Targeting SRC Family Kinases in Mesothelioma: Time to Upgrade
Abstract
1. Introduction
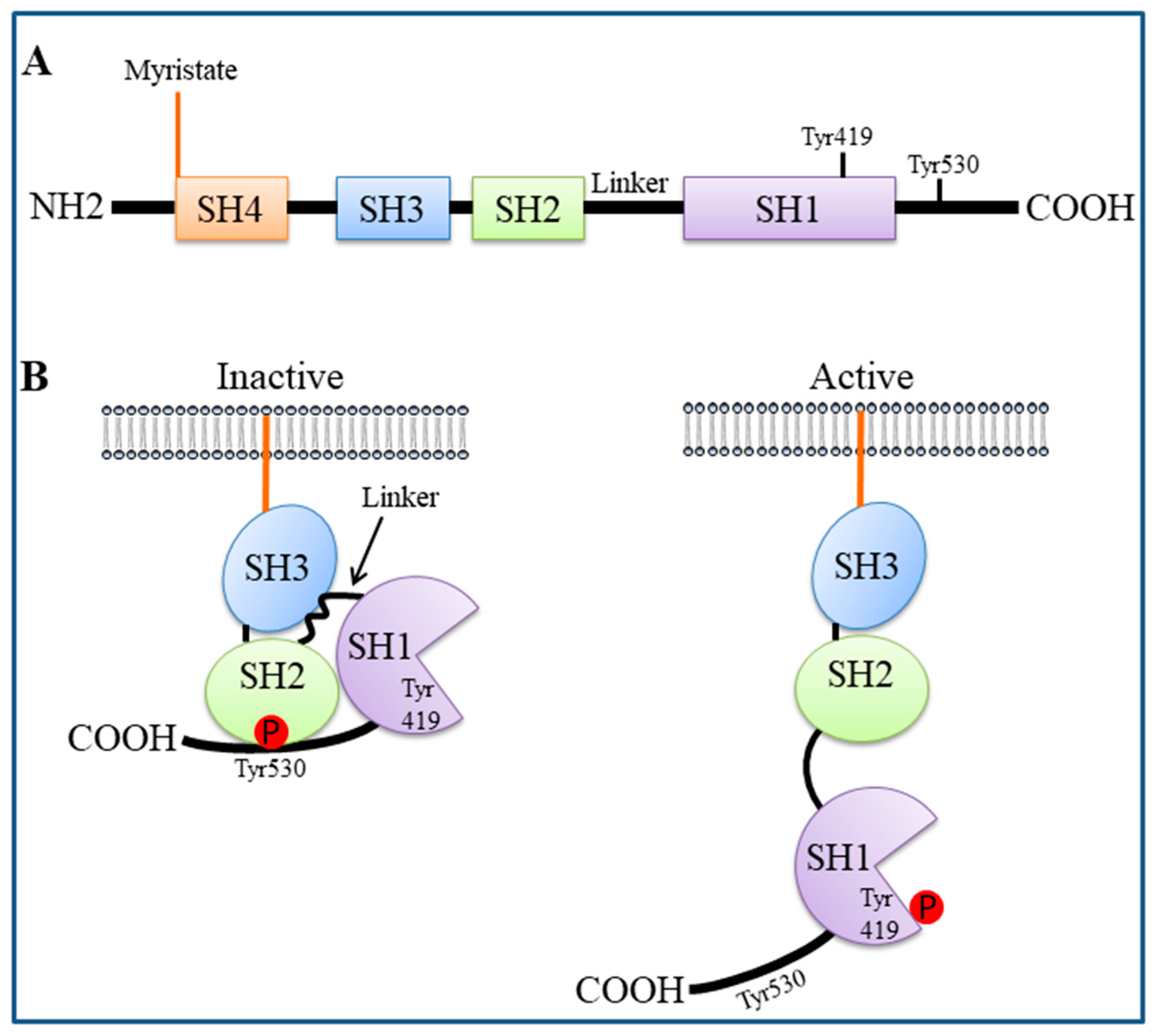
2. SFK Structure and Activation
3. Main Roles of SFKs in Cancer
4. Potential SFK Involvement in MM Development and Progression
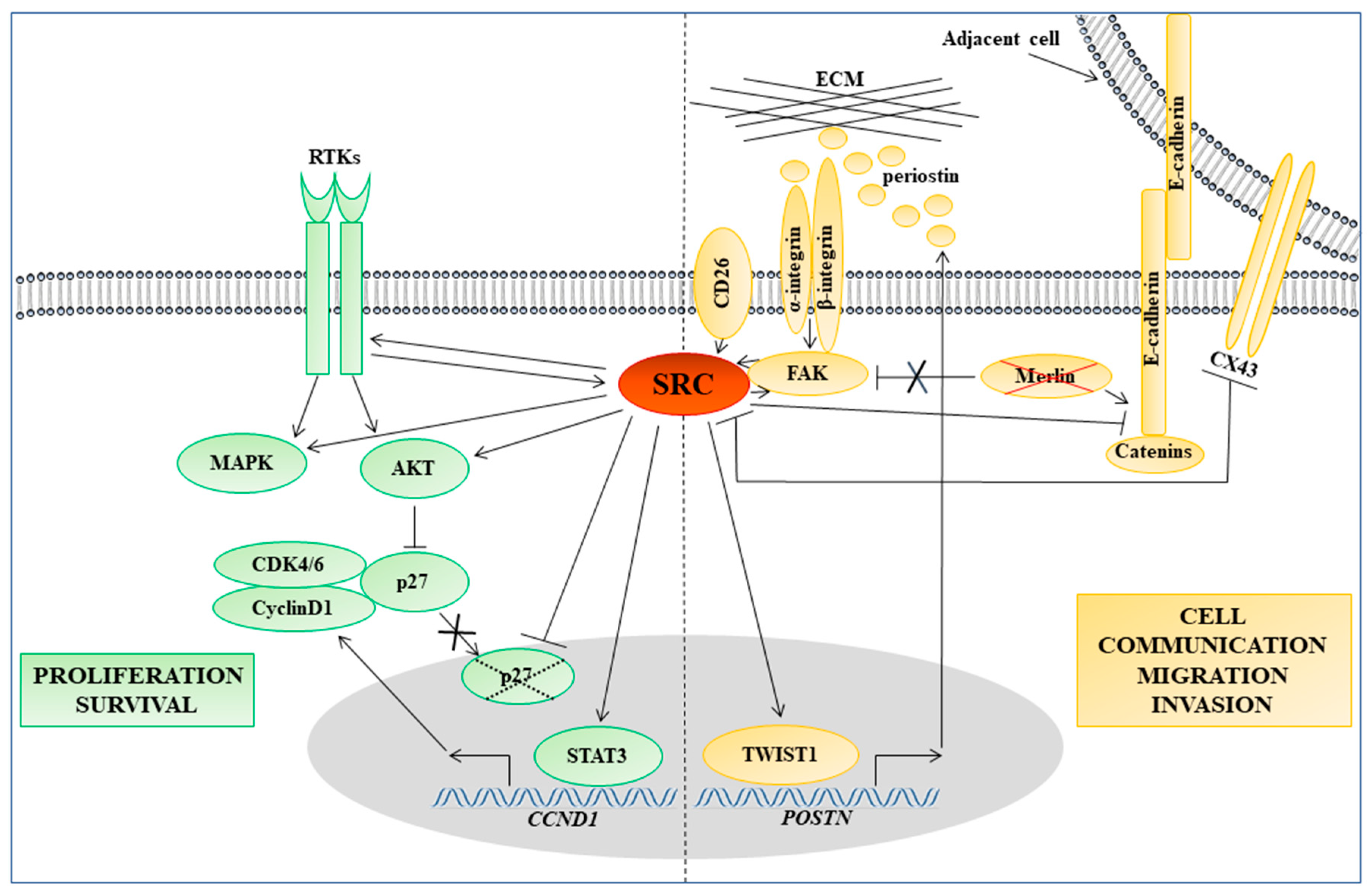
5. Role of SFKs in Molecular Pathways Regulating Cell Adhesion, Motility, and Invasion in MM
6. Antitumor Activity of SFK Inhibitors in MM Cell Lines and Underlying Molecular Mechanisms
7. SFK Inhibitors in Combination with Both Chemotherapeutics and Targeted Drugs in MM Cell Lines
8. Clinical Trials of Dasatinib in MM Patients
9. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hiriart, E.; Deepe, R.; Wessels, A. Mesothelium and Malignant Mesothelioma. J. Dev. Biol. 2019, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Novello, S.; Pinto, C.; Torri, V.; Porcu, L.; Di Maio, M.; Tiseo, M.; Ceresoli, G.; Magnani, C.; Silvestri, S.; Veltri, A.; et al. The Third Italian Consensus Conference for Malignant Pleural Mesothelioma: State of the art and recommendations. Crit. Rev. Oncol. Hematol. 2016, 104, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Scherpereel, A.; Opitz, I.; Berghmans, T.; Psallidas, I.; Glatzer, M.; Rigau, D.; Astoul, P.; Bölükbas, S.; Boyd, J.; Coolen, J.; et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Baumann, F.; Carbone, M. Environmental risk of mesothelioma in the United States: An emerging concern-epidemiological issues. J. Toxicol. Environ. Health. B Crit. Rev. 2016, 19, 231–249. [Google Scholar] [CrossRef]
- Carbone, M.; Yang, H. Mesothelioma: Recent highlights. Ann. Transl. Med. 2017, 5, 238. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Sauter, J.L.; Nowak, A.K.; Kindler, H.L.; Gill, R.R.; Remy-Jardin, M.; Armato, S.G.; Fernandez-Cuesta, L.; Bueno, R.; Alcala, N.; et al. EURACAN/IASLC Proposals for Updating the Histologic Classification of Pleural Mesothelioma: Towards a More Multidisciplinary Approach. J. Thorac. Oncol. 2020, 15, 29–49. [Google Scholar] [CrossRef]
- Galateau-Salle, F.; Churg, A.; Roggli, V.; Travis, W.D. The 2015 world health organization classification of tumors of the pleura: Advances since the 2004 Classification. J. Thorac. Oncol. 2016, 11, 142–154. [Google Scholar] [CrossRef]
- The Lancet Respiratory Medicine. Pleural mesothelioma: Tackling a deadly cancer. Lancet Respir. Med. 2019, 7, 99. [Google Scholar] [CrossRef]
- Robinson, B.W.S.; Musk, A.W.; Lake, R.A. Malignant mesothelioma. Lancet 2005, 366, 397–408. [Google Scholar] [CrossRef]
- Hinz, T.K.; Heasley, L.E. Translating mesothelioma molecular genomics and dependencies into precision oncology-based therapies. Semin. Cancer Biol. 2020, 61, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Quetel, L.; Meiller, C.; Assié, J.B.; Blum, Y.; Imbeaud, S.; Montagne, F.; Tranchant, R.; de Wolf, J.; Caruso, S.; Copin, M.C.; et al. Genetic alterations of malignant pleural mesothelioma: Association with tumor heterogeneity and overall survival. Mol. Oncol. 2020, 14, 1207–1223. [Google Scholar] [CrossRef]
- Oehl, K.; Vrugt, B.; Opitz, I.; Meerang, M. Heterogeneity in malignant pleural mesothelioma. Int. J. Mol. Sci. 2018, 19, 1603. [Google Scholar] [CrossRef] [PubMed]
- Hiddinga, B.I.; Rolfo, C.; van Meerbeeck, J.P. Mesothelioma treatment: Are we on target? A review. J. Adv. Res. 2015, 6, 319–330. [Google Scholar] [CrossRef] [PubMed]
- McCambridge, A.J.; Napolitano, A.; Mansfield, A.S.; Fennell, D.A.; Sekido, Y.; Nowak, A.K.; Reungwetwattana, T.; Mao, W.; Pass, H.I.; Carbone, M.; et al. Progress in the Management of Malignant Pleural Mesothelioma in 2017. J. Thorac. Oncol. 2018, 13, 606–623. [Google Scholar] [CrossRef] [PubMed]
- Kukuyan, A.-M.; Sementino, E.; Kadariya, Y.; Menges, C.W.; Cheung, M.; Tan, Y.; Cai, K.Q.; Slifker, M.J.; Peri, S.; Klein-Szanto, A.J.; et al. Inactivation of Bap1 Cooperates with Losses of Nf2 and Cdkn2a to Drive the Development of Pleural Malignant Mesothelioma in Conditional Mouse Models. Cancer Res. 2019, 79, 4113–4123. [Google Scholar] [CrossRef] [PubMed]
- Barbarino, M.; Cesari, D.; Bottaro, M.; Luzzi, L.; Namagerdi, A.; Bertolino, F.M.; Bellan, C.; Proietti, F.; Somma, P.; Micheli, M.; et al. PRMT5 silencing selectively affects MTAP-deleted mesothelioma: In vitro evidence of a novel promising approach. J. Cell. Mol. Med. 2020, 24, 5565–5577. [Google Scholar] [CrossRef]
- Sato, T.; Sekido, Y. NF2/merlin inactivation and potential therapeutic targets in mesothelioma. Int. J. Mol. Sci. 2018, 19, 988. [Google Scholar] [CrossRef]
- Miyanaga, A.; Masuda, M.; Tsuta, K.; Kawasaki, K.; Nakamura, Y.; Sakuma, T.; Asamura, H.; Gemma, A.; Yamada, T. Hippo pathway gene mutations in malignant mesothelioma: Revealed by RNA and targeted exon sequencing. J. Thorac. Oncol. 2015, 10, 844–851. [Google Scholar] [CrossRef]
- Ladanyi, M.; Zauderer, M.G.; Krug, L.M.; Ito, T.; McMillan, R.; Bott, M.; Giancotti, F. New strategies in pleural mesothelioma: BAP1 and NF2 as novel targets for therapeutic development and risk assessment. Clin. Cancer Res. 2012, 18, 4485–4490. [Google Scholar] [CrossRef]
- Testa, J.R.; Cheung, M.; Pei, J.; Below, J.E.; Tan, Y.; Sementino, E.; Cox, N.J.; Dogan, A.U.; Pass, H.I.; Trusa, S.; et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat. Genet. 2011, 43, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Nasu, M.; Emi, M.; Pastorino, S.; Tanji, M.; Powers, A.; Luk, H.; Baumann, F.; Zhang, Y.A.; Gazdar, A.; Kanodia, S.; et al. High incidence of somatic BAP1 alterations in sporadic malignant mesothelioma. J. Thorac. Oncol. 2015, 10, 565–576. [Google Scholar] [CrossRef]
