Clinical Significance of Adverse Events for Patients with Unresectable Hepatocellular Carcinoma Treated with Lenvatinib: A Multicenter Retrospective Study
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Therapeutic Efficacy of LEN
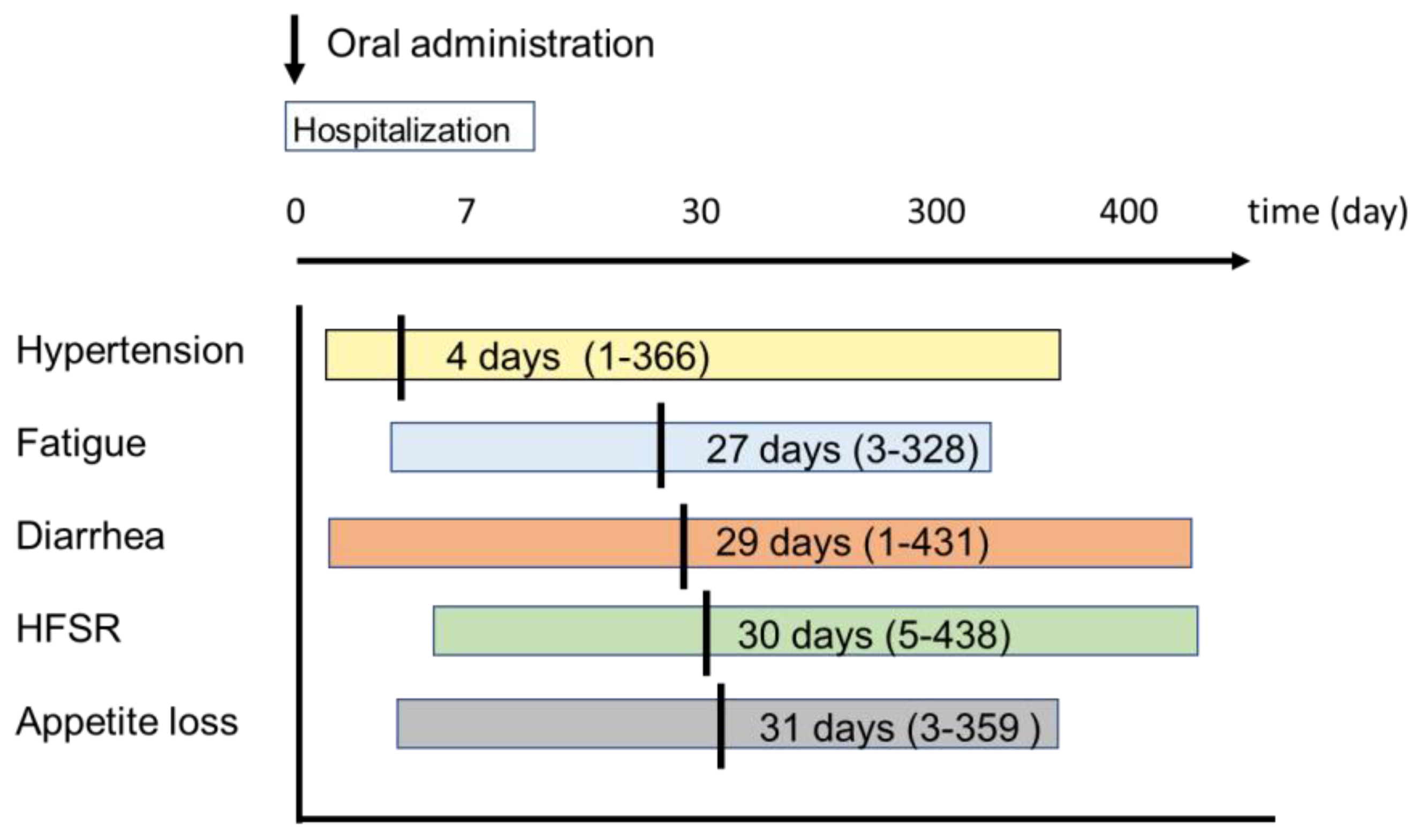
2.3. Type and Timing of AEs
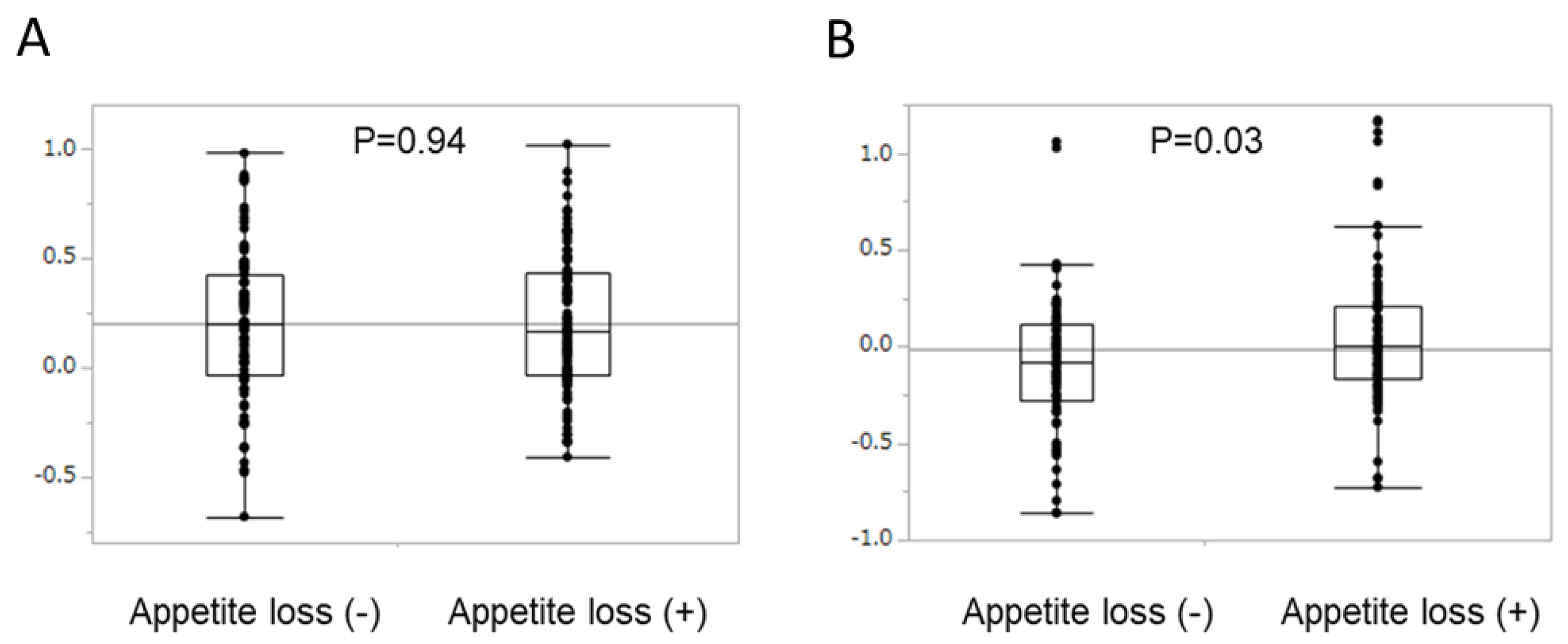
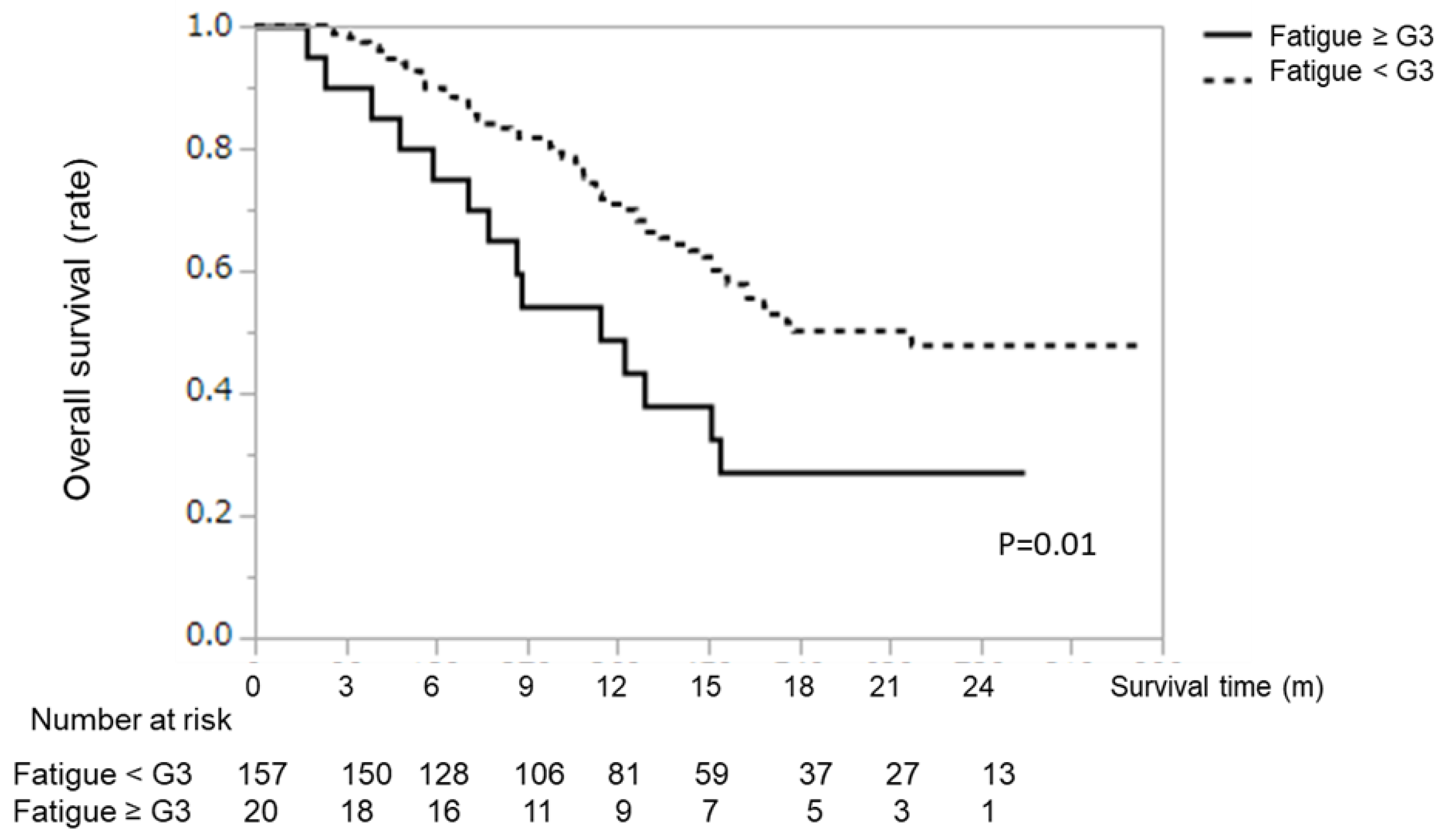
2.4. Survival Analysis According to Type of AE
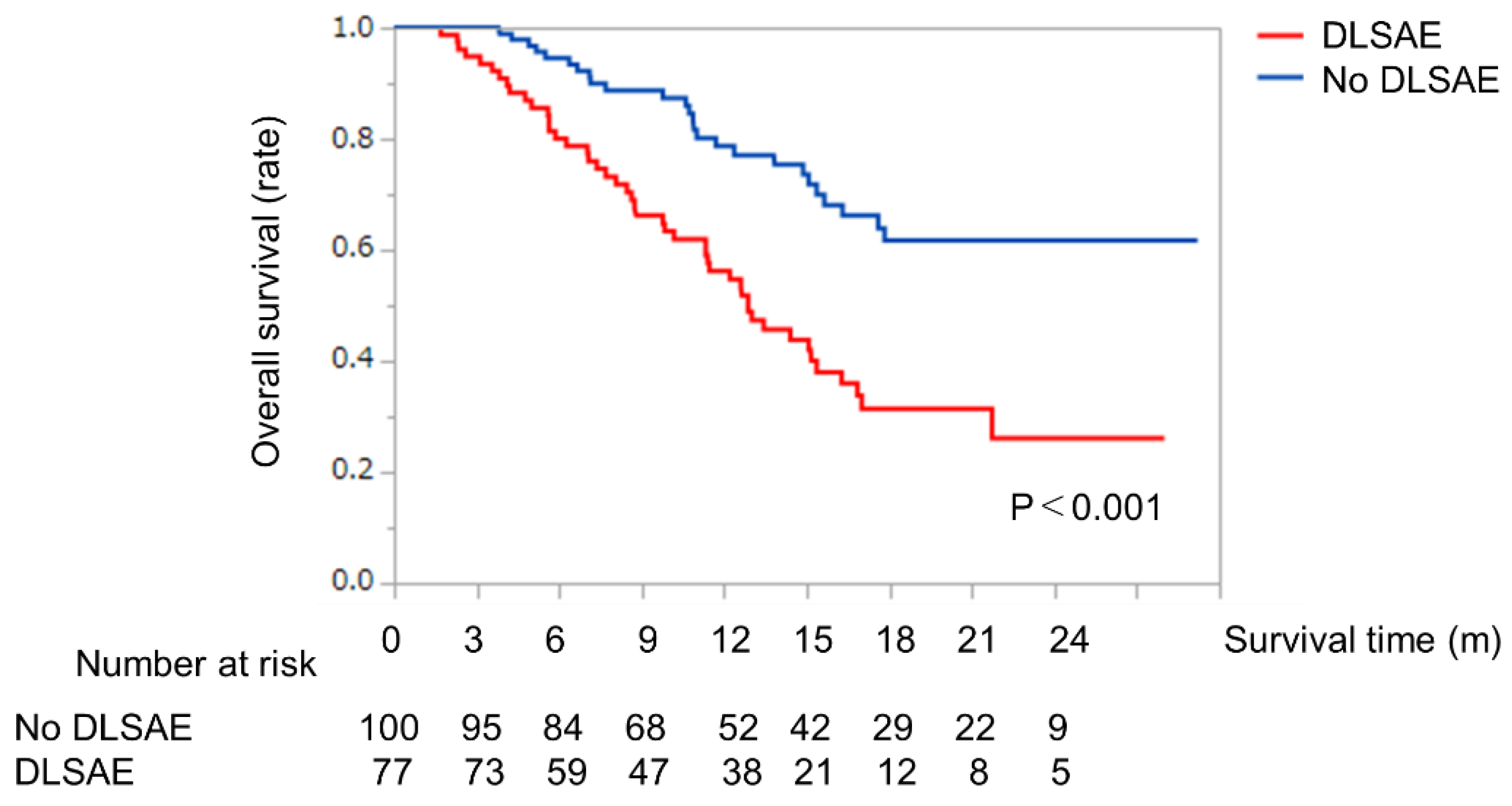
2.5. Survival Analysis with or without Discontinuation of LEN Due to Severe AEs
2.6. Decision-Tree Analysis for Discontinuation of LEN Due to Severe AEs
2.7. Difference in Adverse Events Associated with LEN between the <71 Years and ≥71 Years Groups
2.8. Logistic Regression Analysis for Discontinuation of LEN Due to Severe AEs
3. Discussion
4. Materials and Methods
4.1. Study Design and Patients
4.2. Evaluation of Hepatic Reserve Function
4.3. Treatment Protocol
4.4. Evaluation of the Therapeutic Response and the Follow-Up Schedule
4.5. Safety Evaluation and Assessment of Adverse Events
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Balogh, J.; Victor, D., 3rd; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Papatheodoridis, G.V.; Sypsa, V.; Dalekos, G.N.; Yurdaydin, C.; Van Boemmel, F.; Buti, M.; Calleja, J.L.; Chi, H.; Goulis, J.; Manolakopoulos, S.; et al. Hepatocellular carcinoma prediction beyond year 5 of oral therapy in a large cohort of Caucasian patients with chronic hepatitis B. J. Hepatol. 2020, 72, 1088–1096. [Google Scholar] [CrossRef]
- Grandhi, M.S.; Kim, A.K.; Ronnekleiv-Kelly, S.M.; Kamel, I.R.; Ghasebeh, M.A.; Pawlik, T.M. Hepatocellular carcinoma: From diagnosis to treatment. Surg. Oncol. 2016, 25, 74–85. [Google Scholar] [CrossRef]
- Kudo, M. Lenvatinib May Drastically Change the Treatment Landscape of Hepatocellular Carcinoma. Liver Cancer 2018, 7, 1–19. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Ueshima, K.; Nishida, N.; Hagiwara, S.; Aoki, T.; Minami, T.; Chishina, H.; Takita, M.; Minami, Y.; Ida, H.; Takenaka, M.; et al. Impact of Baseline ALBI Grade on the Outcomes of Hepatocellular Carcinoma Patients Treated with Lenvatinib: A Multicenter Study. Cancers 2019, 11, 952. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; Itobayashi, E.; Tajiri, K.; et al. Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions-Multicenter analysis. Cancer Med. 2019, 8, 3719–3728. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Hiraoka, A.; Michitaka, K.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; et al. Neutrophil-to-lymphocyte ratio is associated with survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Liver Int. 2020, 40, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Shimose, S.; Kawaguchi, T.; Iwamoto, H.; Tanaka, M.; Miyazaki, K.; Ono, M.; Niizeki, T.; Shirono, T.; Okamura, S.; Nakano, M.; et al. Controlling Nutritional Status (CONUT) Score is Associated with Overall Survival in Patients with Unresectable Hepatocellular Carcinoma Treated with Lenvatinib: A Multicenter Cohort Study. Nutrients 2020, 12, 1076. [Google Scholar] [CrossRef] [PubMed]
- Kirino, S.; Tsuchiya, K.; Kurosaki, M.; Kaneko, S.; Inada, K.; Yamashita, K.; Osawa, L.; Hayakawa, Y.; Sekiguchi, S.; Okada, M.; et al. Relative dose intensity over the first four weeks of lenvatinib therapy is a factor of favorable response and overall survival in patients with unresectable hepatocellular carcinoma. PLoS ONE 2020, 15, e0231828. [Google Scholar] [CrossRef]
- Ye, S.L.; Yang, J.; Bie, P.; Zhang, S.; Chen, X.; Liu, F.; Liu, L.; Zhou, J.; Dou, K.; Hao, C.; et al. Safety assessment of sorafenib in Chinese patients with unresectable hepatocellular carcinoma: Subgroup analysis of the GIDEON study. BMC Cancer 2018, 18, 247. [Google Scholar] [CrossRef]
- Chuma, M.; Terashita, K.; Sakamoto, N. New molecularly targeted therapies against advanced hepatocellular carcinoma: From molecular pathogenesis to clinical trials and future directions. Hepatol. Res. 2015, 45, E1–E11. [Google Scholar] [CrossRef]
- Tohyama, O.; Matsui, J.; Kodama, K.; Hata-Sugi, N.; Kimura, T.; Okamoto, K.; Minoshima, Y.; Iwata, M.; Funahashi, Y. Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J. Thyroid Res. 2014, 2014, 638–747. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H.; Suzuki, H.; Shimose, S.; Niizeki, T.; Nakano, M.; Shirono, T.; Okamura, S.; Noda, Y.; Kamachi, N.; Nakamura, T.; et al. Weekends-Off Lenvatinib for Unresectable Hepatocellular Carcinoma Improves Therapeutic Response and Tolerability toward Adverse Events. Cancers 2020, 12, 1010. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Iwamoto, H.; Hosaka, K.; Seki, T.; Andersson, P.; Lim, S.; Fischer, C.; Nakamura, M.; Abe, M.; et al. Discontinuation of anti-VEGF cancer therapy promotes metastasis through a liver revascularization mechanism. Nat. Commun. 2016, 7, 12680. [Google Scholar] [CrossRef]
