Improved Diagnostic Imaging of Brain Tumors by Multimodal Microscopy and Deep Learning
Abstract
1. Introduction
2. Results
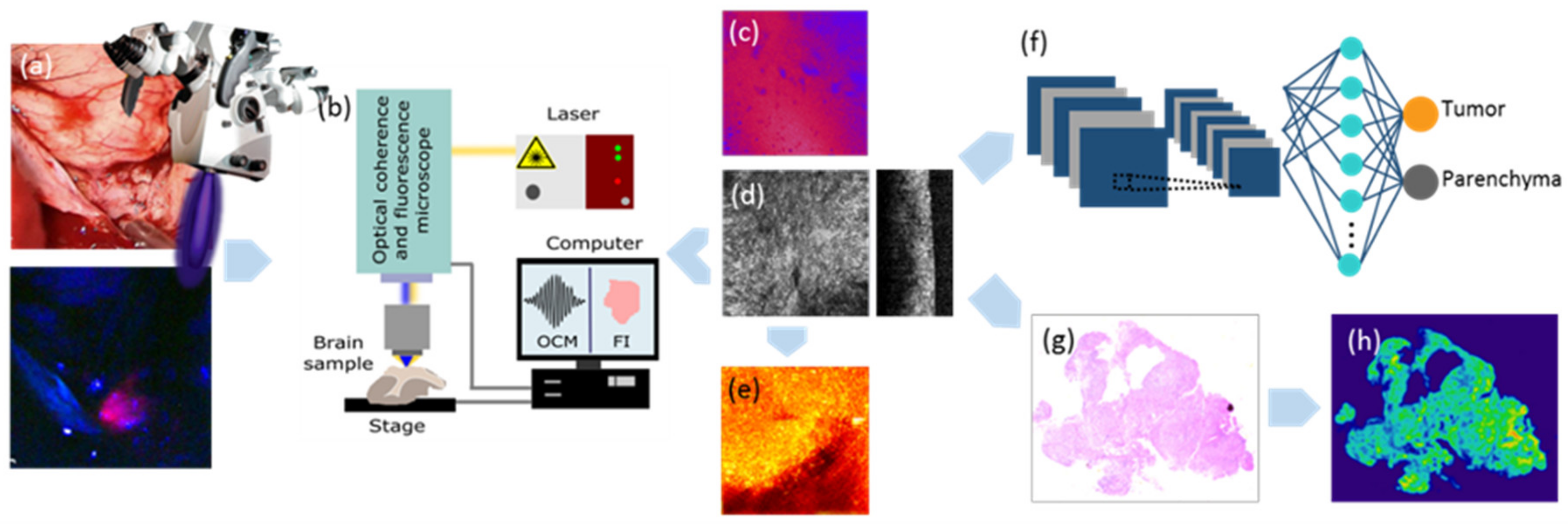
2.1. Comparison of In Vivo 5-ALA Readout with Ex Vivo Fluorescence, OCM and Histology
2.2. Comparison of OCM Attenuation and Quantitative Fluorescence Data
2.3. Investigation of Intra-Tumor Heterogeneity Using Wide-Field OCM Imaging
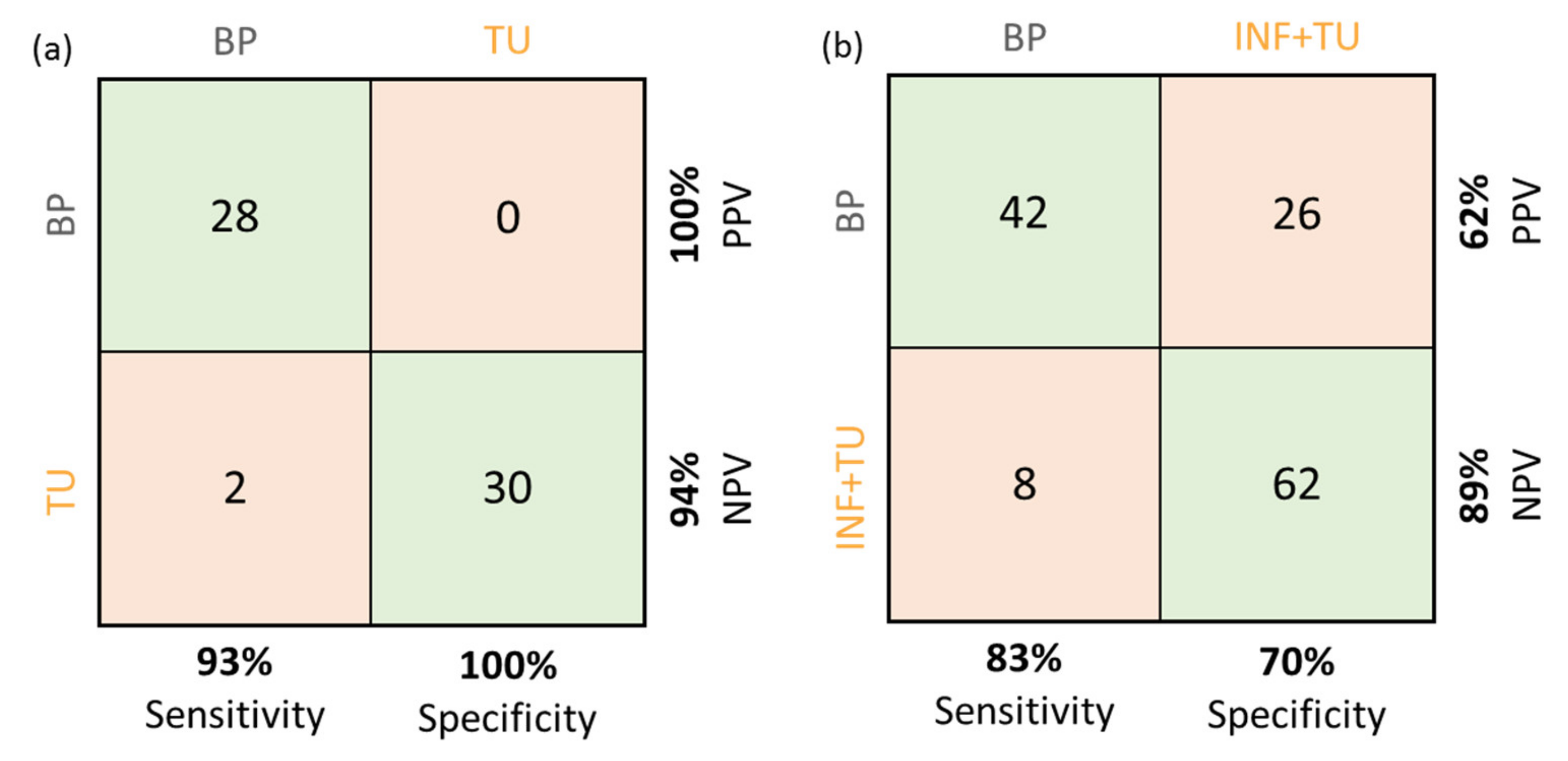
2.4. CNN for Tumor Classification in OCM Intensity B-Scan Data
3. Discussion
4. Materials and Methods
4.1. Tumor Samples
4.2. Optical Coherence Microscopy and Fluorescence Imaging
4.3. Data Acquisition and Post-Processing with the Multimodal OCM Setup
4.4. Large Field-Of-View Scans
4.5. Histopathological Workup
4.6. Cell Density Maps and Statistical Correlation
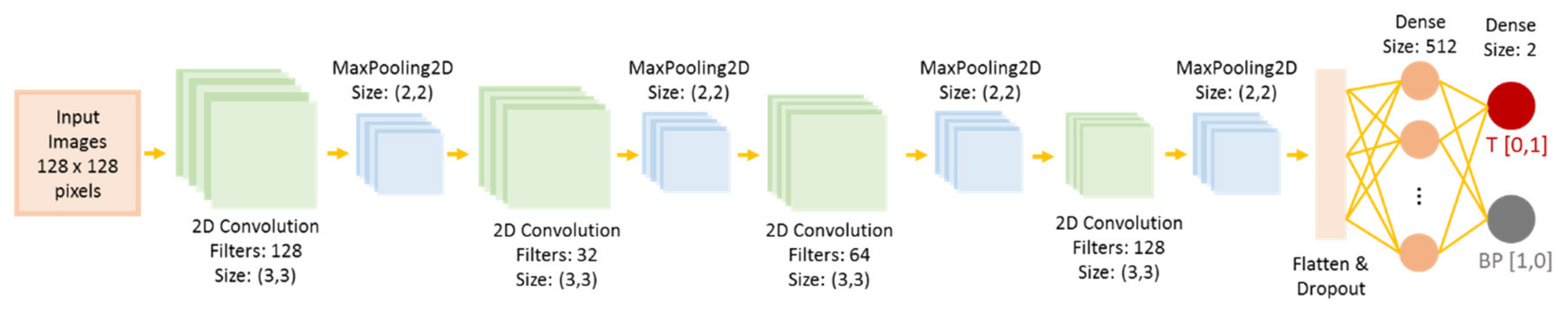
4.7. Convolutional Neural Network
4.8. Data and Code Availability Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Molinaro, A.M.; Taylor, J.W.; Wiencke, J.K.; Wrensch, M.R. Genetic and molecular epidemiology of adult diffuse glioma. Nat. Rev. Neurol. 2019, 15, 405–417. [Google Scholar] [CrossRef]
- Tamimi, A.F.; Juweid, M. Epidemiology and outcome of glioblastoma. In Glioblastoma; Codon Publications: Brisbane, Australia, 2017; pp. 143–155. ISBN 9780323479745. [Google Scholar]
- Davis, M.E. Glioblastoma: Overview of disease and treatment. Clin. J. Oncol. Nurs. 2016, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Wright, C.H.; Barnholtz-Sloan, J.S. Brain Metastases: Epidemiology, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 149, ISBN 9780128111611. [Google Scholar]
- Lowery, F.J.; Yu, D. Brain metastasis: Unique challenges and open opportunities. Biochim. Biophys. Acta 2017, 1867, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Valdés, P.A.; Roberts, D.W.; Lu, F.K.; Golby, A. Optical technologies for intraoperative neurosurgical guidance. Neurosurg. Focus 2016, 40, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Vasefi, F.; MacKinnon, N.; Farkas, D.L.; Kateb, B. Review of the potential of optical technologies for cancer diagnosis in neurosurgery: A step toward intraoperative neurophotonics. Neurophotonics 2016, 4, 011010. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Widhalm, G.; Stummer, W. What is the surgical benefit of utilizing 5-aminolevulinic acid for fluorescence-guided surgery of malignant gliomas? Neurosurgery 2015, 77, 663–673. [Google Scholar] [CrossRef]
