Development of Antibody Immuno-PET/SPECT Radiopharmaceuticals for Imaging of Oncological Disorders—An Update
Abstract
1. Introduction
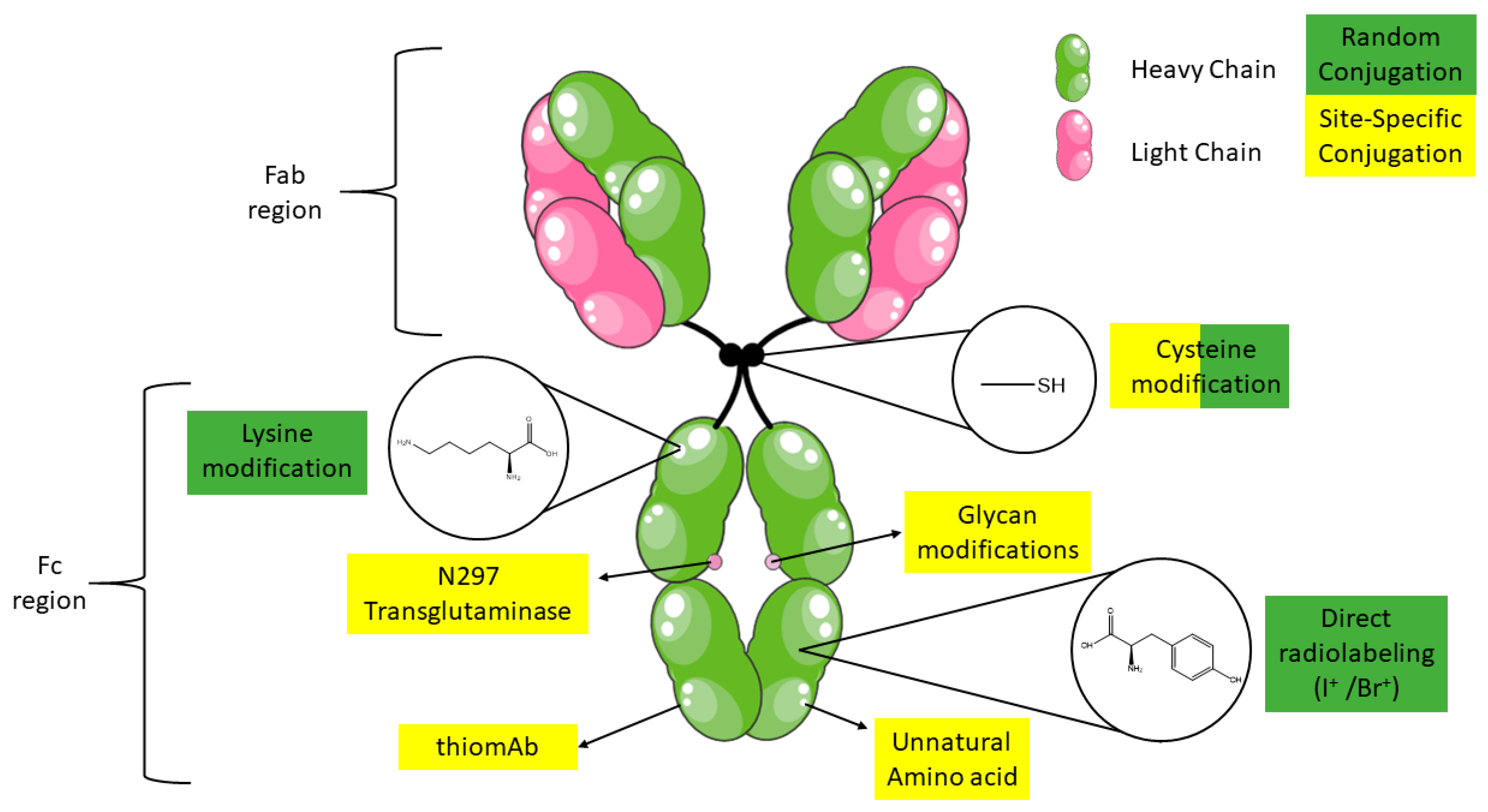
2. Antibody Structure
3. Radiolabeling and Bioconjugation Strategies of Monoclonal Antibodies
3.1. Conjugation Reactions Using Solvent Accessible Amino Acids in Antibodies
3.1.1. Lysine
3.1.2. Cysteine
3.2. Site-Specific Cysteine Bioconjugation
3.3. Glycans
3.4. Antibody Engineering
3.4.1. Protein Engineering
3.4.2. Unnatural Amino Acids (uAA)
3.5. Pretargeting Approach
4. Radionuclides for Antibody Imaging
4.1. Common Radionuclides
4.1.1. Iodine
4.1.2. 76Bromine
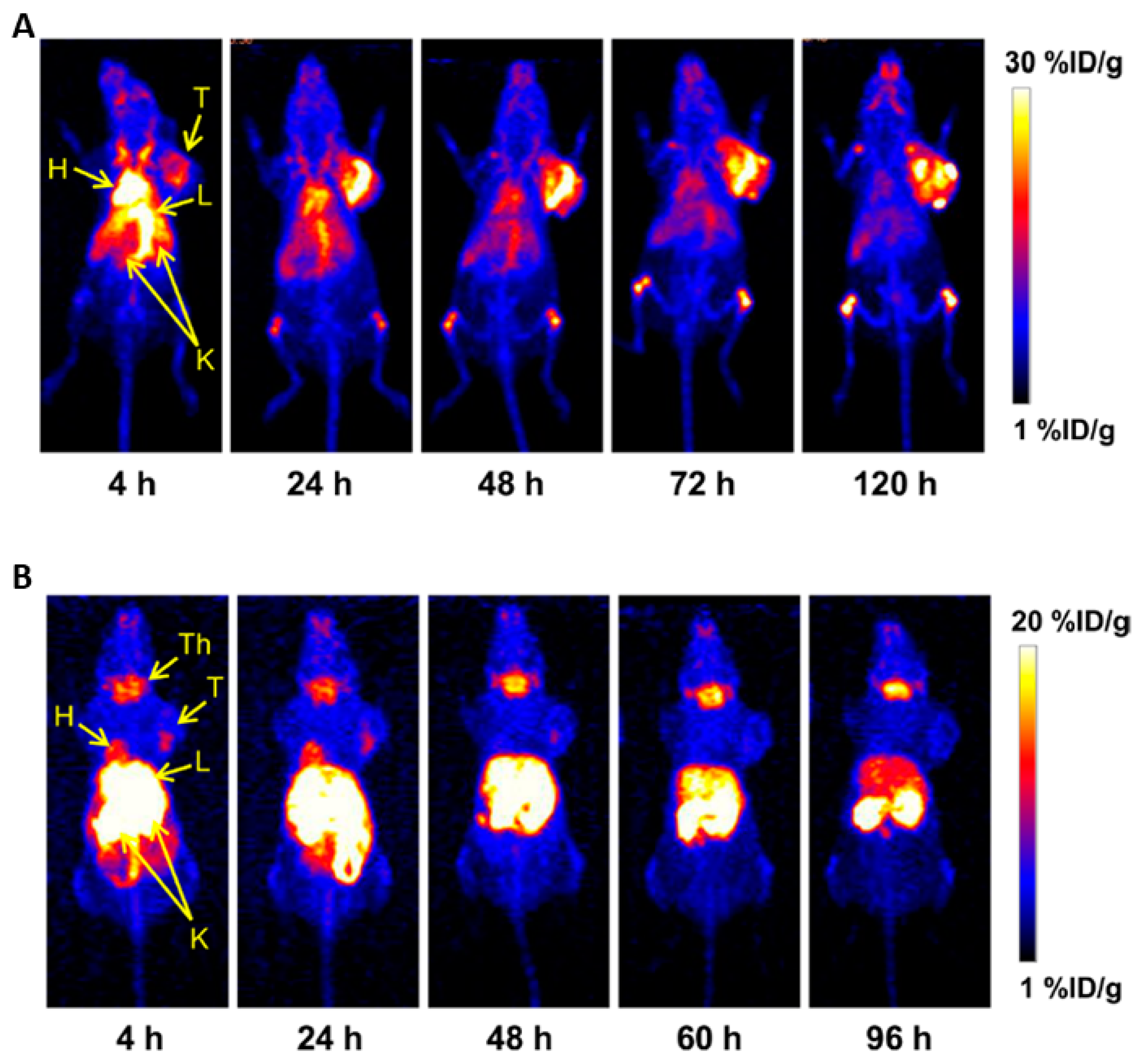
4.1.3. 89Zirconium
4.1.4. 64Copper
4.1.5. 86Yttrium
4.1.6. 111Indium
4.1.7. 99mTechnetium
4.2. Emerging Radionuclides
4.2.1. 52Manganese
4.2.2. Gallium
4.2.3. 90Niobium
4.2.4. Arsenic
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Reddy, S.; Robinson, M.K. Immuno-positron emission tomography in cancer models. Semin. Nucl. Med. 2010, 40, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Dijkers, E.C.; Kosterink, J.G.; Rademaker, A.P.; Perk, L.R.; van Dongen, G.A.; Bart, J.; de Jong, J.R.; de Vries, E.G.; Lub-de Hooge, M.N. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J. Nucl. Med. 2009, 50, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, P.; Incoronato, M. Usefulness of traditional serum biomarkers for management of breast cancer patients. Biomed. Res. Int. 2013, 2013, 685641. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, G.; Lamberts, L.E.; Wimana, Z.; Garcia, C.; Emonts, P.; Ameye, L.; Stroobants, S.; Huizing, M.; Aftimos, P.; Tol, J.; et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): The ZEPHIR trial. Ann. Oncol. 2016, 27, 619–624. [Google Scholar] [CrossRef]
- Gaykema, S.B.; de Jong, J.R.; Perik, P.J.; Brouwers, A.H.; Schroder, C.P.; Oude Munnink, T.H.; Bongaerts, A.H.; de Vries, E.G.; Lub-de Hooge, M.N. (111)In-trastuzumab scintigraphy in HER2-positive metastatic breast cancer patients remains feasible during trastuzumab treatment. Mol. Imaging. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Laforest, R.; Lapi, S.E.; Oyama, R.; Bose, R.; Tabchy, A.; Marquez-Nostra, B.V.; Burkemper, J.; Wright, B.D.; Frye, J.; Frye, S.; et al. [(89)Zr]Trastuzumab: Evaluation of Radiation Dosimetry, Safety, and Optimal Imaging Parameters in Women with HER2-Positive Breast Cancer. Mol. Imaging. Biol. 2016, 18, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Hijnen, N.M.; de Vries, A.; Nicolay, K.; Grull, H. Dual-isotope 111In/177Lu SPECT imaging as a tool in molecular imaging tracer design. Contrast Media Mol. Imaging 2012, 7, 214–222. [Google Scholar] [CrossRef]
- Knight, J.C.; Mosley, M.J.; Kersemans, V.; Dias, G.M.; Allen, P.D.; Smart, S.; Cornelissen, B. Dual-isotope imaging allows in vivo immunohistochemistry using radiolabelled antibodies in tumours. Nucl. Med. Biol. 2019, 70, 14–22. [Google Scholar] [CrossRef]
- Roux, K.H. Immunoglobulin structure and function as revealed by electron microscopy. Int. Arch. Allergy Immunol. 1999, 120, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Adumeau, P.; Sharma, S.K.; Brent, C.; Zeglis, B.M. Site-Specifically Labeled Immunoconjugates for Molecular Imaging—Part 1: Cysteine Residues and Glycans. Mol. Imaging Biol. 2016, 18, 1–17. [Google Scholar] [CrossRef]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.M.; Surana, R.; Wang, S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 2010, 10, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Irani, V.; Guy, A.J.; Andrew, D.; Beeson, J.G.; Ramsland, P.A.; Richards, J.S. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol. Immunol. 2015, 67, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Vivier, D.; Sharma, S.K.; Adumeau, P.; Rodriguez, C.; Fung, K.; Zeglis, B.M. The Impact of FcgammaRI Binding on Immuno-PET. J. Nucl. Med. 2019, 60, 1174–1182. [Google Scholar] [CrossRef]
- Baruah, K.; Bowden, T.A.; Krishna, B.A.; Dwek, R.A.; Crispin, M.; Scanlan, C.N. Selective deactivation of serum IgG: A general strategy for the enhancement of monoclonal antibody receptor interactions. J. Mol. Biol. 2012, 420, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef]
- Ryman, J.T.; Meibohm, B. Pharmacokinetics of Monoclonal Antibodies. CPT Pharmacomet. Syst. Pharm. 2017, 6, 576–588. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Carroll, L.; Yahioglu, G.; Aboagye, E.O.; Miller, P.W. Antibody Fragment and Affibody ImmunoPET Imaging Agents: Radiolabelling Strategies and Applications. ChemMedChem 2018, 13, 2466–2478. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, J.; Perols, A.; Varasteh, Z.; Altai, M.; Braun, A.; Sandstrom, M.; Garske, U.; Tolmachev, V.; Orlova, A.; Karlstrom, A.E. Comparative evaluation of synthetic anti-HER2 Affibody molecules site-specifically labelled with 111In using N-terminal DOTA, NOTA and NODAGA chelators in mice bearing prostate cancer xenografts. Eur. J. Nucl. Med. Mol. Imaging. 2012, 39, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Freise, A.C.; Wu, A.M. In vivo imaging with antibodies and engineered fragments. Mol. Immunol. 2015, 67, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Smith, S.W.; Ghone, S.; Tomczuk, B. Current ADC Linker Chemistry. Pharm. Res. 2015, 32, 3526–3540. [Google Scholar] [CrossRef] [PubMed]
- Ducry, L. Antibody-Drug Conjugates; Humana Press: Totowa, NJ, USA, 2013; Volume 1045. [Google Scholar]