- Ohki, T.; Sato, K.; Kondo, M.; Goto, E.; Sato, T.; Kondo, Y.; Akamatsu, M.; Sato, S.; Yoshida, H.; Koike, Y.; et al. Impact of Adverse Events on the Progression-Free Survival of Patients with Advanced Hepatocellular Carcinoma Treated with Lenvatinib: A Multicenter Retrospective Study. Drugs Real World Outcomes 2020. [Google Scholar] [CrossRef]
- Scartozzi, M.; Galizia, E.; Chiorrini, S.; Giampieri, R.; Berardi, R.; Pierantoni, C.; Cascinu, S. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann. Oncol 2009, 20, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Spano, J.P.; Chodkiewicz, C.; Maurel, J.; Wong, R.; Wasan, H.; Barone, C.; Letourneau, R.; Bajetta, E.; Pithavala, Y.; Bycott, P.; et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: An open-label randomised phase II study. Lancet 2008, 371, 2101–2108. [Google Scholar] [CrossRef]
- Cunningham, D.; Humblet, Y.; Siena, S.; Khayat, D.; Bleiberg, H.; Santoro, A.; Bets, D.; Mueser, M.; Harstrick, A.; Verslype, C.; et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004, 351, 337–345. [Google Scholar] [CrossRef]
- Yamashita, T.; Kudo, M.; Ikeda, K.; Izumi, N.; Tateishi, R.; Ikeda, M.; Aikata, H.; Kawaguchi, Y.; Wada, Y.; Numata, K.; et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: An analysis of Japanese subset. J. Gastroenterol. 2020, 55, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Xia, D.; Bai, W.; Yuan, J.; Li, X.; Niu, J.; Yin, Z.; Xia, J.; Cai, H.; Fan, D.; et al. Tumor Hypervascularity and hand-foot-skin reaction predict better outcomes in combination treatment of TACE and Sorafenib for intermediate hepatocellular carcinoma. BMC Cancer 2019, 19, 409. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Tan, G.; Zhu, M.; Li, W.; Zhai, B.; Sun, X. Hand-foot skin reaction is a beneficial indicator of sorafenib therapy for patients with hepatocellular carcinoma: A systemic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 1–8. [Google Scholar] [CrossRef]
- Reig, M.; Torres, F.; Rodriguez-Lope, C.; Forner, A.; Neus, L.L.; Rimola, J.; Darnell, A.; Rios, J.; Ayuso, C.; Bruix, J. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J. Hepatol. 2014, 61, 318–324. [Google Scholar] [CrossRef]