- Forte, I.M.; Giordano, A.; Pentimalli, F. Insert: Molecular markers f mesothelioma aiding in diagnostic challenge: The combined use of p16 and BAP1. In Malignant Pleural Mesothelioma: A Guide for Clinicians; Giordano, A., Franco, R., Eds.; Academic Press Inc.: Cambridge, MA, USA, 2019; ISBN 978-0-12-812724-7. [Google Scholar]
- Cioce, M.; Canino, C.; Goparaju, C.; Yang, H.; Carbone, M.; Pass, H.I. Autocrine CSF-1R signaling drives mesothelioma chemoresistance via AKT activation. Cell Death Dis. 2014, 5, e1167. [Google Scholar] [CrossRef] [PubMed]
- Yeatman, T.J. A renaissance for SRC. Nat. Rev. Cancer 2004, 4, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Clump, D.A.; Qazi, I.H.; Sudol, M.; Flynn, D.C. c-Yes response to growth factor activation. Growth Factors 2005, 23, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.L.; Iida, M.; Dunn, E.F. The role of Src in solid tumors. Oncologist 2009, 14, 667–678. [Google Scholar] [CrossRef]
- Puls, L.N.; Eadens, M.; Messersmith, W. Current status of SRC inhibitors in solid tumor malignancies. Oncologist 2011, 16, 566–578. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, D. Targeting Src family kinases in anti-cancer therapies: Turning promise into triumph. Trends Pharmacol. Sci. 2012, 33, 122–128. [Google Scholar] [CrossRef]
- Elias, D.; Ditzel, H.J. Fyn is an important molecule in cancer pathogenesis and drug resistance. Pharmacol. Res. 2015, 100, 250–254. [Google Scholar] [CrossRef]
- Menges, C.W.; Chen, Y.; Mossman, B.T.; Chernoff, J.; Yeung, A.T.; Testa, J.R. A Phosphotyrosine Proteomic Screen Identifies Multiple Tyrosine Kinase Signaling Pathways Aberrantly Activated in Malignant Mesothelioma. Genes Cancer 2010, 1, 493–505. [Google Scholar] [CrossRef]
- Tsao, A.S.; He, D.; Saigal, B.; Liu, S.; Lee, J.J.; Bakkannagari, S.; Ordonez, N.G.; Waun, K.H.; Wistuba, I.; Johnson, F.M. Inhibition of c-Src expression and activation in malignant pleural mesothelioma tissues leads to apoptosis, cell cycle arrest, and decreased migration and invasion. Mol. Cancer Ther. 2007, 6, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, L.J.; Lee, F.Y.; Chen, P.; Norris, D.; Barrish, J.C.; Behnia, K.; Castaneda, S.; Cornelius, L.A.M.; Das, J.; Doweyko, A.M.; et al. Discovery of N-(2-chloro-6-methylphenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J. Med. Chem. 2004, 47, 6658–6661. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Reeves, K.; Han, X.; Fairchild, C.; Platero, S.; Wong, T.W.; Lee, F.; Shaw, P.; Clark, E. Identification ofcandidate molecular markers predicting sensitivity in solid tumors to dasatinib: Rationale for patient selection. Cancer Res. 2007, 67, 2226–2238. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, D.; Gouda, M.; Kirii, Y.; Sugiyama, N.; Ishihama, Y.; Fujii, I.; Narumi, Y.; Akita, K.; Yokota, K. Characterization of Kinase Inhibitors Using Different Phosphorylation States of Colony Stimulating factor-1 Receptor Tyrosine Kinase. J. Biochem. 2012, 151, 47–55. [Google Scholar] [CrossRef]
- Abbas, R.; Hsyu, P.H. Clinical Pharmacokinetics and Pharmacodynamics of Bosutinib. Clin. Pharmacokinet. 2016, 55, 1191–1204. [Google Scholar] [CrossRef]
- Tan, F.H.; Putoczki, T.L.; Stylli, S.S.; Luwor, R.B. Ponatinib: A novel multi-tyrosine kinase inhibitor against human malignancies. OncoTargets Ther. 2019, 12, 635–645. [Google Scholar] [CrossRef]
- Hennequin, L.F.; Allen, J.; Breed, J.; Curwen, J.; Fennell, M.; Green, T.P.; Lambert-van der Brempt, C.; Morgentin, R.; Norman, R.A.; Olivier, A.; et al. N-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5- (tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J. Med. Chem. 2006, 49, 6465–6488. [Google Scholar] [CrossRef]
- Woodcock, V.K.; Clive, S.; Wilson, R.H.; Coyle, V.M.; Stratford, M.R.L.; Folkes, L.K.; Eastell, R.; Barton, C.; Jones, P.; Kazmi-Stokes, S.; et al. A first-in-human phase i study to determine the maximum tolerated dose of the oral Src/ABL inhibitor AZD0424. Br. J. Cancer 2018, 118, 770–776. [Google Scholar] [CrossRef]
- Naing, A.; Cohen, R.; Dy, G.K.; Hong, D.S.; Dyster, L.; Hangauer, D.G.; Kwan, R.; Fetterly, G.; Kurzrock, R.; Adjei, A.A. A phase I trial of KX2-391, a novel non-ATP competitive substrate-pocket- directed SRC inhibitor, in patients with advanced malignancies. Investig. New Drugs 2013, 31, 967–973. [Google Scholar] [CrossRef]
- Bartscht, T.; Rosien, B.; Rades, D.; Kaufmann, R.; Biersack, H.; Lehnerta, H.; Ungefroren, H. Inhibition of TGF-β Signaling in Tumor Cells by Small Molecule Src Family Kinase Inhibitors. Anticancer Agents Med. Chem. 2017, 17, 1351–1356. [Google Scholar] [CrossRef]
- Tatton, L.; Morley, G.M.; Chopra, R.; Khwaja, A. The Src-selective kinase inhibitor PP1 also inhibits Kit and Bcr-Abl tyrosine kinases. J. Biol. Chem. 2003, 278, 4847–4853. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.; Plater, L.; Elliott, M.; Shpiro, N.; Hastie, C.J.; Mclauchlan, H.; Klevernic, I.; Arthur, J.S.C.; Alessi, D.R.; Cohen, P. The selectivity of protein kinase inhibitors: A further update. Biochem. J. 2007, 408, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Schenone, S.; Brullo, C.; Musumeci, F.; Botta, M. Novel dual Src/Abl inhibitors for hematologic and solid malignancies. Expert Opin. Investig. Drugs 2010, 19, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Muscella, A.; Cossa, L.G.; Vetrugno, C.; Antonaci, G.; Marsigliante, S. Adenosine diphosphate regulates MMP2 and MMP9 activity in malignant mesothelioma cells. Ann. N. Y. Acad. Sci. 2018, 1431, 72–84. [Google Scholar] [CrossRef]
