study Group. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology 2017, 104, 26–32. [Google Scholar] [CrossRef]
- Yao, J.C.; Lombard-Bohas, C.; Baudin, E.; Kvols, L.K.; Rougier, P.; Ruszniewski, P.; Hoosen, S.; St. Peter, J.; Haas, T.; Lebwohl, D.; et al. Daily Oral Everolimus Activity in Patients with Metastatic Pancreatic Neuroendocrine Tumors After Failure of Cytotoxic Chemotherapy: A Phase II Trial. J. Clin. Oncol. 2010, 28, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.E.; et al. Everolimus for Advanced Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Lombard-Bohas, C.; Yao, J.C.; Hobday, T.; Van Cutsem, E.; Wolin, E.M.; Panneerselvam, A.; Stergiopoulos, S.; Shah, M.H.; Capdevila, J.; Pommier, R. Impact of Prior Chemotherapy Use on the Efficacy of Everolimus in Patients with Advanced Pancreatic Neuroendocrine Tumors: A Subgroup Analysis of the Phase III RADIANT-3 Trial. Pancreas 2015, 44, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Pavel, M.; Lombard-Bohas, C.; Van Cutsem, E.; Voi, M.; Brandt, U.; He, W.; Chen, D.; Capdevila, J.; de Vries, E.G.E.; et al. Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers from the Randomized, Phase III RADIANT-3 Study. J. Clin. Oncol. 2016, 34, 3906–3913. [Google Scholar] [CrossRef] [PubMed]
- Eric, R.; Laetitia, D.; Jean-Luc, R.; Yung-Jue, B.; Ivan, B.; Catherine, L.-B.; Juan, V.; Peter, M.; Denis, S.; Aaron, V.; et al. Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar]
- Faivre, S.; Niccoli, P.; Castellano, D.; Valle, J.W.; Hammel, P.; Raoul, J.-L.; Vinik, A.; Van Cutsem, E.; Bang, Y.-J.; Lee, S.-H.; et al. Sunitinib in Pancreatic Neuroendocrine Tumors: Updated Progression-Free Survival and Final Overall Survival from a Phase III Randomized Study. Ann. Oncol. 2016, mdw561. [Google Scholar] [CrossRef]
- Ducreux, M.; Dahan, L.; Smith, D.; O’Toole, D.; Lepère, C.; Dromain, C.; Vilgrain, V.; Baudin, E.; Lombard-Bohas, C.; Scoazec, J.-Y.; et al. Bevacizumab combined with 5-FU/streptozocin in patients with progressive metastatic well-differentiated pancreatic endocrine tumours (BETTER trial)—A phase II non-randomised trial. Eur. J. Cancer 2014, 50, 3098–3106. [Google Scholar] [CrossRef]
- Dilz, L.-M.; Denecke, T.; Steffen, I.G.; Prasad, V.; von Weikersthal, L.F.; Pape, U.-F.; Wiedenmann, B.; Pavel, M. Streptozocin/5-fluorouracil chemotherapy is associated with durable response in patients with advanced pancreatic neuroendocrine tumours. Eur. J. Cancer 2015, 51, 1253–1262. [Google Scholar] [CrossRef]
- Moertel, C.G.; Lefkopoulo, M.; Lipsitz, S.; Hahn, R.G.; Klaassen, D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N. Engl. J. Med. 1992, 326, 519–523. [Google Scholar] [CrossRef]
- Kunz, P.L.; Catalano, P.J.; Nimeiri, H.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Yao, J.C.; Kulke, M.H.; Hendifar, A.E.; Shanks, J.C.; et al. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: A trial of the ECOG-ACRIN Cancer Research Group (E2211). J. Clin. Oncol. 2018, 36, 4004. [Google Scholar] [CrossRef]
- de Mestier, L.; Walter, T.; Evrard, C.; de Boissieu, P.; Hentic, O.; Cros, J.; Tougeron, D.; Lombard-Bohas, C.; Rebours, V.; Hammel, P.; et al. Temozolomide alone or combined to capecitabine for the treatment of advanced pancreatic NET. Neuroendocrinology 2019. [Google Scholar] [CrossRef]
- Campana, D.; Walter, T.; Pusceddu, S.; Gelsomino, F.; Graillot, E.; Prinzi, N.; Spallanzani, A.; Fiorentino, M.; Barritault, M.; Dall’Olio, F.; et al. Correlation between MGMT promoter methylation and response to temozolomide-based therapy in neuroendocrine neoplasms: An observational retrospective multicenter study. Endocrine 2018, 60, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Fine, R.L.; Choi, J.; Nasir, A.; Coppola, D.; Chen, D.-T.; Helm, J.; Kvols, L. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011, 117, 268–275. [Google Scholar] [CrossRef]
- de Mestier, L.; Walter, T.; Brixi, H.; Evrard, C.; Legoux, J.-L.; de Boissieu, P.; Hentic, O.; Cros, J.; Hammel, P.; Tougeron, D.; et al. Comparison of Temozolomide-Capecitabine to 5-Fluorouracile-Dacarbazine in 247 Patients with Advanced Digestive Neuroendocrine Tumors Using Propensity Score Analyses. Neuroendocrinology 2019, 108, 343–353. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Wolin, E.M.; Chasen, B.A.; Kulke, M.H.; Bushnell, D.L.; Caplin, M.E.; Baum, R.P.; Hobday, T.J.; Hendifar, A.E.; Lopera Sierra, M.; et al. First update on overall survival, progression-free survival, and health-related time-to-deterioration quality of life from the NETTER-1 study: 177Lu-Dotatate vs. high dose octreotide in progressive midgut neuroendocrine tumors. J. Clin. Oncol. 2018, 36, 4099. [Google Scholar] [CrossRef]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; Feelders, R.A.; de Herder, W.W.; van Eijck, C.H.J.; Franssen, G.J.H.; Krenning, E.P.; Kwekkeboom, D.J. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTA0,Tyr3]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin. Cancer Res. 2017, 23, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Garske-Román, U.; Sandström, M.; Fröss Baron, K.; Lundin, L.; Hellman, P.; Welin, S.; Johansson, S.; Khan, T.; Lundqvist, H.; Eriksson, B.; et al. Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): Feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 970–988. [Google Scholar] [CrossRef]
- Sansovini, M.; Severi, S.; Ianniello, A.; Nicolini, S.; Fantini, L.; Mezzenga, E.; Ferroni, F.; Scarpi, E.; Monti, M.; Bongiovanni, A.; et al. Long-term follow-up and role of FDG PET in advanced pancreatic neuroendocrine patients treated with 177Lu-D OTATATE. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 490–499. [Google Scholar] [CrossRef]
- Dumont, R.A.; Seiler, D.; Marincek, N.; Brunner, P.; Radojewski, P.; Müller-Brand, J.; Maecke, H.R.; Briel, M.; Walter, M.A. Survival after somatostatin based radiopeptide therapy with 90Y-DOTATOC vs. 90Y-DOTATOC plus 177Lu-DOTATOC in metastasized gastrinoma. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 46–55. [Google Scholar]
- Bertani, E.; Fazio, N.; Radice, D.; Zardini, C.; Grana, C.; Bodei, L.; Funicelli, L.; Ferrari, C.; Spada, F.; Partelli, S.; et al. Resection of the Primary Tumor Followed by Peptide Receptor Radionuclide Therapy as Upfront Strategy for the Treatment of G1–G2 Pancreatic Neuroendocrine Tumors with Unresectable Liver Metastases. Ann. Surg. Oncol. 2016, 23, 981–989. [Google Scholar] [CrossRef]
- Imhof, A.; Brunner, P.; Marincek, N.; Briel, M.; Schindler, C.; Rasch, H.; Mäcke, H.R.; Rochlitz, C.; Müller-Brand, J.; Walter, M.A. Response, Survival, and Long-Term Toxicity After Therapy with the Radiolabeled Somatostatin Analogue [90 Y-DOTA]-TOC in Metastasized Neuroendocrine Cancers. J. Clin. Oncol. 2011, 29, 2416–2423. [Google Scholar] [CrossRef] [PubMed]
- Ramage, J.; Naraev, B.G.; Halfdanarson, T.R. Peptide receptor radionuclide therapy for patients with advanced pancreatic neuroendocrine tumors. Semin. Oncol. 2018, 45, 236–248. [Google Scholar] [CrossRef]
- Rogowski, W.; Wachuła, E.; Lewczuk, A.; Buscombe, J.R.; Seklecka, N.; Sankowski, A.; Ćwikła, J.B. Long-term efficacy of 90Y-DOTATATE in patients with nonresectable pancreatic and small bowel neuroendocrine neoplasms. Future Oncol. 2016, 12, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Hamiditabar, M.; Ali, M.; Roys, J.; Wolin, E.M.; OʼDorisio, T.M.; Ranganathan, D.; Tworowska, I.; Strosberg, J.R.; Delpassand, E.S. Peptide Receptor Radionuclide Therapy with 177Lu-Octreotate in Patients with Somatostatin Receptor Expressing Neuroendocrine Tumors: Six Years’ Assessment. Clin. Nucl. Med. 2017, 42, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.P.; Kulkarni, H.R.; Singh, A.; Kaemmerer, D.; Mueller, D.; Prasad, V.; Hommann, M.; Robiller, F.C.; Niepsch, K.; Franz, H.; et al. Results and adverse events of personalized peptide receptor radionuclide therapy with 90Yttrium and 177Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget 2018, 9, 16932–16950. [Google Scholar] [CrossRef] [PubMed]
- Hörsch, D.; Ezziddin, S.; Haug, A.; Gratz, K.F.; Dunkelmann, S.; Miederer, M.; Schreckenberger, M.; Krause, B.J.; Bengel, F.M.; Bartenstein, P.; et al. Effectiveness and side-effects of peptide receptor radionuclide therapy for neuroendocrine neoplasms in Germany: A multi-institutional registry study with prospective follow-up. Eur. J. Cancer 2016, 58, 41–51. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Mizuno, N.; Doi, T.; Grande, E.; Delord, J.-P.; Shapira-Frommer, R.; Bergsland, E.K.; Shah, M.H.; Fakih, M.; Takahashi, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results from the Phase 2 KEYNOTE-158 Study. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Rugo, H.S.; O’Neil, B.H.; Santoro, A.; Schellens, J.H.M.; Cohen, R.B.; Doi, T.; Ott, P.A.; Pishvaian, M.J.; Puzanov, I.; et al. 427OPembrolizumab for patients with PD-L1–positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Ann. Oncol. 2017, 28. [Google Scholar] [CrossRef]
- Yao, J.C.; Strosberg, J.; Fazio, N.; Pavel, M.E.; Ruszniewski, P.; Bergsland, E.; Li, D.; Tafuto, S.; Raj, N.; Campana, D.; et al. 1308OActivity & safety of spartalizumab (PDR001) in patients (pts) with advanced neuroendocrine tumors (NET) of pancreatic (Pan), gastrointestinal (GI), or thoracic (T) origin, & gastroenteropancreatic neuroendocrine carcinoma (GEP NEC) who have progressed on prior treatment (Tx). Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Stueven, A.K.; Kayser, A.; Wetz, C.; Amthauer, H.; Wree, A.; Tacke, F.; Wiedenmann, B.; Roderburg, C.; Jann, H. Somatostatin Analogues in the Treatment of Neuroendocrine Tumors: Past, Present and Future. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Papotti, M.; Bongiovanni, M.; Volante, M.; Allìa, E.; Landolfi, S.; Helboe, L.; Schindler, M.; Cole, S.L.; Bussolati, G. Expression of somatostatin receptor types 1-5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch. 2002, 440, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Toumpanakis, C.; Caplin, M.E. Update on the role of somatostatin analogs for the treatment of patients with gastroenteropancreatic neuroendocrine tumors. Semin. Oncol. 2013, 40, 56–68. [Google Scholar] [CrossRef]
- Kvols, L.K.; Oberg, K.E.; O’Dorisio, T.M.; Mohideen, P.; de Herder, W.W.; Arnold, R.; Hu, K.; Zhang, Y.; Hughes, G.; Anthony, L.; et al. Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: Results from a phase II study. Endocr. Relat. Cancer 2012, 19, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Guillermet-Guibert, J.; Lahlou, H.; Pyronnet, S.; Bousquet, C.; Susini, C. Endocrine tumours of the gastrointestinal tract. Somatostatin receptors as tools for diagnosis and therapy: Molecular aspects. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonero, R.; Sorbye, H.; Baudin, E.; Raymond, E.; Wiedenmann, B.; Niederle, B.; Sedlackova, E.; Toumpanakis, C.; Anlauf, M.; Cwikla, J.B.; et al. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology 2016, 103, 186–194. [Google Scholar]
- Klöppel, G.; Couvelard, A.; Perren, A.; Komminoth, P.; McNicol, A.-M.; Nilsson, O.; Scarpa, A.; Scoazec, J.-Y.; Wiedenmann, B.; Papotti, M.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Towards a Standardized Approach to the Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors and Their Prognostic Stratification. Neuroendocrinology 2009, 90, 162–166. [Google Scholar] [CrossRef]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Klöppel, G.; et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef]
- Jann, H.; Denecke, T.; Koch, M.; Pape, U.F.; Wiedenmann, B.; Pavel, M. Impact of octreotide long-acting release on tumour growth control as a first-line treatment in neuroendocrine tumours of pancreatic origin. Neuroendocrinology 2013, 98, 137–143. [Google Scholar] [CrossRef]
- Phan, A.T.; Dasari, A.; Liyanage, N.; Cox, D.; Lowenthal, S.P.; Wolin, E.M. Tumor response in the CLARINET study of lanreotide depot vs. placebo in patients with metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETs). J. Clin. Oncol. 2016, 34, 434. [Google Scholar] [CrossRef]
- Cives, M.; Kunz, P.L.; Morse, B.; Coppola, D.; Schell, M.J.; Campos, T.; Nguyen, P.T.; Nandoskar, P.; Khandelwal, V.; Strosberg, J.R. Phase II clinical trial of pasireotide long-acting repeatable in patients with metastatic neuroendocrine tumors. Endocr. Relat. Cancer 2015, 22, 1–9. [Google Scholar] [CrossRef]