- Cohen, R.; Vugts, D.J.; Stigter-van Walsum, M.; Visser, G.W.; van Dongen, G.A. Inert coupling of IRDye800CW and zirconium-89 to monoclonal antibodies for single- or dual-mode fluorescence and PET imaging. Nat. Protoc. 2013, 8, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Vosjan, M.J.; Perk, L.R.; Visser, G.W.; Budde, M.; Jurek, P.; Kiefer, G.E.; van Dongen, G.A. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat. Protoc. 2010, 5, 739–743. [Google Scholar] [CrossRef]
- Perk, L.R.; Vosjan, M.J.; Visser, G.W.; Budde, M.; Jurek, P.; Kiefer, G.E.; van Dongen, G.A. p-Isothiocyanatobenzyl-desferrioxamine: A new bifunctional chelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur. J. Nucl. Med. Mol. Imaging. 2010, 37, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; May, K. Disulfide bond structures of IgG molecules: Structural variations, chemical modifications and possible impacts to stability and biological function. MAbs 2012, 4, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Adumeau, P.; Davydova, M.; Zeglis, B.M. Thiol-Reactive Bifunctional Chelators for the Creation of Site-Selectively Modified Radioimmunoconjugates with Improved Stability. Bioconjug. Chem. 2018, 29, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Tinianow, J.N.; Gill, H.S.; Ogasawara, A.; Flores, J.E.; Vanderbilt, A.N.; Luis, E.; Vandlen, R.; Darwish, M.; Junutula, J.R.; Williams, S.P.; et al. Site-specifically 89Zr-labeled monoclonal antibodies for ImmunoPET. Nucl. Med. Biol. 2010, 37, 289–297. [Google Scholar] [CrossRef]
- Slinkin, M.A.; Curtet, C.; Sai-Maurel, C.; Gestin, J.F.; Torchilin, V.P.; Chatal, J.F. Site-specific conjugation of chain-terminal chelating polymers to Fab’ fragments of anti-CEA mAb: Effect of linkage type and polymer size on conjugate biodistribution in nude mice bearing human colorectal carcinoma. Bioconjug. Chem. 1992, 3, 477–483. [Google Scholar] [CrossRef]
- Alley, S.C.; Benjamin, D.R.; Jeffrey, S.C.; Okeley, N.M.; Meyer, D.L.; Sanderson, R.J.; Senter, P.D. Contribution of linker stability to the activities of anticancer immunoconjugates. Bioconjug. Chem. 2008, 19, 759–765. [Google Scholar] [CrossRef]
- Baldwin, A.D.; Kiick, K.L. Tunable degradation of maleimide-thiol adducts in reducing environments. Bioconjug. Chem. 2011, 22, 1946–1953. [Google Scholar] [CrossRef]
- Ponte, J.F.; Sun, X.; Yoder, N.C.; Fishkin, N.; Laleau, R.; Coccia, J.; Lanieri, L.; Bogalhas, M.; Wang, L.; Wilhelm, S.; et al. Understanding How the Stability of the Thiol-Maleimide Linkage Impacts the Pharmacokinetics of Lysine-Linked Antibody-Maytansinoid Conjugates. Bioconjug. Chem. 2016, 27, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Asano, S.; Barbas, C.F., 3rd. Rapid, stable, chemoselective labeling of thiols with Julia-Kocienski-like reagents: A serum-stable alternative to maleimide-based protein conjugation. Angew. Chem. Int. Ed. Engl. 2013, 52, 12592–12596. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Glaser, J.M.; Edwards, K.J.; Khozeimeh Sarbisheh, E.; Salih, A.K.; Lewis, J.S.; Price, E.W. A Systematic Evaluation of Antibody Modification and (89)Zr-Radiolabeling for Optimized Immuno-PET. Bioconjug. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Amphlett, G.; Blattler, W.A.; Lambert, J.M.; Zhang, W. Structural characterization of the maytansinoid-monoclonal antibody immunoconjugate, huN901-DM1, by mass spectrometry. Protein Sci. 2005, 14, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, B.S.; Wei, B.; Ohri, R.; Zhou, J.; He, J.; Yu, S.F.; Leipold, D.; Cosino, E.; Yee, S.; Fourie-O’Donohue, A.; et al. Attachment Site Cysteine Thiol pKa Is a Key Driver for Site-Dependent Stability of THIOMAB Antibody-Drug Conjugates. Bioconjug. Chem. 2017, 28, 2538–2548. [Google Scholar] [CrossRef] [PubMed]
- Junutula, J.R.; Raab, H.; Clark, S.; Bhakta, S.; Leipold, D.D.; Weir, S.; Chen, Y.; Simpson, M.; Tsai, S.P.; Dennis, M.S.; et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat. Biotechnol. 2008, 26, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Tavare, R.; Wu, W.H.; Zettlitz, K.A.; Salazar, F.B.; McCabe, K.E.; Marks, J.D.; Wu, A.M. Enhanced immunoPET of ALCAM-positive colorectal carcinoma using site-specific (6)(4)Cu-DOTA conjugation. Protein Eng. Des. Sel. 2014, 27, 317–324. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodwell, J.D.; Alvarez, V.L.; Lee, C.; Lopes, A.D.; Goers, J.W.; King, H.D.; Powsner, H.J.; McKearn, T.J. Site-specific covalent modification of monoclonal antibodies: In vitro and in vivo evaluations. Proc. Natl. Acad. Sci. USA 1986, 83, 2632–2636. [Google Scholar] [CrossRef] [PubMed]
- Chengazi, V.U.; Feneley, M.R.; Ellison, D.; Stalteri, M.; Granowski, A.; Granowska, M.; Nimmon, C.C.; Mather, S.J.; Kirby, R.S.; Britton, K.E. Imaging prostate cancer with technetium-99m-7E11-C5.3 (CYT-351). J. Nucl. Med. 1997, 38, 675–682. [Google Scholar] [PubMed]
- Bejot, R.; Goggi, J.; Moonshi, S.S.; Padmanabhan, P.; Bhakoo, K.K. Aminooxy-functionalized DOTA for radiolabeling of oxidized antibodies: Evaluation of site-specific 111In-labeled trastuzumab. J. Label. Compd. Radiopharm. 2012, 55, 346–353. [Google Scholar] [CrossRef]