- Komiya, E.; Ohnuma, K.; Yamazaki, H.; Hatano, R.; Iwata, S.; Okamoto, T.; Dang, N.H.; Yamada, T.; Morimoto, C. CD26-mediated regulation of periostin expression contributes to migration and invasion of malignant pleural mesothelioma cells. Biochem. Biophys. Res. Commun. 2014, 447, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, K.; Virgona, N.; Yamada, J.; Sato, A.; Ota, M.; Yazawa, T.; Yano, T. Bowman-Birk protease inhibitor from soybeans enhances cisplatin-induced cytotoxicity in human mesothelioma cells. Exp. Ther. Med. 2011, 2, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Iwata, H.; Takano, Y.; Yamada, R.; Okuzawa, H.; Nagashima, Y.; Yamaura, K.; Ueno, K.; Yano, T. Enhanced effect of connexin 43 on cisplatin-induced cytotoxicity in mesothelioma cells. J. Pharmacol. Sci. 2009, 110, 466–475. [Google Scholar] [CrossRef]
- Indovina, P.; Giorgi, F.; Rizzo, V.; Khadang, B.; Schenone, S.; Di Marzo, D.; Forte, I.M.; Tomei, V.; Mattioli, E.; D’Urso, V.; et al. New pyrazolo[3,4-d]pyrimidine SRC inhibitors induce apoptosis in mesothelioma cell lines through p27 nuclear stabilization. Oncogene 2012, 31, 929–938. [Google Scholar] [CrossRef]
- Dudek, A.Z.; Pang, H.; Kratzke, R.A.; Otterson, G.A.; Hodgson, L.; Vokes, E.E.; Kindler, H.L. Cancer and Leukemia Group B Phase II study of dasatinib in patients with previously treated malignant mesothelioma (cancer and leukemia group B 30601): A brief report. J. Thorac. Oncol. 2012, 7, 755–759. [Google Scholar] [CrossRef]
- Tsao, A.S.; Lin, H.; Carter, B.W.; Lee, J.J.; Rice, D.; Vaporcyan, A.; Swisher, S.; Mehran, R.; Heymach, J.; Nilsson, M.; et al. Biomarker-Integrated Neoadjuvant Dasatinib Trial in Resectable Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2018, 13, 246–257. [Google Scholar] [CrossRef]
- Eguchi, R.; Fujita, Y.; Tabata, C.; Ogawa, H.; Wakabayashi, I.; Nakano, T.; Fujimori, Y. Inhibition of Src family kinases overcomes anoikis resistance induced by spheroid formation and facilitates cisplatin-induced apoptosis in human mesothelioma cells. Oncol. Rep. 2015, 34, 2305–2310. [Google Scholar] [CrossRef] [PubMed]
- Monica, V.; Lo Iacono, M.; Bracco, E.; Busso, S.; Di Blasio, L.; Primo, L.; Peracino, B.; Papotti, M.; Scagliotti, G. Dasatinib modulates sensitivity to pemetrexed in malignant pleural mesothelioma cell lines. Oncotarget 2016, 7, 76577–76589. [Google Scholar] [CrossRef] [PubMed]
- Stehelin, D.; Varmus, H.E.; Bishop, J.M.; Vogt, P.K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature 1976, 260, 170–173. [Google Scholar] [CrossRef]
- Okada, M. Regulation of the Src family kinases by Csk. Int. J. Biol. Sci. 2012, 8, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Bromann, P.A.; Korkaya, H.; Courtneidge, S.A. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene 2004, 23, 7957–7968. [Google Scholar] [CrossRef]
- Garmendia, I.; Pajares, M.J.; Hermida-Prado, F.; Ajona, D.; Bértolo, C.; Sainz, C.; Lavín, A.; Remírez, A.B.; Valencia, K.; Moreno, H.; et al. YES1 Drives Lung Cancer Growth and Progression and Predicts Sensitivity to Dasatinib. Am. J. Respir. Crit. Care Med. 2019, 200, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, H.; Xu, D.; Zhu, H. Upregulation of Tyrosine Kinase FYN in Human Thyroid Carcinoma: Role in Modulating Tumor Cell Proliferation, Invasion, and Migration. Cancer Biother. Radiopharm. 2017, 32, 320–326. [Google Scholar] [CrossRef]
- Tornillo, G.; Knowlson, C.; Kendrick, H.; Cooke, J.; Mirza, H.; Aurrekoetxea-Rodríguez, I.; Vivanco, M.; Buckley, N.E.; Grigoriadis, A.; Smalley, M.J. Dual Mechanisms of LYN Kinase Dysregulation Drive Aggressive Behavior in Breast Cancer Cells. Cell Rep. 2018, 25, 3674–3692. [Google Scholar] [CrossRef]
- Zhao, X.; Guan, J.L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 2011, 63, 610–615. [Google Scholar] [CrossRef]
- Yeo, M.G.; Oh, H.J.; Cho, H.S.; Chun, J.S.; Marcantonio, E.E.; Song, W.K. Phosphorylation of Ser 21 in Fyn regulates its kinase activity, focal adhesion targeting, and is required for cell migration. J. Cell. Physiol. 2011, 226, 236–247. [Google Scholar] [CrossRef]
- Huang, R.Y.-J.; Wang, S.-M.; Hsieh, C.-Y.; Wu, J.-C. Lysophosphatidic acid induces ovarian cancer cell dispersal by activating Fyn kinase associated with p120-catenin. Int. J. Cancer 2008, 123, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Chatterji, T.; Varkaris, A.S.; Parikh, N.U.; Song, J.H.; Cheng, C.-J.; Schweppe, R.E.; Alexander, S.; Davis, J.W.; Troncoso, P.; Friedl, P.; et al. Yes-mediated phosphorylation of focal adhesion kinase at tyrosine 861 increases metastatic potential of prostate cancer cells. Oncotarget 2015, 6, 10175–10194. [Google Scholar] [CrossRef] [PubMed]
- Rübsam, M.; Broussard, J.A.; Wickström, S.A.; Nekrasova, O.; Green, K.J.; Niessen, C.M. Adherens Junctions and Desmosomes Coordinate Mechanics and Signaling to Orchestrate Tissue Morphogenesis and Function: An Evolutionary Perspective. Cold Spring Harb. Perspect. Biol. 2018, 1010, a029207. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.S.; Ino, Y.; Sakamoto, M.; Hirohashi, S. Src family kinase inhibitor PP2 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin. Cancer Res. 2002, 8, 2430–2436. [Google Scholar]
- McLachlan, R.W.; Kraemer, A.; Helwani, F.M.; Kovacs, E.M.; Yap, A.S. E-cadherin adhesion activates c-Src signaling at cell-cell contacts. Mol. Biol. Cell 2007, 18, 3214–3223. [Google Scholar] [CrossRef]
- Vlahov, N.; Scrace, S.; Soto, M.S.; Grawenda, A.M.; Bradley, L.; Pankova, D.; Papaspyropoulos, A.; Yee, K.S.; Buffa, F.; Goding, C.R.; et al. Alternate RASSF1 Transcripts Control SRC Activity, E-Cadherin Contacts, and YAP-Mediated Invasion. Curr. Biol. 2015, 25, 3019–3034. [Google Scholar] [CrossRef]