- Cho, J.Y.; Paik, Y.H.; Lim, H.Y.; Kim, Y.G.; Lim, H.K.; Min, Y.W.; Gwak, G.Y.; Choi, M.S.; Lee, J.H.; Koh, K.C.; et al. Clinical parameters predictive of outcomes in sorafenib-treated patients with advanced hepatocellular carcinoma. Liver Int. 2013, 33, 950–957. [Google Scholar] [CrossRef]
- Otsuka, T.; Eguchi, Y.; Kawazoe, S.; Yanagita, K.; Ario, K.; Kitahara, K.; Kawasoe, H.; Kato, H.; Mizuta, T.; Saga Liver Cancer Study, G. Skin toxicities and survival in advanced hepatocellular carcinoma patients treated with sorafenib. Hepatol. Res. 2012, 42, 879–886. [Google Scholar] [CrossRef]
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; LeFevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014, 311, 507–520. [Google Scholar] [CrossRef]
- Tamaki, Y.; Nakade, Y.; Yamauchi, T.; Makino, Y.; Yokohama, S.; Okada, M.; Aso, K.; Kanamori, H.; Ohashi, T.; Sato, K.; et al. Angiotensin II type 1 receptor antagonist prevents hepatic carcinoma in rats with nonalcoholic steatohepatitis. J. Gastroenterol. 2013, 48, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wen, W.; Morgans, A.K.; Pao, W.; Shu, X.O.; Zheng, W. Disparities by Race, Age, and Sex in the Improvement of Survival for Major Cancers: Results From the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol. 2015, 1, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Dale, W.; Mohile, S.G.; Eldadah, B.A.; Trimble, E.L.; Schilsky, R.L.; Cohen, H.J.; Muss, H.B.; Schmader, K.E.; Ferrell, B.; Extermann, M.; et al. Biological, clinical, and psychosocial correlates at the interface of cancer and aging research. J. Natl. Cancer Inst. 2012, 104, 581–589. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Hurria, A. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology Summary. J. Oncol. Pract. 2018, 14, 442–446. [Google Scholar] [CrossRef]
- Inomata, M.; Shimokawa, K.; Tokui, K.; Taka, C.; Okazawa, S.; Kambara, K.; Yamada, T.; Miwa, T.; Hayashi, R.; Kashii, T.; et al. Appetite Loss as an Adverse Effect During Treatment with EGFR-TKIs in Elderly Patients with Non-small Cell Lung Cancer. Anticancer Res. 2016, 36, 4951–4954. [Google Scholar] [CrossRef]
- Tamai, T.; Hayato, S.; Hojo, S.; Suzuki, T.; Okusaka, T.; Ikeda, K.; Kumada, H. Dose Finding of Lenvatinib in Subjects With Advanced Hepatocellular Carcinoma Based on Population Pharmacokinetic and Exposure-Response Analyses. J. Clin. Pharmacol. 2017, 57, 1138–1147. [Google Scholar] [CrossRef]
- Yao, X.; Yan, D.; Liu, D.; Zeng, H.; Li, H. Efficacy and adverse events of transcatheter arterial chemoembolization in combination with sorafenib in the treatment of unresectable hepatocellular carcinoma. Mol. Clin. Oncol. 2015, 3, 929–935. [Google Scholar] [CrossRef]
- Labeur, T.A.; Berhane, S.; Edeline, J.; Blanc, J.F.; Bettinger, D.; Meyer, T.; Van Vugt, J.L.A.; Ten Cate, D.W.G.; De Man, R.A.; Eskens, F.; et al. Improved survival prediction and comparison of prognostic models for patients with hepatocellular carcinoma treated with sorafenib. Liver Int. 2020, 40, 215–228. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Chan, S.; Minami, T.; Chishina, H.; Aoki, T.; Takita, M.; Hagiwara, S.; Minami, Y.; Ida, H.; et al. Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child-Pugh A Liver Function: A Proof-Of-Concept Study. Cancers 2019, 11, 1084. [Google Scholar] [CrossRef]
- Porta, C.; Levy, A.; Hawkins, R.; Castellano, D.; Bellmunt, J.; Nathan, P.; McDermott, R.; Wagstaff, J.; Donnellan, P.; McCaffrey, J.; et al. Impact of adverse events, treatment modifications, and dose intensity on survival among patients with advanced renal cell carcinoma treated with first-line sunitinib: A medical chart review across ten centers in five European countries. Cancer Med. 2014, 3, 1517–1526. [Google Scholar] [CrossRef]
- Morimoto, M.; Numata, K.; Kondo, M.; Hidaka, H.; Takada, J.; Shibuya, A.; Kobayashi, S.; Ohkawa, S.; Okuse, C.; Morita, S.; et al. Higher discontinuation and lower survival rates are likely in elderly Japanese patients with advanced hepatocellular carcinoma receiving sorafenib. Hepatol. Res. 2011, 41, 296–302. [Google Scholar] [CrossRef]
- Labeur, T.A.; van Vugt, J.L.A.; Ten Cate, D.W.G.; Takkenberg, R.B.; JNM, I.J.; Groot Koerkamp, B.; de Man, R.A.; van Delden, O.M.; Eskens, F.; Klumpen, H.J. Body Composition Is an Independent Predictor of Outcome in Patients with Hepatocellular Carcinoma Treated with Sorafenib. Liver Cancer 2019, 8, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F. Forcing the vicious circle: Sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann. Oncol. 