- Wang, W.; Vlasak, J.; Li, Y.; Pristatsky, P.; Fang, Y.; Pittman, T.; Roman, J.; Wang, Y.; Prueksaritanont, T.; Ionescu, R. Impact of methionine oxidation in human IgG1 Fc on serum half-life of monoclonal antibodies. Mol. Immunol. 2011, 48, 860–866. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Qasba, P.K. Structure-based design of beta 1,4-galactosyltransferase I (beta 4Gal-T1) with equally efficient N-acetylgalactosaminyltransferase activity: Point mutation broadens beta 4Gal-T1 donor specificity. J. Biol. Chem. 2002, 277, 20833–20839. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Davis, C.B.; Aggeler, R.; Kang, H.C.; Chen, A.; Agnew, B.J.; Lewis, J.S. Enzyme-mediated methodology for the site-specific radiolabeling of antibodies based on catalyst-free click chemistry. Bioconjug. Chem. 2013, 24, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.K.; Christensen, C.; Jensen, M.M.; Agnew, B.J.; Schjoth-Frydendahl, C.; Kjaer, A.; Nielsen, C.H. Site-specifically labeled (89)Zr-DFO-trastuzumab improves immuno-reactivity and tumor uptake for immuno-PET in a subcutaneous HER2-positive xenograft mouse model. Theranostics 2019, 9, 4409–4420. [Google Scholar] [CrossRef] [PubMed]
- Vivier, D.; Fung, K.; Rodriguez, C.; Adumeau, P.; Ulaner, G.A.; Lewis, J.S.; Sharma, S.K.; Zeglis, B.M. The Influence of Glycans-Specific Bioconjugation on the FcgammaRI Binding and In vivo Performance of (89)Zr-DFO-Pertuzumab. Theranostics 2020, 10, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Agatemor, C.; Buettner, M.J.; Ariss, R.; Muthiah, K.; Saeui, C.T.; Yarema, K.J. Exploiting metabolic glycoengineering to advance healthcare. Nat. Rev. Chem. 2019, 3, 605–620. [Google Scholar] [CrossRef]
- Rochefort, M.M.; Girgis, M.D.; Ankeny, J.S.; Tomlinson, J.S. Metabolic exploitation of the sialic acid biosynthetic pathway to generate site-specifically labeled antibodies. Glycobiology 2013, 24, 62–69. [Google Scholar] [CrossRef]
- Popp, M.W.; Ploegh, H.L. Making and breaking peptide bonds: Protein engineering using sortase. Angew. Chem. Int. Ed. Engl. 2011, 50, 5024–5032. [Google Scholar] [CrossRef]
- Massa, S.; Vikani, N.; Betti, C.; Ballet, S.; Vanderhaegen, S.; Steyaert, J.; Descamps, B.; Vanhove, C.; Bunschoten, A.; van Leeuwen, F.W.; et al. Sortase A-mediated site-specific labeling of camelid single-domain antibody-fragments: A versatile strategy for multiple molecular imaging modalities. Contrast. Media. Mol. Imaging 2016, 11, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Jeger, S.; Zimmermann, K.; Blanc, A.; Grunberg, J.; Honer, M.; Hunziker, P.; Struthers, H.; Schibli, R. Site-specific and stoichiometric modification of antibodies by bacterial transglutaminase. Angew. Chem. Int. Ed. Engl. 2010, 49, 9995–9997. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Vaughn, B.A.; Solis, W.A.; Lupher, M.L., Jr.; Hallam, T.J.; Boros, E. Site-Specific (89)Zr- and (111)In-Radiolabeling and In Vivo Evaluation of Glycan-free Antibodies by Azide-Alkyne Cycloaddition with a Non-natural Amino Acid. Bioconjug. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Abou, D.S.; Ku, T.; Smith-Jones, P.M. In vivo biodistribution and accumulation of 89Zr in mice. Nucl. Med. Biol. 2011, 38, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, D.A. Tumor pretargeting: Almost the bottom line. J. Nucl. Med. 1995, 36, 876–879. [Google Scholar] [PubMed]
- Goodwin, D.; Meares, C.; Diamanti, C.; McCall, M.; Lai, C.; Torti, F.; McTigue, M.; Martin, B. Use of specific antibody for rapid clearance of circulating blood background from radiolabeled tumor imaging proteins. Eur. J. Nucl. Med. 1984, 9, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Reardan, D.T.; Meares, C.F.; Goodwin, D.A.; McTigue, M.; David, G.S.; Stone, M.R.; Leung, J.P.; Bartholomew, R.M.; Frincke, J.M. Antibodies against metal chelates. Nature 1985, 316, 265–268. [Google Scholar] [CrossRef]
- Stickney, D.R.; Anderson, L.D.; Slater, J.B.; Ahlem, C.N.; Kirk, G.A.; Schweighardt, S.A.; Frincke, J.M. Bifunctional antibody: A binary radiopharmaceutical delivery system for imaging colorectal carcinoma. Cancer Res. 1991, 51, 6650–6655. [Google Scholar]
- Hnatowich, D.J.; Virzi, F.; Rusckowski, M. Investigations of avidin and biotin for imaging applications. J. Nucl. Med. 1987, 28, 1294–1302. [Google Scholar]
- Liu, G.; Dou, S.; Liu, Y.; Wang, Y.; Rusckowski, M.; Hnatowich, D.J. 90Y labeled phosphorodiamidate morpholino oligomer for pretargeting radiotherapy. Bioconjug. Chem. 2011, 22, 2539–2545. [Google Scholar] [CrossRef][Green Version]
- Liu, G.; Dou, S.; Cheng, D.; Leif, J.; Rusckowski, M.; Streeter, P.R.; Shultz, L.D.; Hnatowich, D.J.; Greiner, D.L. Human islet cell MORF/cMORF pretargeting in a xenogeneic murine transplant model. Mol. Pharm. 2011, 8, 767–773. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Chang, F.; Zhang, Y.; Liu, N.; Liu, G.; Gupta, S.; Rusckowski, M.; Hnatowich, D.J. Pretargeting with amplification using polymeric peptide nucleic acid. Bioconjug. Chem. 2001, 12, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Altai, M.; Membreno, R.; Cook, B.; Tolmachev, V.; Zeglis, B.M. Pretargeted Imaging and Therapy. J. Nucl. Med. 2017, 58, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Vugts, D.J.; Vervoort, A.; Stigter-van Walsum, M.; Visser, G.W.M.; Robillard, M.S.; Versteegen, R.M.; Vulders, R.C.M.; Herscheid, J.K.D.M.; van Dongen, G.A.M.S. Synthesis of phosphine and antibody-azide probes for in vivo Staudinger ligation in a pretargeted imaging and therapy approach. Bioconjug. Chem. 2011, 22, 2072–2081. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, S.M.; Rossin, R.; Renart Verkerk, P.; Ten Hoeve, W.; Janssen, H.M.; Lub, J.; Robillard, M.S. Evaluation of strained alkynes for Cu-free click reaction in live mice. Nucl. Med. Biol. 2013, 40, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.L.; Guo, Z.; Bernardes, G.J.L. Inverse electron demand Diels–Alder reactions in chemical biology. Chem. Soc. Rev. 2017, 46, 4895–4950. [Google Scholar] [CrossRef]
- Rossin, R.; Verkerk, P.R.; van den Bosch, S.M.; Vulders, R.C.; Verel, I.; Lub, J.; Robillard, M.S. In vivo chemistry for pretargeted tumor imaging in live mice. Angew. Chem. 2010, 49, 3375–3378. [Google Scholar] [CrossRef]
- Houghton, J.L.; Zeglis, B.M.; Abdel-Atti, D.; Sawada, R.; Scholz, W.W.; Lewis, J.S. Pretargeted Immuno-PET of Pancreatic Cancer: Overcoming Circulating Antigen and Internalized Antibody to Reduce Radiation Doses. J. Nucl. Med. 2016, 57, 453–459. [Google Scholar] [CrossRef]
- Evans, H.L.; Nguyen, Q.-D.; Carroll, L.S.; Kaliszczak, M.; Twyman, F.J.; Spivey, A.C.; Aboagye, E.O. A bioorthogonal 68Ga-labelling strategy for rapid in vivo imaging. Chem. Commun. 2014, 50, 9557–9560. [Google Scholar] [CrossRef]
- Meyer, J.P.; Houghton, J.L.; Kozlowski, P.; Abdel-Atti, D.; Reiner, T.; Pillarsetty, N.V.; Scholz, W.W.; Zeglis, B.M.; Lewis, J.S. (18)F-Based Pretargeted PET Imaging Based on Bioorthogonal Diels-Alder Click Chemistry. Bioconjug. Chem. 2016, 27, 298–301. [Google Scholar] [CrossRef]
- Li, Z.; Cai, H.; Hassink, M.; Blackman, M.L.; Brown, R.C.D.; Conti, P.S.; Fox, J.M. Tetrazine–trans-cyclooctene ligation for the rapid construction of 18F labeled probes. Chem. Commun. 2010, 46, 8043–8045. [Google Scholar] [CrossRef]
- Wyffels, L.; Thomae, D.; Waldron, A.-M.; Fissers, J.; Dedeurwaerdere, S.; Van der Veken, P.; Joossens, J.; Stroobants, S.; Augustyns, K.; Staelens, S. In vivo evaluation of 18F-labeled TCO for pre-targeted PET imaging in the brain. Nucl. Med. Biol. 2014, 41, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Darko, A.; Wallace, S.; Dmitrenko, O.; Machovina, M.M.; Mehl, R.A.; Chin, J.W.; Fox, J.M. Conformationally Strained trans-Cyclooctene with Improved Stability and Excellent Reactivity in Tetrazine Ligation. Chem. Sci. 2014, 5, 3770–3776. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Svatunek, D.; Rohlfing, K.; Liu, Y.; Wang, H.; Giglio, B.; Yuan, H.; Wu, Z.; Li, Z.; Fox, J. Conformationally Strained trans-Cyclooctene (sTCO) Enables the Rapid Construction of (18)F-PET Probes via Tetrazine Ligation. Theranostics 2016, 6, 887–895. [Google Scholar] [CrossRef]
- Billaud, E.M.F.; Belderbos, S.; Cleeren, F.; Maes, W.; Van de Wouwer, M.; Koole, M.; Verbruggen, A.; Himmelreich, U.; Geukens, N.; Bormans, G. Pretargeted PET Imaging Using a Bioorthogonal (18)F-Labeled trans-Cyclooctene in an Ovarian Carcinoma Model. Bioconjug. Chem. 2017, 28, 2915–2920. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Sevak, K.K.; Reiner, T.; Mohindra, P.; Carlin, S.D.; Zanzonico, P.; Weissleder, R.; Lewis, J.S. A pretargeted PET imaging strategy based on bioorthogonal Diels-Alder click chemistry. J. Nucl. Med. 2013, 54, 1389–1396. [Google Scholar] [CrossRef]
- Goodwin, D.A.; Meares, C.F.; McCall, M.J.; McTigue, M.; Chaovapong, W. Pre-targeted immunoscintigraphy of murine tumors with indium-111-labeled bifunctional haptens. J. Nucl. Med. 1988, 29, 226–234. [Google Scholar]
- Starling, J.J.; Law, K.L.; Hinson, N.A. Internalization studies using a panel of three monoclonal antibodies (MAbs) directed against the human adenocarcinoma-associated antigen, TAG-72. Antib. Immunoconj Radiopharm 1992, 5, 11. [Google Scholar]
- Ackerman, M.E.; Chalouni, C.; Schmidt, M.M.; Raman, V.V.; Ritter, G.; Old, L.J.; Mellman, I.; Wittrup, K.D. A33 antigen displays persistent surface expression. Cancer Immunol. Immunother. 2008, 57, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Keinanen, O.; Fung, K.; Pourat, J.; Jallinoja, V.; Vivier, D.; Pillarsetty, N.K.; Airaksinen, A.J.; Lewis, J.S.; Zeglis, B.M.; Sarparanta, M. Pretargeting of internalizing trastuzumab and cetuximab with a (18)F-tetrazine tracer in xenograft models. EJNMMI Res. 2017, 7, 95. [Google Scholar] [CrossRef]
- Radford, L.L.; Lapi, S.E. Methods for the Production of Radionuclides for Medicine. In Radiopharmaceutical Chemistry; Lewis, J., Windhorst, A., Zeglis, B., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Synowiecki, M.A.; Perk, L.R.; Nijsen, J.F.W. Production of novel diagnostic radionuclides in small medical cyclotrons. EJNMMI Radiopharm. Chem. 2018, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.M.; Greenwood, F.C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature 1962, 194, 495–496. [Google Scholar] [CrossRef] [PubMed]
- David, G.S. Solid state lactoperoxidase: A highly stable enzyme for simple, gentle iodination of proteins. Biochem. Biophys. Res. Commun. 1972, 48, 464–471. [Google Scholar] [CrossRef]
- Salacinski, P.R.P.; McLean, C.; Sykes, J.E.C.; Clement-Jones, V.V.; Lowry, P.J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3α,6α-diphenyl glycoluril (Iodogen). Anal. Biochem. 1981, 117, 136–146. [Google Scholar] [CrossRef]
- Rudinger, J.; Ruegg, U. Appendix: Preparation of N-succinimidyl 3-(4-hydroxyphenyl)propionate. Biochem. J. 1973, 133, 538–539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilbur, D.S.; Hadley, S.W.; Hylarides, M.D.; Abrams, P.G.; Beaumier, P.A.; Morgan, A.C.; Reno, J.M.; Fritzberg, A.R. Development of a stable radioiodinating reagent to label monoclonal antibodies for radiotherapy of cancer. J. Nucl. Med. 1989, 30, 216–226. [Google Scholar] [PubMed]
- Geissler, F.; Anderson, S.K.; Venkatesan, P.; Press, O. Intracellular catabolism of radiolabeled anti-mu antibodies by malignant B-cells. Cancer Res. 1992, 52, 2907–2915. [Google Scholar]
- Sharkey, R.M.; Behr, T.M.; Mattes, M.J.; Stein, R.; Griffiths, G.L.; Shih, L.B.; Hansen, H.J.; Blumenthal, R.D.; Dunn, R.M.; Juweid, M.E.; et al. Advantage of residualizing radiolabels for an internalizing antibody against the B-cell lymphoma antigen, CD22. Cancer Immunol. Immunother. 1997, 44, 179–188. [Google Scholar] [CrossRef]
- Ono, M.; Watanabe, H.; Ikehata, Y.; Ding, N.; Yoshimura, M.; Sano, K.; Saji, H. Radioiodination of BODIPY and its application to a nuclear and optical dual functional labeling agent for proteins and peptides. Sci. Rep. 2017, 7, 3337. [Google Scholar] [CrossRef]
- Carrasquillo, J.A.; Pandit-Taskar, N.; O’Donoghue, J.A.; Humm, J.L.; Zanzonico, P.; Smith-Jones, P.M.; Divgi, C.R.; Pryma, D.A.; Ruan, S.; Kemeny, N.E.; et al. (124)I-huA33 antibody PET of colorectal cancer. J. Nucl. Med. 2011, 52, 1173–1180. [Google Scholar] [CrossRef]
- Delaloye, B.; Bischof-Delaloye, A.; Buchegger, F.; von Fliedner, V.; Grob, J.P.; Volant, J.C.; Pettavel, J.; Mach, J.P. Detection of colorectal carcinoma by emission-computerized tomography after injection of 123I-labeled Fab or F(ab’)2 fragments from monoclonal anti-carcinoembryonic antigen antibodies. J. Clin. Invest. 1986, 77, 301–311. [Google Scholar] [CrossRef]
- Vose, J.M. Bexxar: Novel radioimmunotherapy for the treatment of low-grade and transformed low-grade non-Hodgkin’s lymphoma. Oncologist 2004, 9, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Lövqvist, A.; Einarsson, L.; Schultz, J.; Lundqvist, H. Production of 76Br by a low-energy cyclotron. Appl. Radiat. Isot. 1998, 49, 1537–1540. [Google Scholar] [CrossRef]
- Wilbur, D.S.; Hylarides, M.D. Radiolabeling of a monoclonal antibody with N-succinimidyl para-(77Br)bromobenzoate. Int. J. Radiat. Appl. Instrum. Part B Nucl. Med. Biol. 1991, 18, 363–365. [Google Scholar] [CrossRef]
- Knight, L.C.; Harwig, S.L.; Welch, M.J. In vitro stability and in vivo clearance of fibrinogen or serum albumin labeled with 77Br, 131I, or 125I by direct or indirect synthetic methods. J. Nucl. Med. 1977, 18, 282–288. [Google Scholar]
- Winberg, K.J.; Persson, M.; Malmstrom, P.U.; Sjoberg, S.; Tolmachev, V. Radiobromination of anti-HER2/neu/ErbB-2 monoclonal antibody using the p-isothiocyanatobenzene derivative of the [76Br]undecahydro-bromo-7,8-dicarba-nido-undecaborate(1-) ion. Nucl. Med. Biol. 2004, 31, 425–433. [Google Scholar] [CrossRef]
- Bruskin, A.; Sivaev, I.; Persson, M.; Lundqvist, H.; Carlsson, J.; Sjoberg, S.; Tolmachev, V. Radiobromination of monoclonal antibody using potassium [76Br] (4 isothiocyanatobenzyl-ammonio)-bromo-decahydro-closo-dodecaborate (Bromo-DABI). Nucl. Med. Biol. 2004, 31, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Lövqvist, A.; Sundin, A.; Ahlström, H.; Carlsson, J.; Lundqvist, H. 76Br-labeled monoclonal anti-CEA antibodies for radioimmuno positron emission tomography. Nucl. Med. Biol. 1995, 22, 125–131. [Google Scholar] [CrossRef]
- Lövqvist, A.; Sundin, A.; Roberto, A.; Ahlström, H.; Carlsson, J.H.L. Comparative PET imaging of experimental tumors with bromine-76-labeled antibodies, fluorine-18-fluorodeoxyglucose and carbon-11-methionine. J. Nucl. Med. 1997, 38. [Google Scholar]
- Holland, J.P.; Sheh, Y.; Lewis, J.S. Standardized methods for the production of high specific-activity zirconium-89. Nucl. Med. Biol. 2009, 36, 729–739. [Google Scholar] [CrossRef]
- Verel, I.; Visser, G.W.; Boerman, O.C.; van Eerd, J.E.; Finn, R.; Boellaard, R.; Vosjan, M.J.; Stigter-van Walsum, M.; Snow, G.B.; van Dongen, G.A. Long-lived positron emitters zirconium-89 and iodine-124 for scouting of therapeutic radioimmunoconjugates with PET. Cancer Biother. Radiopharm. 2003, 18, 655–661. [Google Scholar] [CrossRef]
- Price, E.W.; Carnazza, K.E.; Carlin, S.D.; Cho, A.; Edwards, K.J.; Sevak, K.K.; Glaser, J.M.; de Stanchina, E.; Janjigian, Y.Y.; Lewis, J.S. (89)Zr-DFO-AMG102 Immuno-PET to Determine Local Hepatocyte Growth Factor Protein Levels in Tumors for Enhanced Patient Selection. J. Nucl. Med. 2017, 58, 1386–1394. [Google Scholar] [CrossRef]
- Bensch, F.; Smeenk, M.M.; van Es, S.C.; de Jong, J.R.; Schroder, C.P.; Oosting, S.F.; Lub-de Hooge, M.N.; Menke-van der Houven van Oordt, C.W.; Brouwers, A.H.; Boellaard, R.; et al. Comparative biodistribution analysis across four different (89)Zr-monoclonal antibody tracers-The first step towards an imaging warehouse. Theranostics 2018, 8, 4295–4304. [Google Scholar] [CrossRef] [PubMed]
- Raave, R.; Sandker, G.; Adumeau, P.; Jacobsen, C.B.; Mangin, F.; Meyer, M.; Moreau, M.; Bernhard, C.; Da Costa, L.; Dubois, A.; et al. Direct comparison of the in vitro and in vivo stability of DFO, DFO* and DFOcyclo* for (89)Zr-immunoPET. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1966–1977. [Google Scholar] [CrossRef]
- Rudd, S.E.; Roselt, P.; Cullinane, C.; Hicks, R.J.; Donnelly, P.S. A desferrioxamine B squaramide ester for the incorporation of zirconium-89 into antibodies. Chem. Commun. 2016, 52, 11889–11892. [Google Scholar] [CrossRef]
- Deri, M.A.; Ponnala, S.; Kozlowski, P.; Burton-Pye, B.P.; Cicek, H.T.; Hu, C.; Lewis, J.S.; Francesconi, L.C. p-SCN-Bn-HOPO: A Superior Bifunctional Chelator for (89)Zr ImmunoPET. Bioconjug. Chem. 2015, 26, 2579–2591. [Google Scholar] [CrossRef]
- Rosenkrans, Z.T.; Cai, W. Total-Body PET Imaging for up to 30 Days After Injection of (89)Zr-Labeled Antibodies. J. Nucl. Med. 2020, 61, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Hyman, D.M.; Lyashchenko, S.K.; Lewis, J.S.; Carrasquillo, J.A. 89Zr-Trastuzumab PET/CT for Detection of Human Epidermal Growth Factor Receptor 2-Positive Metastases in Patients with Human Epidermal Growth Factor Receptor 2-Negative Primary Breast Cancer. Clin. Nucl. Med. 2017, 42, 912–917. [Google Scholar] [CrossRef]
- Bensch, F.; Brouwers, A.H.; Lub-de Hooge, M.N.; de Jong, J.R.; van der Vegt, B.; Sleijfer, S.; de Vries, E.G.E.; Schroder, C.P. (89)Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2300–2306. [Google Scholar] [CrossRef] [PubMed]
- Gaykema, S.B.M.; Brouwers, A.H.; Lub-de Hooge, M.N.; Pleijhuis, R.G.; Timmer-Bosscha, H.; Pot, L.; van Dam, G.M.; van der Meulen, S.B.; de Jong, J.R.; Bart, J.; et al. 89Zr-Bevacizumab PET Imaging in Primary Breast Cancer. J. Nucl. Med. 2013, 54, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- van Asselt, S.J.; Oosting, S.F.; Brouwers, A.H.; Bongaerts, A.H.; de Jong, J.R.; Lub-de Hooge, M.N.; Oude Munnink, T.H.; Fiebrich, H.B.; Sluiter, W.J.; Links, T.P.; et al. Everolimus Reduces (89)Zr-Bevacizumab Tumor Uptake in Patients with Neuroendocrine Tumors. J. Nucl. Med. 2014, 55, 1087–1092. [Google Scholar] [CrossRef]
- Bahce, I.; Huisman, M.C.; Verwer, E.E.; Ooijevaar, R.; Boutkourt, F.; Vugts, D.J.; van Dongen, G.A.; Boellaard, R.; Smit, E.F. Pilot study of (89)Zr-bevacizumab positron emission tomography in patients with advanced non-small cell lung cancer. EJNMMI Res. 2014, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Niemeijer, A.N.; Leung, D.; Huisman, M.C.; Bahce, I.; Hoekstra, O.S.; van Dongen, G.; Boellaard, R.; Du, S.; Hayes, W.; Smith, R.; et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat. Commun. 2018, 9, 4664. [Google Scholar] [CrossRef] [PubMed]
- Bensch, F.; van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schroder, C.P.; Hiltermann, T.J.N.; van der Wekken, A.J.; et al. (89)Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef]
- Quinn, B.; Dauer, Z.; Pandit-Taskar, N.; Schoder, H.; Dauer, L.T. Radiation dosimetry of 18F-FDG PET/CT: Incorporating exam-specific parameters in dose estimates. BMC Med. Imaging 2016, 16, 41. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, H.; Engle, J.W.; Bean, J.; Yang, Y.; Leigh, B.R.; Barnhart, T.E.; Cai, W. Positron emission tomography imaging of CD105 expression with a 64Cu-labeled monoclonal antibody: NOTA is superior to DOTA. PLoS ONE 2011, 6, e28005. [Google Scholar] [CrossRef]
- Xie, Q.; Zhu, H.; Wang, F.; Meng, X.; Ren, Q.; Xia, C.; Yang, Z. Establishing Reliable Cu-64 Production Process: From Target Plating to Molecular Specific Tumor Micro-PET Imaging. Molecules 2017, 22, 641. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Xu, X.; Zhao, M.; Zhang, B.; Deng, S.; Wu, Y. (64)Cu-based Radiopharmaceuticals in Molecular Imaging. Technol. Cancer Res. Treat 2019, 18. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef]
- Mortimer, J.E.; Bading, J.R.; Park, J.M.; Frankel, P.H.; Carroll, M.I.; Tran, T.T.; Poku, E.K.; Rockne, R.C.; Raubitschek, A.A.; Shively, J.E.; et al. Tumor Uptake of (64)Cu-DOTA-Trastuzumab in Patients with Metastatic Breast Cancer. J. Nucl. Med. 2018, 59, 38–43. [Google Scholar] [CrossRef]
- Lewis, J.S.; Windhorst, A.D.; Zeglis, B.M. Radiopharmaceutical Chemistry; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Koppe, M.J.; Bleichrodt, R.P.; Soede, A.C.; Verhofstad, A.A.; Goldenberg, D.M.; Oyen, W.J.G.; Boerman, O.C. Biodistribution and Therapeutic Efficacy of 125/131I-, 186Re-, 88/90Y-, or 177Lu-Labeled Monoclonal Antibody MN-14 to Carcinoembryonic Antigen in Mice with Small Peritoneal Metastases of Colorectal Origin. J. Nucl. Med. 2004, 45, 1224–1232. [Google Scholar] [PubMed]
- Camera, L.; Kinuya, S.; Garmestani, K.; Brechbiel, M.W.; Wu, C.; Pai, L.H.; McMurry, T.J.; Gansow, O.A.; Pastan, I.; Paik, C.H.; et al. Comparative biodistribution of indium- and yttrium-labeled B3 monoclonal antibody conjugated to either 2-(p-SCN-Bz)-6-methyl-DTPA (1B4M-DTPA) or 2-(p-SCN-Bz)-1,4,7,10-tetraazacyclododecane tetraacetic acid (2B-DOTA). Eur. J. Nucl. Med. 1994, 21, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Nayak, T.K.; Garmestani, K.; Baidoo, K.E.; Milenic, D.E.; Brechbiel, M.W. PET imaging of tumor angiogenesis in mice with VEGF-A–targeted 86Y-CHX-A″-DTPA-bevacizumab. Int. J. Cancer 2011, 128, 920–926. [Google Scholar] [CrossRef]
- Kang, C.S.; Chen, Y.; Lee, H.; Liu, D.; Sun, X.; Kweon, J.; Lewis, M.R.; Chong, H.S. Synthesis and evaluation of a new bifunctional NETA chelate for molecular targeted radiotherapy using (90)Y or (177)Lu. Nucl. Med. Biol. 2015, 42, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Price, E.W.; Edwards, K.J.; Carnazza, K.E.; Carlin, S.D.; Zeglis, B.M.; Adam, M.J.; Orvig, C.; Lewis, J.S. A comparative evaluation of the chelators H4octapa and CHX-A’’-DTPA with the therapeutic radiometal (90)Y. Nucl. Med. Biol. 2016, 43, 566–576. [Google Scholar] [CrossRef]
- Nayak, T.K.; Regino, C.A.; Wong, K.J.; Milenic, D.E.; Garmestani, K.; Baidoo, K.E.; Szajek, L.P.; Brechbiel, M.W. PET imaging of HER1-expressing xenografts in mice with 86Y-CHX-A’’-DTPA-cetuximab. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1368–1376. [Google Scholar] [CrossRef]
- Lövqvist, A.; Humm, J.L.; Sheikh, A.; Finn, R.D.; Koziorowski, J.; Ruan, S.; Pentlow, K.S.; Jungbluth, A.; Welt, S.; Lee, F.T.; et al. PET imaging of (86)Y-labeled anti-Lewis Y monoclonal antibodies in a nude mouse model: Comparison between (86)Y and (111)In radiolabels. J. Nucl. Med. 2001, 42, 7. [Google Scholar]
- Rizvi, S.N.; Visser, O.J.; Vosjan, M.J.; van Lingen, A.; Hoekstra, O.S.; Zijlstra, J.M.; Huijgens, P.C.; van Dongen, G.A.; Lubberink, M. Biodistribution, radiation dosimetry and scouting of 90Y-ibritumomab tiuxetan therapy in patients with relapsed B-cell non-Hodgkin’s lymphoma using 89Zr-ibritumomab tiuxetan and PET. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 512–520. [Google Scholar] [CrossRef]
- Perk, L.R.; Visser, G.W.; Vosjan, M.J.; Stigter-van Walsum, M.; Tijink, B.M.; Leemans, C.R.; van Dongen, G.A.M.S. (89)Zr as a PET Surrogate Radioisotope for Scouting Biodistribution of the Therapeutic Radiometals (90)Y and (177)Lu in Tumor-Bearing Nude Mice After Coupling to the Internalizing Antibody Cetuximab. J. Nucl. Med. 2005, 46, 1898–1906. [Google Scholar]
- Fairweather, D.S.; Bradwell, A.R.; Dykes, P.W.; Vaughan, A.T.; Watson-James, S.F.; Chandler, S. Improved tumour localisation using indium-111 labelled antibodies. Br. Med. J. (Clin. Res. Ed) 1983, 167–170. [Google Scholar] [CrossRef]
- Sabbah, E.N.; Kadouche, J.; Ellison, D.; Finucane, C.; Decaudin, D.; Mather, S.J. In vitro and in vivo comparison of DTPA- and DOTA-conjugated antiferritin monoclonal antibody for imaging and therapy of pancreatic cancer. Nucl. Med. Biol. 2007, 34, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Perik, P.J.; Lub-De Hooge, M.N.; Gietema, J.A.; van der Graaf, W.T.; de Korte, M.A.; Jonkman, S.; Kosterink, J.G.; van Veldhuisen, D.J.; Sleijfer, D.T.; Jager, P.L.; et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 2006, 24, 2276–2282. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; O‛Donoghue, J.A.; Divgi, C.R.; Wills, E.A.; Schwartz, L.; Gonen, M.; Smith-Jones, P.; Bander, N.H.; Scher, H.I.; Larson, S.M.; et al. Indium 111-labeled J591 anti-PSMA antibody for vascular targeted imaging in progressive solid tumors. EJNMMI Res. 2015, 5, 28. [Google Scholar] [CrossRef]
- Lebowitz, E.; Richards, P. Radionuclide generator systems. Semin. Nucl. Med. 1974, 4, 257–268. [Google Scholar] [CrossRef]
- Schwartz, A.; Steinstrasser, A. Anovel approach to Tc-99m labeled monoclonal antibodies. J. Nucl. Med. 1987, 28, 721. [Google Scholar]
- Mather, S.J.; Ellison, D. Reduction-mediated technetium-99m labeling of monoclonal antibodies. J. Nucl. Med. 1990, 31, 692–697. [Google Scholar]
- Eckelman, W.C.; Paik, C.H.; Steigman, J. Three approaches to radiolabeling antibodies with 99mTc. Int. J. Radiat. Appl. Instrum. Part B. Nucl. Med. Biol. 1989, 16, 171–176. [Google Scholar] [CrossRef]
- Abrams, M.J.; Juweid, M.; tenKate, C.I.; Schwartz, D.A.; Hauser, M.M.; Gaul, F.E.; Fuccello, A.J.; Rubin, R.H.; Strauss, H.W.; Fischman, A.J. Technetium-99m-human polyclonal IgG radiolabeled via the hydrazino nicotinamide derivative for imaging focal sites of infection in rats. J. Nucl. Med. 1990, 31, 2022–2028. [Google Scholar]
- Childs, R.L.; Hnatowich, D.J. Optimum conditions for labeling of DTPA-coupled antibodies with technetium-99m. J. Nucl. Med. 1985, 26, 293–299. [Google Scholar]
- Hnatowich, D.J.; Mardirossian, G.; Rusckowski, M.; Fogarasi, M.; Virzi, F.; Winnard, P., Jr. Directly and indirectly technetium-99m-labeled antibodies—a comparison of in vitro and animal in vivo properties. J. Nucl. Med. 1993, 34, 109–119. [Google Scholar]
- Fritzberg, A.R.; Abrams, P.G.; Beaumier, P.L.; Kasina, S.; Morgan, A.C.; Rao, T.N.; Reno, J.M.; Sanderson, J.A.; Srinivasan, A.; Wilbur, D.S.; et al. Specific and stable labeling of antibodies with technetium-99m with a diamide dithiolate chelating agent. Proc. Natl. Acad. Sci. USA 1988, 85, 4025–4029. [Google Scholar] [CrossRef] [PubMed]
- Gmeiner Stopar, T.; Fettich, J.; Zver, S.; Mlinaric-Rascan, I.; Hojker, S.; Socan, A.; Peitl, P.K.; Mather, S. 99mTc-labelled rituximab, a new non-Hodgkin’s lymphoma imaging agent: First clinical experience. Nucl. Med. Commun. 2008, 29, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.A.; Perera, A.; Batista, J.F.; Hernandez, A.; Crombet, T.; Ramos, M.; Neninger, E.; Perez, M.; Sanchez, E.L.; Romero, S.; et al. Phase I/II clinical trial of the humanized anti-EGF-r monoclonal antibody h-R3 labelled with 99mTc in patients with tumour of epithelial origin. Nucl. Med. Commun. 2005, 26, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.M.; Graves, S.A.; Hernandez, R.; Valdovinos, H.F.; Barnhart, T.E.; Cai, W.; Meyerand, M.E.; Nickles, R.J.; Suzuki, M. (5)(2)Mn production for PET/MRI tracking of human stem cells expressing divalent metal transporter 1 (DMT1). Theranostics 2015, 5, 227–239. [Google Scholar] [CrossRef]
- Fonslet, J.; Tietze, S.; Jensen, A.I.; Graves, S.A.; Severin, G.W. Optimized procedures for manganese-52: Production, separation and radiolabeling. Appl. Radiat. Isot. 2017, 121, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Graves, S.A.; Hernandez, R.; Fonslet, J.; England, C.G.; Valdovinos, H.F.; Ellison, P.A.; Barnhart, T.E.; Elema, D.R.; Theuer, C.P.; Cai, W.; et al. Novel Preparation Methods of (52)Mn for ImmunoPET Imaging. Bioconjug. Chem. 2015, 26, 2118–2124. [Google Scholar] [CrossRef]
- Tsionou, M.I.; Knapp, C.E.; Foley, C.A.; Munteanu, C.R.; Cakebread, A.; Imberti, C.; Eykyn, T.R.; Young, J.D.; Paterson, B.M.; Blower, P.J.; et al. Comparison of macrocyclic and acyclic chelators for gallium-68 radiolabelling. RSC Adv. 2017, 7, 49586–49599. [Google Scholar] [CrossRef] [PubMed]
- Engle, J.W.; Hong, H.; Zhang, Y.; Valdovinos, H.F.; Myklejord, D.V.; Barnhart, T.E.; Theuer, C.P.; Nickles, R.J.; Cai, W. Positron emission tomography imaging of tumor angiogenesis with a 66Ga-labeled monoclonal antibody. Mol. Pharm. 2012, 9, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Goethals, P.; Coene, M.; Slegers, G.; Vogelaers, D.; Everaert, J.; Lemahieu, I.; Colardyn, F.; Heyndrickx, G.R. Production of carrier-free 66Ga and labeling of antimyosin antibody for positron imaging of acute myocardial infarction. Eur. J. Nucl. Med. 1990, 16, 237–240. [Google Scholar] [CrossRef]
- Milani, S.; Ghaemimanesh, F.; Salimi, A.; Hadavi, R.; Bayat, A.A.; Alirezapour, B.; Rabbani, H. Production and evaluation of a 67Ga-labeled anti-Ror1 monoclonal antibody in a mouse model of breast cancer. J. Radioanal. Nucl. Chem. 2018, 316, 267–273. [Google Scholar] [CrossRef]
- Ryser, J.E.; Jones, R.M.; Egeli, R.; Pelegrin, A.; Rose, K.; Kurt, A.M.; Perin, M.; Broquet, P.E.; Ambrosetti, P.; Fisch, I.; et al. Colon carcinoma immunoscintigraphy by monoclonal anti-CEA antibody labeled with gallium-67-aminooxyacetyldeferroxamine. J. Nucl. Med. 1992, 33, 1766–1773. [Google Scholar] [PubMed]
- Radchenko, V.; Hauser, H.; Eisenhut, M.; Vugts, D.J.; van Dongen, G.A.M.S.; Roesch, F. 90Nb—a potential PET nuclide: Production and labeling of monoclonal antibodies. Radiochim. Acta 2012, 100, 857–864. [Google Scholar] [CrossRef]
- Radchenko, V.; Bouziotis, P.; Tsotakos, T.; Paravatou-Petsotas, M.; la Fuente, A.; Loudos, G.; Harris, A.L.; Xanthopoulos, S.; Filosofov, D.; Hauser, H.; et al. Labeling and preliminary in vivo assessment of niobium-labeled radioactive species: A proof-of-concept study. Nucl. Med. Biol. 2016, 43, 280–287. [Google Scholar] [CrossRef]
- Radchenko, V.; Busse, S.; Roesch, F. Desferrioxamine as an appropriate chelator for 90Nb: Comparison of its complexation properties for M-Df-Octreotide (M = Nb, Fe, Ga, Zr). Nucl. Med. Biol. 2014, 41, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Radchenko, V.; Filosofov, D.V.; Bochko, O.K.; Lebedev, N.A.; Rakhimov, A.V.; Hauser, H.; Eisenhut, M.; Aksenov, N.V.; Bozhikov, G.A.; Ponsard, B.; et al. Separation of 90Nb from zirconium target for application in immuno-PET. Radiochim. Acta 2014, 102. [Google Scholar] [CrossRef]
- Jahn, M.; Radchenko, V.; Filosofov, D.V.; Hauser, H.; Eisenhut, M.; Rösch, F.; Jennewein, M. Separation and purification of no-carrier-added arsenic from bulk amounts of germanium for use in radiopharmaceutical labelling. Radiochim. Acta 2010, 98. [Google Scholar] [CrossRef]
- Shen, S.; Li, X.F.; Cullen, W.R.; Weinfeld, M.; Le, X.C. Arsenic binding to proteins. Chem. Rev. 2013, 113, 7769–7792. [Google Scholar] [CrossRef]
- Jennewein, M.; Lewis, M.A.; Zhao, D.; Tsyganov, E.; Slavine, N.; He, J.; Watkins, L.; Kodibagkar, V.D.; O’Kelly, S.; Kulkarni, P.; et al. Vascular imaging of solid tumors in rats with a radioactive arsenic-labeled antibody that binds exposed phosphatidylserine. Clin. Cancer Res. 2008, 14, 1377–1385. [Google Scholar] [CrossRef]
- Ellison, P.A.; Barnhart, T.E.; Chen, F.; Hong, H.; Zhang, Y.; Theuer, C.P.; Cai, W.; Nickles, R.J.; DeJesus, O.T. High Yield Production and Radiochemical Isolation of Isotopically Pure Arsenic-72 and Novel Radioarsenic Labeling Strategies for the Development of Theranostic Radiopharmaceuticals. Bioconjug. Chem. 2016, 27, 179–188. [Google Scholar] [CrossRef] [PubMed]
- DeGraffenreid, A.J.; Feng, Y.; Barnes, C.L.; Ketring, A.R.; Cutler, C.S.; Jurisson, S.S. Trithiols and their arsenic compounds for potential use in diagnostic and therapeutic radiopharmaceuticals. Nucl. Med. Biol. 2016, 43, 288–295. [Google Scholar] [CrossRef] [PubMed]


| Radionuclides | Decay-Life | Production Method [82] *** | Emission | Labeling Strategies |
|---|---|---|---|---|
| PET | β+ (%; EMean) | |||
| 124Iodine | 4.2 days | Cyclotron | β+ (12%; 687 keV) β+ (11%; 975 keV) | Direct radiolabeling |
| 89Zirconium | 3.3 days | Cyclotron | β+ (23%; 396 keV) | DFO, DFO*, DFOSq, HOPO |
| 72Arsenic | 1.1 days | Generator | β+ (6%; 824 keV) β+ (64%; 1117 keV) β+ (16%; 1528 keV) | Direct labelling, SATA, Traut’s reagent |
| 74Arsenic | 17.8 days | High energy Cyclotron | β+ (26%; 408 keV) β− | Direct labelling, SATA, Traut’s reagent |
| 64Copper | 12.7 h | Cyclotron | β+ (18%; 278 keV) β− | NOTA |
| 86Yttrium | 14.7 h | Cyclotron | β+ (32%; 394–1437 keV) * | DOTA, CHX-A″-DTPA |
| 76Bromine | 16.2 h | Cyclotron | β+ (55%; 336–1800 keV) * | Direct radiolabeling |
| 52Manganese | 5.6 days | High energy cyclotron | β+ (29%; 242 keV) | DOTA |
| 90Niobium | 14.6 h | β+ (51%; 662 keV) | DFO | |
| 66Gallium | 9.5 h | Cyclotron | β+ (4%; 397 keV) β+ (51%; 1904 keV) | NOTA |
| SPECT | γ (%; Energy) ** | |||
| 123Iodine | 13.2 h | High energy Cyclotron | γ1 (83%; 159 keV) γ2 (1%; 529 keV) | Direct radiolabeling |
| 131Iodine | 8.0 days | Reactor | γ1 (82%; 364 keV) γ2 (7%; 637 keV) | Direct radiolabeling |
| 67Gallium | 3.3 days | High energy Cyclotron | γ1 (3%; 91 keV) γ2 (39%; 93 keV) γ3 (21%; 184 keV) γ4 (2%; 209 keV) γ5 (17%; 300 keV) γ6 (5%; 394 keV) | NOTA |
| 99mTechnetium | 6 h | Generator | γ (89%; 141 keV) | HYNIC, N2S2 ligand |
| 111Indium | 2.8 days | High energy Cyclotron | γ1 (91%; 171 keV) γ2 (94%; 245 keV) | DOTA, CHX-A″-DTPA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewulf, J.; Adhikari, K.; Vangestel, C.; Wyngaert, T.V.D.; Elvas, F. Development of Antibody Immuno-PET/SPECT Radiopharmaceuticals for Imaging of Oncological Disorders—An Update. Cancers 2020, 12, 1868. https://doi.org/10.3390/cancers12071868
Dewulf J, Adhikari K, Vangestel C, Wyngaert TVD, Elvas F. Development of Antibody Immuno-PET/SPECT Radiopharmaceuticals for Imaging of Oncological Disorders—An Update. Cancers. 2020; 12(7):1868. https://doi.org/10.3390/cancers12071868
Chicago/Turabian StyleDewulf, Jonatan, Karuna Adhikari, Christel Vangestel, Tim Van Den Wyngaert, and Filipe Elvas. 2020. "Development of Antibody Immuno-PET/SPECT Radiopharmaceuticals for Imaging of Oncological Disorders—An Update" Cancers 12, no. 7: 1868. https://doi.org/10.3390/cancers12071868
APA StyleDewulf, J., Adhikari, K., Vangestel, C., Wyngaert, T. V. D., & Elvas, F. (2020). Development of Antibody Immuno-PET/SPECT Radiopharmaceuticals for Imaging of Oncological Disorders—An Update. Cancers, 12(7), 1868. https://doi.org/10.3390/cancers12071868