- Nagathihalli, N.S.; Merchant, N.B. Src-mediated regulation of E-cadherin and EMT in pancreatic cancer. Front. Biosci. 2012, 17, 2059–2069. [Google Scholar] [CrossRef]
- Cavallaro, U.; Christofori, G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer 2004, 4, 118–132. [Google Scholar] [CrossRef]
- Wolfenson, H.; Lavelin, I.; Geiger, B. Dynamic Regulation of the Structure and Functions of Integrin Adhesions. Dev. Cell 2013, 24, 447–458. [Google Scholar] [CrossRef]
- Bachir, A.I.; Horwitz, A.R.; Nelson, W.J.; Bianchini, J.M. Actin-Based Adhesion Modules Mediate Cell Interactions with the Extracellular Matrix and Neighboring Cells. Cold Spring Harb. Perspect. Biol. 2017, 9, a023234. [Google Scholar] [CrossRef]
- Huveneers, S.; Danen, E.H.J. Adhesion signaling—Crosstalk between integrins, Src and Rho. J. Cell Sci. 2009, 122, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef] [PubMed]
- Hsia, D.A.; Mitra, S.K.; Hauck, C.R.; Streblow, D.N.; Nelson, J.A.; Ilic, D.; Huang, S.; Li, E.; Nemerow, G.R.; Leng, J.; et al. Differential regulation of cell motility and invasion by FAK. J. Cell Biol. 2003, 160, 753–767. [Google Scholar] [CrossRef]
- Van Slambrouck, S.; Grijelmo, C.; De Wever, O.; Bruyneel, E.; Emami, S.; Gespach, C.; Steelant, W.F.A. Activation of the FAK-src molecular scaffolds and p130Cas-JNK signaling cascades by α1-integrins during colon cancer cell invasion. Int. J. Oncol. 2007, 31, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, N.; Pang, B.; Tong, D.; Sun, D.; Sun, H.; Zhang, C.; Sun, W.; Meng, X.; Bai, J.; et al. TRIB1 promotes colorectal cancer cell migration and invasion through activation MMP-2 via FAK/Src and ERK pathways. Oncotarget 2017, 8, 47931–47942. [Google Scholar] [CrossRef] [PubMed]
- Olea-Flores, M.; Zuñiga-Eulogio, M.; Tacuba-Saavedra, A.; Bueno-Salgado, M.; Sánchez-Carvajal, A.; Vargas-Santiago, Y.; Mendoza-Catalán, M.A.; Pérez Salazar, E.; García-Hernández, A.; Padilla-Benavides, T.; et al. Leptin Promotes Expression of EMT-Related Transcription Factors and Invasion in a Src and FAK-Dependent Pathway in MCF10A Mammary Epithelial Cells. Cells 2019, 8, 1133. [Google Scholar] [CrossRef]
- Paz, H.; Pathak, N.; Yang, J. Invading one step at a time: The role of invadopodia in tumor metastasis. Oncogene 2014, 33, 4193–4202. [Google Scholar] [CrossRef]
- Ngan, E.; Stoletov, K.; Smith, H.W.; Common, J.; Muller, W.J.; Lewis, J.D.; Siegel, P.M. LPP is a Src substrate required for invadopodia formation and efficient breast cancer lung metastasis. Nat. Commun. 2017, 8, 15059. [Google Scholar] [CrossRef]
- Ogawa, K.; Lin, Q.; Li, L.; Bai, X.; Chen, X.; Chen, H.; Kong, R.; Wang, Y.; Zhu, H.; He, F.; et al. Aspartate β-hydroxylase promotes pancreatic ductal adenocarcinoma metastasis through activation of SRC signaling pathway. J. Hematol. Oncol. 2019, 12, 1–16. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Windham, T.C.; Parikh, N.U.; Siwak, D.R.; Summy, J.M.; McConkey, D.J.; Kraker, A.J.; Gallick, G.E. Src activation regulates anoikis in human colon tumor cell lines. Oncogene 2002, 21, 7797–7807. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Lv, Y.-F.; Yan, G.-N.; Meng, G.; Zhang, X.; Guo, Q.-N. RanBP9/TSSC3 complex cooperates to suppress anoikis resistance and metastasis via inhibiting Src-mediated Akt signaling in osteosarcoma. Cell Death Dis. 2016, 7, e2572. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhao, G.; Ao, J.; Gong, D.; Zhang, J.; Chen, Y.; Li, J.; Huang, L.; Xiang, R.; Hu, J.; et al. ZNF32 induces anoikis resistance through maintaining redox homeostasis and activating Src/FAK signaling in hepatocellular carcinoma. Cancer Lett. 2019, 442, 271–278. [Google Scholar] [CrossRef] [PubMed]
- England, J.M.; Panella, M.J.; Ewert, D.L.; Halpern, M.S. Induction of a diffuse mesothelioma in chickens by intraperitoneal inoculation of v-src DNA. Virology 1991, 182, 423–429. [Google Scholar] [CrossRef]
- Miura, Y.; Nishimura, Y.; Katsuyama, H.; Maeda, M.; Hayashi, H.; Dong, M.; Hyodoh, F.; Tomita, M.; Matsuo, Y.; Uesaka, A.; et al. Involvement of IL-10 and Bcl-2 in resistance against an asbestos-induced apoptosis of T cells. Apoptosis 2006, 11, 1825–1835. [Google Scholar] [CrossRef]
- Eguchi, R.; Kubo, S.; Takeda, H.; Ohta, T.; Tabata, C.; Ogawa, H.; Nakano, T. Deficiency of Fyn Protein Is Prerequisite for Apoptosis Induced by Src Family Kinase Inhibitors in Human Mesothelioma Cells. Carcinogenesis 2012, 33, 969–975. [Google Scholar] [CrossRef]
- Sato, A.; Sekine, M.; Virgona, N.; Ota, M.; Yano, T. Yes is a central mediator of cell growth in malignant mesothelioma cells. Oncol. Rep. 2012, 28, 1889–1893. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Pelletier, S.; Buchdunger, E.; Warmuth, M.; Fabbro, D.; Hallek, M.; Van Etten, R.A.; Li, S. Requirement of Src kinases Lyn, Hck and Fgr for BCR-ABL1-induced B-lymphoblastic leukemia but not chronic myeloid leukemia. Nat. Genet. 2004, 36, 453–461. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Fedorchenko, O.; Rosen, N.; Koch, M.; Barthel, R.; Winarski, T.; Florin, A.; Wunderlich, F.T.; Reinart, N.; Hallek, M. LYN Kinase in the Tumor Microenvironment Is Essential for the Progression of Chronic Lymphocytic Leukemia. Cancer Cell 2016, 30, 610–622. [Google Scholar] [CrossRef]
- Cai, H.; Smith, D.A.; Memarzadeh, S.; Lowell, C.A.; Cooper, J.A.; Witte, O.N. Differential transformation capacity of Src family kinases during the initiation of prostate cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 6579–6584. [Google Scholar] [CrossRef]
- Poh, A.R.; Love, C.G.; Masson, F.; Preaudet, A.; Tsui, C.; Whitehead, L.; Monard, S.; Khakham, Y.; Burstroem, L.; Lessene, G.; et al. Inhibition of Hematopoietic Cell Kinase Activity Suppresses Myeloid Cell-Mediated Colon Cancer Progression. Cancer Cell 2017, 31, 563–575. [Google Scholar] [CrossRef]