- Schucht, P.; Knittel, S.; Slotboom, J.; Seidel, K.; Murek, M.; Jilch, A.; Raabe, A.; Beck, J. 5-ALA complete resections go beyond MR contrast enhancement: Shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir. 2014, 156, 305–312. [Google Scholar] [CrossRef]
- Stummer, W.; Stocker, S.; Novotny, A.; Heimann, A.; Sauer, O.; Kempski, O.; Plesnila, N.; Wietzorrek, J.; Reulen, H.J. In vitro and in vivo porphyrin accumulation by C6 glioma cells after exposure to 5-aminolevulinic acid. J. Photochem. Photobiol. B Biol. 1998, 45, 160–169. [Google Scholar] [CrossRef]
- Díez Valle, R.; Hadjipanayis, C.G.; Stummer, W. Established and emerging uses of 5-ALA in the brain: An overview. J. Neurooncol. 2019, 141, 487–494. [Google Scholar] [CrossRef]
- Valdés, P.A.; Kim, A.; Brantsch, M.; Niu, C.; Moses, Z.B.; Tosteson, D.; Wilson, B.C.; Paulsen, K.D.; Roberts, D.W.; Harris, B.T. δ-aminolevulinic acid-induced protoporphyrin IX concentration correlates with histopathologic markers of malignancy regions of increasing malignancy. Neuro-Oncology 2011, 13, 846–856. [Google Scholar]
- Widhalm, G.; Kiesel, B.; Woehrer, A.; Traub-Weidinger, T.; Preusser, M.; Marosi, C.; Prayer, D.; Hainfellner, J.A.; Knosp, E.; Wolfsberger, S. 5-aminolevulinic acid induced fluorescence is a powerful intraoperative marker for precise histopathological grading of gliomas with non-significant contrast-enhancement. PLoS ONE 2013, 8, e76988. [Google Scholar] [CrossRef]
- Widhalm, G. Intraoperative visualization of brain tumors with 5-aminolevulinic acid-induced fluorescence. Clin. Neuropathol. 2014, 33, 260–278. [Google Scholar] [CrossRef]
- Widhalm, G.; Wolfsberger, S.; Minchev, G.; Woehrer, A.; Krssak, M.; Czech, T.; Prayer, D.; Asenbaum, S.; Hainfellner, J.A.; Knosp, E. 5-Aminolevulinic acid is a promising marker for detection of anaplastic foci in diffusely infiltrating gliomas with nonsignificant contrast enhancement. Cancer 2010, 116, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Marhold, F.; Mercea, P.A.; Scheichel, F.; Berghoff, A.S.; Heicappell, P.; Kiesel, B.; Mischkulnig, M.; Borkovec, M.; Wolfsberger, S.; Woehrer, A.; et al. Detailed analysis of 5-aminolevulinic acid induced fluorescence in different brain metastases at two specialized neurosurgical centers: Experience in 157 cases. J. Neurosurg. 2019, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Seetha, J.; Selvakumar Raja, S. Brain tumor classification using convolutional neural networks. Biomed. Pharmacol. J. 2018, 11, 1457–1461. [Google Scholar] [CrossRef]
