Abstract
Tumor-associated macrophages (TAMs) represent the most abundant innate immune cells in tumors. TAMs, exhibiting anti-inflammatory phenotype, are key players in cancer progression, metastasis and resistance to therapy. A high TAM infiltration is generally associated with poor prognosis, but macrophages are highly plastic cells that can adopt either proinflammatory/antitumor or anti-inflammatory/protumor features in response to tumor microenvironment stimuli. In the context of cancer therapy, many anticancer therapeutics, apart from their direct effect on tumor cells, display different effects on TAM activation status and density. In this review, we aim to evaluate the indirect effects of anticancer therapies in the modulation of TAM phenotypes and pro/antitumor activity.
1. Introduction
TAMs are key players in cancers influencing progression, metastasis and tumor recurrence. TAMs originate mainly from circulating precursor monocytes, however, resident macrophages can later develop in a tumor [1,2]. Specific signals by chemokines, i.e., CCL2 and colony stimulating factors (CSF)-1, cytokines and components of complement recall monocytes to tumor sites [3].
In solid tumors, macrophages represent the main immune population constituting up to 50% of the tumor mass. Macrophage plasticity allows these innate immune cells to adopt their well-known M1–M2 polarization axis. TAMs usually display a protumor/anti-inflammatory phenotype associated with the M2 profile, whereas the antitumor/proinflammatory function is associated with the M1 phenotype. TAMs and M2 macrophages sustain tumor growth and progression, inhibiting immune-stimulatory signals. TAM infiltration in tumor has been associated with poor prognosis [3]. Numerous investigations suggest TAMs as an interesting therapeutic target since strategies aiming to deplete TAM, inhibit their recruitment and influence their polarization status might be employed to promote their antitumor effects. Additionally, TAMs are mainly responsible for resistance to well-known antitumor treatments like chemotherapy and radiotherapy, indeed they limit the efficacy of immunotherapy, i.e., anti-PD1 treatment [3,4,5]. However, depending on the tumor type and treatment adopted it might be possible to identify novel approaches, i.e., combinatory therapies also taking advantage of more recent treatments as those based on the use of oncolytic viruses (OV) [6,7,8,9]. In this review, we will highlight TAMs as therapeutic target and their modulation by anticancer therapies.
2. Classically Activated vs. Alternatively Activated Macrophages
2.1. Proinflammatory/Antitumor M1 TAMs
Classically activated macrophages (M1) are crucial for host defense and cancer cell killing. M1 TAMs are stimulated by toll-like receptor (TLR) ligands, microbial substrates such as lipopolysaccharide (LPS) and Th1 cytokines. Once activated, M1 macrophages secrete proinflammatory cytokines and express markers typically used to identify the M1 phenotype [10] (Figure 1).
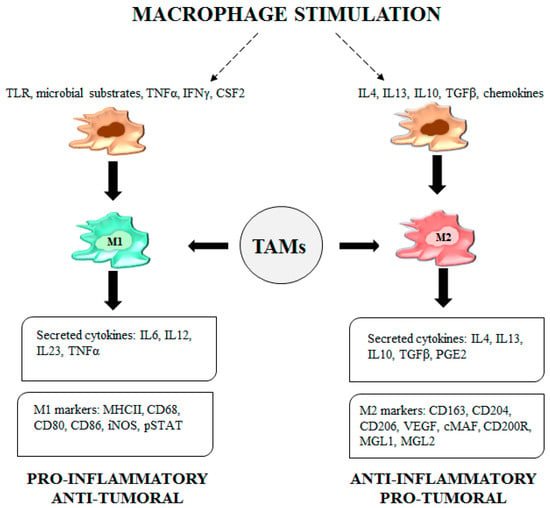
Figure 1.
Legend. Tumor-associated macrophage (TAM) functions. Upon stimulation by a TLR, microbial substrates, IFNγ and CSF2, M1-TAMs secrete cytokines such as IL6, IL12, IL23 and TNFα and express specific M1 markers like MHCII molecules, CD68, CD80, CD86, iNOS and pSTAT. The adopted phenotype confers proinflammatory/antitumor features to TAMs. Upon stimulation by IL4, IL13, IL10, TGFβ and chemokines, M2-TAMs secrete IL4, IL13, IL10, TGFβ and PGE2. M2- TAMs express CD163, CD204, CD206, VEGF, cMAF, CD200R, macrophage galactose-type lectin (MGL) 1 and MGL2, all markers representative of the M2 phenotype characterized by anti-inflammatory/protumor functions.
2.2. Anti-Inflammatory/Protumor M2 TAMs
The alternatively activated macrophages (M2) are involved in the resolution of inflammation and suppress the immunity against parasites and tumor cells, thus enabling the tumor microenvironment to promote cancer progression and metastasis. M2 TAMs are activated by Th2 cytokines, transforming growth factor β (TGFβ), chemokines and prostaglandin E2 (PGE2). M2 TAMs secrete anti-inflammatory cytokines, TGFβ and express M2 phenotype markers (Figure 1). Additionally, the M2 profile is characterized by upregulation of genes such as arginase 1 (Arg1), resistin-like molecule α (FIZZ1), macrophage mannose receptor (MMR) 1, (Mrc1) and chitinase-like protein Ym1 [10].
3. Role of TAMs in the Tumor Microenvironment
The dynamic and heterogeneous interactions between cancer cells and tumor microenvironment determine a range of opposite functions of macrophages even within the same type of tumor. Tumor and immune cells secrete growth factors, cytokines and metabolites that promote TAM protumor polarization. In particular, mediators like CSF-1, CCL2 and vascular endothelial growth factor (VEGF) promote TAM accumulation in the tumor microenvironment [11,12,13]. The protumor activation status of macrophages includes secretion of growth factors, promotion of angiogenesis, release of proteases and molecules for extracellular matrix remodeling, production of immune-suppressive molecules. Among molecules affecting cancer cell proliferation, TAMs express epidermal growth factor (EGF), members of the TGFβ and fibroblast growth factors (FGF) [4,14]. TAMs also promote vessel growth by upregulation and release of proangiogenic factors such as VEGF, tumor necrosis factor (TNFα), FGF, urokinase plasminogen activator (uPA), thymidine phosphorylase (TP), adrenomedullin (ADM) and semoforin 4D (Sema4D) [14,15]. TAMs release proteolytic enzymes such as matrix metalloproteinases (MMPs) and cathepsins able to degrade the extracellular matrix activating and releasing angiogenic factors [3,16,17]. Additionally, TAMs, by the release of proangiogenic chemokines and cytokines such as IL10, TGFβ, CCL3, CCL4, CCL5 and CCL22, mediate the immunosuppressive activity on T cells by repression of genes encoding granzymes, perforins and cytotoxins [18,19,20]. Indeed, secreting proinflammatory cytokines, such as IL6 and TNFα, TAMs, contribute to the “dormant inflammation” determining immunosuppression in the tumor microenvironment.
4. Exploiting TAMs as a Therapeutic Target
TAM subpopulations are implicated in all the steps of tumor growth and progression, including epithelial-mesenchymal transition, invasion, angiogenic switch, intravasation, extravasation, priming of the metastatic niche [16,21]. The complex interplay between tumor cells and TAMs orchestrate tumor progression and metastasis formation through the secretion by both cell types of cytokines, chemokines and growth factors sustaining tumor growth. Among the rational approaches to counteract tumor progression, the study of TAM targeting and reprogramming is aimed at creating an unfavorable environment for tumor cells thus preventing their exploitation of hosting immune cells. Therefore, TAM targeting may help not only to reduce the growth of the primary tumor, but also to hinder metastases formation. Strategies able to target TAMs and modulate their reprogramming are below reported and showed in Figure 2.
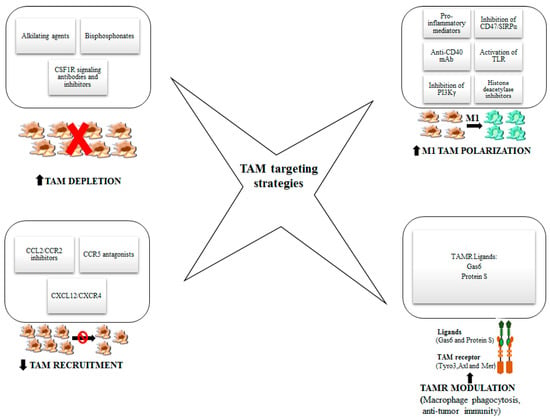
Figure 2.
Legend. Strategies to target TAMs and modulate their reprogramming. In this figure, different approaches to target TAMs are represented with the aim of enhancing antitumor efficacy: the depletion of macrophages, the reduction of monocyte recruitment, the polarization into M1-like macrophages and TAM receptor modulation.
4.1. Strategies Aimed at TAM Depletion
The first strategy of TAM targeting is based on TAM depletion. Several molecules tested in preclinical models showed a promising efficacy in clinical trials.
Trabectidin is an antineoplastic alkylating agent of marine origin, approved for the treatment of advanced soft tissue sarcoma and ovarian cancer relapse and under testing in clinical trials for other solid tumors. Beyond a direct cytotoxic effect on tumor cells, its antitumor activity relies also on the depletion of TAMs (in a range from 30 to 77% as observed in clinical studies) through induction of apoptosis [22]. Structurally and functionally similar to trabectidin, lurbinectedin has shown promising clinical results in the treatment of small cell lung cancer, alone or in combination with checkpoint inhibitors or conventional chemotherapeutics [23,24]. Another class of drugs that showed secondary efficacy in depleting TAMs when used in chemotherapeutic regimens, are bisphosphonates, originally developed as inhibitors of osteoclastic bone resorption, now used in both hematological and solid tumors to contrast bone metastases and skeletal negative events [25]. Liposomal formulations of clodronate have been reported to target macrophage survival and functions reducing bone and visceral metastases in breast cancer and others [16,26]. The antitumor properties of zoledronate rely also on the impairment of TAM polarization, neoangiogenesis and metastatization. Lipid-coated calcium zoledronate nanoparticles, beyond inducing TAM apoptosis, were able to inhibit angiogenesis and improve antitumor immune response [27].
Another interesting target is the CSF1 receptor (CSF1R) that is expressed on circulating monocytes and tissue macrophages and controls their survival, proliferation, differentiation and chemotaxis. CSF1/CSF1R signaling axis is overexpressed in several tumors and associated to a worse prognosis [28]. Monoclonal antibodies or small tyrosine kinase inhibitors to block CSF1R signaling showed efficacy in preclinical models, successfully depleting TAM and enhancing the percentage of intratumor T cells. Three CSF1R-blocking mAb, RG7155 (emactuzumab), IMC-CS4 and FPA008 are in clinical evaluation, alone or in combination therapy, in several solid tumors [29,30]. In mice models of breast cancer and melanoma, CSF1R-blocking antibodies were not able to arrest the growth of the primary tumors but significantly reduced the development of metastases [31]. Only a partial response was observed for emactuzumab in combination with paclitaxel for the treatment of advanced solid tumors, even if M2 TAM depletion and repolarization toward M1 was obtained [32]. Small molecule tyrosine kinase inhibitors PLX3397 (pexidartinib) and BLZ945 provided more encouraging results both in preclinical models and in the clinic. Pexidartinib has been recently authorized by the FDA for the chemotherapy of inoperable or relapsing diffuse-type tenosynovial giant-cell tumor, a rare aggressive tumor of the connective tissue caused by a translocation associated to CSF1/CSF1R overexpression [33]. The clinical use of both antibodies and small molecules was associated to the occurrence of severe adverse events, such as fatigue, asthenia, anemia, nausea, facial and periorbital oedema, lupus erythematosus and hepatotoxicity [34]. Such side effects are likely due to unwanted disruption of perivascular macrophages in non-target tissues and organs. Indeed, none of above reported approaches leads to the selective elimination of protumorigenic M2-like macrophages without affecting the protective populations that maintain their natural antitumoral function or perform specific protective actions in various body’s districts. More selective strategies are thus being developed, like the promising M2-macrophage-targeting peptide (M2pep) able to preferentially identify and kill M2 TAM saving up the antitumor M1 subpopulation [35]. Another possibility is to target the scavenging receptor CD163, an M2 and TAM marker, depleting selectively such subpopulation reported to inhibit melanoma progression in mice through the promotion of M1 and T cell-mediated antitumor immunity [36].
4.2. Strategies Aimed at Inhibiting TAM Recruitment
Several primary tumors showed high expression of both CCL2 and its receptor CCR2 that were associated to TAM recruitment [37]. Preclinical studies with CCL2 blocking antibodies or small molecule inhibitors supported the notion of their key role in the intratumor accumulation of TAMs, through signaling pathways that are strictly dependent on the tumor type [38]. The inhibitors were efficacious alone or in combination with other chemotherapeutics in various tumor models, even if drug removal elicited the recruitment of new macrophages and the rapid development of metastases in breast cancer [39]. Despite their promising efficacy in preclinical studies, clinical trials with CCL2/CCR2 inhibitors were partially disappointing. The anti-CCL2 monoclonal antibody carlumab failed to inhibit tumor growth in early stage clinical trials in prostate cancer, since CCL2 levels increased just one week after the treatment through the induction of compensatory mechanisms [40]. Better results were obtained in combination regimens with the CCR2 inhibitor PF-04136309 and FOLFIRINOX chemotherapy (leucovorin, fluorouracil, irinotecan, oxaliplatin) in advanced pancreatic cancer, where reduced numbers of both circulating and intratumoral CCR2+ monocytes were measured. The treatment was well tolerated, and an objective tumor response was reported in half of the patients. In the FOLFIRINOX group none objective response was observed [41]. On the contrary, the combination of PF-04136309 with paclitaxel and gemcitabine in metastatic pancreatic cancer displayed safety issues and had no therapeutic advantage with respect to the standard treatment [42]. The partial response to CCL2/CCR2 targeting strategies highlights that other chemokines, cytokines and growth factors contribute to the process, or may compensate the absence of CCL2/CCR2, with differences depending on the stage and the type of tumor, that hence need to be carefully evaluated for the development of effective strategies.
Other monoclonal antibodies and small molecule inhibitors targeting chemokine signaling pathways are already available for the treatment of autoimmune pathologies, like CCR5 antagonists. CCR5 is overexpressed in various solid cancers and in lymphoma, being also related to a worse prognosis. CCR5 antagonists, like maraviroc and vicriviroc, used as antiretroviral drugs in HIV combination regimens (since CCR5 acts as a coreceptor for HIV-1 entry into CD4+ lymphocytes) have been repurposed in cancer [43]. Both maraviroc and vicriviroc were efficacious in preclinical models of breast and colon cancer through the induction of antitumor immunity [44]. In colorectal cancer patients, refractory to other therapeutic regimens, maraviroc showed a good safety profile [45]. CXCL12/CXCR4 and CCL20/CCR6 are alternative signaling axes that could be successfully targeted in cancer to prevent TAM accumulation. The CXCR4 antagonist AMD3100 (plerixafor) has been reported to restrain tumor growth also through the inhibition of immunosuppressive mechanisms [46]. Several clinical trials are ongoing to test plerixafor in solid tumors, in order to assess its safety profile alone or in combinatorial regimens.
Development of dual-antagonists targeting simultaneously two different chemokine receptors is a strategy that could lead to more effective TAM targeting. The dual CCR2/CCR5 antagonist BMS-813160 is actually under clinical investigation in combination with nivolumab or standard chemotherapy in pancreatic, lung, renal and hepatocellular cancers.
Chemokine-targeting agents need to be accurately selected in order to not affect the recruitment of other immune cells like natural killer (NK) and T cells that are fundamental for the efficacy of immunotherapeutic approaches. Of note, recent studies have reported that cytotoxic cells mainly use CXCR3 for intratumor homing [38]. Thus, targeting chemokine receptors other than CXCR3 may be effective in inhibiting TAM recruitment alone.
Understanding the interplay among different factors in the microenvironment and their correlation to TAM homing in different tumors, will help the development of efficient targeted therapies aimed at inhibiting TAM recruitment or retention both in the primary tumor and at metastatic sites avoiding the priming of compensatory mechanisms.
4.3. Strategies to Influence TAM Polarization
The above-reported strategies indiscriminately target all macrophages in the tumor and other districts, inducing undesired side effects and long-term toxicities. Reprogramming TAMs has the advantage to address them specifically toward the antitumor phenotype. Modulation of cytokines, chemokines, growth factors and their receptors can direct TAM functional program. Many of these factors are particularly abundant in hypoxic conditions, a frequent characteristic of solid tumors. The direct or indirect induction of proinflammatory mediators, like IFNγ, IL4, IL6, IL13, VEGF, GM-CSF, CSF-1, Ang-2, CCL2 and other chemokines in the tumor milieu, has an antitumor effect linked to M1 polarization of TAM. An oncolytic virus encoding the proinflammatory cytokine IL12 has been reported to efficiently kill glioblastoma tumors also through the restoration of the antitumor immune response [47].
Among the numerous approaches in preclinical models, including small molecules, antibodies and RNA, some are giving promising results also in clinical testing, alone or in combination with classical chemotherapeutic drugs or immune checkpoint inhibitors.
An interesting strategy aims to enhance TAM phagocytic properties blocking the CD47/SIRPα ‘don’t eat’ signal by target cancer cells that express CD47 at high levels as an acquired mechanism of resistance to clearing by phagocytosis [48]. Pharmacological inhibition of CD47 signaling by the antibodies Hu5F9-G4 and CC-90002 or the engineered fusion protein SIRPα-Fc (TTI-621) was effective in enhancing tumor cell phagocytosis. The combinatorial treatment with monoclonal antibodies like rituximab further improved the antitumor response through phagocytosis induction in lymphoma [49]. Another way to reprogram TAMs toward M1 phenotype and augment phagocytosis is through the anti-CD40 mAb selicrelumab that is now under testing in Phase I trials in several solid tumors alone or in combination with standard chemotherapy or immunotherapy. Moreover, its combination with emactuzumab has been reported to be particularly effective in the repolarization of TAMs without undesired depletion of all macrophages [50].
The activation of TLR by bacterial products or viral nucleic acids is able to induce the immune response, addressing macrophages toward the M1 phenotype. Encouraging results were obtained in mice tumor models of melanoma, breast cancer and others, with TLR3, TLR7, TLR8 and TLR9 ligands [16]. The TLR7 agonist imiquimod, approved for the topical treatment of squamous and basal cell carcinoma, was reported to be safe also in the treatment of melanoma and skin lesions of metastatic breast cancer, enhancing accumulation of immune cells in the lesions [51]. Poly-I:C stimulation of TLR3 efficiently repolarized TAMs in melanoma and now is under evaluation as a cancer vaccine to improve the antitumor immune response in advanced cancers [52]. The scavenger receptor MARCO (Macrophage Receptor with Collagenous Structure), of which engagement is fundamental for pattern recognition receptor responses, is highly expressed by TAMs in patients with melanoma and breast cancer and found to be related to a worse prognosis. An antibody specifically directed to MARCO receptor has been reported to redirect TAMs toward the inflammatory phenotype, increasing the antitumor immune response also in combination with anti-CTLA-4 immune checkpoint inhibitor [53].
Several small molecule inhibitors exerted their antitumor action activating the immune system through the reprogramming of macrophages. The inhibition of PI3Kγ blocked tumor growth through the induction of a proinflammatory response by M1 TAMs that recruited CTLs into the tumor [54]. Mice treated with PI3Kγ inhibitor in combination with anti-PD1 checkpoint inhibitor had improved response and overall survival [55].
Another promising strategy is TAM repolarization at the epigenetic or genetic level. The histone deacetylase inhibitor TMP195 is able to address TAMs toward M1 profile, modifying their epigenetic signature and inducing their accumulation and phagocytic activity in the microenvironment of breast tumors [56]. miRNA modulation through the genetic deletion in macrophages of DICER, an enzyme fundamental for miRNA synthesis, was shown to inhibit tumor growth in preclinical models of breast, colon and lung tumors, reprogramming TAMs to express IFNγ and activate STAT1 proinflammatory signaling [57].
Undoubtedly, all approaches targeting TAMs need to be based on a solid mechanistic knowledge of TAM plasticity and ability to switch from a phenotype to another in different phases of tumor growth and progression and in response to therapy.
4.4. Targeting TAM Receptors (TAMR)
An interesting druggable target is represented by the so-called TAMR, a family of three known tyrosine kinase receptors, Tyro3, Axl and MerTK, whose initials form the acronym TAM (unintentionally identical to TAM cells’ acronym), expressed by several cell types including tumor cells and immune cells. These receptors display multiple roles in cell fate, proliferation, migration and in modulating processes like tissue homeostasis and inflammation [58].
The best-known ligands for TAMR are Gas6 and Protein S that function as adaptors connecting phosphatidylserine proapoptotic signal on target cells with TAMR, thus favoring phagocytosis by macrophages. The activated downstream signaling pathways converge on the PI3K/Akt axis that is involved in TAM activation and polarization towards the M2 phenotype, associated with the promotion of efferocytosis, which limits the inflammatory response, fostering the production of immunosuppressive cytokines and preventing the immune cell activation in response to cancer cell death in solid tumors. For these reasons, blocking TAMR may be a promising anticancer strategy [59]. Several small-molecule inhibitors of TAMR along with antibody–drug conjugates, engineered CAR-T cells and Axl proteins fused with Fc, have been developed as anticancer agents in different preclinical models and some are already in clinical trials. These drugs have been originally designed to target cancer cells expressing TAMR that are upregulated, as well as their partner protein Gas6, in several tumors, like leukemia, melanoma and glioblastoma [60]. Axl expression levels correlated with worse prognosis in glioblastoma and pancreatic cancer, where Axl is overactivated in the 70% of cases and is responsible of resistance to chemotherapy [61,62,63]. Therefore, the action of these molecules on TAM recruitment in animal models and their evaluation as a secondary endpoint in clinical studies is worth of investigation. MerTK is the most abundant receptor of the family on macrophages and microglia, whereas Axl is prevalently expressed by dendritic cells (DCs) [58]. MerTK blocking with a specific antibody leads to inhibition of TAM-mediated efferocytosis of apoptotic tumor cells that activate an inflammatory type I IFN response through the induction of the stimulator of interferon genes (STING)-controlled innate immune pathway, with subsequent CD8+ activation and a synergistic effect with anti-PD1/PDL1 checkpoint inhibitors [64]. The blood–brain barrier permeable small molecule UNC2025 has been observed to inhibit glioblastoma growth in combination with radiotherapy [60]. The Axl inhibitor BGB324 showed promising efficacy in a preclinical model of glioblastoma in combination with nivolumab anti-PD1 antibody [62]. A pan-TAM inhibitor, RXDX-106, able to block all the receptors of the family, was effective in inhibiting tumor progression in several models, boosting antitumor immunity [65]. Strategies to target Gas6 have been developed, by blocking antibodies or drugs like warfarin, and are currently in an early Phase 1 clinical trial in patients with pancreatic cancer. Beyond inhibiting epithelial mesenchymal transition of pancreatic tumor cells, targeting Gas6 promotes the antitumor immune response mediated by NK cells [66].
5. TAMs in Cancer Treatment: Chemotherapy, Radiotherapy, ICB Therapy and Virotherapy
The ideal cancer treatment is expected to switch TAMs toward an antitumor phenotype. Here, we discuss the main findings describing the effect of conventional and more recent anticancer therapies on TAM reprogramming.
5.1. TAMs and Chemotherapy
TAM behavior during chemotherapeutic treatment is controversial, and their potentiated or reduced effect on chemotherapy depends on the chemotherapeutic agent and type of tumor (Table 1). Three major mechanisms seem to drive the editing of macrophages by chemotherapeutics: (i) polarization, (ii) recruitment and migration and (iii) depletion.

Table 1.
Effects of Chemotherapeutic Agents on Monocytes/Macrophages.
5.1.1. Polarization
Numerous chemotherapeutics prompt the antitumor M1 phenotype switch from the M2 population. In vitro and in vivo, paclitaxel induced M1 macrophage polarization in a TLR4-dependent manner [79]. In addition, paclitaxel enabled macrophages to activate genes encoding inflammatory mediators (see Table 1) and cytokines able to activate NKs, DCs and tumor-specific cytotoxic T-lymphocytes (CTLs) [67].
In mammary tumors 4T1-Neu in mice, docetaxel caused the activation of M1-like TAMs and depletion of M2-like TAMs, enhancing CTL response [69]. Cisplatin treatment of murine peritoneal macrophages cocultured with L929 cells, favored the release of cancer cell-specific cytotoxic factors such as FasL and TNF and facilitated the apoptosis of tumor cells, confirmed by the activation of caspase-8, caspase-3, cytochrome c, Bid, Bax, along with DNA fragmentation and downregulation of Bcl-2 [80]. Indeed, cisplatin induced the secretion of IL1, IL6, IL8 and TNFα by peritoneal macrophages isolated from BALB/c mice [81]. On the other hand, in ovarian and cervical cancer cell lines, cisplatin and carboplatin, by increasing the production of PGE2 and IL6 by cancer cells, skewed monocyte differentiation towards the M2 phenotype leading to chemoresistance [70].
In vivo, in mouse peritoneal macrophages, cyclophosphamide enhanced the production of IL6, and IL12 and decreased anti-inflammatory cytokines like IL10 and TGFβ, thus activating Th1 cells and macrophages [82]. Combinatory approaches including the cotreatment with chemotherapy (cyclophosphamide, doxorubicin, vincristine) and immunotherapy (anti-CD40+ cytosine-phosphate-guanosine-containing oligodeoxynucleotide 1826) prompted the enrichment of an M1 polarized TAM subset. Thus, molecules associated with the M1 phenotype were upregulated whereas M2-associated molecules (see Table 1) were downregulated [76].
Chemotherapeutics mostly are effective in reprogramming TAMs versus the M1 phenotype, however, depending on tumor type and therapy schedule, can promote the M2 phenotype. The interaction between TAMs and chemotherapeutics deserves a careful investigation, addressing patient-to-patient differences, tumor types and type of treatment. Nonetheless, in the M1 phenotype prevalence, it must be taken into account whether the proinflammatory effect of TAMs can promote an antitumor response or favor a low-grade inflammation. Further investigations are required to address open questions.
5.1.2. Recruitment and Migration
Chemotherapeutics induce the recall of monocytes to tumor site as consequence of chemotherapy’s damaging effects. TAMs initiating the regenerative program, contribute to support cancer cell proliferation [83]. Among these agents, cyclophosphamide was shown to recruit DCs, macrophages and NKs in mouse models and in cancer patients [71,72]. Combinatory approaches using chemotherapeutics and other agents have been studied [68,77,78,84]. In HER2/Neu-driven mammary carcinoma, doxorubicin in combination with lapatinib, favored the infiltration of immature macrophages and the reduction of mature TAMs at the tumor site [74]. In addition, in a mouse model of breast cancer metastasis, doxorubicin increased the recruitment of monocytes; in particular, CD206+ macrophages favored an increased vascular leakage that prompted a better doxorubicin response, whereas CCR2-dependent monocyte recruitment was associated with tumor relapse [73]. In vivo, in murine breast tumor, abraxane (albumin + paclitaxel) evoked high infiltration of CD45+CD169+ macrophages, an increased F4/80+ macrophage population was detected in MDA-MB-435 tumors but not in the correspondent paclitaxel resistant tumors [68]. The treatment with gemcitabine of patients with pancreatic cancer induced increase of CD14+ monocytes, CD123+ plasmacytoid DCs and CD11c+ myeloid DCs [75]. The conventional chemotherapy (oxaliplatin, docetaxel, irinotecan with folinic acid and 5-fluorouracile, 5-FU) combined with an anti-CCL2 to block monocyte migration provided an increased antitumor response in pancreatic and prostate cancer [77,78]. Overall, the pro/antitumor role of infiltrating monocytes/macrophages at tumor sites depends on the type of chemotherapeutic and the local tumor microenvironment.
5.1.3. Depletion
Chemotherapy is known to lead to monocytopenia [85,86]. The effect of docetaxel in 4T1-Neu mammary tumors involves the depletion of the M2 TAMs and activation of M1 monocytes and myeloid-derived suppressor cells that can promote antitumor cytotoxic T cell response [69]. In murine tumors, trabectedin exerted cytotoxic effects on monocytes/macrophage in the bloodstream, bone marrow and spleen (Table 1). Patients with soft tissue sarcoma treated with trabectedin showed a strong reduction of TAM density [22].
5.2. TAMs and Radiotherapy
Radiotherapy can affect TAM phenotype and polarization according to the dosage used (Table 2). Radiotherapy induces the production of antioxidant molecules conferring resistance to macrophages that, in humans, are considered the most radio-resistant cells [87]. Radiotherapy prompts tumor regression, however, the recruitment of macrophages and myeloid cells at tumor site might lead to tumor recurrence [88,89,90]. Liposamol clodronate used to deplete macrophages before ionizing radiation renders efficient the antitumor effects of radiotherapy and highlights the protumor role of TAMs [91]. Macrophage infiltration and accumulation by radiotherapy is mainly mediated by CCL2 and CSF1 [90,92].

Table 2.
Macrophage Reprogramming after Low, Intermediate and High Dose of Irradiation.
5.2.1. Low Doses
Low doses (below 1Gy) evoke low toxicity and favor the M2 phenotype of TAMs. In Raw264.7 macrophages, low irradiation doses decreased iNOS level and NO production, repolarizing M1 macrophages towards the M2 phenotype [93]. In addition, in the same cells stimulated with LPS, irradiation decreased p38 phosphorylation and TNFα secretion [94]. An anti-inflammatory phenotype was also observed in the LPS-stimulated human THP-1 macrophage cell line with a decrease of IL1β [95,96]. Doses under 2Gy do not affect macrophages’ viability and phagocytic ability but induce an anti-inflammatory cytokine milieu, i.e., increased TGFβ secretion favoring an M2 phenotype [97]. In whole-body-irradiated mice [101], different effects were observed depending on mouse strain (see Table 2).
5.2.2. Intermediate Doses
Intermediate doses between 1 to 10 Gy induce a shift of unpolarized macrophages toward the M1 phenotype. These macrophages show upregulation of proinflammatory markers and downregulation of M2 anti-inflammatory markers [98] (Table 2). Indeed, the observed M1 phenotype correlated with increased phagocytosis, and irradiation did not affect in macrophage-tumor cell coculture the promotion of invasiveness and angiogenesis [98]. A proinflammatory profile was also associated with increased mRNA levels of IFNγ, TNFα, IL23, IL6 and higher protein levels of IL8 and IL1β elicited by the transcriptional expression of IRF5 and mediated by ataxia telangiectasia mutated (ATM) [102]. Moderate doses can also potentiate an already acquired M1 profile, whereas, did not affect a prompted M2 profile, (Table 2) [98]. Additionally, in coculture experiments using human unpolarized macrophages with radiosensitive and/or radio-resistant colon cancer cells, the irradiation induced different effects. The irradiation in the coculture with radiosensitive cells prompted the decrease of proinflammatory markers (CXCL8, CCR7, IL1β) while anti-inflammatory marker expression was not affected. In the coculture with radio-resistant cells, proinflammatory (CD80, CCR7) and anti-inflammatory (CCL18, IL10) markers were upregulated, these findings suggest that the interaction with different cancer cell types can affect the macrophage phenotype [103].
In vivo experiments performed in whole-body-irradiated mice, intermediate doses increased the M1 markers and reduced M2 markers, and in contrast, local irradiation did not change M1 and M2 marker expression. The use of local irradiation determined a reduction of T cell infiltration, whereas local irradiation associated with CTL transfer enhanced M1 and reduced M2 markers (Table 2). Indeed, T cell infiltration was proinflammatory-macrophage-dependent. These data suggest a role of CD8+ T cells in macrophage reprogramming [89]. A shift toward M1 phenotype was observed by systemic irradiation (2Gy/week for two weeks) in mice (Table 2). In addition, whole-body irradiation enhanced of iNOS levels and NO production, inducing tumor vasculature normalization [88]. It was proposed that whole-body irradiation differently from local irradiation favors the infiltration of fresh reprogrammed macrophages from different lymphoid organs
5.2.3. High Doses
Doses higher than 10 Gy switch macrophages toward the M2 phenotype. In vitro, irradiation (20 Gy) of Raw264.7 macrophages induced the M2-like phenotype. This effect was confirmed in mice with Panc02 xenograft (Table 2) [99]. High irradiation increased M2-like TAMs also in a murine prostate cancer model (Table 2), prompting angiogenesis and tumor growth [87]. Secretion of proangiogenic factors and M2 macrophage recolonization was observed also in an oral cancer model [100]. The acceleration of tumor growth after high doses of irradiation was also observed in murine pancreatic tumor models, high M2-like TAM infiltration was revealed associated with a T cell suppressive response. Pancreatic dysplasia rapidly progressed toward invasive pancreatic ductal carcinoma. TAMs were characterized by decrease of IRF5, iNOS and H2eb1 mRNA expression and increase of CD206, Arg-1 and PD-L1 [104]. These findings suggest that high-dose-irradiated TAMs promote tumor progression and tumor radio-resistance. It might be of interest to investigate the possibility to prevent macrophage recruitment by blocking M-CSF to avoid tumor promotion and maintain the efficacy of radiotherapy.
5.3. TAMs and Immune Checkpoint Blocking (ICB) Therapy
The T cell surface presents a family of proteins, the immune checkpoints that interact with specific ligands on antigen presenting cells or tumor cells and block T cell receptor-mediated activation. In recent years, the use of immune checkpoint inhibitors provided interesting clinical responses in some type of cancer. TAMs are known to limit the efficacy of ICB therapy [105,106] because they express ligands of checkpoint receptors like PD-L1, PD-L2, CD80, CD86, the V-domain immunoglobulin suppressor of T cell activation (VISTA). These ligands are able to sequester immune checkpoint inhibitors (i.e. anti-PD-L1 monoclonal antibody). TAMs can also capture anti-PD-1 successfully competing with T cells that can bind anti-PD-1 only for a short time [105]. T cells, mast cells and basophils express the ligand of the CD40 receptor (CD40L). CD40 is the receptor of the TNF receptor superfamily and is expressed on the surface of macrophages, DCs. The interaction CD40L-CD40 receptor upregulates the expression of MHC molecules and the production of proinflammatory cytokines that promote T cell activation [107]. In murine tumor models, the use of anti-CD40 antibodies prompted the recovery of tumor immune surveillance via TAM repolarization towards the M1 phenotype favoring an enhanced antitumor activity [108]. Checkpoint immunotherapy in combination with anti-CD40 monoclonal antibody represents a novel alternative that is under investigation. Indeed, the combination of anti-CD40 antibodies with anti CSF1R antibodies has been demonstrated to modify a “cold” tumor in a “hot” tumor enhancing T cell infiltration and the antitumor activity [109].
5.4. TAMs and Virotherapy
The use of virotherapy is emerging as a valid therapeutic treatment for several type of cancer. It is based on the use of OVs that, beyond their direct lytic effect on tumor cells, function through a multitude of events like modification of the tumor micro/macro-environment, modulation of the antitumor immune response [6,7,8,9]. The role of macrophages on virotherapy efficacy varies according to the tumor model. M1 macrophages are often considered allies of OV. Despite their potential for increasing virus clearance, they favor tumor shrinkage. In contrast, M2 macrophages seem to be foes, they may promote cancer growth that prevails over any effect on preventing immune clearance of the OV. We report TAM modulation in virotherapy applied to colorectal cancer, glioblastoma, pancreatic and breast cancers (Table 3).

Table 3.
Tumor-associated macrophages in virotherapy.
In colorectal cancers treated with virotherapy, the activation of inflammatory macrophage is beneficial. In this tumor model, the oncolytic poxvirus vvDD-CCL11 increased immunogenic programmed necrosis; the antitumor acquired immune response correlated with enhanced levels of IFNγ following virus infection [110]. The oncolytic vaccinia virus, GLV-1h68, was associated with enhanced infiltration of NKs and macrophages and increased levels of proinflammatory cytokines and chemokines (Table 3) [111] involved in antiviral and antitumor immune response. Additionally, inflammatory macrophages express macrophage metallelastase endowed with anti-angiogenic activity and able to improve oncolytic adenovirus spread in colorectal cancers [125].
In contrast, in glioblastoma multiforme, virotherapy is improved with the suppression of the innate immune response. The antitumor efficacy of oncolytic herpes simplex virus (oHSV) in glioma was inhibited by inflammatory macrophage activation partly because of the TNFβ mediated arrest of virus replication [112,113]. Indeed, cysteine-rich 61 protein (CCN1) activation inhibited OV antitumor effects via the activation and infiltration of TAMs and NKs expressing CXCL10, MCP-1/3, IFNγ, IL1β [114,115]. In glioblastoma, the activation of M2 macrophages with TGFβ inhibited innate inflammatory macrophages, NKs and microglia, prompting an enhanced virus replication and oHSV antitumor activity [116]. On the other hand, the use of TNFα inhibitors may significantly improve the efficacy of oHSV in glioblastoma. Virus treatment increases macrophage infiltration polarized toward a M1, proinflammatory phenotype, indeed, macrophages/microglia secreted significant levels of TNFα in response to infected glioma cells in vitro and in vivo [113].
In the Netherlands, the oncolytic virus DNX-2401 (formerly Delta-24-RGD), a replication-competent adenovirus modified to increase tropism to glioma cells and replicate in tumor cells that have a defective Rb pathway, entered a phase I/II clinical trial for recurrent glioblastoma (NCT01582516) [117]. In some patients, the virus enhanced the cerebrospinal fluid concentrations of cytokines such as IFNγ, TNF, IL6 and increased levels of CD64, a marker of M1 polarization was observed on macrophages in vitro.
Another study used the rodent H-1 parvovirus (H-1PV), which is the smallest among all OVs, is endowed with natural anticancer activity and is nonpathogenic for humans. The lack of pre-existing immunity in humans and its capacity to cross the blood–brain barrier, made this virus suitable for central nervous system tumors. In glioblastoma patients, H-1PV showed the switch of an immunosuppressed tumor microenvironment towards immunogenicity. The tumor was infiltrated with CTLs, indeed, induction of cathepsin B and iNOS expression (marker of M1 phenotype) in TAMs and accumulation of activated TAMs in CD40L-positive glioblastoma regions was observed [118]. Combination of virotherapy with ICB therapy was investigated in glioblastoma. In particular, the triple combination of anti-CTLA-4, anti-PD-1, and oHSV G47Δ expressing murine IL12 (G47Δ-mIL12) cured most mice in two glioma models. This treatment was associated with influx of macrophages and M1-like polarization [119].
In pancreatic ductal adenocarcinoma, the release of IFNγ seems to mediate the anticancer effect of H-1PV. In rats, the injection of H-1PV with coapplication of IFNγ extended animal survival and improved the H-1PV induced peritoneal macrophage antitumor response. The authors also suggest that IFNγ may induce antigen presentation by enhancing MHC-II molecule expression on the surface of macrophages and DCs [120]. In the same tumor model, the oncolytic adenovirus expressing TNFα and IL2 (OAd-TNFa-IL2) used in combination with mesothelin-redirected chimeric antigen receptor T cell (meso-CAR T cell) therapy induced significant tumor regression in mice engrafted with highly aggressive and immunosuppressive pancreatic ductal adenocarcinoma. This approach increased CAR T cell and host T cell infiltration to the tumor and polarized macrophages toward the M1 phenotype and increased DC maturation [121].
In pancreatic cancer, a combination of immunotherapy based on CD40 as target and oncolytic adenovirus showed antitumor efficacy. A novel oncolytic adenovirus, TMZ-CD40L, armed with a trimerized membrane-bound extracellular CD40L was able to control tumor progression and, indeed, potently increased tumor-infiltrating T cells and promoted the switch from M2 (the classical phenotype of pancreatic tumors) to M1 macrophages [122].
In a model of anaplastic thyroid carcinoma the oncolytic adenovirus dl922-947 decreased monocyte chemotaxis in vitro and tumor macrophage density in vivo and induced the switch of TAMs toward the M1 phenotype, likely by increasing IFNγ [126].
In breast cancer, the presence of TAMs and immune cells is often correlated with poor prognosis, these cells increase tumor resistance to chemotherapy and elicit immunosuppression. Tumor infiltrating immune cells constitutively express low levels of IFN inducing antiviral activity. In particular, breast cancers have constitutive activation of IFN–stimulated genes, activation of antiviral JAK/STAT signaling and are heavily infiltrated with CD68+ macrophages. The use of JAK inhibitors was able to reverse the macrophage-induced antiviral status [127]. The antitumor efficacy of the oncolytic paramyxoviruses’ (measles/mumps) was increased by human monocyte-derived macrophages independently of the initial polarization status of macrophages and viral replication [123]. Furthermore, an oncolytic adenovirus expressing soluble TGFβ receptor II –Fc fused, inhibited TGFβ in bone metastasis of breast cancer reducing M2 osteoclast activity and tumor progression [124].
6. Conclusions
Therapeutic strategies targeting TAMs have the advantage of hitting simultaneously all the processes in which they are negatively involved in the tumor microenvironment beyond tumor growth and progression, which are immune regulation and mechanisms of resistance to chemotherapy. Several key enzymes involved in processes of tumor progression are suitably druggable, and several molecules are already available. However, a better depiction of the mechanisms underlying the relationship between TAMs and cancer and the peculiarities of this dangerous liaison in different tumor types, will help to implement effective targeted strategies on immunosuppressive M2 subpopulations, in order to avoid the undesired side effects due to depletion of all macrophages. Undoubtedly, this approach can be optimized through appropriate combination regimens with chemotherapy, immunotherapy, radiotherapy, ICB therapy and virotherapy designed to drive an antitumor TAM profile and tailored to patient characteristics. Thus, optimization of TAM-targeted therapies and combination with current antineoplastic therapies might be addressed to avoid the overcome of adaptive or intrinsic therapy resistance due to TAM plasticity and improve the efficacy of antineoplastic treatments.
Funding
This research was carried out in the frame of Programme STAR, financially supported by UniNA and Compagnia San Paolo. (to AMM). S.D. was supported by AIRC fellowship for Italy. S.P. was funded by Associazione Italiana per la Ricerca sul Cancro (AIRC) and Fondazione Cariplo (AIRC TRIDEO No. 17216).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| TAM | tumor-associated macrophages |
| CCL2 | C-C motif chemokine ligand 2 |
| CSF | colony-stimulating factor |
| PD-1 | programmed cell death 1 |
| OV | oncolytic virus |
| TLR | toll-like receptors |
| LPS | lipopolysaccharide |
| TGFβ | transforming growth factor β |
| PGE2 | prostaglandin E, |
| Arg 1 | arginase 1 |
| MMR | macrophage mannose receptor |
| IFN | interferon |
| IL | interleukin |
| TNFα | tumor necrosis factor alpha |
| MHC II | class of major histocompatibility complex |
| iNOS | inducible nitric oxide synthase |
| pSTAT | phosphorylated signal transducer and activator of transcription |
| MGL | macrophage galactose-type lectin |
| VEGF | vascular endothelial growth factor |
| EGF | epidermal growth factor |
| FGF | fibroblast growth factors |
| uPA | urokinase plasminogen activator |
| TP | thymidine phosphorylase |
| ADM | adrenomedullin, |
| Sema4D | semaforin 4D |
| MMPs | metalloproteinases |
| CCL 3,4,5, 22 | C-C motif chemokine ligand 3,4,5,22 |
| CSF1R | CSF1 receptor |
| FDA | Food and Drug Administration |
| M2pep | macrophage-targeting peptide |
| CCL | C-C motif chemokine ligand |
| CCR | C-C motif chemokine receptor |
| HIV1 | human immunodeficiency virus 1 |
| CXCL | C–X–C motif chemokine ligand |
| NK | natural killer |
| CXCR | C-X-C motif chemokine receptor |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| Ang-2 | Angiopoietin 2 |
| SIRPα | signal-regulatory protein α |
| MARCO | macrophage receptor with collagenous structure |
| CTLA4 | cytotoxic T-lymphocytes antigen 4 |
| PI3Kγ | phosphoinositide 3 kinase γ |
| STAT1 | signal transducer and activator of transcription 1 |
| CAR-T | chimeric antigen receptor T cell therapies |
| STING | stimulator of interferon genes |
| COX | cyclooxygenase-2 |
| DCs | dendritic cells |
| CTLs | cytotoxic T lymphocytes |
| 5-FU | 5-fluorouracile |
| Gy | gray |
| NO | nitric oxide |
| HLA-DR | human leukocyte antigen-cell surface receptor |
| IRF | interferon-regulatory factor |
| ATM | ataxia telangiectasia mutated |
| ICB | immune check point blocking |
| PD-L1-2 | programmed death ligand 1-2 |
| VISTA | V-domain immunoglobulin suppressor of T cell activation |
| CD40L | ligand of the CD40 receptor |
| GCP-2 | granulocyte chemotactic protein 2 |
| MIP-1 | macrophage inflammatory protein -1 |
| MCP 1,3,5 | monocyte chemoattractant protein 1,3,5 |
| CCN1 | cellular communication network factor1 |
| mesoCA T cell | mesothelin-redirected chimeric antigen receptor T cell |
References
- Cortez-Retamozo, V.; Etzrodt, M.; Newton, A.; Rauch, P.J.; Chudnovskiy, A.; Berger, C.; Ryan, R.J.; Iwamoto, Y.; Marinelli, B.; Gorbatov, R.; et al. Origins of tumor-associated macrophages and neutrophils. Proc. Natl. Acad. Sci. USA 2012, 109, 2491–2496. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, K.; Van Ginderachter, J.A. The ontogeny and microenvironmental regulation of tumor-associated macrophages. Antioxid. Redox Signal. 2016, 25, 775–791. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Mantovani, A. Immunology in the clinic review series; focus on cancer: Tumour-associated macrophages: Undisputed stars of the inflammatory tumour microenvironment. Clin. Exp. Immunol. 2012, 167, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, V.; Schmid, M.C. Macrophage-Mediated subversion of anti-tumour immunity. Cells 2019, 8, 747. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, S.; Iannuzzi, C.A.; Passaro, C.; Forte, I.M.; Iannone, R.; Gigantino, V.; Indovina, P.; Botti, G.; Giordano, A.; Formisano, P.; et al. The Oncolytic Virus dl922-947 Triggers Immunogenic Cell Death in Mesothelioma and Reduces Xenograft Growth. Front. Oncol. 2019, 9, 564. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Di Somma, S.; Iannuzzi, C.A.; Pentimalli, F.; Portella, G. Virotherapy: From single agents to combinatorial treatments. Biochem. Pharmacol. 2020, 177, 113986. [Google Scholar] [CrossRef] [PubMed]
- Malfitano, A.M.; Di Somma, S.; Prevete, N.; Portella, G. Reply to comment on “Malfitano, A.M. et al. Virotherapy as a potential therapeutic approach for the treatment of aggressive thyroid cancer” cancers 2019, 11, 1532. Cancers 2020, 12, 281. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Somma, S.D.; Prevete, N.; Portella, G. Virotherapy as a potential therapeutic approach for the treatment of aggressive thyroid cancer. Cancers 2019, 11, 1532. [Google Scholar] [CrossRef]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Mat Zin, A.A.; Ang, K.C.; Ch’ng, E.S. Evaluating the polarization of tumor-associated macrophages into M1 and M2 phenotypes in human cancer tissue: Technicalities and challenges in routine clinical practice. Front. Oncol. 2019, 9, 1512. [Google Scholar] [CrossRef]
- Biswas, S.K. Metabolic Reprogramming of immune cells in cancer progression. Immunity 2015, 43, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Linde, N.; Lederle, W.; Depner, S.; van Rooijen, N.; Gutschalk, C.M.; Mueller, M.M. Vascular endothelial growth factor-induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J. Pathol. 2012, 227, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Riabov, V.; Gudima, A.; Wang, N.; Mickley, A.; Orekhov, A.; Kzhyshkowska, J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front. Physiol. 2014, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Schioppa, T.; Porta, C.; Allavena, P.; Sica, A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006, 25, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Pollard, J.W. Targeting macrophages: Therapeutic approaches in cancer. Nat. Rev. Drug Discov. 2018, 17, 887–904. [Google Scholar] [CrossRef]
- Prenen, H.; Mazzone, M. Tumor-associated macrophages: A short compendium. Cell. Mol. Life Sci. 2019, 76, 1447–1458. [Google Scholar] [CrossRef]
- Thomas, D.A.; Massagué, J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005, 8, 369–380. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, S.J.; Broxmeyer, H.E. Phagocytosis, a potential mechanism for myeloid-derived suppressor cell regulation of CD8+ T cell function mediated through programmed cell death-1 and programmed cell death-1 ligand interaction. J. Immunol. (Baltim. MD 1950) 2011, 187, 2291–2301. [Google Scholar] [CrossRef]
- De Palma, M.; Lewis, C.E. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013, 23, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Germano, G.; Frapolli, R.; Belgiovine, C.; Anselmo, A.; Pesce, S.; Liguori, M.; Erba, E.; Uboldi, S.; Zucchetti, M.; Pasqualini, F.; et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 2013, 23, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Forveille, S.; Iribarren, K.; Sauvat, A.; Senovilla, L.; Wang, Y.; Humeau, J.; Perez-Lanzon, M.; Zhou, H.; Martínez-Leal, J.F.; et al. Lurbinectedin synergizes with immune checkpoint blockade to generate anticancer immunity. Oncoimmunology 2019, 8, e1656502. [Google Scholar] [CrossRef]
- Arrieta, O.; Zatarain-Barrón, Z.L.; Cardona, A.F. New opportunities in a challenging disease: Lurbinectedin for relapsed small-cell lung cancer. Lancet. Oncol. 2020, 21, 605–607. [Google Scholar] [CrossRef]
- Van Acker, H.H.; Anguille, S.; Willemen, Y.; Smits, E.L.; Van Tendeloo, V.F. Bisphosphonates for cancer treatment: Mechanisms of action and lessons from clinical trials. Pharmacol. Ther. 2016, 158, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Diel, I.J.; Solomayer, E.F.; Costa, S.D.; Gollan, C.; Goerner, R.; Wallwiener, D.; Kaufmann, M.; Bastert, G. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N. Engl. J. Med. 1998, 339, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Zhang, X.; Hu, H.; Qiao, M.; Zhao, X.; Deng, Y.; Chen, D. Targeted delivery of zoledronate to tumor-associated macrophages for cancer immunotherapy. Mol. Pharm. 2019, 16, 2249–2258. [Google Scholar] [CrossRef]
- Anfray, C.; Ummarino, A.; Andón, F.T.; Allavena, P. Current strategies to target tumor-associated-macrophages to improve anti-tumor immune responses. Cells 2019, 9, 46. [Google Scholar] [CrossRef]
- Zhu, Y.; Knolhoff, B.L.; Meyer, M.A.; Nywening, T.M.; West, B.L.; Luo, J.; Wang-Gillam, A.; Goedegebuure, S.P.; Linehan, D.C.; DeNardo, D.G. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014, 74, 5057–5069. [Google Scholar] [CrossRef] [PubMed]
- Pradel, L.P.; Ooi, C.H.; Romagnoli, S.; Cannarile, M.A.; Sade, H.; Rüttinger, D.; Ries, C.H. Macrophage susceptibility to Emactuzumab (RG7155) treatment. Mol. Cancer Ther. 2016, 15, 3077–3086. [Google Scholar] [CrossRef] [PubMed]
- Beltraminelli, T.; De Palma, M. Biology and therapeutic targeting of tumour-associated macrophages. J. Pathol. 2020, 250, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roca, C.A.; Italiano, A.; Le Tourneau, C.; Cassier, P.A.; Toulmonde, M.; D’Angelo, S.P.; Campone, M.; Weber, K.L.; Loirat, D.; Cannarile, M.A.; et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, M.; Cassier, P.; Dufresne, A.; Chabaud, S.; Karanian, M.; Meurgey, A.; Bouhamama, A.; Gouin, F.; Vaz, G.; Garret, J.; et al. Long term term follow-up of tyrosine kinase inhibitors treatments in inoperable or relapsing diffuse type tenosynovial giant cell tumors (dTGCT). PLoS ONE 2020, 15, e0233046. [Google Scholar] [CrossRef] [PubMed]
- Cassier, P.A.; Italiano, A.; Gomez-Roca, C.A.; Le Tourneau, C.; Toulmonde, M.; Cannarile, M.A.; Ries, C.; Brillouet, A.; Müller, C.; Jegg, A.M.; et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: A dose-escalation and dose-expansion phase 1 study. Lancet. Oncol. 2015, 16, 949–956. [Google Scholar] [CrossRef]
- Cieslewicz, M.; Tang, J.; Yu, J.L.; Cao, H.; Zavaljevski, M.; Motoyama, K.; Lieber, A.; Raines, E.W.; Pun, S.H. Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival. Proc. Natl. Acad. Sci. USA 2013, 110, 15919–15924. [Google Scholar] [CrossRef]
- Etzerodt, A.; Tsalkitzi, K.; Maniecki, M.; Damsky, W.; Delfini, M.; Baudoin, E.; Moulin, M.; Bosenberg, M.; Graversen, J.H.; Auphan-Anezin, N.; et al. Specific targeting of CD163(+) TAMs mobilizes inflammatory monocytes and promotes T cell-mediated tumor regression. J. Exp. Med. 2019, 216, 2394–2411. [Google Scholar] [CrossRef]
- Peña, C.G.; Nakada, Y.; Saatcioglu, H.D.; Aloisio, G.M.; Cuevas, I.; Zhang, S.; Miller, D.S.; Lea, J.S.; Wong, K.K.; DeBerardinis, R.J.; et al. LKB1 loss promotes endometrial cancer progression via CCL2-dependent macrophage recruitment. J. Clin. Investig. 2015, 125, 4063–4076. [Google Scholar] [CrossRef]
- Argyle, D.; Kitamura, T. Targeting macrophage-recruiting chemokines as a novel therapeutic strategy to prevent the progression of solid tumors. Front. Immunol. 2018, 9, 2629. [Google Scholar] [CrossRef]
- Bonapace, L.; Coissieux, M.M.; Wyckoff, J.; Mertz, K.D.; Varga, Z.; Junt, T.; Bentires-Alj, M. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature 2014, 515, 130–133. [Google Scholar] [CrossRef]
- Pienta, K.J.; Machiels, J.P.; Schrijvers, D.; Alekseev, B.; Shkolnik, M.; Crabb, S.J.; Li, S.; Seetharam, S.; Puchalski, T.A.; Takimoto, C.; et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Investig. New Drugs 2013, 31, 760–768. [Google Scholar] [CrossRef]
- Nywening, T.M.; Wang-Gillam, A.; Sanford, D.E.; Belt, B.A.; Panni, R.Z.; Cusworth, B.M.; Toriola, A.T.; Nieman, R.K.; Worley, L.A.; Yano, M.; et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: A single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet. Oncol. 2016, 17, 651–662. [Google Scholar] [CrossRef]
- Noel, M.; O’Reilly, E.M.; Wolpin, B.M.; Ryan, D.P.; Bullock, A.J.; Britten, C.D.; Linehan, D.C.; Belt, B.A.; Gamelin, E.C.; Ganguly, B.; et al. Phase 1b study of a small molecule antagonist of human chemokine (C-C motif) receptor 2 (PF-04136309) in combination with nab-paclitaxel/gemcitabine in first-line treatment of metastatic pancreatic ductal adenocarcinoma. Investig. New Drugs 2020, 38, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Horuk, R. Chemokine receptor antagonists: Overcoming developmental hurdles. Nat. Rev. Drug Discov. 2009, 8, 23–33. [Google Scholar] [CrossRef]
- Upadhyaya, C.; Jiao, X.; Ashton, A.; Patel, K.; Kossenkov, A.V.; Pestell, R.G. The G protein coupled receptor CCR5 in cancer. Adv. Cancer Res. 2020, 145, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Halama, N.; Zoernig, I.; Berthel, A.; Kahlert, C.; Klupp, F.; Suarez-Carmona, M.; Suetterlin, T.; Brand, K.; Krauss, J.; Lasitschka, F.; et al. Tumoral Immune Cell exploitation in colorectal cancer metastases can be targeted effectively by Anti-CCR5 therapy in cancer patients. Cancer Cell 2016, 29, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.K.; Seo, Y.D.; Pillarisetty, V.G. The CXCL12-CXCR4/CXCR7 axis as a mechanism of immune resistance in gastrointestinal malignancies. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- Kerkar, S.P.; Goldszmid, R.S.; Muranski, P.; Chinnasamy, D.; Yu, Z.; Reger, R.N.; Leonardi, A.J.; Morgan, R.A.; Wang, E.; Marincola, F.M.; et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J. Clin. Investig. 2011, 121, 4746–4757. [Google Scholar] [CrossRef]
- Veillette, A.; Chen, J. SIRPα-CD47 Immune checkpoint blockade in anticancer therapy. Trends Immunol. 2018, 39, 173–184. [Google Scholar] [CrossRef]
- Weiskopf, K.; Jahchan, N.S.; Schnorr, P.J.; Cristea, S.; Ring, A.M.; Maute, R.L.; Volkmer, A.K.; Volkmer, J.P.; Liu, J.; Lim, J.S.; et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J. Clin. Investig. 2016, 126, 2610–2620. [Google Scholar] [CrossRef]
- Piechutta, M.; Berghoff, A.S. New emerging targets in cancer immunotherapy: The role of Cluster of Differentiation 40 (CD40/TNFR5). ESMO Open 2019, 4, e000510. [Google Scholar] [CrossRef]
- Adams, S.; Kozhaya, L.; Martiniuk, F.; Meng, T.C.; Chiriboga, L.; Liebes, L.; Hochman, T.; Shuman, N.; Axelrod, D.; Speyer, J.; et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 6748–6757. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, M.E.; Perez-Gracia, J.L.; Rodríguez, I.; Alfaro, C.; Oñate, C.; Pérez, G.; Gil-Bazo, I.; Benito, A.; Inogés, S.; López-Diaz de Cerio, A.; et al. Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. 2018, 29, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Georgoudaki, A.M.; Prokopec, K.E.; Boura, V.F.; Hellqvist, E.; Sohn, S.; Östling, J.; Dahan, R.; Harris, R.A.; Rantalainen, M.; Klevebring, D.; et al. Reprogramming Tumor-Associated Macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. 2016, 15, 2000–2011. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, S.L.; Careddu, G.; Serio, S.; Consonni, F.M.; Maeda, A.; Viswanadha, S.; Vakkalanka, S.; Castagna, L.; Santoro, A.; Allavena, P.; et al. Targeting cancer cells and tumor microenvironment in preclinical and clinical models of hodgkin lymphoma using the dual PI3Kδ/γ inhibitor RP6530. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 1098–1112. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, M.M.; Messer, K.S.; Ralainirina, N.; Li, H.; Leem, C.J.; Gorjestani, S.; Woo, G.; Nguyen, A.V.; Figueiredo, C.C.; Foubert, P.; et al. PI3Kγ is a molecular switch that controls immune suppression. Nature 2016, 539, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, J.L.; Sotayo, A.; Ponichtera, H.E.; Castrillon, J.A.; Pourzia, A.L.; Schad, S.; Johnson, S.F.; Carrasco, R.D.; Lazo, S.; Bronson, R.T.; et al. Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature 2017, 543, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Baer, C.; Squadrito, M.L.; Laoui, D.; Thompson, D.; Hansen, S.K.; Kiialainen, A.; Hoves, S.; Ries, C.H.; Ooi, C.H.; De Palma, M. Suppression of microRNA activity amplifies IFN-γ-induced macrophage activation and promotes anti-tumour immunity. Nat. Cell Biol. 2016, 18, 790–802. [Google Scholar] [CrossRef]
- Lemke, G. Biology of the TAM receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a009076. [Google Scholar] [CrossRef]
- Myers, K.V.; Amend, S.R.; Pienta, K.J. Targeting Tyro3, Axl and MerTK (TAM receptors): Implications for macrophages in the tumor microenvironment. Mol. Cancer 2019, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Frady, L.N.; Bash, R.E.; Cohen, S.M.; Schorzman, A.N.; Su, Y.T.; Irvin, D.M.; Zamboni, W.C.; Wang, X.; Frye, S.V.; et al. MerTK as a therapeutic target in glioblastoma. Neuro Oncol. 2018, 20, 92–102. [Google Scholar] [CrossRef]
- Song, X.; Wang, H.; Logsdon, C.D.; Rashid, A.; Fleming, J.B.; Abbruzzese, J.L.; Gomez, H.F.; Evans, D.B.; Wang, H. Overexpression of receptor tyrosine kinase Axl promotes tumor cell invasion and survival in pancreatic ductal adenocarcinoma. Cancer 2011, 117, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Sadahiro, H.; Kang, K.D.; Gibson, J.T.; Minata, M.; Yu, H.; Shi, J.; Chhipa, R.; Chen, Z.; Lu, S.; Simoni, Y.; et al. Activation of the Receptor Tyrosine Kinase AXL Regulates the Immune Microenvironment in Glioblastoma. Cancer Res. 2018, 78, 3002–3013. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, G.; Alonso-Nocelo, M.; Vallespinos, M.; Hermann, P.C.; Alcalá, S.; García, C.P.; Martin-Hijano, L.; Valle, S.; Earl, J.; Cassiano, C.; et al. Tumor-associated macrophage-secreted 14-3-3ζ signals via AXL to promote pancreatic cancer chemoresistance. Oncogene 2019, 38, 5469–5485. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fei, M.; Zhang, G.; Liang, W.C.; Lin, W.; Wu, Y.; Piskol, R.; Ridgway, J.; McNamara, E.; Huang, H.; et al. Blockade of the phagocytic receptor MerTK on tumor-associated macrophages enhances P2X7R-dependent STING activation by tumor-derived cGAMP. Immunity 2020, 52, 357–373 e359. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Lew, E.D.; Seelige, R.; Tindall, E.A.; Walsh, C.; Fagan, P.C.; Lee, J.Y.; Nevarez, R.; Oh, J.; Tucker, K.D.; et al. Immuno-oncological Efficacy of RXDX-106, a Novel TAM (TYRO3, AXL, MER) Family Small-Molecule Kinase Inhibitor. Cancer Res. 2019, 79, 1996–2008. [Google Scholar] [CrossRef]
- Ireland, L.; Luckett, T.; Schmid, M.C.; Mielgo, A. Blockade of stromal Gas6 Alters Cancer Cell Plasticity, activates NK Cells, and inhibits pancreatic cancer metastasis. Front. Immunol. 2020, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Javeed, A.; Ashraf, M.; Riaz, A.; Ghafoor, A.; Afzal, S.; Mukhtar, M.M. Paclitaxel and immune system. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2009, 38, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Yan, X.; Chen, K.; Huang, Q.; Melancon, M.P.; Lopez, G.; Cheng, Z.; Li, C. Macrophages as a potential tumor-microenvironment target for noninvasive imaging of early response to anticancer therapy. Biomaterials 2018, 152, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Kodumudi, K.N.; Woan, K.; Gilvary, D.L.; Sahakian, E.; Wei, S.; Djeu, J.Y. A novel chemoimmunomodulating property of docetaxel: Suppression of myeloid-derived suppressor cells in tumor bearers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 4583–4594. [Google Scholar] [CrossRef] [PubMed]
- Dijkgraaf, E.M.; Heusinkveld, M.; Tummers, B.; Vogelpoel, L.T.; Goedemans, R.; Jha, V.; Nortier, J.W.; Welters, M.J.; Kroep, J.R.; van der Burg, S.H. Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res. 2013, 73, 2480–2492. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Menard, C.; Puig, P.E.; Ladoire, S.; Roux, S.; Martin, F.; Solary, E.; Le Cesne, A.; Zitvogel, L.; Chauffert, B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007, 56, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Jaffar, J.; Hellstrom, I.; Hellstrom, K.E. Administration of cyclophosphamide changes the immune profile of tumor-bearing mice. J. Immunother. (Hagerstown MD 1997) 2010, 33, 53–59. [Google Scholar] [CrossRef]
- Nakasone, E.S.; Askautrud, H.A.; Kees, T.; Park, J.H.; Plaks, V.; Ewald, A.J.; Fein, M.; Rasch, M.G.; Tan, Y.X.; Qiu, J.; et al. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell 2012, 21, 488–503. [Google Scholar] [CrossRef] [PubMed]
- Hannesdóttir, L.; Tymoszuk, P.; Parajuli, N.; Wasmer, M.H.; Philipp, S.; Daschil, N.; Datta, S.; Koller, J.B.; Tripp, C.H.; Stoitzner, P.; et al. Lapatinib and doxorubicin enhance the Stat1-dependent antitumor immune response. Eur. J. Immunol. 2013, 43, 2718–2729. [Google Scholar] [CrossRef] [PubMed]
- Soeda, A.; Morita-Hoshi, Y.; Makiyama, H.; Morizane, C.; Ueno, H.; Ikeda, M.; Okusaka, T.; Yamagata, S.; Takahashi, N.; Hyodo, I.; et al. Regular dose of gemcitabine induces an increase in CD14+ monocytes and CD11c+ dendritic cells in patients with advanced pancreatic cancer. Jpn. J. Clin. Oncol. 2009, 39, 797–806. [Google Scholar] [CrossRef]
- Buhtoiarov, I.N.; Sondel, P.M.; Wigginton, J.M.; Buhtoiarova, T.N.; Yanke, E.M.; Mahvi, D.A.; Rakhmilevich, A.L. Anti-tumour synergy of cytotoxic chemotherapy and anti-CD40 plus CpG-ODN immunotherapy through repolarization of tumour-associated macrophages. Immunology 2011, 132, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Kinn, J.; Zirakzadeh, A.A.; Sherif, A.; Norstedt, G.; Wikström, A.C.; Winqvist, O. The effects of chemotherapeutic drugs on human monocyte-derived dendritic cell differentiation and antigen presentation. Clin. Exp. Immunol. 2013, 172, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Loberg, R.D.; Ying, C.; Craig, M.; Day, L.L.; Sargent, E.; Neeley, C.; Wojno, K.; Snyder, L.A.; Yan, L.; Pienta, K.J. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007, 67, 9417–9424. [Google Scholar] [CrossRef] [PubMed]
- Wanderley, C.W.; Colón, D.F.; Luiz, J.P.M.; Oliveira, F.F.; Viacava, P.R.; Leite, C.A.; Pereira, J.A.; Silva, C.M.; Silva, C.R.; Silva, R.L.; et al. Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1 profile in a TLR4-Dependent manner. Cancer Res. 2018, 78, 5891–5900. [Google Scholar] [CrossRef]
- Chauhan, P.; Sodhi, A.; Tarang, S. Cisplatin-treated murine peritoneal macrophages induce apoptosis in L929 cells: Role of Fas-Fas ligand and tumor necrosis factor-tumor necrosis factor receptor 1. Anti-Cancer Drugs 2007, 18, 187–196. [Google Scholar] [CrossRef]
- Singh, R.A.; Sodhi, A. Antigen presentation by cisplatin-activated macrophages: Role of soluble factor(s) and second messengers. Immunol. Cell Biol. 1998, 76, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Bryniarski, K.; Szczepanik, M.; Ptak, M.; Zemelka, M.; Ptak, W. Influence of cyclophosphamide and its metabolic products on the activity of peritoneal macrophages in mice. Pharmacol. Rep. PR 2009, 61, 550–557. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Gazzerro, P.; Malfitano, A.M.; Proto, M.C.; Santoro, A.; Pisanti, S.; Caruso, M.G.; Notarnicola, M.; Messa, C.; Laezza, C.; Misso, G.; et al. Synergistic inhibition of human colon cancer cell growth by the cannabinoid CB1 receptor antagonist rimonabant and oxaliplatin. Oncol. Rep. 2010, 23, 171–175. [Google Scholar]
- Nazir, T.; Taha, N.; Islam, A.; Abraham, S.; Mahmood, A.; Mustafa, M. Monocytopenia; Induction by Vinorelbine, cisplatin and doxorubicin in breast, non-small cell lung and cervix cancer patients. Int. J. Health Sci. 2016, 10, 542–547. [Google Scholar] [CrossRef]
- Saeed, L.; Patnaik, M.M.; Begna, K.H.; Al-Kali, A.; Litzow, M.R.; Hanson, C.A.; Ketterling, R.P.; Porrata, L.F.; Pardanani, A.; Gangat, N.; et al. Prognostic relevance of lymphocytopenia, monocytopenia and lymphocyte-to-monocyte ratio in primary myelodysplastic syndromes: A single center experience in 889 patients. Blood Cancer J. 2017, 7, e550. [Google Scholar] [CrossRef]
- Tsai, C.S.; Chen, F.H.; Wang, C.C.; Huang, H.L.; Jung, S.M.; Wu, C.J.; Lee, C.C.; McBride, W.H.; Chiang, C.S.; Hong, J.H. Macrophages from irradiated tumors express higher levels of iNOS, arginase-I and COX-2, and promote tumor growth. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 499–507. [Google Scholar] [CrossRef]
- Prakash, H.; Klug, F.; Nadella, V.; Mazumdar, V.; Schmitz-Winnenthal, H.; Umansky, L. Low doses of gamma irradiation potentially modifies immunosuppressive tumor microenvironment by retuning tumor-associated macrophages: Lesson from insulinoma. Carcinogenesis 2016, 37, 301–313. [Google Scholar] [CrossRef]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-dose irradiation programs macrophage differentiation to an iNOS⁺/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef]
- Stafford, J.H.; Hirai, T.; Deng, L.; Chernikova, S.B.; Urata, K.; West, B.L.; Brown, J.M. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol. 2016, 18, 797–806. [Google Scholar] [CrossRef]
- Meng, Y.; Beckett, M.A.; Liang, H.; Mauceri, H.J.; van Rooijen, N.; Cohen, K.S.; Weichselbaum, R.R. Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Res. 2010, 70, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Kalbasi, A.; Komar, C.; Tooker, G.M.; Liu, M.; Lee, J.W.; Gladney, W.L.; Ben-Josef, E.; Beatty, G.L. Tumor-Derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, G.; Radlingmayr, A.; Rosenthal, S.; Rothe, R.; Jahns, J.; Hindemith, M.; Rödel, F.; Kamprad, F. Low-dose radiotherapy (LD-RT) and the modulation of iNOS expression in adjuvant-induced arthritis in rats. Int. J. Radiat. Biol. 2003, 79, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Tsukimoto, M.; Homma, T.; Mutou, Y.; Kojima, S. 0.5 Gy gamma radiation suppresses production of TNF-alpha through up-regulation of MKP-1 in mouse macrophage RAW264.7 cells. Radiat. Res. 2009, 171, 219–224. [Google Scholar] [CrossRef]
- Lödermann, B.; Wunderlich, R.; Frey, S.; Schorn, C.; Stangl, S.; Rödel, F.; Keilholz, L.; Fietkau, R.; Gaipl, U.S.; Frey, B. Low dose ionising radiation leads to a NF-κB dependent decreased secretion of active IL-1β by activated macrophages with a discontinuous dose-dependency. Int. J. Radiat. Biol. 2012, 88, 727–734. [Google Scholar] [CrossRef]
- Frey, B.; Hehlgans, S.; Rödel, F.; Gaipl, U.S. Modulation of inflammation by low and high doses of ionizing radiation: Implications for benign and malign diseases. Cancer Lett. 2015, 368, 230–237. [Google Scholar] [CrossRef]
- Wunderlich, R.; Ernst, A.; Rödel, F.; Fietkau, R.; Ott, O.; Lauber, K.; Frey, B.; Gaipl, U.S. Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clin. Exp. Immunol. 2015, 179, 50–61. [Google Scholar] [CrossRef]
- Teresa Pinto, A.; Laranjeiro Pinto, M.; Patrícia Cardoso, A.; Monteiro, C.; Teixeira Pinto, M.; Filipe Maia, A.; Castro, P.; Figueira, R.; Monteiro, A.; Marques, M.; et al. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci. Rep. 2016, 6, 18765. [Google Scholar] [CrossRef]
- Crittenden, M.R.; Cottam, B.; Savage, T.; Nguyen, C.; Newell, P.; Gough, M.J. Expression of NF-κB p50 in tumor stroma limits the control of tumors by radiation therapy. PLoS ONE 2012, 7, e39295. [Google Scholar] [CrossRef]
- Okubo, M.; Kioi, M.; Nakashima, H.; Sugiura, K.; Mitsudo, K.; Aoki, I.; Taniguchi, H.; Tohnai, I. M2-polarized macrophages contribute to neovasculogenesis, leading to relapse of oral cancer following radiation. Sci. Rep. 2016, 6, 27548. [Google Scholar] [CrossRef]
- Nowosielska, E.M.; Cheda, A.; Wrembel-Wargocka, J.; Janiak, M.K. Effect of low doses of low-let radiation on the innate anti-tumor reactions in radioresistant and radiosensitive mice. Dose-Response A Publ. Int. Hormesis Soc. 2012, 10, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Allouch, A.; Paoletti, A.; Leteur, C.; Mirjolet, C.; Martins, I.; Voisin, L.; Law, F.; Dakhli, H.; Mintet, E.; et al. NOX2-dependent ATM kinase activation dictates pro-inflammatory macrophage phenotype and improves effectiveness to radiation therapy. Cell Death Differ. 2017, 24, 1632–1644. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.T.; Pinto, M.L.; Velho, S.; Pinto, M.T.; Cardoso, A.P.; Figueira, R.; Monteiro, A.; Marques, M.; Seruca, R.; Barbosa, M.A.; et al. Intricate Macrophage-Colorectal Cancer Cell Communication in Response to Radiation. PLoS ONE 2016, 11, e0160891. [Google Scholar] [CrossRef]
- Seifert, L.; Werba, G.; Tiwari, S.; Giao Ly, N.N.; Nguy, S.; Alothman, S.; Alqunaibit, D.; Avanzi, A.; Daley, D.; Barilla, R.; et al. Radiation Therapy induces macrophages to suppress T-Cell responses against pancreatic tumors in mice. Gastroenterology 2016, 150, 1659–1672 e1655. [Google Scholar] [CrossRef] [PubMed]
- Arlauckas, S.P.; Garris, C.S.; Kohler, R.H.; Kitaoka, M.; Cuccarese, M.F.; Yang, K.S.; Miller, M.A.; Carlson, J.C.; Freeman, G.J.; Anthony, R.M.; et al. In Vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Zeng, S.; Vitiello, G.A.; Seifert, A.M.; Medina, B.D.; Beckman, M.J.; Loo, J.K.; Santamaria-Barria, J.; Maltbaek, J.H.; Param, N.J.; et al. Macrophages and CD8(+) T Cells mediate the antitumor efficacy of combined CD40 Ligation and imatinib therapy in gastrointestinal stromal tumors. Cancer Immunol. Res. 2018, 6, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Vonderheide, R.H. CD40 Agonist antibodies in cancer immunotherapy. Ann. Rev. Med. 2020, 71, 47–58. [Google Scholar] [CrossRef]
- Wiehagen, K.R.; Girgis, N.M.; Yamada, D.H.; Smith, A.A.; Chan, S.R.; Grewal, I.S.; Quigley, M.; Verona, R.I. Combination of CD40 Agonism and CSF-1R Blockade Reconditions Tumor-Associated Macrophages and Drives Potent Antitumor Immunity. Cancer Immunol. Res. 2017, 5, 1109–1121. [Google Scholar] [CrossRef]
- Francis, L.; Guo, Z.S.; Liu, Z.; Ravindranathan, R.; Urban, J.A.; Sathaiah, M.; Magge, D.; Kalinski, P.; Bartlett, D.L. Modulation of chemokines in the tumor microenvironment enhances oncolytic virotherapy for colorectal cancer. Oncotarget 2016, 7, 22174–22185. [Google Scholar] [CrossRef] [PubMed]
- Ehrig, K.; Kilinc, M.O.; Chen, N.G.; Stritzker, J.; Buckel, L.; Zhang, Q.; Szalay, A.A. Growth inhibition of different human colorectal cancer xenografts after a single intravenous injection of oncolytic vaccinia virus GLV-1h68. J. Transl. Med. 2013, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chen, W.Y.; Lin, S.F.; Wong, R.J. Epithelial-mesenchymal transition enhances response to oncolytic herpesviral therapy through nectin-1. Hum. Gene Ther. 2014, 25, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Meisen, W.H.; Wohleb, E.S.; Jaime-Ramirez, A.C.; Bolyard, C.; Yoo, J.Y.; Russell, L.; Hardcastle, J.; Dubin, S.; Muili, K.; Yu, J.; et al. The Impact of Macrophage- and Microglia-Secreted TNFα on Oncolytic HSV-1 Therapy in the Glioblastoma Tumor Microenvironment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 3274–3285. [Google Scholar] [CrossRef]
- Thorne, A.H.; Meisen, W.H.; Russell, L.; Yoo, J.Y.; Bolyard, C.M.; Lathia, J.D.; Rich, J.; Puduvalli, V.K.; Mao, H.; Yu, J.; et al. Role of cysteine- erich 61 protein (CCN1) in macrophage-mediated oncolytic herpes simplex virus clearance. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 1678–1687. [Google Scholar] [CrossRef]
- Jacobsen, K.; Russell, L.; Kaur, B.; Friedman, A. Effects of CCN1 and Macrophage Content on Glioma Virotherapy: A Mathematical Model. Bull. Math. Biol. 2015, 77, 984–1012. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chen, X.; Chu, J.; Xu, B.; Meisen, W.H.; Chen, L.; Zhang, L.; Zhang, J.; He, X.; Wang, Q.E.; et al. TGFβ treatment enhances glioblastoma virothrapy by inhibiting the innate immune response. Cancer Res. 2015, 75, 5273–5282. [Google Scholar] [CrossRef] [PubMed]
- van den Bossche, W.B.L.; Kleijn, A.; Teunissen, C.E.; Voerman, J.S.A.; Teodosio, C.; Noske, D.P.; van Dongen, J.J.M.; Dirven, C.M.F.; Lamfers, M.L.M. Oncolytic virotherapy in glioblastoma patients induces a tumor macrophage phenotypic shift leading to an altered glioblastoma microenvironment. Neuro Oncol. 2018, 20, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.L.; Barf, M.; Geletneky, K.; Unterberg, A.; Rommelaere, J. Immunotherapeutic potential of Oncolytic H-1 parvovirus: Hints of glioblastoma microenvironment conversion towards immunogenicity. Viruses 2017, 9, 382. [Google Scholar] [CrossRef]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell 2017, 32, 253–267 e255. [Google Scholar] [CrossRef]
- Grekova, S.P.; Aprahamian, M.; Daeffler, L.; Leuchs, B.; Angelova, A.; Giese, T.; Galabov, A.; Heller, A.; Giese, N.A.; Rommelaere, J.; et al. Interferon γ improves the vaccination potential of oncolytic parvovirus H-1PV for the treatment of peritoneal carcinomatosis in pancreatic cancer. Cancer Biol. Ther. 2011, 12, 888–895. [Google Scholar] [CrossRef][Green Version]
- Watanabe, K.; Luo, Y.; Da, T.; Guedan, S.; Ruella, M.; Scholler, J.; Keith, B.; Young, R.M.; Engels, B.; Sorsa, S.; et al. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Moreno, R.; Milenova, I.; Liljenfeldt, L.; Dieterich, L.C.; Christiansson, L.; Karlsson, H.; Ullenhag, G.; Mangsbo, S.M.; Dimberg, A.; et al. Activation of myeloid and endothelial cells by CD40L gene therapy supports T-cell expansion and migration into the tumor microenvironment. Gene Ther. 2017, 24, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.Q.; Zhang, L.; Ohba, K.; Ye, M.; Ichiyama, K.; Yamamoto, N. Macrophage response to oncolytic paramyxoviruses potentiates virus-mediated tumor cell killing. Eur. J. Immunol. 2016, 46, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Gerseny, H.; Zhang, Z.; Chen, Y.J.; Berg, A.; Zhang, Z.; Stock, S.; Seth, P. Oncolytic adenovirus expressing soluble TGFβ receptor II-Fc-mediated inhibition of established bone metastases: A safe and effective systemic therapeutic approach for breast cancer. Mol. Ther. J. Am. Soc. Gene Ther. 2011, 19, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Lavilla-Alonso, S.; Bauer, M.M.; Abo-Ramadan, U.; Ristimäki, A.; Halavaara, J.; Desmond, R.A.; Wang, D.; Escutenaire, S.; Ahtiainen, L.; Saksela, K.; et al. Macrophage metalloelastase (MME) as adjuvant for intra-tumoral injection of oncolytic adenovirus and its influence on metastases development. Cancer Gene Ther. 2012, 19, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Passaro, C.; Borriello, F.; Vastolo, V.; Di Somma, S.; Scamardella, E.; Gigantino, V.; Franco, R.; Marone, G.; Portella, G. The oncolytic virus dl922-947 reduces IL-8/CXCL8 and MCP-1/CCL2 expression and impairs angiogenesis and macrophage infiltration in anaplastic thyroid carcinoma. Oncotarget 2016, 7, 1500–1515. [Google Scholar] [CrossRef]
- Liu, Y.P.; Suksanpaisan, L.; Steele, M.B.; Russell, S.J.; Peng, K.W. Induction of antiviral genes by the tumor microenvironment confers resistance to virotherapy. Sci. Rep. 2013, 3, 2375. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).