- Kanteti, R.; Mirzapoiazova, T.; Riehm, J.J.; Dhanasingh, I.; Mambetsariev, B.; Wang, J.; Kulkarni, P.; Kaushik, G.; Seshacharyulu, P.; Ponnusamy, M.P.; et al. Focal adhesion kinase a potential therapeutic target for pancreatic cancer and malignant pleural mesothelioma. Cancer Biol. Ther. 2018, 19, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, I.M.; Kolev, V.N.; Vidal, C.M.; Kadariya, Y.; Ring, J.E.; Wright, Q.; Weaver, D.T.; Menges, C.; Padval, M.; McClatchey, A.I.; et al. Merlin deficiency predicts FAK inhibitor sensitivity: A synthetic lethal relationship. Sci. Transl. Med. 2014, 6, 237ra68. [Google Scholar] [CrossRef] [PubMed]
- Poulikakos, P.I.; Xiao, G.H.; Gallagher, R.; Jablonski, S.; Jhanwar, S.C.; Testa, J.R. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene 2006, 25, 5960–5968. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, T.; Yamada, T.; Ohnuma, K.; Kina, S.; Takahashi, N.; Yamochi, T.; Inamoto, S.; Katsuoka, Y.; Hosono, O.; Tanaka, H.; et al. Humanized anti-CD26 monoclonal antibody as a treatment for malignant mesothelioma tumors. Clin. Cancer Res. 2007, 13, 4191–4200. [Google Scholar] [CrossRef] [PubMed]
- González-González, L.; Alonso, J. Periostin: A Matricellular Protein with Multiple Functions in Cancer Development and Progression. Front. Oncol. 2018, 8, 225. [Google Scholar] [CrossRef]
- Schramm, A.; Opitz, I.; Thies, S.; Seifert, B.; Moch, H.; Weder, W.; Soltermann, A. Prognostic significance of epithelial-mesenchymal transition in malignant pleural mesothelioma. Eur. J. Cardio-Thorac. Surg. 2010, 37, 566–572. [Google Scholar] [CrossRef]
- Liu, Z.; Ivanoff, A.; Klominek, J. Expression and activity of matrix metalloproteases in human malignant mesothelioma cell lines. Int. J. Cancer 2001, 91, 638–643. [Google Scholar] [CrossRef]
- Hirano, H.; Tsuji, M.; Kizaki, T.; Sashikata, T.; Yoshi, Y.; Okada, Y.; Mori, H. Expression of matrix metalloproteinases, tissue inhibitors of metalloproteinase, collagens, and Ki67 antigen in pleural malignant mesothelioma: An immunohistochemical and electron microscopic study. Med. Electron Microsc. 2002, 35, 16–23. [Google Scholar] [CrossRef]
- Edwards, J.G.; McLaren, J.; Jones, J.L.; Waller, D.A.; O’Byrne, K.J. Matrix metalloproteinases 2 and 9 (gelatinases A and B) expression in malignant mesothelioma and benign pleura. Br. J. Cancer 2003, 88, 1553–1559. [Google Scholar] [CrossRef]
- Mannello, F.; Medda, V. Nuclear localization of Matrix metalloproteinases. Prog. Histochem. Cytochem. 2012, 47, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Sépult, C.; Bellefroid, M.; Rocks, N.; Donati, K.; Gérard, C.; Gilles, C.; Ludwig, A.; Duysinx, B.; Noël, A.; Cataldo, D. ADAM10 mediates malignant pleural mesothelioma invasiveness. Oncogene 2019, 38, 3521–3534. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Pan, Y.; Hu, G.; Sun, W.; Jiang, L.; Wang, P.; Ding, X. SRC fine-tunes ADAM10 shedding activity to promote pituitary adenoma cell progression. FEBS J. 2020, 287, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Schwager, S.C.; Taufalele, P.V.; Reinhart-King, C.A. Cell–Cell Mechanical Communication in Cancer. Cell. Mol. Bioeng. 2019, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Y.; Li, Q.Q.; Gao, Y.F.; Zhou, H.H.; Liu, Z.Q.; Jin, W.L. Gap junction as an intercellular glue: Emerging roles in cancer EMT and metastasis. Cancer Lett. 2016, 381, 133–137. [Google Scholar] [CrossRef]
- Linnainmaa, K.; Pelin, K.; Vanhala, E.; Tuomi, T.; Piccoli, C.; Fitzgerald, D.J.; Yamasaki, H. Gap junctional intercellular communication of primary and asbestos-associated malignant human mesothelial cells. Carcinogenesis 1993, 14, 1597–1602. [Google Scholar] [CrossRef]
- Pelin, K.; Hirvonen, A.; Linnainmaa, K. Expression of cell adhesion molecules and connexins in gap junctional intercellular communication deficient human mesothelioma tumour cell lines and communication competent primary mesothelial cells. Carcinogenesis 1994, 15, 2673–2675. [Google Scholar] [CrossRef]
- Loo, L.W.; Berestecky, J.M.; Kanemitsu, M.Y.; Lau, A.F. pp60src-mediated phosphorylation of connexin 43, a gap junction protein. J. Biol. Chem. 1995, 270, 12751–12761. [Google Scholar] [CrossRef]
- Giepmans, B.N.G.; Hengeveld, T.; Postma, F.R.; Moolenaar, W.H. Interaction of c-Src with gap junction protein connexin-43. Role in the regulation of cell-cell communication. J. Biol. Chem. 2001, 276, 8544–8549. [Google Scholar] [CrossRef]
- Fallacara, A.L.; Passannanti, R.; Mori, M.; Iovenitti, G.; Musumeci, F.; Greco, C.; Crespan, E.; Kissova, M.; Maga, G.; Tarantelli, C.; et al. Identification of a new family of pyrazolo[3,4-d]pyrimidine derivatives as multitarget Fyn-Blk-Lyn inhibitors active on B- and T-lymphoma cell lines. Eur. J. Med. Chem. 2019, 181, 111545. [Google Scholar] [CrossRef]
- Cammarata, F.P.; Torrisi, F.; Forte, G.I.; Minafra, L.; Bravatà, V.; Pisciotta, P.; Savoca, G.; Calvaruso, M.; Petringa, G.; Cirrone, G.A.P.; et al. Proton therapy and src family kinase inhibitor combined treatments on U87 human glioblastoma multiforme cell line. Int. J. Mol. Sci. 2019, 20, 4745. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, M.; Giorgi, F.; Marcelli, E.; Pentimalli, F.; Forte, I.M.; Schenone, S.; D’Urso, V.; De Falco, G.; Botta, M.; Giordano, A.; et al. Antitumor activity of new pyrazolo[3,4-d] pyrimidine SRC kinase inhibitors in Burkitt lymphoma cell lines and its enhancement by WEE1 inhibition. Cell Cycle 2012, 11, 1029–1039. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ceccherini, E.; Indovina, P.; Zamperini, C.; Dreassi, E.; Casini, N.; Cutaia, O.; Forte, I.M.; Pentimalli, F.; Esposito, L.; Polito, M.S.; et al. SRC family kinase inhibition through a new pyrazolo[3,4-d]pyrimidine derivative as a feasible approach for glioblastoma treatment. J. Cell. Biochem. 2015, 116, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Casini, N.; Forte, I.M.; Mastrogiovanni, G.; Pentimalli, F.; Angelucci, A.; Festuccia, C.; Tomei, V.; Ceccherini, E.; Di Marzo, D.; Schenone, S.; et al. SRC family kinase (SFK) inhibition reduces rhabdomyosarcoma cell growth in vitro and in vivo and triggers p38 MAP kinase-mediated differentiation. Oncotarget 2015, 6, 12421–12435. [Google Scholar] [CrossRef][Green Version]
- Indovina, P.; Casini, N.; Forte, I.M.; Garofano, T.; Cesari, D.; Iannuzzi, C.A.; Del Porro, L.; Pentimalli, F.; Napoliello, L.; Boffo, S.; et al. SRC Family Kinase Inhibition in Ewing Sarcoma Cells Induces p38 MAP Kinase-Mediated Cytotoxicity and Reduces Cell Migration. J. Cell. Physiol. 2017, 232, 129–135. [Google Scholar] [CrossRef]
- Calgani, A.; Vignaroli, G.; Zamperini, C.; Coniglio, F.; Festuccia, C.; Di Cesare, E.; Gravina, G.L.; Mattei, C.; Vitale, F.; Schenone, S.; et al. Suppression of SRC signaling is effective in reducing synergy between glioblastoma and stromal cells. Mol. Cancer Ther. 2016, 15, 1535–1544. [Google Scholar] [CrossRef]
- Laurenzana, I.; Caivano, A.; La Rocca, F.; Trino, S.; De Luca, L.; D’Alessio, F.; Schenone, S.; Falco, G.; Botta, M.; Del Vecchio, L.; et al. A pyrazolo[3,4-d]pyrimidine compound reduces cell viability and induces apoptosis in different hematological malignancies. Front. Pharmacol. 2016, 7, 416. [Google Scholar] [CrossRef]
- Laschi, M.; Bernardini, G.; Geminiani, M.; Manetti, F.; Mori, M.; Spreafico, A.; Campanacci, D.; Capanna, R.; Schenone, S.; Botta, M.; et al. Differentially activated Src kinase in chemo-naïve human primary osteosarcoma cells and effects of a Src kinase inhibitor. BioFactors 2017, 43, 801–811. [Google Scholar] [CrossRef]
- Fallacara, A.L.; Zamperini, C.; Podolski-Renić, A.; Dinić, J.; Stanković, T.; Stepanović, M.; Mancini, A.; Rango, E.; Iovenitti, G.; Molinari, A.; et al. A new strategy for glioblastoma treatment: In vitro and in vivo preclinical characterization of Si306, a pyrazolo[3,4-d]pyrimidine dual Src/P-Glycoprotein inhibitor. Cancers 2019, 11, 848. [Google Scholar] [CrossRef]
- Johnson, F.M.; Saigal, B.; Tran, H.; Donato, N.J. Abrogation of signal transducer and activator of transcription 3 reactivation after Src kinase inhibition results in synergistic antitumor effects. Clin. Cancer Res. 2007, 13, 4233–4244. [Google Scholar] [CrossRef]
- Chen, R.; Kim, O.; Yang, J.; Sato, K.; Eisenmann, K.M.; McCarthy, J.; Chen, H.; Qiu, Y. Regulation of Akt/PKB Activation by Tyrosine Phosphorylation. J. Biol. Chem. 2001, 276, 31858–31862. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Qiu, Y. Interaction between Src and a C-terminal proline-rich motif of Akt is required for Akt activation. J. Biol. Chem. 2003, 278, 15789–15793. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.A.; You, H.; Xiao, G.H.; Ramos-Nino, M.E.; Skele, K.L.; De Rienzo, A.; Jhanwar, S.C.; Mossman, B.T.; Kane, A.B.; Testa, J.R. Human and mouse mesotheliomas exhibit elevated AKT/PKB activity, which can be targeted pharmacologically to inhibit tumor cell growth. Oncogene 2005, 24, 6080–6089. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, L.; Li, H.; Eilers, G.; Kuang, Y.; Shi, S.; Yan, Z.; Li, X.; Corson, J.M.; Meng, F.; et al. Multipoint targeting of the PI3K/mTOR pathway in mesothelioma. Br. J. Cancer 2014, 110, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Pentimalli, F.; Forte, I.M.; Esposito, L.; Indovina, P.; Iannuzzi, C.A.; Alfano, L.; Costa, C.; Barone, D.; Rocco, G.; Giordano, A. RBL2/p130 is a direct AKT target and is required to induce apoptosis upon AKT inhibition in lung cancer and mesothelioma cell lines. Oncogene 2018, 37, 3657–3671. [Google Scholar] [CrossRef] [PubMed]
- Ventura, E.; Pentimalli, F.; Giordano, A. RBL2/p130: A direct AKT substrate and mediator of AKT inhibition-induced apoptosis. Oncoscience 2018, 5, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Chu, I.; Sun, J.; Arnaout, A.; Kahn, H.; Hanna, W.; Narod, S.; Sun, P.; Tan, C.K.; Hengst, L.; Slingerland, J. p27 Phosphorylation by Src Regulates Inhibition of Cyclin E-Cdk2. Cell 2007, 128, 281–294. [Google Scholar] [CrossRef]
- Chu, I.M.; Hengst, L.; Slingerland, J.M. The Cdk inhibitor p27 in human cancer: Prognostic potential and relevance to anticancer therapy. Nat. Rev. Cancer 2008, 8, 253–267. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Zhao, R.; Wen, Y.Y.; Fournier, K.; Wu, H.B.; Yang, H.Y.; Diaz, J.; Laronga, C.; Lee, M.H. Negative cell cycle regulator 14-3-3σ stabilizes p27 Kip1 by inhibiting the activity of PKB/Akt. Oncogene 2006, 25, 4585–4594. [Google Scholar] [CrossRef]
- Kops, G.J.P.L.; Medema, R.H.; Glassford, J.; Essers, M.A.G.; Dijkers, P.F.; Coffer, P.J.; Lam, E.W.-F.; Burgering, B.M.T. Control of Cell Cycle Exit and Entry by Protein Kinase B-Regulated Forkhead Transcription Factors. Mol. Cell. Biol. 2002, 22, 2025–2036. [Google Scholar] [CrossRef]
- Sinibaldi, D.; Wharton, W.; Turkson, J.; Bowman, T.; Pledger, W.J.; Jove, R. Induction of p21(WAF1/CIP1) and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: Role of activated STAT3 signaling. Oncogene 2000, 19, 5419–5427. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Roberts, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.W.; Shepherd, P.; Pullinger, N.C. p27 immunostaining is related to prognosis in malignant mesothelioma. Histopathology 2001, 38, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, M.; Cassoni, P.; De Giuli, P.; Viberti, L.; Cappia, S.; Ivaldi, C.; Chiusa, L.; Bussolati, G. p27(kip1) Immunoreactivity Correlates with Long-Term Survival in Pleural Malignant Mesothelioma. Cancer 2001, 92, 1245–1250. [Google Scholar] [CrossRef]
- Baldi, A.; Santini, D.; Vasaturo, F.; Santini, M.; Vicidomini, G.; Pia Di Marino, M.; Esposito, V.; Groeger, A.M.; Liuzzi, G.; Vincenzi, B.; et al. Prognostic significance of cyclooxygenase-2 (COX-2) and expression of cell cycle inhibitors p21 and p27 in human pleural malignant mesothelioma. Thorax 2004, 59, 428–433. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daubriac, J.; Fleury-Feith, J.; Kheuang, L.; Galipon, J.; Saint-Albin, A.; Renier, A.; Giovannini, M.; Galateau-Sallé, F.; Jaurand, M.C. Malignant pleural mesothelioma cells resist anoikis as quiescent pluricellular aggregates. Cell Death Differ. 2009, 16, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Xiang, L.; FitzGerald, D.J.; Pastan, I. Antitumor effects of immunotoxins are enhanced by lowering HCK or treatment with Src kinase inhibitors. Mol. Cancer Ther. 2013, 13, 82–89. [Google Scholar] [CrossRef]
- Liu, R.; Ferguson, B.D.; Zhou, Y.; Naga, K.; Salgia, R.; Gill, P.S.; Krasnoperov, V. EphB4 as a therapeutic target in mesothelioma. BMC Cancer 2013, 13, 269. [Google Scholar] [CrossRef][Green Version]
- Kashiwagi, K.; Virgona, N.; Harada, K.; Kido, W.; Yano, Y.; Ando, A.; Hagiwara, K.; Yano, T. A redox-silent analogue of tocotrienol acts as a potential cytotoxic agent against human mesothelioma cells. Life Sci. 2009, 84, 650–656. [Google Scholar] [CrossRef]
- Bantscheff, M.; Eberhard, D.; Abraham, Y.; Bastuck, S.; Boesche, M.; Hobson, S.; Mathieson, T.; Perrin, J.; Raida, M.; Rau, C.; et al. Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nat. Biotechnol. 2007, 25, 1035–1044. [Google Scholar] [CrossRef]
- Rix, U.; Hantschel, O.; Dürnberger, G.; Remsing Rix, L.L.; Planyavsky, M.; Fernbach, N.V.; Kaupe, I.; Bennett, K.L.; Valent, P.; Colinge, J.; et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood 2007, 110, 4055–4063. [Google Scholar] [CrossRef]
- Li, J.; Rix, U.; Fang, B.; Bai, Y.; Edwards, A.; Colinge, J.; Bennett, K.L.; Gao, J.; Song, L.; Eschrich, S.; et al. A chemical and phosphoproteomic characterization of dasatinib action in lung cancer. Nat. Chem. Biol. 2010, 6, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.W.-M.; States, D.J. Both Src-Dependent and -Independent Mechanisms Mediate Phosphatidylinositol 3-Kinase Regulation of Colony-Stimulating Factor 1-Activated Mitogen-Activated Protein Kinases in Myeloid Progenitors. Mol. Cell. Biol. 2000, 20, 6779–6798. [Google Scholar] [CrossRef]
- Bourgin-Hierle, C.; Gobert-Gosse, S.; Thérier, J.; Grasset, M.F.; Mouchiroud, G. Src-family kinases play an essential role in differentiation signaling downstream of macrophage colony-stimulating factor receptors mediating persistent phosphorylation of phospholipase C-γ2 and MAP kinases ERK1 and ERK2. Leukemia 2008, 22, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Blanquart, C.; Jaurand, M.-C.; Jean, D. The Biology of Malignant Mesothelioma and the Relevance of Preclinical Models. Front. Oncol. 2020, 10, 388. [Google Scholar] [CrossRef] [PubMed]
- Blum, Y.; Meiller, C.; Quetel, L.; Elarouci, N.; Ayadi, M.; Tashtanbaeva, D.; Armenoult, L.; Montagne, F.; Tranchant, R.; Renier, A.; et al. Dissecting heterogeneity in malignant pleural mesothelioma through histo-molecular gradients for clinical applications. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tsao, A.S.; Lindwasser, O.W.; Adjei, A.A.; Adusumilli, P.S.; Beyers, M.L.; Blumenthal, G.M.; Bueno, R.; Burt, B.M.; Carbone, M.; Dahlberg, S.E.; et al. Current and Future Management of Malignant Mesothelioma: A Consensus Report from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation. J. Thorac. Oncol. 2018, 13, 1655–1667. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201–218. [Google Scholar] [CrossRef]
- Finn, R. Targeting Src in Breast Cancer. Ann. Oncol. 2008, 8, 1379–1386. [Google Scholar] [CrossRef]
- Bild, A.H.; Yao, G.; Chang, J.T.; Wang, Q.; Potti, A.; Chasse, D.; Joshi, M.B.; Harpole, D.; Lancaster, J.M.; Berchuck, A.; et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 2006, 439, 353–357. [Google Scholar] [CrossRef]
- De Wang, X.; Reeves, K.; Luo, F.R.; Xu, L.A.; Lee, F.; Clark, E.; Huang, F. Identification of candidate predictive and surrogate molecular markers for dasatinib in prostate cancer: Rationale for patient selection and efficacy monitoring. Genome Biol. 2007, 8, R255. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Dering, J.; Ginther, C.; Wilson, C.A.; Glaspy, P.; Tchekmedyian, N.; Slamon, D.J. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/”triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res. Treat. 2007, 105, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Konecny, G.E.; Glas, R.; Dering, J.; Manivong, K.; Qi, J.; Finn, R.S.; Yang, G.R.; Hong, K.L.; Ginther, C.; Winterhoff, B.; et al. Activity of the multikinase inhibitor dasatinib against ovarian cancer cells. Br. J. Cancer 2009, 101, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hu, W.; Bottsford-Miller, J.; Liu, T.; Han, H.D.; Zand, B.; Pradeep, S.; Roh, J.W.; Thanapprapasr, D.; Dalton, H.J.; et al. Cross-talk between EphA2 and BRaf/CRaf is a key determinant of response to dasatinib. Clin. Cancer Res. 2014, 20, 1846–1855. [Google Scholar] [CrossRef]
- Klammer, M.; Kaminski, M.; Zedler, A.; Oppermann, F.; Blencke, S.; Marx, S.; Müller, S.; Tebbe, A.; Godl, K.; Schaab, C. Phosphosignature predicts dasatinib response in non-small cell lung cancer. Mol. Cell. Proteom. 2012, 11, 651–668. [Google Scholar] [CrossRef]
- Brosseau, S.; Assoun, S.; Naltet, C.; Steinmetz, C.; Gounant, V.; Zalcman, G. A review of bevacizumab in the treatment of malignant pleural mesothelioma. Future Oncol. 2017, 13, 2537–2546. [Google Scholar] [CrossRef]
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet 2016, 387, 1405–1414. [Google Scholar] [CrossRef]
- Weis, S.; Cui, J.; Barnes, L.; Cheresh, D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J. Cell Biol. 2004, 167, 223–229. [Google Scholar] [CrossRef]
- Huveldt, D.; Lewis-Tuffin, L.J.; Carlson, B.L.; Schroeder, M.A.; Rodriguez, F.; Giannini, C.; Galanis, E.; Sarkaria, J.N.; Anastasiadis, P.Z. Targeting Src Family Kinases Inhibits Bevacizumab-Induced Glioma Cell Invasion. PLoS ONE 2013, 8, e56505. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-S.; Wang, Z.-F.; Dai, L.-M.; Chu, S.-H.; Gong, L.-L.; Yang, M.-H.; Li, Z.-Q. Induction of proline-rich tyrosine kinase 2 activation-mediated C6 glioma cell invasion after anti-vascular endothelial growth factor therapy. J. Transl. Med. 2014, 12, 148. [Google Scholar] [CrossRef][Green Version]
- Bai, L.; Yang, J.C.; Ok, J.; Mack, P.C.; Kung, H.-J.; Evans, C.P. Simultaneous targeting of Src kinase and receptor tyrosine kinase results in synergistic inhibition of renal cell carcinoma proliferation and migration. Int. J. Cancer 2012, 130, 2693–2702. [Google Scholar] [CrossRef] [PubMed]
- Lowell, C.A. Src-family kinases: Rheostats of immune cell signaling. Mol. Immunol. 2004, 41, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.-T.; Mao, L.; Wu, L.; Deng, W.-W.; Bu, L.-L.; Liu, J.-F.; Chen, L.; Yang, L.-L.; Wu, H.; Zhang, W.-F.; et al. Inhibition of SRC family kinases facilitates anti-CTLA4 immunotherapy in head and neck squamous cell carcinoma. Cell. Mol. Life Sci. 2018, 75, 4223–4234. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.R.; Greenland, E.L.; Pixley, F.J. Promotion of tumor invasion by tumor-associated macrophages: The role of CSF-1-activated phosphatidylinositol 3 kinase and Src family kinase motility signaling. Cancers 2017, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Cannarile, M.A.; Weisser, M.; Jacob, W.; Jegg, A.M.; Ries, C.H.; Rüttinger, D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J. Immunother. Cancer 2017, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Hoves, S.; Ooi, C.H.; Wolter, C.; Sade, H.; Bissinger, S.; Schmittnaegel, M.; Ast, O.; Giusti, A.M.; Wartha, K.; Runza, V.; et al. Rapid activation of tumor-associated macrophages boosts preexisting tumor immunity. J. Exp. Med. 2018, 215, 859–876. [Google Scholar] [CrossRef]
- Enomoto, M.; Vaughen, J.; Igaki, T. Non-autonomous overgrowth by oncogenic niche cells: Cellular cooperation and competition in tumorigenesis. Cancer Sci. 2015, 106, 1651–1658. [Google Scholar] [CrossRef]
| Drug | Main Targets | Status | References |
|---|---|---|---|
| DASATINIB * (BMS354825) N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-yl]amino]-1,3-thiazole-5-carboxamide | BCR-ABL, CSF-1R, EPHA2, KIT, PDGFRB, SFKs | Clinical trials/FDA approved for CML and Ph+ ALL | [33,34,35,50,51] |
| BOSUTINIB (SKI-606) 4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl)propoxy]-3-quinolinecarbonitrile | BCR-ABL, SFKs | Clinical trials/FDA approved for CML | [36] |
| PONATINIB (AP24534) 3-(imidazo [1,2-b]pyridazin3-ylethynyl)-4-methyl-N-19benzamide hydrochloride benzamide | BCR-ABL, KIT, FGFR1, FLT1, PDGFR, RET, SFKs | Clinical trials/FDA approved for CML and Ph+ ALL | [37] |
| SARACATINIB (AZD0530) N-(5-chloro-1,3-benzodioxol-4-yl)-7- [2-(4-methylpiperazin-1-yl)ethoxy]-5-(oxan-4-yloxy) quinazolin-4-amine | BCR-ABL, SFKs | Clinical trials | [38] |
| AZD0424 1-[4-[2-[4-[(6-chloro-[1,3]dioxolo [4,5-b]pyridin-7-yl)amino]-5-propan-2-yloxyquinazolin-7-yl]oxyethyl]piperazin-1-yl]ethanone | BCR-ABL, SFKs | Clinical trials | [39] |
| KXO1† (KX2-391) N-benzyl-2-[5-[4-(2-morpholin-4-ylethoxy)phenyl]pyridin-2-yl]acetamide | SFKs, Tubulin | Clinical trials | [40] |
| PP1 * 1-tert-butyl-3-(4-methylphenyl)pyrazolo [3,4d] pyrimidin-4-amine | EPHA2, FGFR1, KIT, MAPK, RIP2, TGF-β type I, SFKs | Preclinical studies | [41,42,43,45] |
| PP2 * 1-tert-butyl-3-(4-chlorophenyl)pyrazolo [3,4-d]pyrimidin-4-amine | CK1δ, EPHA2, FGFR1, KIT, MAPK, RIP2,TGF-β type I, SFKs | Preclinical studies | [41,42,43,46] |
| SU6656 * (3Z)-N,N-dimethyl-2-oxo-3-(4,5,6,7-tetrahydro-1H-indol-2-ylmethylidene)-1H-indole-5-sulfonamide | AMPK, AURORA B/C, BRSK2, MST2, SFKs | Preclinical studies | [43,47,48] |
| SI83 * and SI91 * pyrazolo [3,4-d]pyrimidines derivatives | BCR-ABL, SFKs | Preclinical studies | [44,49] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indovina, P.; Forte, I.M.; Pentimalli, F.; Giordano, A. Targeting SRC Family Kinases in Mesothelioma: Time to Upgrade. Cancers 2020, 12, 1866. https://doi.org/10.3390/cancers12071866
Indovina P, Forte IM, Pentimalli F, Giordano A. Targeting SRC Family Kinases in Mesothelioma: Time to Upgrade. Cancers. 2020; 12(7):1866. https://doi.org/10.3390/cancers12071866
Chicago/Turabian StyleIndovina, Paola, Iris Maria Forte, Francesca Pentimalli, and Antonio Giordano. 2020. "Targeting SRC Family Kinases in Mesothelioma: Time to Upgrade" Cancers 12, no. 7: 1866. https://doi.org/10.3390/cancers12071866
APA StyleIndovina, P., Forte, I. M., Pentimalli, F., & Giordano, A. (2020). Targeting SRC Family Kinases in Mesothelioma: Time to Upgrade. Cancers, 12(7), 1866. https://doi.org/10.3390/cancers12071866