- Mohsen, H.; El-Dahshan, E.-S.A.; El-Horbaty, E.-S.M.; Salem, A.-B.M. Classification using deep learning neural networks for brain tumors. Future Comput. Inform. J. 2018, 3, 68–71. [Google Scholar] [CrossRef]
- Hollon, T.C.; Pandian, B.; Adapa, A.R.; Urias, E.; Save, A.V.; Khalsa, S.S.S.; Eichenberg, D.G.; D’Amico, R.; Farooq, Z.U.; Lewis, S.; et al. Near real-time intraoperative brain tumor diagnosis using stimulated Raman histology and deep neural networks. Nat. Med. 2020, 26, 52–58. [Google Scholar] [CrossRef]
- Marusyk, A.; Polyak, K. Tumor heterogeneity: Causes and consequences. Biochim. Biophys. Acta 2010, 1805, 1–28. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Math, D.; Gronroos, E.; Martinez, P.; Matthews, N.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef]
- Navin, N.; Krasnitz, A.; Rodgers, L.; Cook, K.; Meth, J.; Kendall, J.; Riggs, M.; Eberling, Y.; Troge, J.; Grubor, V.; et al. Inferring tumor progression from genomic heterogeneity. Genome Res. 2010, 20, 68–80. [Google Scholar] [CrossRef]
- Sottoriva, A.; Spiteri, I.; Piccirillo, S.G.M.; Touloumis, A.; Collins, V.P.; Marioni, J.C.; Curtis, C.; Watts, C.; Tavaré, S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc. Natl. Acad. Sci. USA 2013, 110, 4009–4014. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Drexler, W.; Fujimoto, J.G. Optical Coherence Tomography: Technology and Applications; Springer Science & Business Media: Berlin, Germany, 2008; ISBN 9783540775492. [Google Scholar]
- Liu, G.; Chen, Z. Optical coherence tomography for brain imaging. In Optical Methods and Instrumentation in Brain Imaging and Therapy; Springer: Berlin, Germany, 2013; pp. 157–172. [Google Scholar]
- Leitgeb, R.A.; Baumann, B. Multimodal optical medical imaging concepts based on optical coherence tomography. Front. Phys. 2018, 6, 1–17. [Google Scholar] [CrossRef]
- Marchand, P.J.; Bouwens, A.; Szlag, D.; Hguyen, D.; Descloux, A.; Sison, M.; Coquoz, S.; Extermann, J.; Lasser, T. Visible spectrum extended-focus optical coherence microscopy for label-free sub-cellular tomography. Biomed. Opt. Express 2017, 8, 3343–3359. [Google Scholar] [CrossRef] [PubMed]
- Lichtenegger, A.; Harper, D.J.; Augustin, M.; Eugui, P.; Muck, M.; Gesperger, J.; Hitzenberger, C.K.; Woehrer, A.; Baumann, B. Spectroscopic imaging with spectral domain visible light optical coherence microscopy in Alzheimer’s disease brain samples. Biomed. Opt. Express 2017, 8, 4007–4025. [Google Scholar] [CrossRef] [PubMed]
- Lichtenegger, A.; Muck, M.; Eugui, P.; Harper, D.J.; Augustin, M.; Leskovar, K.; Hitzenberger, C.K.; Woehrer, A.; Baumann, B. Assessment of pathological features in Alzheimer’s disease brain tissue with a large field-of-view visible-light optical coherence microscope. Neurophotonics 2018, 5, 035002. [Google Scholar] [CrossRef] [PubMed]
- Baumann, B.; Woehrer, A.; Ricken, G.; Augustin, M.; Mitter, C.; Pircher, M.; Kovacs, G.G.; Hitzenberger, C.K. Visualization of neuritic plaques in Alzheimer’s disease by polarization-sensitive optical coherence microscopy. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kut, C.; Chaichana, K.L.; Xi, J.; Raza, S.M.; Ye, X.; McVeigh, E.R.; Rodriguez, F.J.; Quinones-Hinojosa, A.; Li, X. Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci. Transl. Med. 2015, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Assayag, O.; Grieve, K.; Devaux, B.; Harms, F.; Pallud, J.; Chretien, F.; Boccara, C.; Varlet, P. Imaging of non-tumorous and tumorous human brain tissues with full-field optical coherence tomography. NeuroImage Clin. 2013, 2, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Böhringer, H.J.; Boller, D.; Leppert, J.; Knopp, U.; Lankenau, E.; Reusche, E.; Hüttmann, G.; Giese, A. Time-domain and spectral-domain optical coherence tomography in the analysis of brain tumor tissue. Lasers Surg. Med. 2006, 38, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, P.V.; Dolganova, I.N.; Chernomyrdin, N.; Musina, G.; Beshplav, S.-I.; Kosyrkova, A.; Reshetov, I.; Tuchin, V.; Zaytsev, K. Optical coherence tomography of human brain glioma as a promising tool for intraoperative diagnostics in neurosurgery. Eur. Conf. Biomed. Opt. 2019, 11078, 1107829. [Google Scholar]
- Böhringer, H.J.; Lankenau, E.; Stellmacher, F.; Reusche, E.; Hüttmann, G.; Giese, A. Imaging of human brain tumor tissue by near-infrared laser coherence tomography. Acta Neurochir. 2009, 151, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Vakoc, B.J.; Lanning, R.M.; Tyrrell, J.A.; Padera, T.P.; Bartlett, L.A.; Stylianopoulos, T.; Munn, L.L.; Tearney, G.J.; Fukumura, D.; Jain, R.K.; et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat. Med. 2009, 15, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Bizheva, K.; Unterhuber, A.; Hermann, B.; Považay, B.; Sattmann, H.; Fercher, A.F.; Drexler, W.; Preusser, M.; Budka, H.; Stingl, A.; et al. Imaging ex vivo healthy and pathological human brain tissue with ultra-high-resolution optical coherence tomography. J. Biomed. Opt. 2005, 10, 011006. [Google Scholar] [CrossRef]
- Almasian, M.; Wilk, L.S.; Bloemen, P.R.; van Leeuwen, T.G.; ter Laan, M.; Aalders, M.C.G. Pilot feasibility study of in vivo intraoperative quantitative optical coherence tomography of human brain tissue during glioma resection. J. Biophotonics 2019, 12, e201900037. [Google Scholar] [CrossRef]
- Juarez-Chambi, R.M.; Kut, C.; Rico-Jimenez, J.J.; Chaichana, K.L.; Xi, J.; Campos-Delgado, D.U.; Rodriguez, F.J.; Quiñones-Hinojosa, A.; Li, X.; Jo, J.A. AI-assisted in situ detection of human glioma infiltration using a novel computational method for optical coherence tomography. Clin. Cancer Res. 2019, 25, 6329–6338. [Google Scholar] [CrossRef]
- Lenz, M.; Krug, R.; Dillmann, C.; Stroop, R.; Gerhardt, N.C. Automated differentiation between meningioma and healthy brain tissue based on optical coherence tomography ex vivo images using texture features. J. Biomed. Opt. 2018, 23, 071205. [Google Scholar] [CrossRef]
- Yashin, K.S.; Kiseleva, E.B.; Gubarkova, E.V.; Moiseev, A.A.; Kuznetsov, S.S.; Shilyagin, P.A.; Gelikonov, G.V.; Medyanik, I.A.; Kravets, L.Y.; Potapov, A.A.; et al. Cross-polarization optical coherence tomography for brain tumor imaging. Front. Oncol. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Lichtenegger, A.; Gesperger, J.; Kiesel, B.; Muck, M.; Hitzenberger, C.K.; Widhalm, G.; Woehrer, A.; Baumann, B. Revealing brain pathologies with multimodal visible light optical coherence microscopy and fluorescence imaging. J. Biomed. Opt. 2019, 24, 066010. [Google Scholar] [CrossRef]
- Salas, M.; Augustin, M.; Felberer, F.; Wartak, A.; Laslandes, M.; Ginner, L.; Niederleithner, M.; Ensher, J.; Minneman, M.P.; Leitgeb, R.A.; et al. Compact akinetic swept source optical coherence tomography angiography at 1060 nm supporting a wide field of view and adaptive optics imaging modes of the posterior eye. Biomed. Opt. Express 2018, 9, 1871–1892. [Google Scholar] [CrossRef]
- Zhou, X.; Liao, X.; Zhang, B.; He, H.; Shui, Y.; Xu, W.; Jiang, C.; Shen, L.; Wei, Q. Recurrence patterns in patients with high-grade glioma following temozolomide-based chemoradiotherapy. Mol. Clin. Oncol. 2016, 5, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Delgado-López, P.D.; Corrales-García, E.M. Survival in glioblastoma: A review on the impact of treatment modalities. Clin. Transl. Oncol. 2016, 18, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Orringer, D.; Lau, D.; Khatri, S.; Zamora-Berridi, G.J.; Zhang, K.; Wu, C.; Chaudhary, N.; Sagher, O. Extent of resection in patients with glioblastoma: Limiting factors, perception of resectability, and effect on survival. J. Neurosurg. 2012, 117, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Suki, D.; Hess, K.; Sawaya, R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J. Neurosurg. 2016, 124, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Chang, E.; Lamborn, K.R.; Chang, S.M.; Prados, M.D.; Cha, S.; Tihan, T.; VandenBerg, S.; McDermott, M.; Berger, M.S. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J. Clin. Oncol. 2008, 26, 1338–1345. [Google Scholar] [CrossRef]
- Stummer, W.; Reulen, H.J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.-C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Clin. Res. 2008, 62, 564–576. [Google Scholar]
- Jolesz, F.; Kettenbach, J.; Kikinis, R. Image-Guided Neurosurgery with Intraoperative MRI; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Nabavi, A.; Thurm, H.; Zountsas, B.; Pietsch, T.; Lanfermann, H.; Pichlmeier, U.; Mehdorn, M. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: A phase II study. Neurosurgery 2009, 65, 1070–1077. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Ishihara, R.; Katayama, Y.; Watanabe, T.; Yoshino, A.; Fukushima, T.; Sakatani, K. Quantitative spectroscopic analysis of 5-aminolevulinic acid-induced protoporphyrin IX fluorescence intensity in diffusely infiltrating astrocytomas. Neurol. Med. Chir. 2007, 47, 53–57. [Google Scholar] [CrossRef]
- Marbacher, S.; Klinger, E.; Schwyzer, L.; Fischer, I.; Nevzati, E.; Diepers, M.; Roelcke, U.; Fathi, A.R.; Coluccia, D.; Fandino, J. Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg. Focus 2014, 36, 1–10. [Google Scholar] [CrossRef]
- Sanai, N.; Snyder, L.A.; Honea, N.J.; Coons, S.W.; Eschbacher, J.M.; Smith, K.A.; Spetzler, R.F. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas: Clinical article. J. Neurosurg. 2011, 115, 740–748. [Google Scholar] [CrossRef]
- Valdés, P.A.; Kim, A.; Leblond, F.; Conde, O.M.; Harris, B.T.; Paulsen, K.D.; Wilson, B.C.; Roberts, D.W. Combined fluorescence and reflectance spectroscopy for in vivo quantification of cancer biomarkers in low- and high-grade glioma surgery. J. Biomed. Opt. 2011, 16, 116007. [Google Scholar] [CrossRef]
- Tonn, J.C.; Stummer, W. Fluorescence-guided resection of malignant gliomas using 5-aminolevulinic acid: Practical use, risks, and pitfalls. Clin. Neurosurg. 2008, 55, 20–26. [Google Scholar] [PubMed]
- Stummer, W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-guided resection of glioblastoma multiforme utilizing 5-ALA-induced porphyrins: A prospective study in 52 consecutive patients. J. Neurosurg. 2000, 93, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Berger, M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008, 62, 753–766. [Google Scholar] [CrossRef] [PubMed]
- McGirt, M.J.; Chaichana, K.L.; Attenello, F.J.; Weingart, J.D.; Than, K.; Burger, P.C.; Olivi, A.; Brem, H.; Quinoñes-Hinojosa, A. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery 2008, 63, 700–708. [Google Scholar] [CrossRef]
- Hart, M.G.; Garside, R.; Rogers, G.; Stein, K.; Grant, R. Temozolomide for high grade glioma. Cochrane Database Syst. Rev. 2013, 2013, 1–60. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; van den Bent, M.J.; Mason, W.P.; Weller, M.; Mirimanoff, R.O.; Cairncross, J.G. Changing paradigms—An update on the multidisciplinary management of malignant glioma. Oncologist 2006, 11, 165–180. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Vermeer, K.A.; Mo, J.; Weda, J.J.A.; Lemij, H.G.; De Boer, J.F. Depth-resolved model-based reconstruction of attenuation coefficients in optical coherence tomography. Biomed. Opt. Express 2014, 5, 322–337. [Google Scholar] [CrossRef]
- Chang, S.; Bowden, A.K. Review of methods and applications of attenuation coefficient measurements with optical coherence tomography. J. Biomed. Opt. 2019, 24, 090901. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji—An open source platform for biological image analysis. Nat. Methods 2013, 9, 1–15. [Google Scholar] [CrossRef]
- Roetzer, T.; Leskovar, K.; Peter, N.; Furtner, J.; Muck, M.; Augustin, M.; Lichtenegger, A.; Nowosielski, M.; Hainfellner, J.A.; Baumann, B.; et al. Evaluating cellularity and structural connectivity on whole brain slides using a custom-made digital pathology pipeline. J. Neurosci. Methods 2019, 311, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Deroulers, C.; Ameisen, D.; Badoual, M.; Gerin, C.; Granier, A.; Lartaud, M. Analyzing huge pathology images with open source software. Diagn. