Determinants of Sensitivity to Radiotherapy in Endometrial Cancer
Abstract
1. Introduction
2. Current State of the Art in Treatment of Endometrial Cancer
3. Signaling Pathways and Cellular Processes Modulating Radiotherapy Response in Endometrial Cancer and Preclinical Investigations
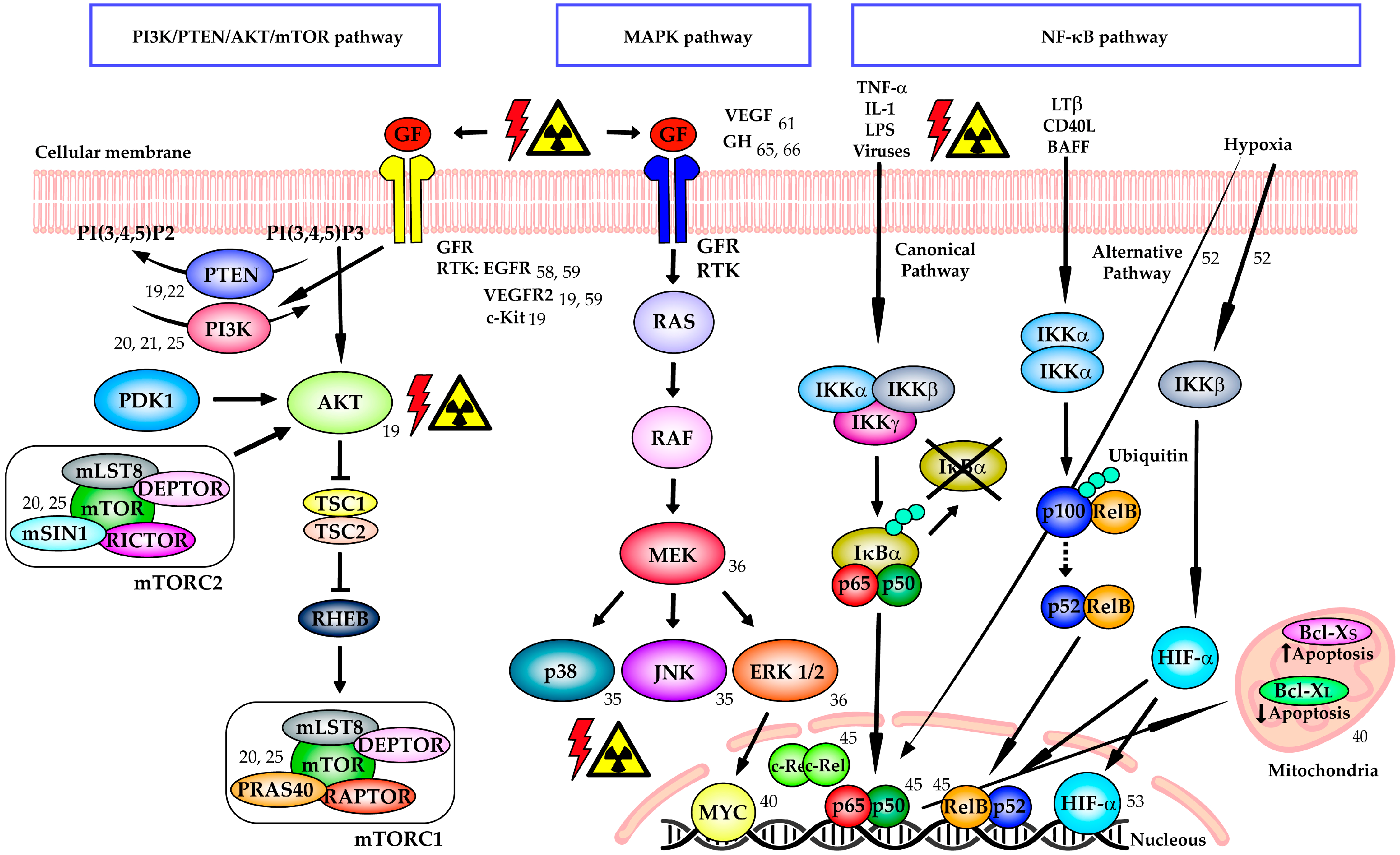
3.1. PI3K/PTEN/AKT/mTOR Signaling Pathway
3.2. MAPK Signaling Pathway
3.3. NF-κB Signaling Pathway
3.4. Growth Factor Receptors and Growth Hormone
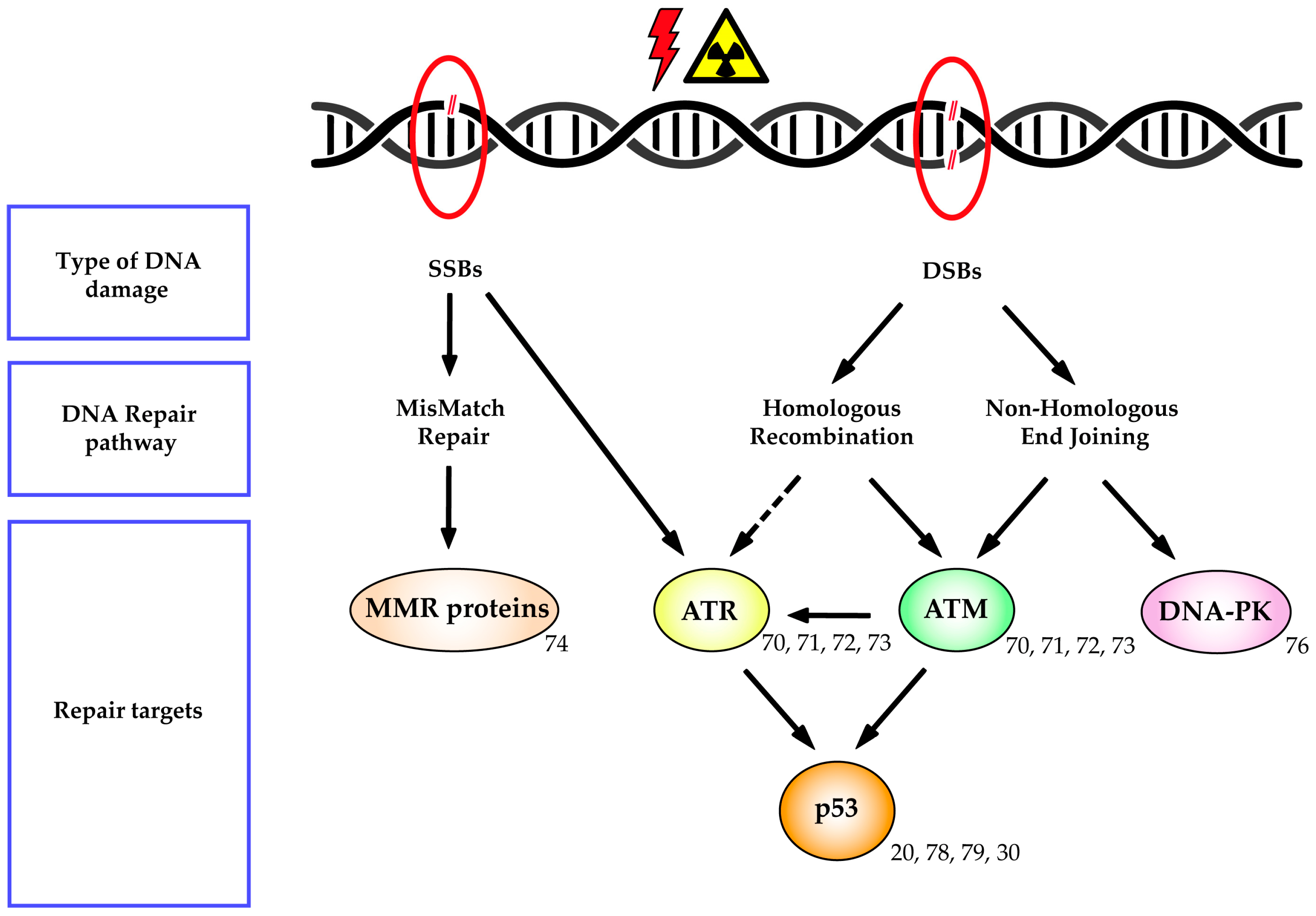
3.5. DNA Repair Mechanisms
3.6. Immune System
3.7. Others
4. Functional Assays that Assess Radiosensitivity
5. Current Clinical Trials Employing Agents with Radiosensitization Properties in Endometrial Cancer
6. Cutting-Edge Advances in Radiotherapy for Endometrial Cancer
6.1. Modern Radiotherapy
6.2. Radiogenomics
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Group, A.E.S.; Blake, P.; Swart, A.M.; Orton, J.; Kitchener, H.; Whelan, T.; Lukka, H.; Eisenhauer, E.; Bacon, M.; Tu, D.; et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): Pooled trial results, systematic review, and meta-analysis. Lancet 2009, 373, 137–146. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N.; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; D’Amico, R.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): Patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019, 20, 1273–1285. [Google Scholar] [CrossRef]
- Geisler, H.E. The use of megestrol acetate in the treatment of advanced malignant lesions of the endometrium. Gynecol. Oncol. 1973, 1, 340–344. [Google Scholar] [CrossRef]
- Makker, V.; Taylor, M.H.; Aghajanian, C.; Oaknin, A.; Mier, J.; Cohn, A.L.; Romeo, M.; Bratos, R.; Brose, M.S.; DiSimone, C.; et al. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef]
- Shepherd, J.H. Revised FIGO staging for gynaecological cancer. Br. J. Obs. Gynaecol. 1989, 96, 889–892. [Google Scholar] [CrossRef]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obs. 2009, 105, 103–104. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; Gonzalez-Martin, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Randall, M.E.; Filiaci, V.; McMeekin, D.S.; von Gruenigen, V.; Huang, H.; Yashar, C.M.; Mannel, R.S.; Kim, J.W.; Salani, R.; DiSilvestro, P.A.; et al. Phase III Trial: Adjuvant Pelvic Radiation Therapy Versus Vaginal Brachytherapy Plus Paclitaxel/Carboplatin in High-Intermediate and High-Risk Early Stage Endometrial Cancer. J. Clin. Oncol. 2019, 37, 1810–1818. [Google Scholar] [CrossRef] [PubMed]
- Matei, D.; Filiaci, V.; Randall, M.E.; Mutch, D.; Steinhoff, M.M.; DiSilvestro, P.A.; Moxley, K.M.; Kim, Y.M.; Powell, M.A.; O’Malley, D.M.; et al. Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. N. Engl. J. Med. 2019, 380, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309. [Google Scholar] [CrossRef]
- Risinger, J.I.; Hayes, K.; Maxwell, G.L.; Carney, M.E.; Dodge, R.K.; Barrett, J.C.; Berchuck, A. PTEN mutation in endometrial cancers is associated with favorable clinical and pathologic characteristics. Clin. Cancer Res. 1998, 4, 3005–3010. [Google Scholar]
- Salvesen, H.B.; Carter, S.L.; Mannelqvist, M.; Dutt, A.; Getz, G.; Stefansson, I.M.; Raeder, M.B.; Sos, M.L.; Engelsen, I.B.; Trovik, J.; et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc. Natl. Acad. Sci. USA 2009, 106, 4834–4839. [Google Scholar] [CrossRef]
- Cheung, L.W.; Hennessy, B.T.; Li, J.; Yu, S.; Myers, A.P.; Djordjevic, B.; Lu, Y.; Stemke-Hale, K.; Dyer, M.D.; Zhang, F.; et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 2011, 1, 170–185. [Google Scholar] [CrossRef]
- Shoji, K.; Oda, K.; Nakagawa, S.; Hosokawa, S.; Nagae, G.; Uehara, Y.; Sone, K.; Miyamoto, Y.; Hiraike, H.; Hiraike-Wada, O.; et al. The oncogenic mutation in the pleckstrin homology domain of AKT1 in endometrial carcinomas. Br. J. Cancer 2009, 101, 145–148. [Google Scholar] [CrossRef]
- Wang, E.; Sorolla, A. Sensitizing endometrial cancer to ionizing radiation by multi-tyrosine kinase inhibition. J. Gynaecol. Oncol. 2020, 31, e29. [Google Scholar] [CrossRef]
- Miyasaka, A.; Oda, K.; Ikeda, Y.; Sone, K.; Fukuda, T.; Inaba, K.; Makii, C.; Enomoto, A.; Hosoya, N.; Tanikawa, M.; et al. PI3K/mTOR pathway inhibition overcomes radioresistance via suppression of the HIF1-alpha/VEGF pathway in endometrial cancer. Gynaecol. Oncol. 2015, 138, 174–180. [Google Scholar] [CrossRef]
- Yard, B.D.; Adams, D.J.; Chie, E.K.; Tamayo, P.; Battaglia, J.S.; Gopal, P.; Rogacki, K.; Pearson, B.E.; Phillips, J.; Raymond, D.P.; et al. A genetic basis for the variation in the vulnerability of cancer to DNA damage. Nat. Commun. 2016, 7, 11428. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Patterson, A.L.; George, J.W.; Carpenter, T.J.; Madaj, Z.B.; Hostetter, G.; Risinger, J.I.; Teixeira, J.M. Nuclear PTEN Localization Contributes to DNA Damage Response in Endometrial Adenocarcinoma and Could Have a Diagnostic Benefit for Therapeutic Management of the Disease. Mol. Cancer 2018, 17, 1995–2003. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Holst, F.; Werner, H.M.J.; Mjos, S.; Hoivik, E.A.; Kusonmano, K.; Wik, E.; Berg, A.; Birkeland, E.; Gibson, W.J.; Halle, M.K.; et al. PIK3CA Amplification Associates with Aggressive Phenotype but Not Markers of AKT-MTOR Signaling in Endometrial Carcinoma. Clin. Cancer Res. 2019, 25, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Kourea, H.P.; Nikolaou, M.; Tzelepi, V.; Adonakis, G.; Kardamakis, D.; Tsapanos, V.; Scopa, C.D.; Kalofonos, C.; Decavalas, G. Expression of phosphorylated Akt, mTOR and MAPK in type I endometrial carcinoma: Clinical significance. Anticancer Res. 2015, 35, 2321–2331. [Google Scholar]
- Lin, D.I. Improved survival associated with somatic PIK3CA mutations in copy-number low endometrioid endometrial adenocarcinoma. Oncol. Lett. 2015, 10, 2743–2748. [Google Scholar] [CrossRef]
- McIntyre, J.B.; Nelson, G.S.; Ghatage, P.; Morris, D.; Duggan, M.A.; Lee, C.H.; Doll, C.M.; Köbel, M. PIK3CA missense mutation is associated with unfavorable outcome in grade 3 endometrioid carcinoma but not in serous endometrial carcinoma. Gynaecol. Oncol. 2014, 132, 188–193. [Google Scholar] [CrossRef]
- Catasus, L.; Gallardo, A.; Cuatrecasas, M.; Prat, J. Concomitant PI3K-AKT and p53 alterations in endometrial carcinomas are associated with poor prognosis. Mod. Pathol. 2009, 22, 522–529. [Google Scholar] [CrossRef]
- Jeck, W.; Roque, D.R.; Hayes, D.N.; Dizon, A.M.; Clark, L.H.; Pierce, S.; Wysham, W.Z.; Gehrig, P.A.; Bae-Jump, V.L. Frequency of multiple PIK3CA and PK3R1 concurrent mutations in endometrial cancers. J. Clin. Oncol. 2015, 33, e16522. [Google Scholar] [CrossRef]
- Akiyama, A.; Minaguchi, T.; Fujieda, K.; Hosokawa, Y.; Nishida, K.; Shikama, A.; Tasaka, N.; Sakurai, M.; Ochi, H.; Satoh, T. Abnormal accumulation of p53 predicts radioresistance leading to poor survival in patients with endometrial carcinoma. Oncol. Lett. 2019, 18, 5952–5958. [Google Scholar] [CrossRef]
- Cohen, Y.; Shalmon, B.; Korach, J.; Barshack, I.; Fridman, E.; Rechavi, G. AKT1 pleckstrin homology domain E17K activating mutation in endometrial carcinoma. Gynaecol. Oncol. 2010, 116, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Banno, K.; Yanokura, M.; Iida, M.; Masuda, K.; Aoki, D. Carcinogenic mechanisms of endometrial cancer: Involvement of genetics and epigenetics. J. Obs. Gynaecol. Res. 2014, 40, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.J.; Der, C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007, 26, 3291–3310. [Google Scholar] [CrossRef]
- Aslan, O.; Cremona, M.; Morgan, C.; Cheung, L.W.; Mills, G.B.; Hennessy, B.T. Preclinical evaluation and reverse phase protein Array-based profiling of PI3K and MEK inhibitors in endometrial carcinoma in vitro. BMC Cancer 2018, 18, 168. [Google Scholar] [CrossRef] [PubMed]
- Dent, P.; Yacoub, A.; Fisher, P.B.; Hagan, M.P.; Grant, S. MAPK pathways in radiation responses. Oncogene 2003, 22, 5885–5896. [Google Scholar] [CrossRef] [PubMed]
- Marampon, F.; Gravina, G.L.; Popov, V.M.; Scarsella, L.; Festuccia, C.; La Verghetta, M.E.; Parente, S.; Cerasani, M.; Bruera, G.; Ficorella, C.; et al. Close correlation between MEK/ERK and Aurora-B signaling pathways in sustaining tumorigenic potential and radioresistance of gynecological cancer cell lines. Int. J. Oncol. 2014, 44, 285–294. [Google Scholar] [CrossRef]
- Wang, E.; Sorolla, A.; Cunningham, P.T.; Bogdawa, H.M.; Beck, S.; Golden, E.; Dewhurst, R.E.; Florez, L.; Cruickshank, M.N.; Hoffmann, K.; et al. Tumor penetrating peptides inhibiting MYC as a potent targeted therapeutic strategy for triple-negative breast cancers. Oncogene 2019, 38, 140–150. [Google Scholar] [CrossRef]
- Sorolla, A.; Wang, E.; Golden, E.; Duffy, C.; Henriques, S.T.; Redfern, A.D.; Blancafort, P. Precision medicine by designer interference peptides: Applications in oncology and molecular therapeutics. Oncogene 2020, 39, 1167–1184. [Google Scholar] [CrossRef]
- Borst, M.P.; Baker, V.V.; Dixon, D.; Hatch, K.D.; Shingleton, H.M.; Miller, D.M. Oncogene alterations in endometrial carcinoma. Gynaecol. Oncol. 1990, 38, 364–366. [Google Scholar] [CrossRef]
- Manning, G.; Tichy, A.; Sirak, I.; Badie, C. Radiotherapy-Associated Long-term Modification of Expression of the Inflammatory Biomarker Genes ARG1, BCL2L1, and MYC. Front. Immunol. 2017, 8, 412. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Cao, Y.; Greten, F.R.; Li, Z.W. NF-kappaB in cancer: From innocent bystander to major culprit. Nat. Rev. Cancer 2002, 2, 301–310. [Google Scholar] [CrossRef]
- Pallares, J.; Martinez-Guitarte, J.L.; Dolcet, X.; Llobet, D.; Rue, M.; Palacios, J.; Prat, J.; Matias-Guiu, X. Abnormalities in the NF-kappaB family and related proteins in endometrial carcinoma. J. Pathol. 2004, 204, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sung, B. NF-kappaB in cancer: A matter of life and death. Cancer Discov. 2011, 1, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Santacana, M.; Yeramian, A.; Velasco, A.; Bergada, L.; Gatius, S.; Garcia, V.; Azueta, A.; Palacios, J.; Dolcet, X.; Oliva, E.; et al. Immunohistochemical features of post-radiation vaginal recurrences of endometrioid carcinomas of the endometrium: Role for proteins involved in resistance to apoptosis and hypoxia. Histopathology 2012, 60, 460–471. [Google Scholar] [CrossRef]
- Baud, V.; Karin, M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef]
- Gray, L.H.; Conger, A.D.; Ebert, M.; Hornsey, S.; Scott, O.C. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br. J. Radiol. 1953, 26, 638–648. [Google Scholar] [CrossRef]
- Overgaard, J.; Hansen, H.S.; Overgaard, M.; Bastholt, L.; Berthelsen, A.; Specht, L.; Lindeløv, B.; Jørgensen, K. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiother. Oncol. 1998, 46, 135–146. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A.; Sivridis, E.; Fezoulidis, I. Cancer vascularization: Implications in radiotherapy? Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 545–553. [Google Scholar] [CrossRef]
- Sivridis, E.; Giatromanolaki, A.; Gatter, K.C.; Harris, A.L.; Koukourakis, M.I. Association of hypoxia-inducible factors 1alpha and 2alpha with activated angiogenic pathways and prognosis in patients with endometrial carcinoma. Cancer 2002, 95, 1055–1063. [Google Scholar] [CrossRef]
- Rius, J.; Guma, M.; Schachtrup, C.; Akassoglou, K.; Zinkernagel, A.S.; Nizet, V.; Johnson, R.S.; Haddad, G.G.; Karin, M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 2008, 453, 807–811. [Google Scholar] [CrossRef]
- Yeramian, A.; Santacana, M.; Sorolla, A.; Llobet, D.; Encinas, M.; Velasco, A.; Bahi, N.; Eritja, N.; Domingo, M.; Oliva, E.; et al. Nuclear factor-kappaB2/p100 promotes endometrial carcinoma cell survival under hypoxia in a HIF-1alpha independent manner. Lab. Investig. 2011, 91, 859–871. [Google Scholar] [CrossRef]
- Pijnenborg, J.M.; Wijnakker, M.; Hagelstein, J.; Delvoux, B.; Groothuis, P.G. Hypoxia contributes to development of recurrent endometrial carcinoma. Int. J. Gynaecol. Cancer 2007, 17, 897–904. [Google Scholar] [CrossRef]
- Chakravarti, A.; Chakladar, A.; Delaney, M.A.; Latham, D.E.; Loeffler, J.S. The epidermal growth factor receptor pathway mediates resistance to sequential administration of radiation and chemotherapy in primary human glioblastoma cells in a RAS-dependent manner. Cancer Res. 2002, 62, 4307–4315. [Google Scholar] [PubMed]
- Uzawa, K.; Ishigami, T.; Fushimi, K.; Kawata, T.; Shinozuka, K.; Kasamatsu, A.; Sakamoto, Y.; Ogawara, K.; Shiiba, M.; Bukawa, H.; et al. Targeting fibroblast growth factor receptor 3 enhances radiosensitivity in human squamous cancer cells. Oncogene 2011, 30, 4447–4452. [Google Scholar] [CrossRef] [PubMed]
- Slomovitz, B.M.; Broaddus, R.R.; Schmandt, R.; Wu, W.; Oh, J.C.; Ramondetta, L.M.; Burke, T.W.; Gershenson, D.M.; Lu, K.H. Expression of imatinib mesylate-targeted kinases in endometrial carcinoma. Gynaecol. Oncol. 2004, 95, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Westin, S.N.; Broaddus, R.R. Personalized therapy in endometrial cancer: Challenges and opportunities. Cancer Biol. 2012, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, N.; Lai, W.; Yan, B.; Chen, L.; Liu, S.; Liu, S.; Wang, X.; Xiao, D.; Liu, X.; et al. Nuclear EGFR-PKM2 axis induces cancer stem cell-like characteristics in irradiation-resistant cells. Cancer Lett. 2018, 422, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Rhee, Y.H.; Kim, J.S. The Anticancer Effects of Radachlorin-mediated Photodynamic Therapy in the Human Endometrial Adenocarcinoma Cell Line HEC-1-A. Anticancer Res. 2017, 37, 6251–6258. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Limberis, V.; Tentes, I.; Kontomanolis, E.; Kortsaris, A.; Sivridis, E.; Giatromanolaki, A. Serum VEGF levels and tissue activation of VEGFR2/KDR receptors in patients with breast and gynecologic cancer. Cytokine 2011, 53, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Lee, H.; Berkowitz, R.; Berlin, S.; Campos, S.; Feltmate, C.; Horowitz, N.; Muto, M.; Sadow, C.A.; Matulonis, U. A prospective feasibility study of radiation and concurrent bevacizumab for recurrent endometrial cancer. Gynaecol. Oncol. 2014, 132, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.S.; Yang, K.; Wan, Y.; Qian, P.X.; Perry, J.K.; Chiesa, J.; Mertani, H.C.; Zhu, T.; Lobie, P.E. Tumor expression of human growth hormone and human prolactin predict a worse survival outcome in patients with mammary or endometrial carcinoma. J. Clin. Endocrinol. Metab. 2011, 96, E1619–E1629. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Perry, J.K.; Mohankumar, K.M.; Kong, X.J.; Liu, S.M.; Wu, Z.S.; Mitchell, M.D.; Zhu, T.; Lobie, P.E. Autocrine human growth hormone stimulates oncogenicity of endometrial carcinoma cells. Endocrinology 2008, 149, 3909–3919. [Google Scholar] [CrossRef] [PubMed]
- Bougen, N.M.; Yang, T.; Chen, H.; Lobie, P.E.; Perry, J.K. Autocrine human growth hormone reduces mammary and endometrial carcinoma cell sensitivity to mitomycin C. Oncol. Rep. 2011, 26, 487–493. [Google Scholar] [CrossRef]
- Bougen, N.M.; Steiner, M.; Pertziger, M.; Banerjee, A.; Brunet-Dunand, S.E.; Zhu, T.; Lobie, P.E.; Perry, J.K. Autocrine human GH promotes radioresistance in mammary and endometrial carcinoma cells. Endocr. Relat. Cancer 2012, 19, 625–644. [Google Scholar] [CrossRef][Green Version]
- Evans, A.; Jamieson, S.M.; Liu, D.X.; Wilson, W.R.; Perry, J.K. Growth hormone receptor antagonism suppresses tumour regrowth after radiotherapy in an endometrial cancer xenograft model. Cancer Lett. 2016, 379, 117–123. [Google Scholar] [CrossRef]
- Rantanen, V.; Grenman, S.; Kulmala, J.; Alanen, K.; Lakkala, T.; Grenman, R. Sublethal damage repair after fractionated irradiation in endometrial cancer cell lines tested with the 96-well plate clonogenic assay. J. Cancer Res. Clin. Oncol. 1994, 120, 712–716. [Google Scholar] [CrossRef]
- Biau, J.; Chautard, E.; Verrelle, P.; Dutreix, M. Altering DNA Repair to Improve Radiation Therapy: Specific and Multiple Pathway Targeting. Front. Oncol. 2019, 9, 1009. [Google Scholar] [CrossRef]
- Awasthi, P.; Foiani, M.; Kumar, A. ATM and ATR signaling at a glance. J. Cell Sci. 2016, 129, 1285. [Google Scholar] [CrossRef]
- Teng, P.N.; Bateman, N.W.; Darcy, K.M.; Hamilton, C.A.; Maxwell, G.L.; Bakkenist, C.J.; Conrads, T.P. Pharmacologic inhibition of ATR and ATM offers clinically important distinctions to enhancing platinum or radiation response in ovarian, endometrial, and cervical cancer cells. Gynaecol. Oncol. 2015, 136, 554–561. [Google Scholar] [CrossRef]
- Takeuchi, M.; Tanikawa, M.; Nagasaka, K.; Oda, K.; Kawata, Y.; Oki, S.; Agapiti, C.; Sone, K.; Miyagawa, Y.; Hiraike, H.; et al. Anti-Tumor Effect of Inhibition of DNA Damage Response Proteins, ATM and ATR, in Endometrial Cancer Cells. Cancers 2019, 11, 1913. [Google Scholar] [CrossRef]
- de Jonge, M.M.; Auguste, A.; van Wijk, L.M.; Schouten, P.C.; Meijers, M.; Ter Haar, N.T.; Smit, V.; Nout, R.A.; Glaire, M.A.; Church, D.N.; et al. Frequent Homologous Recombination Deficiency in High-grade Endometrial Carcinomas. Clin. Cancer Res. 2019, 25, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, D.A.; Angelakis, E.; Koumantakis, E.E.; Spandidos, D.A. Allelic imbalance in hMLH1 or BRCA2 loci associated with response of cervical and endometrial cancer to radiotherapy. Int. J. Mol. Med. 2002, 10, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Reijnen, C.; Kusters-Vandevelde, H.V.N.; Prinsen, C.F.; Massuger, L.; Snijders, M.; Kommoss, S.; Brucker, S.Y.; Kwon, J.S.; McAlpine, J.N.; Pijnenborg, J.M.A. Mismatch repair deficiency as a predictive marker for response to adjuvant radiotherapy in endometrial cancer. Gynaecol. Oncol. 2019, 154, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.C.; Jackson, S.P. The DNA-dependent protein kinase. Genes Dev. 1999, 13, 916–934. [Google Scholar] [CrossRef]
- Saygili, U.; Gorkay, I.B.; Koyuncuoglu, M.; Gol, M.; Uslu, T.; Erten, O. The relationship between expression of Ku70 and survival in irradiated patients with endometrial carcinoma. Gynaecol. Oncol. 2004, 95, 518–522. [Google Scholar] [CrossRef]
- Toufektchan, E.; Toledo, F. The Guardian of the Genome Revisited: p53 Downregulates Genes Required for Telomere Maintenance, DNA Repair, and Centromere Structure. Cancers 2018, 10, 135. [Google Scholar] [CrossRef]
- Rantanen, V.; Grenman, S.; Kurvinen, K.; Hietanen, S.; Raitanen, M.; Syrjanen, S. p53 mutations and presence of HPV DNA do not correlate with radiosensitivity of gynecological cancer cell lines. Gynaecol. Oncol. 1998, 71, 352–358. [Google Scholar] [CrossRef]
- Saffari, B.; Bernstein, L.; Hong, D.C.; Sullivan-Halley, J.; Runnebaum, I.B.; Grill, H.J.; Jones, L.A.; El-Naggar, A.; Press, M.F. Association of p53 mutations and a codon 72 single nucleotide polymorphism with lower overall survival and responsiveness to adjuvant radiotherapy in endometrioid endometrial carcinomas. Int. J. Gynaecol. Cancer 2005, 15, 952–963. [Google Scholar] [CrossRef]
- Mandeville, R.; Sidrac-Ghali, S.; Ajdukovic, I.; Vidal, D.; Ayoub, J. Early inhibition of natural and interferon-activated killers in endometrial cancer patients treated with local radiotherapy. Cancer Detect. Prev. 1987, 10, 129–139. [Google Scholar]
- Son, C.H.; Fleming, G.F.; Moroney, J.W. Potential role of radiation therapy in augmenting the activity of immunotherapy for gynecologic cancers. Cancer Manag. Res. 2017, 9, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Frenel, J.-S.; Tourneau, C.L.; O’Neil, B.H.; Ott, P.A.; Piha-Paul, S.A.; Gomez-Roca, C.A.; Brummelen, E.V.; Rugo, H.S.; Thomas, S.; Saraf, S.; et al. Pembrolizumab in patients with advanced cervical squamous cell cancer: Preliminary results from the phase Ib KEYNOTE-028 study. J. Clin. Oncol. 2016, 34, 5515. [Google Scholar] [CrossRef]
- Ott, P.A.; Bang, Y.-J.; Berton-Rigaud, D.; Elez, E.; Pishvaian, M.J.; Rugo, H.S.; Puzanov, I.; Morgan, M.A.; Mehnert, J.M.; Aung, K.L.; et al. Pembrolizumab in advanced endometrial cancer: Preliminary results from the phase Ib KEYNOTE-028 study. J. Clin. Oncol. 2016, 34, 5581. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Howitt, B.E.; Shukla, S.A.; Sholl, L.M.; Ritterhouse, L.L.; Watkins, J.C.; Rodig, S.; Stover, E.; Strickland, K.C.; D’Andrea, A.D.; Wu, C.J.; et al. Association of Polymerase e-Mutated and Microsatellite-Instable Endometrial Cancers With Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015, 1, 1319–1323. [Google Scholar] [CrossRef]
- Lee, L.; Matulonis, U. Immunotherapy and radiation combinatorial trials in gynecologic cancer: A potential synergy? Gynaecol. Oncol. 2019, 154, 236–245. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef]
- Lin, L.L.; Lakomy, D.S.; Ning, M.S.; Simpkins, F.; Jhingran, A. Combining novel agents with radiotherapy for gynecologic malignancies: Beyond the era of cisplatin. Int. J. Gynaecol. Cancer 2020, 30, 409–423. [Google Scholar] [CrossRef]
- Salehi, F.; Scheithauer, B.W.; Sharma, S.; Kovacs, K.; Lloyd, R.V.; Cusimano, M.D.; Munoz, D.G. Immunohistochemical expression of PTTG in brain tumors. Anticancer Res. 2013, 33, 119–122. [Google Scholar]
- Zhang, X.; Horwitz, G.A.; Heaney, A.P.; Nakashima, M.; Prezant, T.R.; Bronstein, M.D.; Melmed, S. Pituitary tumor transforming gene (PTTG) expression in pituitary adenomas. J. Clin. Endocrinol. Metab. 1999, 84, 761–767. [Google Scholar] [CrossRef]
- Zhang, S.X.; Shan, W.X.; Yuan, L.P.; Liu, Y.L.; Sun, L.Z. Effects of silencing PTTG expression by small interference RNA. Eur. Rev. Med. Pharm. Sci. 2016, 20, 2835–2841. [Google Scholar]
- Murphy, M.E. The HSP70 family and cancer. Carcinogenesis 2013, 34, 1181–1188. [Google Scholar] [CrossRef]
- Du, X.L.; Jiang, T.; Wen, Z.Q.; Gao, R.; Cui, M.; Wang, F. Silencing of heat shock protein 70 expression enhances radiotherapy efficacy and inhibits cell invasion in endometrial cancer cell line. Croat. Med. J. 2009, 50, 143–150. [Google Scholar] [CrossRef]
- Gao, Q.; Huang, X.; Tang, D.; Cao, Y.; Chen, G.; Lu, Y.; Zhuang, L.; Wang, S.; Xu, G.; Zhou, J.; et al. Influence of chk1 and plk1 silencing on radiation- or cisplatin-induced cytotoxicity in human malignant cells. Apoptosis 2006, 11, 1789–1800. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Yang, Z.; Liu, Y.; Gong, D.; Ji, T.; Wang, J.; Xi, B.; Zhou, J.; Ma, D.; Gao, Q. Co-abrogation of Chk1 and Chk2 by potent oncolytic adenovirus potentiates the antitumor efficacy of cisplatin or irradiation. Cancer Gene 2014, 21, 209–217. [Google Scholar] [CrossRef]
- Brown, L.F.; Berse, B.; Van de Water, L.; Papadopoulos-Sergiou, A.; Perruzzi, C.A.; Manseau, E.J.; Dvorak, H.F.; Senger, D.R. Expression and distribution of osteopontin in human tissues: Widespread association with luminal epithelial surfaces. Mol. Biol. Cell 1992, 3, 1169–1180. [Google Scholar] [CrossRef]
- Chang, S.H.; Minai-Tehrani, A.; Shin, J.Y.; Park, S.; Kim, J.E.; Yu, K.N.; Hong, S.H.; Hong, C.M.; Lee, K.H.; Beck, G.R., Jr.; et al. Beclin1-induced autophagy abrogates radioresistance of lung cancer cells by suppressing osteopontin. J. Radiat. Res. 2012, 53, 422–432. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, W.; Zuo, W.S.; Wei, L.; Song, X.R.; Wang, X.W.; Zheng, G.; Zheng, M.Z. Silencing of osteopontin promotes the radiosensitivity of breast cancer cells by reducing the expression of hypoxia inducible factor 1 and vascular endothelial growth factor. Chin. Med. J. (Engl.) 2012, 125, 293–299. [Google Scholar]
- Hahne, J.C.; Meyer, S.R.; Kranke, P.; Dietl, J.; Guckenberger, M.; Polat, B.; Honig, A. Studies on the role of osteopontin-1 in endometrial cancer cell lines. Strahlenther. Onkol. 2013, 189, 1040–1048. [Google Scholar] [CrossRef]
- Overgaard, J.; Eriksen, J.G.; Nordsmark, M.; Alsner, J.; Horsman, M.R.; Danish, H.; Neck Cancer Study, G. Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: Results from the DAHANCA 5 randomised double-blind placebo-controlled trial. Lancet Oncol. 2005, 6, 757–764. [Google Scholar] [CrossRef]
- Singhal, H.; Bautista, D.S.; Tonkin, K.S.; O’Malley, F.P.; Tuck, A.B.; Chambers, A.F.; Harris, J.F. Elevated plasma osteopontin in metastatic breast cancer associated with increased tumor burden and decreased survival. Clin. Cancer Res. 1997, 3, 605–611. [Google Scholar] [PubMed]
- Fujimoto, J.; Sakaguchi, H.; Aoki, I.; Tamaya, T. Steroid receptors and metastatic potential in endometrial cancers. Eur. J. Cancer 2000, 36 (Suppl. 4), S33. [Google Scholar] [CrossRef]
- Jongen, V.; Briet, J.; de Jong, R.; ten Hoor, K.; Boezen, M.; van der Zee, A.; Nijman, H.; Hollema, H. Expression o-alpha and -beta and progesterone receptor-A and -B in a large cohort of patients with endometrioid endometrial cancer. Gynaecol. Oncol. 2009, 112, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Huber, H.; Husslein, P.; Michalica, W.; Wagenbichler, P. Radiosensitizing effect of medroxyprogesterone acetate on endometrial cancer cells in vitro. Cancer 1984, 54, 999–1001. [Google Scholar] [CrossRef]
- You, J.; Chen, W.; Chen, J.; Zheng, Q.; Dong, J.; Zhu, Y. The Oncogenic Role of ARG1 in Progression and Metastasis of Hepatocellular Carcinoma. Biomed. Res. Int. 2018, 2018, 2109865. [Google Scholar] [CrossRef]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2018, 61, 5108–5121. [Google Scholar] [CrossRef]
- Terek, M.C.; Zekioglu, O.; Sendag, F.; Akercan, F.; Ozsaran, A.; Erhan, Y. MDR1 gene expression in endometrial carcinoma. Int. J. Gynaecol. Cancer 2003, 13, 673–677. [Google Scholar] [CrossRef]
- Wenz, F.; Engling, A.; Fruehauf, S.; Weber, K.J. Transfection of Human Cell Lines with the Human Multi-Drug-Resistence (MDR-1) Gene Suppresses Radiation-Induced Apoptosis and Increases Radioresistance. In Proceedings of the 43rd Annual ASTRO Meeting, San Francisco, CA, USA, 4–8 November 2001; p. 229. [Google Scholar]
- Rosa, R.; D’Amato, V.; De Placido, S.; Bianco, R. Approaches for targeting cancer stem cells drug resistance. Expert Opin. Drug Discov. 2016, 11, 1201–1212. [Google Scholar] [CrossRef]
- Baumann, M.; Krause, M.; Hill, R. Exploring the role of cancer stem cells in radioresistance. Nat. Rev. Cancer 2008, 8, 545–554. [Google Scholar] [CrossRef]
- Giannone, G.; Attademo, L.; Scotto, G.; Genta, S.; Ghisoni, E.; Tuninetti, V.; Aglietta, M.; Pignata, S.; Valabrega, G. Endometrial Cancer Stem Cells: Role, Characterization and Therapeutic Implications. Cancers 2019, 11, 1820. [Google Scholar] [CrossRef]
- Dong, P.; Konno, Y.; Watari, H.; Hosaka, M.; Noguchi, M.; Sakuragi, N. The impact of microRNA-mediated PI3K/AKT signaling on epithelial-mesenchymal transition and cancer stemness in endometrial cancer. J. Transl. Med. 2014, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Streffer, C.; Müller, W.-U.; Wuttke, K. The Formation of Micronuclei after Exposure to Ionizing Radiation. In Chromosomal Alterations; Obe, G., Natarajan, A.T., Eds.; Springer: Berlin/Heidelberg, Germany, 1994. [Google Scholar] [CrossRef]
- Countryman, P.I.; Heddle, J.A. The production of micronuclei from chromosome aberrations in irradiated cultures of human lymphocytes. Mutat. Res. 1976, 41, 321–332. [Google Scholar] [CrossRef]
- Habash, M.; Bohorquez, L.C.; Kyriakou, E.; Kron, T.; Martin, O.A.; Blyth, B.J. Clinical and Functional Assays of Radiosensitivity and Radiation-Induced Second Cancer. Cancers 2017, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Emons, G.; Vordermark, D. Adjuvant treatment for endometrial cancer. Curr. Opin. Oncol. 2019, 31, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Raposo, C.; Merino Salvador, M.; Aguayo Zamora, C.; Casado Saenz, E. Adjuvant chemotherapy in endometrial cancer. Cancer Chemother. Pharm. 2020, 85, 477–486. [Google Scholar] [CrossRef]
- Kunos, C.A.; Sherertz, T.M.; Mislmani, M.; Ellis, R.J.; Lo, S.S.; Waggoner, S.E.; Zanotti, K.M.; Herrmann, K.; Debernardo, R.L. Phase I Trial of Carboplatin and Gemcitabine Chemotherapy and Stereotactic Ablative Radiosurgery for the Palliative Treatment of Persistent or Recurrent Gynecologic Cancer. Front. Oncol. 2015, 5, 126. [Google Scholar] [CrossRef]
- Grigsby, P.W.; Graham, M.V.; Perez, C.A.; Galakatos, A.E.; Camel, H.M.; Kao, M.S. Prospective phase I/II studies of definitive irradiation and chemotherapy for advanced gynecologic malignancies. Am. J. Clin. Oncol. 1996, 19, 1–6. [Google Scholar] [CrossRef]
- Martenson, J.A.; Halyard, M.Y.; Sloan, J.A.; Proulx, G.M.; Miller, R.C.; Deming, R.L.; Dick, S.J.; Johnson, H.A.; Tai, T.H.; Zhu, A.W.; et al. Phase III, double-blind study of depot octreotide versus placebo in the prevention of acute diarrhea in patients receiving pelvic radiation therapy: Results of North Central Cancer Treatment Group N00CA. J. Clin. Oncol. 2008, 26, 5248–5253. [Google Scholar] [CrossRef]
- Maton, P.N.; O’Dorisio, T.M.; Howe, B.A.; McArthur, K.E.; Howard, J.M.; Cherner, J.A.; Malarkey, T.B.; Collen, M.J.; Gardner, J.D.; Jensen, R.T. Effect of a long-acting somatostatin analogue (SMS 201-995) in a patient with pancreatic cholera. N. Engl. J. Med. 1985, 312, 17–21. [Google Scholar] [CrossRef]
- Kaczmarski, R.S.; Mufti, G.J. Low-dose filgrastim therapy for chronic neutropenia. N. Engl. J. Med. 1993, 329, 1280–1281. [Google Scholar] [CrossRef]
- Homesley, H.D.; Filiaci, V.; Gibbons, S.K.; Long, H.J.; Cella, D.; Spirtos, N.M.; Morris, R.T.; DeGeest, K.; Lee, R.; Montag, A. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: A Gynecologic Oncology Group study. Gynaecol. Oncol. 2009, 112, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Lemons, J.M.; Karrison, T.G.; Pitroda, S.P.; Melotek, J.M.; Zha, Y.; Al-Hallaq, H.A.; Arina, A.; Khodarev, N.N.; Janisch, L.; et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2018, 36, 1611–1618. [Google Scholar] [CrossRef]
- Kandula, S.; Zhu, X.; Garden, A.S.; Gillin, M.; Rosenthal, D.I.; Ang, K.K.; Mohan, R.; Amin, M.V.; Garcia, J.A.; Wu, R.; et al. Spot-scanning beam proton therapy vs intensity-modulated radiation therapy for ipsilateral head and neck malignancies: A treatment planning comparison. Med. Dosim. 2013, 38, 390–394. [Google Scholar] [CrossRef]
- Temelli, O.; Demirtas, M.; Sisecioglu, M.S.; Pepele, E.K. Dosimetric Comparison of Adjuvant Pelvic Radiotherapy for Endometrial Cancer using Intensity-Modulated Radiotherapy (IMRT), Volumetric Modulated Arc Therapy (VMAT) and Helical Tomotherapy (HT). Eurasian J. Med. Oncol. 2019, 3, 203–210. [Google Scholar] [CrossRef]
- Chen, J.L.-Y.; Huang, Y.-S.; Huang, C.-Y.; Hsu, C.-Y.; Lan, K.-H.; Cheng, W.-F.; Kuo, S.-H. Impact of adjuvant radiotherapy on the survival of women with optimally resected stage III endometrial cancer in the era of modern radiotherapy: A retrospective study. Radiat. Oncol. 2020, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Teoh, M.; Clark, C.H.; Wood, K.; Whitaker, S.; Nisbet, A. Volumetric modulated arc therapy: A review of current literature and clinical use in practice. Br. J. Radiol. 2011, 84, 967–996. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, C.M.; Adams, Q.; Flynn, R.T.; Wu, X.; Xu, W.; Kim, Y. Systematic Review of Intensity-Modulated Brachytherapy (IMBT): Static and Dynamic Techniques. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 206–221. [Google Scholar] [CrossRef]
- Lee, L.J.; Damato, A.L.; Viswanathan, A.N. Clinical outcomes following 3D image-guided brachytherapy for vaginal recurrence of endometrial cancer. Gynaecol. Oncol. 2013, 131, 586–592. [Google Scholar] [CrossRef]
- Fokdal, L.; Ortoft, G.; Hansen, E.S.; Rohl, L.; Pedersen, E.M.; Tanderup, K.; Lindegaard, J.C. Toward four-dimensional image-guided adaptive brachytherapy in locally recurrent endometrial cancer. Brachytherapy 2014, 13, 554–561. [Google Scholar] [CrossRef]
- Jordan, S.E.; Micaily, I.; Hernandez, E.; Ferriss, J.S.; Miyamoto, C.T.; Li, S.; Micaily, B. Image-guided high-dose-rate intracavitary brachytherapy in the treatment of medically inoperable early-stage endometrioid type endometrial adenocarcinoma. Brachytherapy 2017, 16, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
| Trial Name, Identifier And [Status] | Phase | N | Official Name | Type of EC Included | Drugs and Treatment Scheme | Radiation Regimen and Schedule | Primary Outcomes | Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|
| Growth factors—Growth factor inhibitors | ||||||||
| NCT01005329 [Completed] | II | 34 | A Phase II Study of Postoperative Intensity Modulated Radiation Therapy (IMRT) With Concurrent Cisplatin and Bevacizumab Followed by Carboplatin and Paclitaxel for Patients With Endometrial Cancer | Resected high-risk stage I-IV EC | Bevacizumab (anti-VEGF), and cisplatin, followed by carboplatin and paclitaxel | Pelvic IMRT once daily, 5 days a week, for 5 weeks (45 Gy in 25 fractions) with optional nodal boost RT and/or vaginal BT boost. Concurrently with bevacizumab+cisplatin. | Adverse events by grade within 90 days after the treatment starts | Adverse events by grade within 90 days within 1 year treatment start and from start of treatment to end of follow-up, up to 43.4 months; OS, DFS, pelvic failure rate and distant failure |
| NCT00545792 [Completed] | II | 21 | A Pilot Study Evaluating The Safety Of Avastin And Pelvic Radiation In Women With Pelvic-Confined Recurrence of Gynecological Cancers | Recurrent pelvic-confined EC | Bevacizumab | Daily Pelvic RT. Concurrently with bevacizumab. | PFS OS | Thrombosis and one embolic event in the setting of metastatic disease |
| NCT00033605 [Completed] | III | 130 | Phase III Double-Blind Study Of Depot Octreotide Versus Placebo In The Prevention Of Acute Diarrhea In Patients Receiving Pelvic Radiation Therapy | EC | Octreotide (GH inhibitor) Arm I: Short-acting octreotide SC on day 1 and long-acting octreotide IM on days 2 and 29. Arm II: Patients receive placebo SC on day 1 and IM on days 2 and 29 | Prior planned cumulative dose of RT, including boost fields (4500–5350 cGy vs. 5351–6000 cGy vs. more than 6000 cGy) and planned intracavitary BT. RT starts maximum 4 days before octreotide. | Reduction of diarrhea measured weekly during pelvic RT up to 2 years | Reduction of patient-reported bowel dysfunction, toxicity and importance that patients attach to various measures of bowel dysfunction as assessed by questionnaire |
| DNA repair pathway—PARP inhibitors | ||||||||
| NCT03968406 [Recruiting] | I | 24 | Phase I Study of Talazoparib in Combination With Radiation Therapy for Locally Recurrent Gynecologic Cancers | Stage IV or recurrent EC | Talazoparib (PARP inhibitor) | Fractionated RT 5 days per week for up to 7 weeks. Concurrently with talazoparib. | MTD | Adverse events, RR, PFS, OS. Others: PAR inhibition levels, ɣ-H2AX and RAD51 foci formation levels, OQL |
| Immunotherapy—Immune system activators and immune checkpoints inhibitors | ||||||||
| GOG-0184 NCT00006011 [Completed] | III | 659 | A Randomized Phase III Study of Tumor Volume Directed Pelvic Plus or Minus Para-Aortic Irradiation Followed by Cisplatin and Doxorubicin or Cisplatin, Doxorubicin and Paclitaxel for Advanced Endometrial Carcinoma | Stage III or IV EC | Filgrastim (G-CSF analog) or pegfilgrastim (pG-CSF analog) Arm I: doxorubicin and cisplatin, followed by G-GSF or pGCSF Arm II: doxorubicin and cisplatin, followed by paclitaxel and followed by G-GSF or pGCSF | Pelvic or extended field RT. Within 8 weeks after surgery, patients receive tumor VDPR with or without PNR once daily for 5 consecutive days for up to 16 weeks after surgery. Within 8 weeks of completing RT, patients receive Arm I or Arm II. | RFS | Not provided |
| PRIMMO NCT03192059 [Recruiting] | II | 43 | A Phase II Investigation of Pembrolizumab (Keytruda) in Combination With Radiation and an Immune Modulatory Cocktail in Patients With Cervical and Uterine Cancer (PRIMMO Trial) | Advanced and refractory EC | Pembrolizumab (anti-PD-1) vitamin D, aspirin, cyclophosphamide, lansoprazole and curcumin | EBR 24 Gy in 3 fractions, a fraction every 8 h Concurrently with drug scheme. | ORR at week 26 | Incidence of adverse events, ORR, best OR, PFS, OS and OQL assessment |
| FIERCE NCT03932409 [Recruiting] | I | 20 | A Phase Ib Trial of Vaginal Cuff Brachytherapy + Pembrolizumab (MK3475) Followed by 3 Cycles of Dose Dense Paclitaxel/q 21 Day Carboplatin + Pembrolizumab (MK3475) in High Intermediate Risk Endometrial Cancer | High and intermediate-risk EC | Pembrolizumab (anti-PD-1) and chemoradiation consisting of dose dense paclitaxel and carboplatin and, BT | Vaginal cuff BT given one week after pembrolizumab. | Patients completing 3 cycles of pembrolizumab | PFS OS Adverse event frequency |
| NCT04214067 [Recruiting] | III | 168 | A Phase III Randomized Trial of Radiation ± MK-3475 (Pembrolizumab) for Newly Diagnosed Early Stage High Intermediate Risk Mismatch Repair Deficient (dMMR) Endometrioid Endometrial Cancer | High-intermediate risk stage I-II dMMR EC | Pembrolizumab (anti-PD-1) Arm 1: EBRT + BT Arm 2: ERBT + BT + Pembrolizumab administered 7 days prior to the start of RT, every 3 weeks for up to 1 year (17 cycles) | ERBT daily for 5–6 weeks and vaginal BT completed within 7 days after completion of EBRT. Pembrolizumab given 7 days prior to the start of RT. | 3-year RFS | Incidence of adverse effects, recurrence patterns, 5-year RFS, OS, patients reported outcomes |
| NCT03277482 [Recruiting] | I | 23 | A Phase 1 Study of Durvalumab, Tremelimumab and Radiotherapy in Recurrent Gynecologic Cancer | Recurrent and metastatic EC | Durvalumab (anti-/PD-L1) + Tremelimumab (anti-CTLA-4) | EBT hypofractionated short course (either 1 or 5 days). Concurrently with the immunotherapies. | MTD | ORR, LRR, LCR, ARR, RD, PFS, OS |
| NCT03955978 [Recruiting] | I | 12 | A Phase I Study of PD-1 Inhibition With TSR-042 in Addition to Standard of Care Definitive Radiation for Inoperable Endometrial Cancer | Inoperable EC | TSR-042 (anti-PD-1) | BT 36 Gy in 6 fractions, given weekly. The first dose of TSR-042 is administered 21 days prior to the first BT fraction. | Adverse event at six weeks | PFS |
| Trial Name, Identifier and [Status] | Phase | N | Official Name | Type of EC Included | Drugs and Treatment Scheme | Radiation Regimen and Schedule | Primary Outcomes | Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|
| Recently completed clinical trials | ||||||||
| NCT00285415 [Completed] | II | 46 | A Phase II Evaluation of Docetaxel and Carboplatin Followed by Tumor Volume Directed Pelvic Plus or Minus Para-Aortic Irradiation for Stage III/IV Endometrial Carcinoma | Advanced stage III and pelvis-confined stage IV or recurrent EC | Docetaxel + carboplatin: every 3 weeks for 6 cycles | Tumor Volume Directed Pelvic ± Para-Aortic Irradiation. After chemotherapy | ORR | OS, PFS, safety and tolerability |
| Active clinical trials | ||||||||
| NCT03935256 [Recruiting] | II | 24 | Phase II Study of Concurrent and Sequential Carboplatin and Paclitaxel With Adjuvant Radiotherapy for High Risk Endometrial Cancer | Locally advanced stage III-IVA EC | Carboplatin + paclitaxel: Arm 1: carboplatin + paclitaxel 4 cycles weeks 1, 10, 13 and 16. Arm 2: carboplatin + paclitaxel 2 cycles weeks 4 and 7 + RT | EBPR of 45 Gy in 25 fractions followed by vaginal BT boost at doses of 12–18 Gy in 2–3 fractions. Sequentially and concurrent with carboplatin + paclitaxel | Acute toxicities | Treatment delays, chronic toxicities, local control, pelvic failure-free survival, distant metastasis-free survival, cause-specific survival, DFS and OS |
| DeCRESCEndo NCT04386993 [Not yet recruiting] | II | 25 | De-escalated Conformal Radiation Expedited Sequentially With Chemotherapy for Endometrial Cancer (DeCRESCEndo) | Stage III-IVA EC | Chemotherapy: regimen not determined | IMRT: 5-Gy in 5 fractions with elective simultaneous boost to any suspicious lymph node or residual disease to 30 Gy in 1–2 weeks. Sequentially with chemotherapy | Adverse effects incidence | Change in patient-reported toxicity, change in QOL, Loco-regional control, distant control, DFS and OS |
| PORTEC-3 NCT00411138 [Active, not recruiting] | III | 670 | Randomized Phase III Trial Comparing Concurrent Chemoradiation and Adjuvant Chemotherapy With Pelvic Radiation Alone in High Risk and Advanced Stage Endometrial Carcinoma: PORTEC-3 | High risk stage I-III EC | Cisplatin + paclitaxel + carboplatin: Arm 1: EBPR combined with 2 cycles of cisplatin followed by 4 cycles of carboplatin + paclitaxel Am 2: ERBT and vaginal BT in case of cervical involvement | EBPR: 48.6 Gy in 1.8 Gy fractions up to 6 weeks and vaginal BT boost in case of cervical involvement. Alone or concurrently with chemotherapy | OS, failure-free survival | QOL, severe treatment-related morbidity, vaginal or pelvic relapse and distant metastasis |
| NCT00942357 [Active, not recruiting] | III | 813 | A Randomized Phase III Trial of Cisplatin and Tumor Volume Directed Irradiation Followed by Carboplatin and Paclitaxel vs. Carboplatin and Paclitaxel for Optimally Debulked, Advanced Endometrial Carcinoma | Stage I-IVA EC | Carboplatin + paclitaxel ± cisplatin: Arm 1: cisplatin + VDRT or BT. After chemoradiotherapy, paclitaxel + carboplatin Arm 2: paclitaxel + carboplatin | VDRT 5 days a week for 5–6 weeks or BT over 2–3 weeks. Concurrent with chemotherapy | Number of participants with recurrence, progression or death | Number of participants with acute late adverse effects, OS, patient-reported-neuropathy symptoms and QOL |
| NCT00807768 [Active, not recruiting] | III | 601 | A Phase III Trial of Pelvic Radiation Therapy Versus Vaginal Cuff Brachytherapy Followed by Paclitaxel/Carboplatin Chemotherapy in Patients With High Risk, Early Stage Endometrial Carcinoma | High risk stage I-II EC | Carboplatin + paclitaxel: Arm 1: IMRT ± BT when specified Arm 2: BT + carboplatin + paclitaxel | IRMT: 25–28 fractions during 5–6 weeks. BT: 3–5 high-dose rate treatments over 2 weeks or 1–2 low-dose rate over 1–2 days. Chemotherapy given within 3 weeks after initiating BT | Number of participants with recurrence or death events at primary analysis | Number of participants with death events and with sites of recurrence, patient-reported fatigue and neurotoxicity and QOL |
| NCT00492778 [recruiting] | II | 164 | A Randomized Trial of Pelvic Irradiation With or Without Concurrent Weekly Cisplatin in Patients With Pelvic-Only Recurrence of Carcinoma of the Uterine Corpus | Recurrent EC | Cisplatin: Arm 1: EBRT followed by intracavitary or interstitial rate interstitial BT Arm 2: EBRT + cisplatin, followed by BT | EBRT on days 1–5 for 5 weeks. Intracavitary low-dose or high-dose rate BT or low-dose rate interstitial BT. Concurrently with chemotherapy | PFS | OS, Prognostic significance of tumor size, tumor location (vaginal only vs. all others) and histology and incidence of adverse effects |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorolla, M.A.; Parisi, E.; Sorolla, A. Determinants of Sensitivity to Radiotherapy in Endometrial Cancer. Cancers 2020, 12, 1906. https://doi.org/10.3390/cancers12071906
Sorolla MA, Parisi E, Sorolla A. Determinants of Sensitivity to Radiotherapy in Endometrial Cancer. Cancers. 2020; 12(7):1906. https://doi.org/10.3390/cancers12071906
Chicago/Turabian StyleSorolla, Maria Alba, Eva Parisi, and Anabel Sorolla. 2020. "Determinants of Sensitivity to Radiotherapy in Endometrial Cancer" Cancers 12, no. 7: 1906. https://doi.org/10.3390/cancers12071906
APA StyleSorolla, M. A., Parisi, E., & Sorolla, A. (2020). Determinants of Sensitivity to Radiotherapy in Endometrial Cancer. Cancers, 12(7), 1906. https://doi.org/10.3390/cancers12071906





