The Density and Length of Filopodia Associate with the Activity of Hyaluronan Synthesis in Tumor Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Transfections
2.3. Actin Staining
2.4. Confocal Microscopy
2.5. FiloQuant Analysis
2.6. Hyaluronan Assay
2.7. Statistical Analyses
3. Results
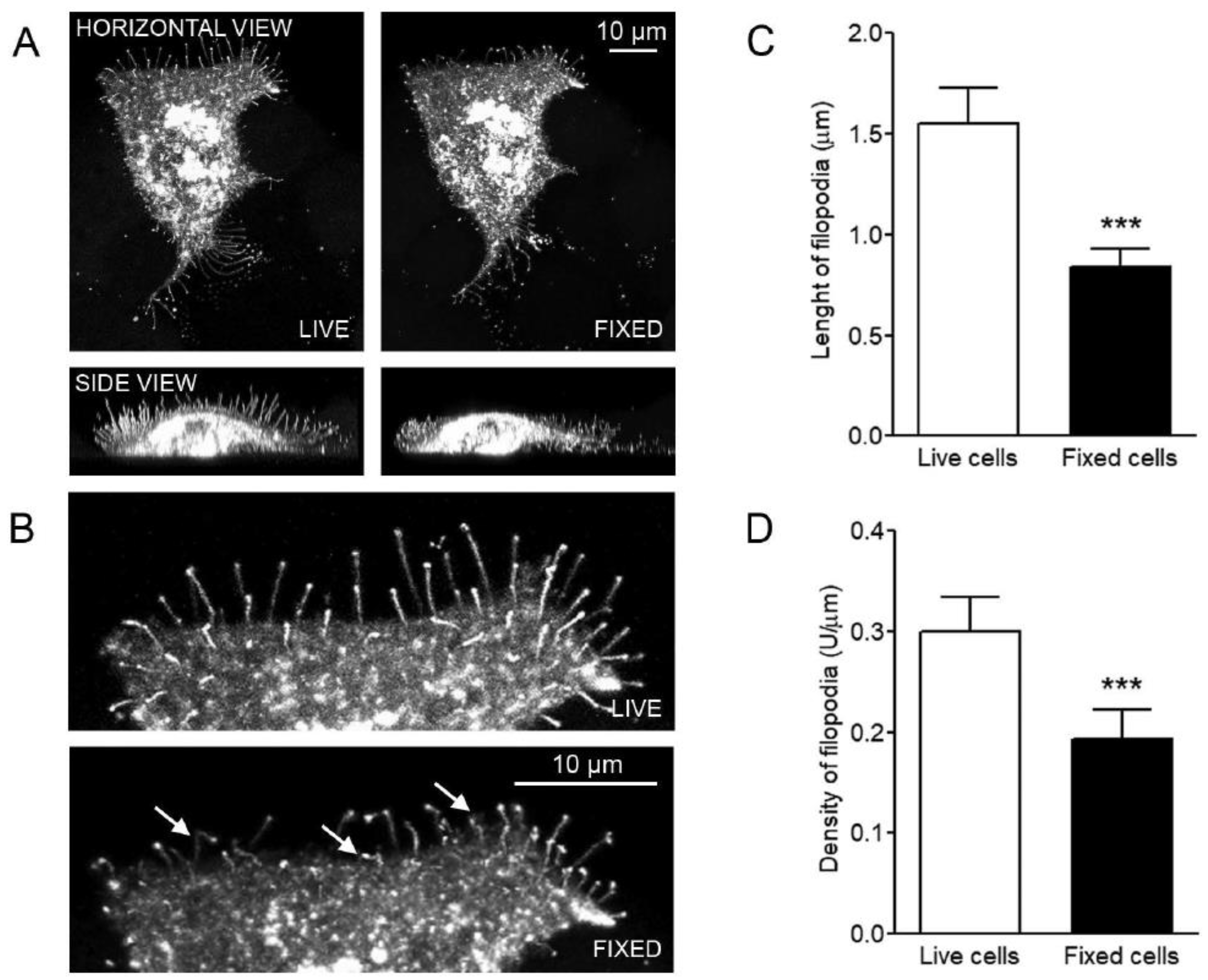
3.1. GFP-HAS3-induced Filopodia are Sensitive to Fixation
3.2. Comparison of EGFP-HAS3 and Phalloidin Signal in the Detection of Filopodia with FiloQuant Software
3.3. Quantification of the Effect of HAS3 Expression on the Density and Average Length of Filopodia
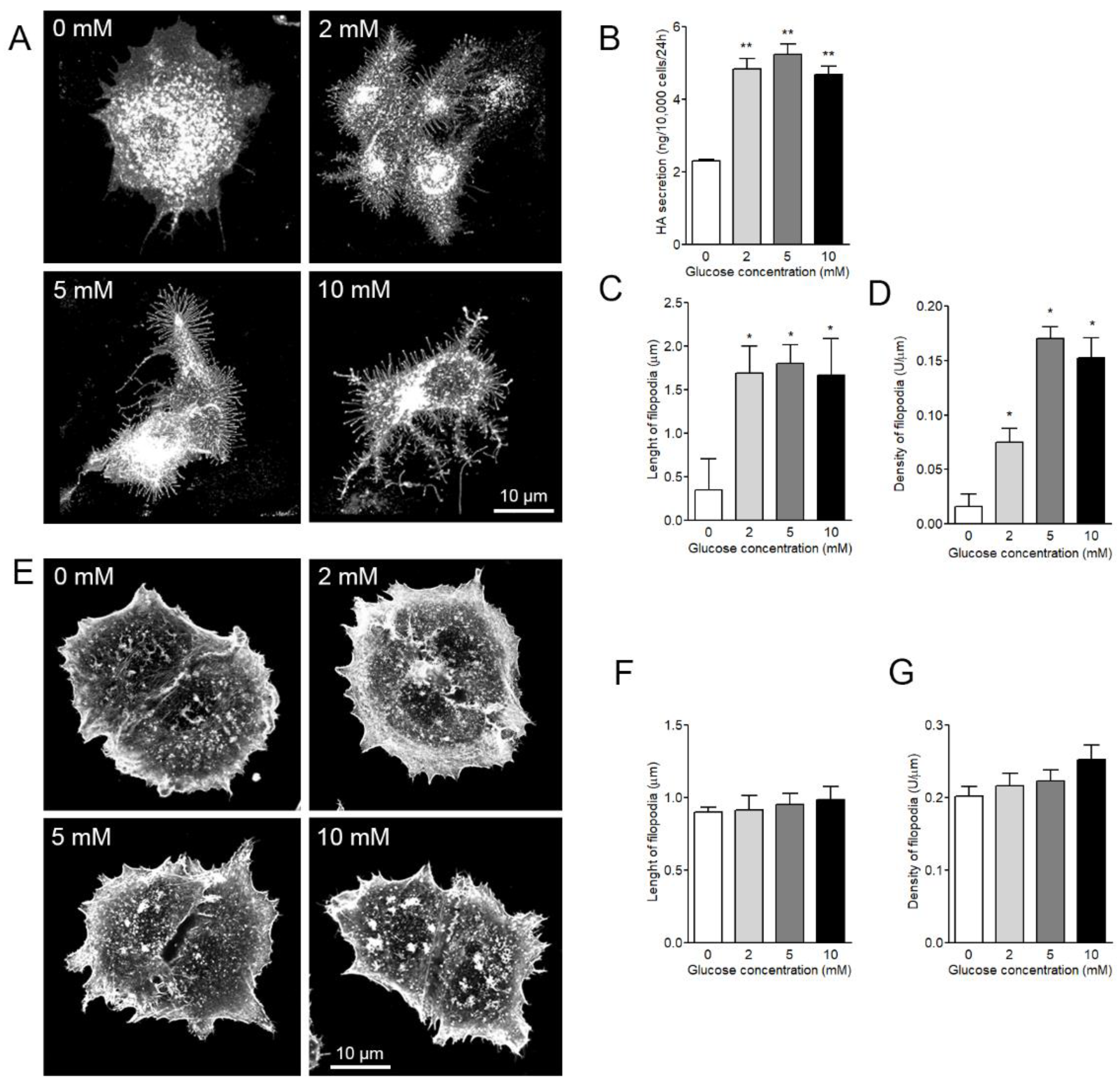
3.4. Both Length and Density of HAS3-Induced Filopodia are Dependent on Glucose Levels
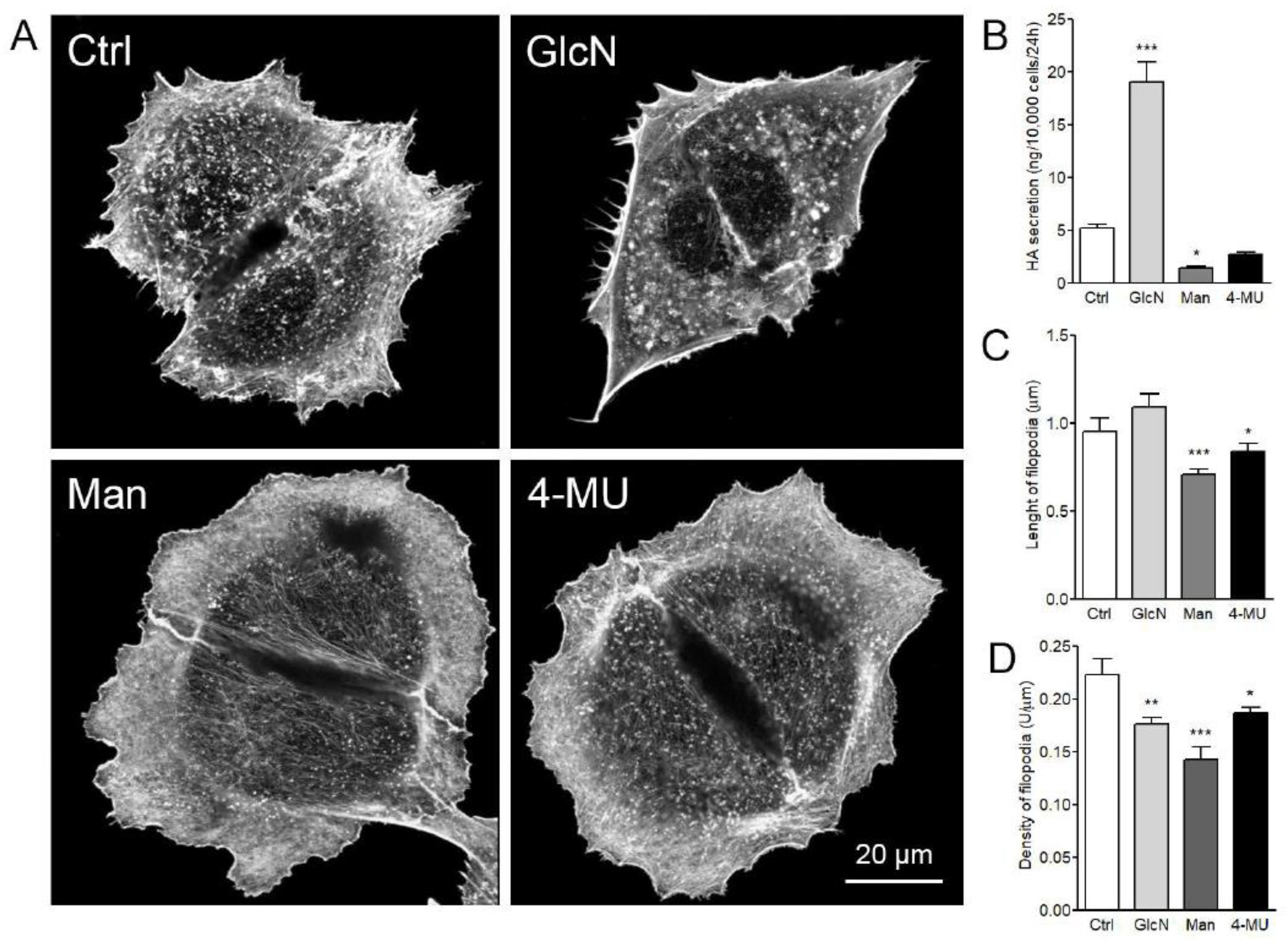
3.5. Effect of Glucosamine, Mannose, and 4-MU Treatment on HAS3-Induced Filopodia Formation
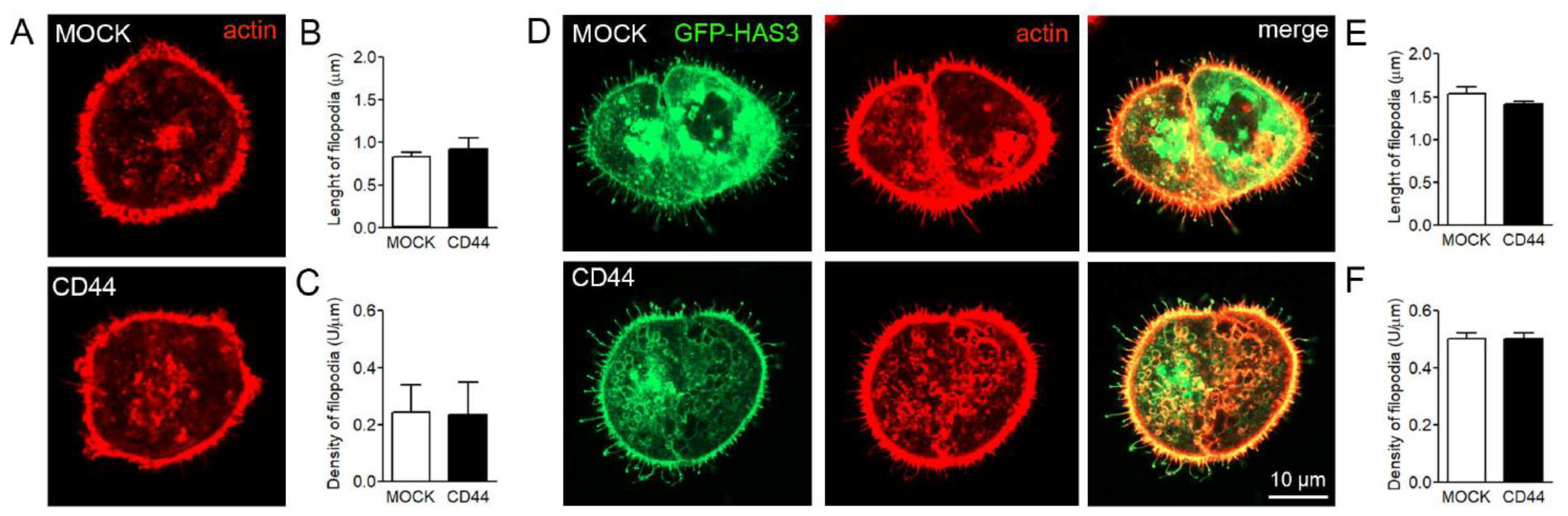
3.6. Effect of CD44 Expression on Filopodia Length and Numbers in MKN74 Cells
3.7. HA Secretion and Filopodial Growth in MCF10A and MCF10CA Breast Cancer Cell Lines
4. Discussion
4.1. FiloQuant Analysis of GFP-HAS3-Induced Filopodia
4.2. Filopodial Growth Is Associated with Hyaluronan Synthesis Activity
4.3. Involvement of CD44 in Filopodia Formation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chhabra, E.S.; Higgs, H.N. The many faces of actin: Matching assembly factors with cellular structures. Nat. Cell Biol. 2007, 9, 1110–1121. [Google Scholar] [CrossRef]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef]
- Sato, Y.; Nagatoshi, K.; Hamano, A.; Imamura, Y.; Huss, D.; Uchida, S.; Lansford, R. Basal filopodia and vascular mechanical stress organize fibronectin into pillars bridging the mesoderm-endoderm gap. Development 2017, 144, 281–291. [Google Scholar] [CrossRef]
- Sagar; Pröls, F.; Wiegreffe, C.; Scaal, M. Communication between distant epithelial cells by filopodia-like protrusions during embryonic development. Development 2015, 142, 665–671. [Google Scholar] [CrossRef]
- Khurana, S.; George, S.P. The role of actin bundling proteins in the assembly of filopodia in epithelial cells. Cell Adhes. Migr. 2011, 5, 1–12. [Google Scholar] [CrossRef]
- Korobova, F.; Svitkina, T.M. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol. Biol. Cell 2010, 21, 165–176. [Google Scholar] [CrossRef]
- Machesky, L.M.; Lia, A. Fascin: Invasive filopodia promoting metastasis. Commun. Integr. Biol. 2010, 3, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, T.; Aishima, S.; Taketomi, A.; Nishihara, Y.; Fujita, N.; Sanefuji, K.; Sugimachi, K.; Yamashita, Y.; Maehara, Y.; Tsuneyoshi, M. Fascin overexpression is involved in carcinogenesis and prognosis of human intrahepatic cholangiocarcinoma: Immunohistochemical and molecular analysis. Hum. Pathol. 2009, 40, 174–180. [Google Scholar] [CrossRef]
- Daponte, A.; Ioannou, M.; Mylonis, I.; Simos, G.; Minas, M.; Messinis, I.E.; Koukoulis, G. Prognostic significance of hypoxia-inducible factor 1 alpha (HIF-1alpha) expression in serous ovarian cancer: An immunohistochemical study. BMC Cancer 2008, 8, 1310–1320. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Rilla, K.; Siiskonen, H.; Spicer, A.P.; Hyttinen, J.M.T.; Tammi, M.I.; Tammi, R.H. Plasma membrane residence of hyaluronan synthase is coupled to its enzymatic activity. J. Biol. Chem. 2005, 280, 31890–31897. [Google Scholar] [CrossRef]
- Kultti, A.; Rilla, K.; Tiihonen, R.; Spicer, A.P.; Tammi, R.H.; Tammi, M.I. Hyaluronan synthesis induces microvillus-like cell surface protrusions. J. Biol. Chem. 2006, 281, 15821–15828. [Google Scholar] [CrossRef]
- Koistinen, V.; Kärnä, R.; Koistinen, A.; Arjonen, A.; Tammi, M.; Rilla, K. Cell protrusions induced by hyaluronan synthase 3 (HAS3) resemble mesothelial microvilli and share cytoskeletal features of filopodia. Exp. Cell Res. 2015, 337, 179–191. [Google Scholar] [CrossRef]
- Twarock, S.; Tammi, M.I.; Savani, R.C.; Fischer, J.W. Hyaluronan stabilizes focal adhesions, filopodia, and the proliferative phenotype in esophageal squamous carcinoma cells. J. Biol. Chem. 2010, 285, 23276–23284. [Google Scholar] [CrossRef]
- Rilla, K.; Tiihonen, R.; Kultti, A.; Tammi, M.; Tammi, R. Pericellular hyaluronan coat visualized in live cells with a fluorescent probe is scaffolded by plasma membrane protrusions. J. Histochem. Cytochem. 2008, 56, 901–910. [Google Scholar] [CrossRef]
- Rilla, K.; Koistinen, A. Correlative Light and Electron Microscopy Reveals the HAS3-Induced Dorsal Plasma Membrane Ruffles. Int. J. Cell Biol. 2015, 2015, 769163. [Google Scholar] [CrossRef]
- Nilufar, S.; Morrow, A.A.; Lee, J.M.; Perkins, T.J. FiloDetect: Automatic detection of filopodia from fluorescence microscopy images. BMC Syst. Biol. 2013, 7, 66. [Google Scholar] [CrossRef]
- Tsygankov, D.; Bilancia, C.G.; Vitriol, E.A.; Hahn, K.M.; Peifer, M.; Elston, T.C. CellGeo: A computational platform for the analysis of shape changes in cells with complex geometries. J. Cell Biol. 2014, 204, 443–460. [Google Scholar] [CrossRef]
- Barry, D.J.; Durkin, C.H.; Abella, J.V.; Way, M. Open source software for quantification of cell migration, protrusions, and fluorescence intensities. J. Cell Biol. 2015, 209, 163–180. [Google Scholar] [CrossRef]
- Jacquemet, G.; Paatero, I.; Carisey, A.F.; Padzik, A.; Orange, J.S.; Hamidi, H.; Ivaska, J. FiloQuant reveals increased filopodia density during breast cancer progression. J. Cell Biol. 2017, 216, 3387–3403. [Google Scholar] [CrossRef]
- Siiskonen, H.; Rilla, K.; Kärnä, R.; Bart, G.; Jing, W.; Haller, M.F.; Deangelis, P.L.; Tammi, R.H.; Tammi, M.I. Hyaluronan in cytosol-Microinjection-based probing of its existence and suggested functions. Glycobiology 2013, 23, 222–231. [Google Scholar] [CrossRef]
- Härkönen, K.; Oikari, S.; Kyykallio, H.; Capra, J.; Hakkola, S.; Ketola, K.; Thanigai Arasu, U.; Daaboul, G.; Malloy, A.; Oliveira, C.; et al. CD44s Assembles Hyaluronan Coat on Filopodia and Extracellular Vesicles and Induces Tumorigenicity of MKN74 Gastric Carcinoma Cells. Cells 2019, 8, 276. [Google Scholar] [CrossRef]
- Rilla, K.; Pasonen-Seppänen, S.; Kärnä, R.; Karjalainen, H.M.; Törrönen, K.; Koistinen, V.; Tammi, M.I.; Tammi, R.H.; Teräväinen, T.; Manninen, A. HAS3-induced accumulation of hyaluronan in 3D MDCK cultures results in mitotic spindle misorientation and disturbed organization of epithelium. Histochem. Cell Biol. 2012, 137, 153–164. [Google Scholar] [CrossRef]
- Rilla, K.; Oikari, S.; Jokela, T.A.; Hyttinen, J.M.T.; Kärnä, R.; Tammi, R.H.; Tammi, M.I. Hyaluronan synthase 1 (HAS1) requires higher cellular udp-glcnac concentration than HAS2 and HAS3. J. Biol. Chem. 2013, 288, 5973–5983. [Google Scholar] [CrossRef]
- Deen, A.J.; Arasu, U.T.; Pasonen-Seppänen, S.; Hassinen, A.; Takabe, P.; Wojciechowski, S.; Kärnä, R.; Rilla, K.; Kellokumpu, S.; Tammi, R.; et al. UDP-sugar substrates of HAS3 regulate its O-GlcNAcylation, intracellular traffic, extracellular shedding and correlate with melanoma progression. Cell. Mol. Life Sci. 2016, 73, 3183–3204. [Google Scholar] [CrossRef]
- Wang, A.; Hascall, V.C. Hyaluronan Structures Synthesized by Rat Mesangial Cells in Response to Hyperglycemia Induce Monocyte Adhesion. J. Biol. Chem. 2004, 279, 10279–10285. [Google Scholar] [CrossRef]
- Lin, W.; Shuster, S.; Maibach, H.I.; Stern, R. Patterns of Hyaluronan Staining Are Modified by Fixation Techniques. J. Histochem. Cytochem. 1997, 45, 1157–1163. [Google Scholar] [CrossRef]
- Arjonen, A.; Kaukonen, R.; Ivaska, J. Filopodia and adhesion in cancer cell motility. Cell Adh. Migr. 2011, 5, 421–430. [Google Scholar] [CrossRef]
- Passi, A.; Vigetti, D.; Buraschi, S.; Iozzo, R.V. Dissecting the role of hyaluronan synthases in the tumor microenvironment. FEBS J. 2019, 286, 2937–2949. [Google Scholar] [CrossRef] [PubMed]
- Pusch, A.; Boeckenhoff, A.; Glaser, T.; Kaminski, T.; Kirfel, G.; Hans, M.; Steinfarz, B.; Swandulla, D.; Kubitscheck, U.; Gieselmann, V.; et al. CD44 and hyaluronan promote invasive growth of B35 neuroblastoma cells into the brain. Biochim. Biophys. Acta-Mol. Cell Res. 2010, 1803, 261–274. [Google Scholar] [CrossRef]
- Evanko, S.P.; Potter-Perigo, S.; Johnson, P.Y.; Wight, T.N. Organization of hyaluronan and versican in the extracellular matrix of human fibroblasts treated with the viral mimetic poly I:C. J. Histochem. Cytochem. 2009, 57, 1041–1060. [Google Scholar] [CrossRef] [PubMed]
- Meran, S.; Martin, J.; Luo, D.D.; Steadman, R.; Phillips, A. Interleukin-1β induces hyaluronan and CD44-dependent cell protrusions that facilitate fibroblast-monocyte binding. Am. J. Pathol. 2013, 182, 2223–2240. [Google Scholar] [CrossRef] [PubMed]
- Docampo, M.J.; Zanna, G.; Fondevila, D.; Cabrera, J.; López-Iglesias, C.; Carvalho, A.; Cerrato, S.; Ferrer, L.; Bassols, A. Increased HAS2-driven hyaluronic acid synthesis in shar-pei dogs with hereditary cutaneous hyaluronosis (mucinosis). Vet. Dermatol. 2011, 22, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, G.; Hamidi, H.; Ivaska, J. Filopodia in cell adhesion, 3D migration and cancer cell invasion. Curr. Opin. Cell Biol. 2015, 36, 23–31. [Google Scholar] [CrossRef]
- Cowman, M.K.; Schmidt, T.A.; Raghavan, P.; Stecco, A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Research 2015, 4, 622. [Google Scholar] [CrossRef]
- Richter, R.P.; Baranova, N.S.; Day, A.J.; Kwok, J.C. Glycosaminoglycans in extracellular matrix organisation: Are concepts from soft matter physics key to understanding the formation of perineuronal nets? Curr. Opin. Struct. Biol. 2018, 50, 65–74. [Google Scholar] [CrossRef]
- Shurer, C.R.; Kuo, J.C.-H.; Roberts, L.M.; Gandhi, J.G.; Colville, M.J.; Enoki, T.A.; Pan, H.; Su, J.; Noble, J.M.; Hollander, M.J.; et al. Physical Principles of Membrane Shape Regulation by the Glycocalyx. Cell 2019, 177, 1757–1770. [Google Scholar] [CrossRef]
- Yonemura, S.; Hirao, M.; Doi, Y.; Takahashi, N.; Kondo, T.; Tsukita, S.; Tsukita, S. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J. Cell Biol. 1998, 140, 885–895. [Google Scholar] [CrossRef]
- Deboux, C.; Ladraa, S.; Cazaubon, S.; Ghribi-Mallah, S.; Weiss, N.; Chaverot, N.; Couraud, P.O.; Evercooren, A.B. Van Overexpression of CD44 in Neural Precursor Cells Improves Trans- Endothelial Migration and Facilitates Their Invasion of Perivascular Tissues In Vivo. PLoS ONE 2013, 8, e57430. [Google Scholar] [CrossRef]
- Roszkowska, M.; Skupien, A.; Wójtowicz, T.; Konopka, A.; Gorlewicz, A.; Kisiel, M.; Bekisz, M.; Ruszczycki, B.; Dolezyczek, H.; Rejmak, E.; et al. CD44: A novel synaptic cell adhesion molecule regulating structural and functional plasticity of dendritic spines. Mol. Biol. Cell 2016, 27, 4055–4066. [Google Scholar] [CrossRef]
- Wolf, K.J.; Shukla, P.; Springer, K.; Lee, S.; Coombes, J.D.; Choy, C.J.; Kenny, S.J.; Xu, K.; Kumar, S. A mode of cell adhesion and migration facilitated by CD44-dependent microtentacles. Proc. Natl. Acad. Sci. USA 2020, 117, 11432–11443. [Google Scholar] [CrossRef]


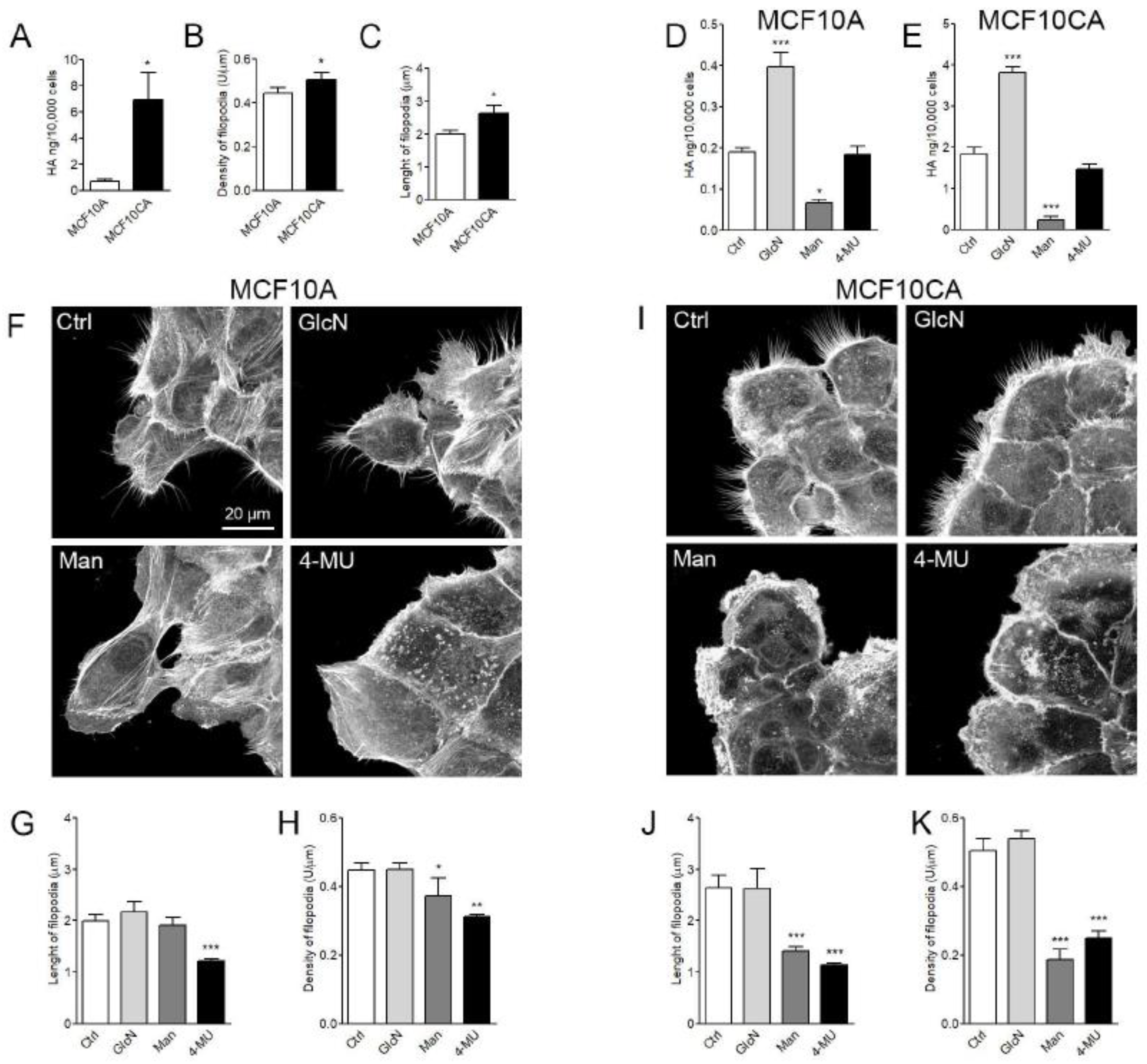
| Cell Line | Hyaluronan Secretion (ng/10,000 cells/24 h) | Average Density of Filopodia (U/µm) | Average Length of Filopodia (µm) |
|---|---|---|---|
| MCF-7-HAS3 Dox- | 0.11 | 0.18 | 0.82 |
| MCF-7-HAS3 0 mM Glc | 2.30 | 0.20 | 0.90 |
| MCF-7-HAS3 2 mM Glc | 4.84 | 0.21 | 0.91 |
| MCF-7-HAS3 5 mM Glc | 5.23 | 0.22 | 0.95 |
| MCF-7-HAS3 10 mM Glc | 4.69 | 0.25 | 0.98 |
| MCF-7-HAS3 + GlcN | 19.0 | 0.18 | 1.10 |
| MCF-7-HAS3 + Man | 1.41 | 0.14 | 0.70 |
| MCF-7-HAS3 + 4-MU | 2.70 | 0.19 | 0.84 |
| MKN74 MOCK | 2.50 | 0.25 | 0.80 |
| MKN74 CD44 | 1.90 | 0.24 | 0.90 |
| MCF10A | 0.19 | 0.45 | 1.99 |
| MCF10A + GlcN | 0.40 | 0.45 | 2.16 |
| MCF10A + Man | 0.07 | 0.37 | 1.92 |
| MCF10A + 4-MU | 0.18 | 0.31 | 1.22 |
| MCF10CA | 1.84 | 0.50 | 2.64 |
| MCF10CA + GlcN | 3.81 | 0.54 | 2.62 |
| MCF10CA + Man | 0.24 | 0.19 | 1.40 |
| MCF10CA + 4-MU | 1.46 | 0.25 | 1.14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyykallio, H.; Oikari, S.; Bueno Álvez, M.; Gallardo Dodd, C.J.; Capra, J.; Rilla, K. The Density and Length of Filopodia Associate with the Activity of Hyaluronan Synthesis in Tumor Cells. Cancers 2020, 12, 1908. https://doi.org/10.3390/cancers12071908
Kyykallio H, Oikari S, Bueno Álvez M, Gallardo Dodd CJ, Capra J, Rilla K. The Density and Length of Filopodia Associate with the Activity of Hyaluronan Synthesis in Tumor Cells. Cancers. 2020; 12(7):1908. https://doi.org/10.3390/cancers12071908
Chicago/Turabian StyleKyykallio, Heikki, Sanna Oikari, María Bueno Álvez, Carlos José Gallardo Dodd, Janne Capra, and Kirsi Rilla. 2020. "The Density and Length of Filopodia Associate with the Activity of Hyaluronan Synthesis in Tumor Cells" Cancers 12, no. 7: 1908. https://doi.org/10.3390/cancers12071908
APA StyleKyykallio, H., Oikari, S., Bueno Álvez, M., Gallardo Dodd, C. J., Capra, J., & Rilla, K. (2020). The Density and Length of Filopodia Associate with the Activity of Hyaluronan Synthesis in Tumor Cells. Cancers, 12(7), 1908. https://doi.org/10.3390/cancers12071908