- Kulke, M.H.; Ruszniewski, P.; Van Cutsem, E.; Lombard-Bohas, C.; Valle, J.W.; De Herder, W.W.; Pavel, M.; Degtyarev, E.; Brase, J.C.; Bubuteishvili-Pacaud, L.; et al. A randomized, open-label, phase 2 study of everolimus in combination with pasireotide LAR or everolimus alone in advanced, well-differentiated, progressive pancreatic neuroendocrine tumors: COOPERATE-2 trial. Ann. Oncol. 2017, 28, 1309–1315. [Google Scholar] [CrossRef]
- Salazar, R.; Garcia-Carbonero, R.; Libutti, S.K.; Hendifar, A.E.; Custodio, A.; Guimbaud, R.; Lombard-Bohas, C.; Ricci, S.; Klümpen, H.; Capdevila, J.; et al. Phase II Study of BEZ235 versus Everolimus in Patients with Mammalian Target of Rapamycin Inhibitor-Naïve Advanced Pancreatic Neuroendocrine Tumors. Oncologist 2018, 23, 766-e90. [Google Scholar] [CrossRef] [PubMed]
- Kulke, M.H.; Lenz, H.-J.; Meropol, N.J.; Posey, J.; Ryan, D.P.; Picus, J.; Bergsland, E.; Stuart, K.; Tye, L.; Huang, X.; et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J. Clin. Oncol. 2008, 26, 3403–3410. [Google Scholar] [CrossRef] [PubMed]
- Rinzivillo, M.; Fazio, N.; Pusceddu, S.; Spallanzani, A.; Ibrahim, T.; Campana, D.; Marconcini, R.; Partelli, S.; Badalamenti, G.; Brizzi, M.P.; et al. Sunitinib in patients with pre-treated pancreatic neuroendocrine tumors: A real-world study. Pancreatology 2018, 18, 198–203. [Google Scholar] [CrossRef]
- Sato, K.; Toyoshima, Y.; Moriyama, S.; Endo, Y.; Ito, T.; Ohki, E. Real-world use of sunitinib in Japanese patients with pancreatic neuroendocrine tumors: Results from a post-marketing surveillance study. Cancer Chemother. Pharmacol. 2019, 83, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Kulke, M.H.; Qin, S.; Yu, X.; Schenker, M.; Cubillo, A.; Lou, W.; Tomasek, J.; Thiis-Evensen, E.; Xu, J.-M.; et al. Efficacy and Safety of Sunitinib in Patients with Well-Differentiated Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2018, 107, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Ramage, J.K.; Punia, P.; Faluyi, O.; Frilling, A.; Meyer, T.; Saharan, R.; Valle, J.W. Observational Study to Assess Quality of Life in Patients with Pancreatic Neuroendocrine Tumors Receiving Treatment with Everolimus: The OBLIQUE Study (UK Phase IV Trial). Neuroendocrinology 2019, 108, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Novartis Pharmaceuticals Phase IV, Open-Label, Multi-Center, Single-Arm Study of the Safety and Efficacy of Everolimus (Afinitor) in Adult Patients with Local Advanced or Metastatic, Well Differentiated Progressive Pancreatic Neuroendocrine Tumors (pNET) in China. Available online: https://clinicaltrials.gov/ct2/show/NCT02842749 (accessed on 20 November 2019).
- Grande, E.; Capdevila, J.; Castellano, D.; Teulé, A.; Durán, I.; Fuster, J.; Sevilla, I.; Escudero, P.; Sastre, J.; García-Donas, J.; et al. Pazopanib in pretreated advanced neuroendocrine tumors: A phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE). Ann. Oncol. 2015, 26, 1987–1993. [Google Scholar] [CrossRef]
- Chan, J.A.; Faris, J.E.; Murphy, J.E.; Blaszkowsky, L.S.; Kwak, E.L.; McCleary, N.J.; Fuchs, C.S.; Meyerhardt, J.A.; Ng, K.; Zhu, A.X.; et al. Phase II trial of cabozantinib in patients with carcinoid and pancreatic neuroendocrine tumors (pNET). J. Clin. Oncol. 2017, 35, 228. [Google Scholar] [CrossRef]
- Capdevila, J.; Fazio, N.; Lopez Lopez, C.; Teule, A.; Valle, J.W.; Tafuto, S.; Custodio, A.B.; Reed, N.; Raderer, M.; Grande, E.; et al. Final results of the TALENT trial (GETNE1509): A prospective multicohort phase II study of lenvatinib in patients (pts) with G1/G2 advanced pancreatic (panNETs) and gastrointestinal (giNETs) neuroendocrine tumors (NETs). J. Clin. Oncol. 2019, 37, 4106. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Bai, C.; Xu, N.; Zhou, Z.; Li, Z.; Zhou, C.; Jia, R.; Lu, M.; Cheng, Y.; et al. Surufatinib in Advanced Well-Differentiated Neuroendocrine Tumors: A Multicenter, Single-Arm, Open-Label, Phase Ib/II Trial. Clin. Cancer Res. 2019, 25, 3486–3494. [Google Scholar] [CrossRef]
- Grande Pulido, E.; Teule, A.; Alonso-Gordoa, T.; Jiménez-Fonseca, P.; Benavent, M.; Capdevila, J.; Custodio, A.; Vera, R.; Munarriz, J.; La Casta-Muñoa, A.; et al. 429OA phase II trial of palbociclib in metastatic grade 1/2 pancreatic neuroendocrine tumors: The PALBONET study on behalf of the Spanish Taskforce Group of Neuroendocrine Tumors (GETNE). Ann. Oncol. 2017, 28. [Google Scholar] [CrossRef]
- Halperin, D.M.; Lee, J.J.; Ng, C.S.; Strosberg, J.R.; Estrella, J.S.; Dagohoy, C.G.; Dasari, A.; Yao, J.C. A Phase II Trial of Ziv-Aflibercept in Patients with Advanced Pancreatic Neuroendocrine Tumors. Pancreas 2019, 48, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Lubner, S.J.; Mulkerin, D.L.; Rajguru, S.; Carmichael, L.; Chenv, H.; Holen, K.D.; LoConte, N.K. A Phase II Trial of a Histone Deacetylase Inhibitor Panobinostat in Patients with Low-Grade Neuroendocrine Tumors. Oncologist 2016, 21, 785–786g. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Chan, J.A.; Ryan, D.P.; Meyerhardt, J.A.; Fuchs, C.S.; Abrams, T.; Regan, E.; Brady, R.; Weber, J.; Campos, T.; et al. A multi-institutional, phase II open-label study of ganitumab (AMG 479) in advanced carcinoid and pancreatic neuroendocrine tumors. Endocr.-Rel. Cancer 2013, 20, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Grande, E.; Castellano, D.E.; Custodio, A.B.; Garcia-Carbonero, R.; González, E.; López-López, C.; Munarriz, J.; Sevilla, I.; Teule, A.; Benavent Viñuales, M.; et al. A phase II trial to assess the activity and safety of the hypoxia-activated prodrug evofosfamide (TH-302) in combination with sunitinib in patients with disseminated grade 1 and 2 pancreatic neuroendocrine tumors (pNET) as a first-line approach: The GETNE-1408 trial. J. Clin. Oncol. 2016, 34, TPS479. [Google Scholar] [CrossRef]
- Novartis Announces Presentation of New Lutathera® NETTER-1 Data at ESMO Demonstrating Significant Improvement in PFS Regardless of Baseline Liver Tumor Burden. Available online: https://www.novartis.com/news/media-releases/novartis-announces-presentation-new-lutathera-netter-1-data-esmo-demonstrating-significant-improvement-pfs-regardless-baseline-liver-tumor-burden (accessed on 1 May 2020).
- EMA, E. Lutathera. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lutathera (accessed on 7 January 2020).
- Lamarca, A.; Valle, J.W. Looking Beyond Chemotherapy in Patients with Advanced, Well-differentiated, Pancreatic Neuroendocrine Tumors. J. Oncopathol. 2014, 11. [Google Scholar] [CrossRef]
- Lamarca, A.; Elliott, E.; Barriuso, J.; Backen, A.; McNamara, M.G.; Hubner, R.; Valle, J.W. Chemotherapy for advanced non-pancreatic well-differentiated neuroendocrine tumours of the gastrointestinal tract, a systematic review and meta-analysis: A lost cause? Cancer Treat. Rev. 2016, 44, 26–41. [Google Scholar]
- Delaunoit, T.; Ducreux, M.; Boige, V.; Dromain, C.; Sabourin, J.-C.; Duvillard, P.; Schlumberger, M.; de Baere, T.; Rougier, P.; Ruffie, P.; et al. The doxorubicin-streptozotocin combination for the treatment of advanced well-differentiated pancreatic endocrine carcinoma; a judicious option? Eur. J. Cancer 2004, 40, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Kouvaraki, M.A.; Ajani, J.A.; Hoff, P.; Wolff, R.; Evans, D.B.; Lozano, R.; Yao, J.C. Fluorouracil, Doxorubicin, and Streptozocin in the Treatment of Patients with Locally Advanced and Metastatic Pancreatic Endocrine Carcinomas. J. Clin. Oncol. 2004, 22, 4762–4771. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Strauss, S.J.; Sarker, D.; Gillmore, R.; Kirkwood, A.; Hackshaw, A.; Papadopoulou, A.; Bell, J.; Kayani, I.; Toumpanakis, C.; et al. Chemotherapy with 5-fluorouracil, cisplatin and streptozocin for neuroendocrine tumours. Br. J. Cancer 2010, 102, 1106–1112. [Google Scholar] [CrossRef]
- Ramanathan, R.K.; Cnaan, A.; Hahn, R.G.; Carbone, P.P.; Haller, D.G. Phase II trial of dacarbazine (DTIC) in advanced pancreatic islet cell carcinoma. Study of the Eastern Cooperative Oncology Group-E6282. Ann. Oncol. 2001, 12, 1139–1143. [Google Scholar] [CrossRef]
- Venook, A.P.; Ko, A.H.; Tempero, M.A.; Uy, J.; Weber, T.; Korn, M.; Bergsland, E.K. Phase II trial of FOLFOX plus bevacizumab in advanced, progressive neuroendocrine tumors. J. Clin. Oncol. 2008, 26, 15545. [Google Scholar] [CrossRef]
- Kunz, P.L.; Kuo, T.; Zahn, J.M.; Kaiser, H.L.; Norton, J.A.; Visser, B.C.; Longacre, T.A.; Ford, J.M.; Balise, R.R.; Fisher, G.A. A phase II study of capecitabine, oxaliplatin, and bevacizumab for metastatic or unresectable neuroendocrine tumors. J. Clin. Oncol. 2010, 28, 4104. [Google Scholar] [CrossRef]
- Kunz, P.L.; Balise, R.R.; Fehrenbacher, L.; Pan, M.; Venook, A.P.; Fisher, G.A.; Tempero, M.A.; Ko, A.H.; Korn, W.M.; Hwang, J.; et al. Oxaliplatin-Fluoropyrimidine Chemotherapy Plus Bevacizumab in Advanced Neuroendocrine Tumors: An Analysis of 2 Phase II Trials. Pancreas 2016, 45, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Kulke, M.H.; Stuart, K.; Enzinger, P.C.; Ryan, D.P.; Clark, J.W.; Muzikansky, A.; Vincitore, M.; Michelini, A.; Fuchs, C.S. Phase II Study of Temozolomide and Thalidomide in Patients with Metastatic Neuroendocrine Tumors. J. Clin. Oncol. 2006, 24, 401–406. [Google Scholar] [CrossRef]
- Chan, J.A.; Stuart, K.; Earle, C.C.; Clark, J.W.; Bhargava, P.; Miksad, R.; Blaszkowsky, L.; Enzinger, P.C.; Meyerhardt, J.A.; Zheng, H.; et al. Prospective Study of Bevacizumab Plus Temozolomide in Patients with Advanced Neuroendocrine Tumors. J. Clin. Oncol. 2012, 30, 2963–2968. [Google Scholar] [CrossRef]
- Chan, J.A.; Blaszkowsky, L.; Stuart, K.; Zhu, A.X.; Allen, J.; Wadlow, R.; Ryan, D.P.; Meyerhardt, J.; Gonzalez, M.; Regan, E.; et al. A prospective, phase 1/2 study of everolimus and temozolomide in patients with advanced pancreatic neuroendocrine tumor: Everolimus, Temozolomide in Pancreatic NET. Cancer 2013, 119, 3212–3218. [Google Scholar] [CrossRef] [PubMed]
- Fine, R.L.; Gulati, A.P.; Krantz, B.A.; Moss, R.A.; Schreibman, S.; Tsushima, D.A.; Mowatt, K.B.; Dinnen, R.D.; Mao, Y.; Stevens, P.D.; et al. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: The Pancreas Center at Columbia University experience. Cancer Chemother. Pharmacol. 2013, 71, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; van Brakel, B.; Vercherat, C.; Hervieu, V.; Forestier, J.; Chayvialle, J.-A.; Molin, Y.; Lombard-Bohas, C.; Joly, M.-O.; Scoazec, J.-Y. O6-Methylguanine-DNA methyltransferase status in neuroendocrine tumours: Prognostic relevance and association with response to alkylating agents. Br. J. Cancer 2015, 112, 523–531. [Google Scholar] [CrossRef]
- O6-Methylguanine-DNA Methyltransferase (MGMT) in Normal Tissues and Tumors: Enzyme Activity, Promoter Methylation and Immunohistochemistry—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0304419X11000382?via%3Dihub (accessed on 14 November 2019).
- Lamarca, A.; Barriuso, J.; McNamara, M.G.; Hubner, R.A.; Manoharan, P.; Mansoor, W.; Valle, J.W. Temozolomide-Capecitabine Chemotherapy for Neuroendocrine Neoplasms: The Dilemma of Treatment Duration. Neuroendocrinology 2020, 110, 155–157. [Google Scholar] [CrossRef]
- Oberg, K. Interferon in the management of neuroendocrine GEP-tumors: A review. Digestion 2000, 62 (Suppl. S1), 92–97. [Google Scholar] [CrossRef]
- Yao, J.C.; Guthrie, K.A.; Moran, C.; Strosberg, J.R.; Kulke, M.H.; Chan, J.A.; LoConte, N.; McWilliams, R.R.; Wolin, E.M.; Mattar, B.; et al. Phase III Prospective Randomized Comparison Trial of Depot Octreotide Plus Interferon Alfa-2b Versus Depot Octreotide Plus Bevacizumab in Patients with Advanced Carcinoid Tumors: SWOG S0518. J. Clin. Oncol. 2017, 35, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.; Jörg, A.-C.; Glatz, K.; Bubendorf, L.; Radojewski, P.; Umlauft, M.; Marincek, N.; Spanjol, P.-M.; Krause, T.; Dumont, R.A.; et al. The prognostic and predictive value of sstr2-immunohistochemistry and sstr2-targeted imaging in neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 468–475. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Jin, K.; Fang, C.; Lin, Y.; Xue, L.; Feng, S.; Zhou, Z.; Shao, C.; Chen, M.; et al. Somatostatin receptor expression indicates improved prognosis in gastroenteropancreatic neuroendocrine neoplasm, and octreotide long-acting release is effective and safe in Chinese patients with advanced gastroenteropancreatic neuroendocrine tumors. Oncol. Lett. 2017, 13, 1165–1174. [Google Scholar] [CrossRef]
- Nielsen, K.; Binderup, T.; Langer, S.W.; Kjaer, A.; Knigge, P.; Grøndahl, V.; Melchior, L.; Federspiel, B.; Knigge, U. P53, Somatostatin receptor 2a and Chromogranin A immunostaining as prognostic markers in high grade gastroenteropancreatic neuroendocrine neoplasms. BMC Cancer 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Phan, A.T.; Chang, D.Z.; Wolff, R.A.; Hess, K.; Gupta, S.; Jacobs, C.; Mares, J.E.; Landgraf, A.N.; Rashid, A.; et al. Efficacy of RAD001 (Everolimus) and Octreotide LAR in Advanced Low- to Intermediate-Grade Neuroendocrine Tumors: Results of a Phase II Study. J. Clin. Oncol. 2008, 26, 4311–4318. [Google Scholar] [CrossRef]
- Yao, J.C.; Oh, D.-Y.; Qian, J.; Park, Y.S.; Herbst, F.; Ridolfi, A.; Izquierdo, M.; Ito, T.; Jia, L.; Komoto, I.; et al. Everolimus for the treatment of advanced gastrointestinal or lung nonfunctional neuroendocrine tumors in East Asian patients: A subgroup analysis of the RADIANT-4 study. Oncol. Targets Ther. 2019, 12, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Grande, E.; Lopez, C.; Alonso-Gordoa, T.; Benavent, M.; Capdevila, J.; Teule, A.; Custodio, A.; Sevilla, I.; Gajate, P.; Molina-Cerrillo, J.; et al. The SUNEVO (GETNE-1408) trial to evaluate the activity and safety of thecombination of sunitinib with evofosfamide (TH-302) in patients with G1/G2 metastatic pancreatic neuroendocrine tumours (pNETs) naïve forsystemic treatment: A phase II study of the Spanish Task Force Group for Neuroendocrine and Endocrine Tumors (GETNE). J. Clin. Oncol. 2019, 37 (Suppl. S15), 4105. [Google Scholar]
- Bendell, J.C.; Zakari, A.; Lang, E.; Waterhouse, D.; Flora, D.; Alguire, K.; McCleod, M.; Peacock, N.; Ruehlman, P.; Lane, C.M.; et al. A Phase II Study of the Combination of Bevacizumab, Pertuzumab, and Octreotide LAR for Patients with Advanced Neuroendocrine Cancers. Cancer Investig. 2016, 34, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177 Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Pulvirenti, A.; Coppa, J. Neuroendocrine tumors metastatic to the liver: How to select patients for liver transplantation? J. Hepatol. 2007, 47, 460–466. [Google Scholar] [CrossRef]
- Mayo, S.C.; de Jong, M.C.; Pulitano, C.; Clary, B.M.; Reddy, S.K.; Gamblin, T.C.; Celinksi, S.A.; Kooby, D.A.; Staley, C.A.; Stokes, J.B.; et al. Surgical management of hepatic neuroendocrine tumor metastasis: Results from an international multi-institutional analysis. Ann. Surg. Oncol. 2010, 17, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhang, Y.; Cai, Z.; Lin, X. The impact of surgery in metastatic pancreatic neuroendocrine tumors: A competing risk analysis. Endocr. Connect. 2019, 8, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, D.J.; Turrini, O.; Vigano, L.; Russolillo, N.; Autret, A.; Moutardier, V.; Capussotti, L.; Le Treut, Y.-P.; Delpero, J.-R.; Hardwigsen, J. Surgical management of advanced pancreatic neuroendocrine tumors: Short-term and long-term results from an international multi-institutional study. Ann. Surg. Oncol. 2015, 22, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux, S.; Gonen, M.; Tang, L.; Klimstra, D.; Brennan, M.F.; D’Angelica, M.; Dematteo, R.; Allen, P.J.; Jarnagin, W.; Fong, Y. Synchronous resection of primary and liver metastases for neuroendocrine tumors. Ann. Surg. Oncol. 2012, 19, 4270–4277. [Google Scholar] [CrossRef]
- Maire, F.; Hammel, P.; Kianmanesh, R.; Hentic, O.; Couvelard, A.; Rebours, V.; Zappa, M.; Raymond, E.; Sauvanet, A.; Louvet, C.; et al. Is adjuvant therapy with streptozotocin and 5-fluorouracil useful after resection of liver metastases from digestive endocrine tumors? Surgery 2009, 145, 69–75. [Google Scholar] [CrossRef]
- Kunz, P.L.; Reidy-Lagunes, D.; Anthony, L.B.; Bertino, E.M.; Brendtro, K.; Chan, J.A.; Chen, H.; Jensen, R.T.; Kim, M.K.; Klimstra, D.S.; et al. Consensus Guidelines for the Management and Treatment of Neuroendocrine Tumors. Pancreas 2013, 42, 557–577. [Google Scholar] [CrossRef]
- NCCN Guidelines: Neuroendocrine and Adrenal Tumors. Available online: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf (accessed on 18 November 2019).
- Magi, L.; Mazzuca, F.; Rinzivillo, M.; Arrivi, G.; Pilozzi, E.; Prosperi, D.; Iannicelli, E.; Mercantini, P.; Rossi, M.; Pizzichini, P.; et al. Multidisciplinary Management of Neuroendocrine Neoplasia: A Real-World Experience from a Referral Center. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef]
- Tamagno, G.; Sheahan, K.; Skehan, S.; Geoghegan, J.; Fennelly, D.; Collins, C.; Maguire, D.; Traynor, O.; Brophy, D.; Cantwell, C.; et al. Initial Impact of a Systematic Multidisciplinary Approach on the Management of Patients with Gastroenteropancreatic Neuroendocrine Tumor. Endocrine 2013, 44. [Google Scholar] [CrossRef]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef]
- Ezziddin, S.; Attassi, M.; Yong-Hing, C.J.; Sabet, A.; Ahmadzadehfar, H.; Willinek, W.; Gruenwald, F.; Guhlke, S.; Biersack, H.-J. Factors predicting outcome of G1/2 GEP NET after PRRT with Lu177-octreotate. J. Clin. Oncol. 2012, 30, e14565. [Google Scholar] [CrossRef]
- Somatostatin Analogs for Pancreatic Neuroendocrine Tumors: Is There Any Benefit When Ki-67 Is ≥ 10%? Available online: https://www.enets.org/somatostatin-analogs-for-pancreatic-neuroendocrine-tumors-is-there-any-benefit-when-ki-67-is-10.html (accessed on 1 May 2020).
- Impact of Baseline Ki-67 index and Other Baseline Characteristics on Outcome in a Study of Sunitinib (SU) for the Treatment of Advanced, Progressive Pancreatic Neuroendocrine Tumor (NET). Available online: https://www.enets.org/impact-of-baseline-ki-67-index-and-other-baseline-characteristics-on-outcome-in-a-study-of-sunitinib-su-for-the-treatment-of-advanced-progressive-pancreatic-neuroendocrine-tumor-net.html (accessed on 18 November 2019).
- Blumenthal, G.M.; Cortazar, P.; Zhang, J.J.; Tang, S.; Sridhara, R.; Murgo, A.; Justice, R.; Pazdur, R. FDA Approval Summary: Sunitinib for the Treatment of Progressive Well-Differentiated Locally Advanced or Metastatic Pancreatic Neuroendocrine Tumors. Oncologist 2012, 17, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Trikalinos, N.A.; Tan, B.R.; Amin, M.; Liu, J.; Govindan, R.; Morgensztern, D. Effect of metastatic site on survival in patients with neuroendocrine neoplasms (NENs). An analysis of SEER data from 2010 to 2014. BMC Endocr. Disord. 2020, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Ronot, M.; Moalla, S.; Crona, J.; Opalinska, M.; Lopez, C.L.; Pezzutti, D.; Najran, P.; Carvalho, L.; Bezerra, R.O.F.; et al. Tumour Growth Rate as a validated early radiological biomarker able to reflect treatment-induced changes in Neuroendocrine Tumours; the GREPONET-2 study. Clin. Cancer Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Raj, N.; Klimstra, D.S.; Horvat, N.; Zhang, L.; Chou, J.F.; Capanu, M.; Basturk, O.; Do, R.K.G.; Allen, P.J.; Reidy-Lagunes, D. O6-Methylguanine DNA Methyltransferase Status Does Not Predict Response or Resistance to Alkylating Agents in Well-Differentiated Pancreatic Neuroendocrine Tumors. Pancreas 2017, 46, 758–763. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. Available online: https://www.nejm.org/doi/10.1056/NEJMoa1606774?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dwww.ncbi.nlm.nih.gov (accessed on 29 December 2019).
- Lopes, G.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.; Cho, B.C.; Castro, G.; Srimuninnimit, V.; Bondarenko, I.; Kubota, K.; Lubiniecki, G.M.; et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: Open-label, phase 3 KEYNOTE-042 study. J. Clin. Oncol. 2018, 36, LBA4. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- PD-L1 Is Expressed in a Subset of Pancreatic Neuroendocrine Tumors (pNET). Available online: https://www.enets.org/pd-l1-is-expressed-in-a-subset-of-pancreatic-neuroendocrine-tumors-pnet.html (accessed on 19 November 2019).
- Goldberg, R.M.; Rothenberg, M.L.; Cutsem, E.V.; Benson, A.B.; Blanke, C.D.; Diasio, R.B.; Grothey, A.; Lenz, H.-J.; Meropol, N.J.; Ramanathan, R.K.; et al. The Continuum of Care: A Paradigm for the Management of Metastatic Colorectal Cancer. Oncologist 2007, 12, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Perysinakis, I.; Aggeli, C.; Kaltsas, G.; Zografos, G.N. Neoadjuvant therapy for advanced pancreatic neuroendocrine tumors: An emerging treatment modality? Hormones 2016, 15, 15–22. [Google Scholar] [CrossRef]
- da Silva, T.N.; van Velthuysen, M.L.F.; van Eijck, C.H.J.; Teunissen, J.J.; Hofland, J.; de Herder, W.W. Successful neoadjuvant peptide receptor radionuclide therapy for an inoperable pancreatic neuroendocrine tumour. Endocrinol Diabetes Metab. Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Sowa-Staszczak, A.; Pach, D.; Chrzan, R.; Trofimiuk, M.; Stefańska, A.; Tomaszuk, M.; Kołodziej, M.; Mikołajczak, R.; Pawlak, D.; Hubalewska-Dydejczyk, A. Peptide receptor radionuclide therapy as a potential tool for neoadjuvant therapy in patients with inoperable neuroendocrine tumours (NETs). Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1669–1674. [Google Scholar] [CrossRef]
- Partelli, S.; Bertani, E.; Bartolomei, M.; Perali, C.; Muffatti, F.; Grana, C.M.; Schiavo Lena, M.; Doglioni, C.; Crippa, S.; Fazio, N.; et al. Peptide receptor radionuclide therapy as neoadjuvant therapy for resectable or potentially resectable pancreatic neuroendocrine neoplasms. Surgery 2018, 163, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Muffatti, F.; Partelli, S.; Andreasi, V.; Piccioli, A.; Bertani, E.; Bartolomei, M.; Grana, M.C.; Zamboni, G.; Doglioni, C.; Fazio, N.; et al. Outcome of Surgical Resection after Neoadjuvant Peptide Receptor Radionuclide Therapy (PRRT) for Pancreatic Neuroendocrine Neoplasms: A case-matched analysis. Pancreatology 2017, 17, S83. [Google Scholar] [CrossRef]
- Efficacy and Safety of Everolimus and (STZ-5FU) Given One Upfront the Other Upon Progression in Advanced pNET—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02246127 (accessed on 18 November 2019).
- COMPETE Trial—Peptide Receptor Radionuclide Therapy (PRRT) with 177Lu-Edotreotide vs. Everolimus in Progressive GEP-NET | OncologyPRO. Available online: https://oncologypro.esmo.org/Meeting-Resources/ESMO-2018-Congress/COMPETE-trial-Peptide-Receptor-Radionuclide-Therapy-PRRT-with-177Lu-Edotreotide-vs.-Everolimus-in-progressive-GEP-NET (accessed on 29 December 2019).
- Yordanova, A.; Wicharz, M.M.; Mayer, K.; Brossart, P.; Gonzalez-Carmona, M.A.; Strassburg, C.P.; Fimmers, R.; Essler, M.; Ahmadzadehfar, H. The Role of Adding Somatostatin Analogues to Peptide Receptor Radionuclide Therapy as a Combination and Maintenance Therapy. Clin. Cancer Res. 2018, 24, 4672–4679. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, C.; Lasfargues, C.; Chalabi, M.; Billah, S.M.; Susini, C.; Vezzosi, D.; Caron, P.; Pyronnet, S. Clinical review: Current scientific rationale for the use of somatostatin analogs and mTOR inhibitors in neuroendocrine tumor therapy. J. Clin. Endocrinol. Metab. 2012, 97, 727–737. [Google Scholar] [CrossRef]
- Capdevila, J.; Teulé, A.; Barriuso, J.; Castellano, D.; Lopez, C.; Manzano, J.L.; Alonso, V.; García-Carbonero, R.; Dotor, E.; Matos, I.; et al. Phase II Study of Everolimus and Octreotide LAR in Patients with Nonfunctioning Gastrointestinal Neuroendocrine Tumors: The GETNE1003_EVERLAR Study. Oncologist 2019, 24, 38–46. [Google Scholar] [CrossRef]
- Koumarianou, A.; Antoniou, S.; Kanakis, G.; Economopoulos, N.; Rontogianni, D.; Ntavatzikos, A.; Tsavaris, N.; Pectasides, D.; Dimitriadis, G.; Kaltsas, G. Combination treatment with metronomic temozolomide, bevacizumab and long-acting octreotide for malignant neuroendocrine tumours. Endocr. Relat. Cancer 2012, 19, L1–L4. [Google Scholar] [CrossRef]
- Berruti, A.; Fazio, N.; Ferrero, A.; Brizzi, M.P.; Volante, M.; Nobili, E.; Tozzi, L.; Bodei, L.; Torta, M.; D’Avolio, A.; et al. Bevacizumab plus octreotide and metronomic capecitabine in patients with metastatic well-to-moderately differentiated neuroendocrine tumors: The xelbevoct study. BMC Cancer 2014, 14, 184. [Google Scholar] [CrossRef]
- Valle, J.W.; Eatock, M.; Clueit, B.; Gabriel, Z.; Ferdinand, R.; Mitchell, S. A systematic review of non-surgical treatments for pancreatic neuroendocrine tumours. Cancer Treat. Rev. 2014, 40, 376–389. [Google Scholar] [CrossRef]
- Merino-Casabiel, X.; Aller, J.; Arbizu, J.; García-Figueiras, R.; González, C.; Grande, E.; Jiménez-Fonseca, P.; Sevilla, M.I.; Capdevila, J. Consensus document on the progression and treatment response criteria in gastroenteropancreatic neuroendocrine tumors. Clin. Transl. Oncol. 2018, 20, 1522–1528. [Google Scholar] [CrossRef]
- Sharma, R.; Wang, W.M.; Yusuf, S.; Evans, J.; Ramaswami, R.; Wernig, F.; Frilling, A.; Mauri, F.; Al-Nahhas, A.; Aboagye, E.O.; et al. 68Ga-DOTATATE PET/CT parameters predict response to peptide receptor radionuclide therapy in neuroendocrine tumours. Radiother. Oncol. 2019, 141, 108–115. [Google Scholar] [CrossRef]
- Lamarca, A.; Barriuso, J.; Kulke, M.; Borbath, I.; Lenz, H.-J.; Raoul, J.L.; Meropol, N.J.; Lombard-Bohas, C.; Posey, J.; Faivre, S.; et al. Determination of an optimal response cut-off able to predict progression-free survival in patients with well-differentiated advanced pancreatic neuroendocrine tumours treated with sunitinib: An alternative to the current RECIST-defined response. Br. J. Cancer 2018, 118, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Dromain, C.; Pavel, M.E.; Ruszniewski, P.; Langley, A.; Massien, C.; Baudin, E.; Caplin, M.E. Tumor growth rate as a metric of progression, response, and prognosis in pancreatic and intestinal neuroendocrine tumors. BMC Cancer 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Crona, J.; Ronot, M.; Opalinska, M.; Lopez Lopez, C.; Pezzutti, D.; Najran, P.; Carvhalo, L.; Franca Bezerra, R.O.; Borg, P.; et al. Value of Tumor Growth Rate (TGR) as an Early Biomarker Predictor of Patients’ Outcome in Neuroendocrine Tumors (NET)-The GREPONET Study. Oncologist 2019, 24, e1082–e1090. [Google Scholar] [CrossRef]
- Bello, C.; Deprimo, S.E.; Friece, C.; Smeraglia, J.; Sherman, L.; Tye, L.; Baum, C.; Meropol, N.J.; Lenz, H.; Kulke, M.H. Analysis of circulating biomarkers of sunitinib malate in patients with unresectable neuroendocrine tumors (NET): VEGF, IL-8, and soluble VEGF receptors 2 and 3. J. Clin. Oncol. 2006, 24, 4045. [Google Scholar] [CrossRef]
- Herrera-Martínez, A.D.; Hofland, L.J.; Moreno, M.A.G.; Castaño, J.P.; de Herder, W.W.; Feelders, R.A. Neuroendocrine neoplasms: Current and potential diagnostic, predictive and prognostic markers. Endocr.-Relat. Cancer 2019, 26, R157–R179. [Google Scholar] [CrossRef] [PubMed]
- Hilfenhaus, G.; Göhrig, A.; Pape, U.-F.; Neumann, T.; Jann, H.; Zdunek, D.; Hess, G.; Stassen, J.M.; Wiedenmann, B.; Detjen, K.; et al. Placental growth factor supports neuroendocrine tumor growth and predicts disease prognosis in patients. Endocr. Relat. Cancer 2013, 20, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Horn, M.; Magee, G.; Hodges, K.; Evers, M.; Arnold, S.; Anthony, L. Immune checkpoint inhibitors in neuroendocrine tumors: A single institution experience with review of literature. Oncotarget 2017, 9, 8801–8809. [Google Scholar] [CrossRef][Green Version]
- Pentheroudakis, G.; Fotopoulos, G.; Gousia, A.; Bobos, M.; Chrysafi, S.; Fountzilas, G.; Pavlidis, N. Activation Status and Prognostic Significance of the Wnt/B Catenin and Hedgehog/Smoothened Signalling Pathways in Patients with Cancer of Unknown Primary (Cup): A Translational Research Study of the Hellenic Cooperative Oncology Group (Hecog). In Proceedings of the Abstract Book of the 39th ESMO Congress (ESMO 2014), Madrid, Spain, 26–30 September 2014; European Society for Medical Oncology: Lugano, Switzerland, 2014; Volume 1138PD, p. iv396. [Google Scholar]
- Mujica-Mota, R.; Varley-Campbell, J.; Tikhonova, I.; Cooper, C.; Griffin, E.; Haasova, M.; Peters, J.; Lucherini, S.; Talens-Bou, J.; Long, L.; et al. Everolimus, lutetium-177 DOTATATE and sunitinib for advanced, unresectable or metastatic neuroendocrine tumours with disease progression: A systematic review and cost-effectiveness analysis. Health Technol. Assess. 2018, 22, 1–326. [Google Scholar] [CrossRef] [PubMed]
- Hirmas, N.; Jadaan, R.; Al-Ibraheem, A. Peptide Receptor Radionuclide Therapy and the Treatment of Gastroentero-pancreatic Neuroendocrine Tumors: Current Findings and Future Perspectives. Nucl. Med. Mol. Imaging 2018, 52, 190–199. [Google Scholar] [CrossRef]
- Study to Evaluate the Safety and Preliminary Efficacy of 177Lu-OPS201 in NETs—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02592707 (accessed on 29 December 2019).
- Huizing, D.M.V.; de Wit-van der Veen, B.J.; Verheij, M.; Stokkel, M.P.M. Dosimetry methods and clinical applications in peptide receptor radionuclide therapy for neuroendocrine tumours: A literature review. EJNMMI Res. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.; Hayward, J.; McLeod, C.; Chen, H.X.; Sharon, E.; Mayerson, E.; Ryan, C.W.; et al. SWOG 1609 (DART): A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors. J. Clin. Oncol. 2019, 37, TPS2658. [Google Scholar] [CrossRef]
- Lee, S.-H.; Chu, S.Y.; Rashid, R.; Phung, S.; Leung, I.W.; Muchhal, U.S.; Moore, G.L.; Bernett, M.J.; Schubbert, S.; Ardila, C.; et al. Abstract 3633: Anti-SSTR2 × anti-CD3 bispecific antibody induces potent killing of human tumor cells in vitro and in mice, and stimulates target-dependent T cell activation in monkeys: A potential immunotherapy for neuroendocrine tumors. Cancer Res. 2017, 77, 3633. [Google Scholar] [CrossRef]


| Tumour Features | G1 NET | G2 NET | G3 NET | NEC * |
|---|---|---|---|---|
| Ki-67 | <3 | 3–20 | >20 | >20 |
| Mitosis | <2 | 2–20 | >20 | >20 |
| Differentiation | Well differentiated | Well differentiated | Well differentiated | Poorly differentiated |
| Approved Treatments | EMA | FDA |
|---|---|---|
| Sandostatin LAR | V | V |
| Lanreotide Autogel | V | V |
| Pasireotide LAR | - | - |
| Sunitinib | V | V |
| Everolimus | V | V |
| Pazopanib | - | - |
| Surufatinib | - | - |
| Cabozantinib | - | - |
| Lenvatinib | - | - |
| Efosfamide +/− Sunitinib | - | - |
| Palbociclib | - | - |
| Ziv-Aflibercept | - | - |
| Bevacizumab +/− Pertuzumab | - | - |
| BEZ235 | - | - |
| Panobinostat | - | - |
| Ganitumab | - | - |
| 177Lu-Dotatate | V | V |
| 90Y-DOTATOC | - | - |
| 90Y-DOTATOC plus 177Lu-DOTATOC | - | - |
| 177Lu-Oxodotreotide | - | - |
| Temozolomide | V | V |
| Capecitabine | V | V |
| Streptozocine | V | V |
| Dacarbazine | V | V |
| 5-Fu | V | V |
| Oxaliplatin | V | V |
| Pembrolizumab | - | - |
| Spartalizumab | - | - |
| Interferon alpha 2b | V | V |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Megdanova-Chipeva, V.G.; Lamarca, A.; Backen, A.; McNamara, M.G.; Barriuso, J.; Sergieva, S.; Gocheva, L.; Mansoor, W.; Manoharan, P.; Valle, J.W. Systemic Treatment Selection for Patients with Advanced Pancreatic Neuroendocrine Tumours (PanNETs). Cancers 2020, 12, 1988. https://doi.org/10.3390/cancers12071988
Megdanova-Chipeva VG, Lamarca A, Backen A, McNamara MG, Barriuso J, Sergieva S, Gocheva L, Mansoor W, Manoharan P, Valle JW. Systemic Treatment Selection for Patients with Advanced Pancreatic Neuroendocrine Tumours (PanNETs). Cancers. 2020; 12(7):1988. https://doi.org/10.3390/cancers12071988
Chicago/Turabian StyleMegdanova-Chipeva, Vera G., Angela Lamarca, Alison Backen, Mairéad G. McNamara, Jorge Barriuso, Sonia Sergieva, Lilia Gocheva, Was Mansoor, Prakash Manoharan, and Juan W. Valle. 2020. "Systemic Treatment Selection for Patients with Advanced Pancreatic Neuroendocrine Tumours (PanNETs)" Cancers 12, no. 7: 1988. https://doi.org/10.3390/cancers12071988
APA StyleMegdanova-Chipeva, V. G., Lamarca, A., Backen, A., McNamara, M. G., Barriuso, J., Sergieva, S., Gocheva, L., Mansoor, W., Manoharan, P., & Valle, J. W. (2020). Systemic Treatment Selection for Patients with Advanced Pancreatic Neuroendocrine Tumours (PanNETs). Cancers, 12(7), 1988. https://doi.org/10.3390/cancers12071988