2017, 28, 2107–2118. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, S.; Ooka, Y.; Itokawa, N.; Inoue, M.; Okabe, S.; Seki, A.; Haga, Y.; Obu, M.; Atsukawa, M.; Itobayashi, E.; et al. Sequential therapy with sorafenib and regorafenib for advanced hepatocellular carcinoma: A multicenter retrospective study in Japan. Invest. New Drugs 2020, 38, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Gerolami, R.; Caparello, C.; et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J. Hepatol. 2018, 69, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Cholongitas, E.; Papatheodoridis, G.V.; Vangeli, M.; Terreni, N.; Patch, D.; Burroughs, A.K. Systematic review: The model for end-stage liver disease--should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment. Pharmacol. Ther. 2005, 22, 1079–1089. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Shiozawa, K.; Watanabe, M.; Ikehara, T.; Matsukiyo, Y.; Kogame, M.; Kishimoto, Y.; Okubo, Y.; Makino, H.; Tsukamoto, N.; Igarashi, Y.; et al. Comparison of percutaneous radiofrequency ablation and CyberKnife((R)) for initial solitary hepatocellular carcinoma: A pilot study. World J. Gastroenterol. 2015, 21, 13490–13499. [Google Scholar] [CrossRef]
- Shimose, S.; Tanaka, M.; Iwamoto, H.; Niizeki, T.; Shirono, T.; Aino, H.; Noda, Y.; Kamachi, N.; Okamura, S.; Nakano, M.; et al. Prognostic impact of transcatheter arterial chemoembolization (TACE) combined with radiofrequency ablation in patients with unresectable hepatocellular carcinoma: Comparison with TACE alone using decision-tree analysis after propensity score matching. Hepatol. Res. 2019, 49, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Shimose, S.; Kawaguchi, T.; Iwamoto, H.; Niizeki, T.; Shirono, T.; Tanaka, M.; Koga, H.; Torimura, T. Indication of suitable transarterial chemoembolization and multikinase inhibitors for intermediate stage hepatocellular carcinoma. Oncol. Lett. 2020, 19, 2667–2676. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Tokushige, K.; Hyogo, H.; Aikata, H.; Nakajima, T.; Ono, M.; Kawanaka, M.; Sawada, K.; Imajo, K.; Honda, K.; et al. A Data Mining-based Prognostic Algorithm for NAFLD-related Hepatoma Patients: A Nationwide Study by the Japan Study Group of NAFLD. Sci. Rep. 2018, 8, 10434. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | All Patients |
|---|---|
| N | 177 |
| Age (years) | 74 (38–90) |
| Sex (female/male) | 35/142 |
| BMI | 22.5 (15–38.9) |
| Etiology (HBV/HCV/Others) | 34/82/61 |
| Child-Pugh score (5/6) | 135/42 |
| ALBI grade (1/2) | 73/104 |
| AST (U/L) | 37 (13–160) |
| ALT (U/L) | 29 (6–126) |
| Diabetes mellitus (+/-) | (75/102) |
| Maximum tumor diameter (mm) | 32 (10–127) |
| Number of tumors | |
| <5/≥5 | 54/123 |
| BCLC stage (B/C) | 105/72 |
| AFP (ng/mL) | 51.2 (1.0–146,260) |
| DCP (mAU/mL) | 233.5 (3.3–524,068) |
| Prior treatment of MTAs | 41 |
| (SORA/SORA+REGO) | (29/12) |
| Follow-up duration (month) | 12.2 (2.1–29.2) |
| Response Category | Patients with HCC Treated with Lenvatinib (n = 177) |
|---|---|
| CR | 6 (3%) |
| PR | 57 (32%) |
| SD | 72 (41%) |
| PD | 42 (24%) |
| ORR | 63 (38%) |
| DCR | 135 (76%) |
| Adverse Event | Any n (%) | Grade 1 n (%) | Grade 2 n (%) | Grade 3 ≥, n (%) |
|---|---|---|---|---|
| Hypertension | 94 (53.1%) | 12 (6.8%) | 56 (31.6%) | 26 (14.7%) |
| Appetite loss | 93 (52.5%) | 40 (22.6%) | 43 (24.2%) | 10 (5.6%) |
| Fatigue | 90 (50.8%) | 24 (13.6%) | 46 (25.9%) | 20 (11.3%) |
| Hypothyroidism | 86 (48.6%) | 28 (15.8%) | 58 (31.0%) | 0 (0% |
| Proteinuria | 67 (37.9%) | 27 (15.6%) | 18 (10.2%) | 22 (12.4%) |
| HFSR | 50 (28.2%) | 22 (12.7%) | 17 (9.6%) | 11 (6.2%) |
| Diarrhea | 34 (19.2%) | 11 (6.2%) | 11 (6.2%) | 12 (6.8%) |
| Liver disorder | 49 (27.7%) | 39 (22.0%) | 6 (3.3%) | 4 (2.3%) |
| Hoarseness | 45 (25.4%) | 42 (23.7%) | 3 (1.7%) | 0 (0%) |
| Thrombocytopenia | 34 (19.2%) | 4 (2.3%) | 21 (11.9%) | 9 (5.0%) |
| Hepatic encephalopathy | 18 (10.1%) | 2 (1.1%) | 15 (8.4%) | 1 (0.5%) |
| Ascites | 7 (3.9%) | 5 (2.8%) | 2(1.1%) | 0 (0%) |
| Drug-induced pneumoniae | 3 (1.7%) | 0 (%) | 3 (1.7%) | 0.0 (0) |
| Characteristic | All Patients | Age < 71 | Age ≥ 71 | p |
|---|---|---|---|---|
| n | 177 | 70 | 107 | |
| Hypertension (Presence/Absence) | 94 (53.1%) | 34/36 (48.6%/51.4%) | 60/47 (56.0%/44.0%) | 0.32 |
| Appetite loss (Presence/Absence) | 93 (52.5%) | 29/41 (41.4%/58.6%) | 64/43 (59.8%/40.2%) | 0.01 |
| Fatigue (Presence/Absence) | 90 (50.8%) | 35/35 (50.0%/50.0%) | 55/52 (51.4%/48.6%) | 0.86 |
| Hypothyroidism (Presence/Absence) | 86 (48.6%) | 31/39 (44.3%/55.7%) | 55/52 (51.4%/48.6%) | 0.35 |
| Proteinuria (Presence/Absence) | 67 (37.9%) | 19/51 (27.1%/72.9%) | 48/59 (44.9%/55.1%) | 0.01 |
| HFSR (Presence/Absence) | 50 (28.2%) | 25/45 (35.7%/64.3%) | 25/82 (23.4%/76.6%) | 0.08 |
| Diarrhea (Presence/Absence) | 34 (19.2%) | 14/56 (20.0%/80.0%) | 20/87 (18.7%/81.3%) | 0.82 |
| Appetite loss grade ≥2 (Presence/Absence) | 53 (30.8%) | 14/56 (20.0%/80.0%) | 39/68 (36.4%/63.6%) | 0.01 |
| Fatigue grade ≥3 (Presence/Absence) | 20 (11.3%) | 4/66 (5.7%/94.3%) | 16/91 (14.9%/84.1%) | 0.04 |
| Factors | Unit | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|---|
| Age | 1 | 1.12 | 1.04–1.12 | <0.001 |
| ALBI grade 2 | N/A | 3.15 | 1.54–6.69 | 0.001 |
| Fatigue grade ≥3 | N/A | 4.49 | 1.34–18.4 | 0.008 |
| Appetite loss grade ≥2 | N/A | 2.69 | 1.24–5.97 | 0.012 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimose, S.; Iwamoto, H.; Niizeki, T.; Shirono, T.; Noda, Y.; Kamachi, N.; Okamura, S.; Nakano, M.; Suga, H.; Kuromatsu, R.; et al. Clinical Significance of Adverse Events for Patients with Unresectable Hepatocellular Carcinoma Treated with Lenvatinib: A Multicenter Retrospective Study. Cancers 2020, 12, 1867. https://doi.org/10.3390/cancers12071867
Shimose S, Iwamoto H, Niizeki T, Shirono T, Noda Y, Kamachi N, Okamura S, Nakano M, Suga H, Kuromatsu R, et al. Clinical Significance of Adverse Events for Patients with Unresectable Hepatocellular Carcinoma Treated with Lenvatinib: A Multicenter Retrospective Study. Cancers. 2020; 12(7):1867. https://doi.org/10.3390/cancers12071867
Chicago/Turabian StyleShimose, Shigeo, Hideki Iwamoto, Takashi Niizeki, Tomotake Shirono, Yu Noda, Naoki Kamachi, Shusuke Okamura, Masahito Nakano, Hideya Suga, Ryoko Kuromatsu, and et al. 2020. "Clinical Significance of Adverse Events for Patients with Unresectable Hepatocellular Carcinoma Treated with Lenvatinib: A Multicenter Retrospective Study" Cancers 12, no. 7: 1867. https://doi.org/10.3390/cancers12071867
APA StyleShimose, S., Iwamoto, H., Niizeki, T., Shirono, T., Noda, Y., Kamachi, N., Okamura, S., Nakano, M., Suga, H., Kuromatsu, R., Yamaguchi, T., Kawaguchi, T., Tanaka, M., Noguchi, K., Koga, H., & Torimura, T. (2020). Clinical Significance of Adverse Events for Patients with Unresectable Hepatocellular Carcinoma Treated with Lenvatinib: A Multicenter Retrospective Study. Cancers, 12(7), 1867. https://doi.org/10.3390/cancers12071867








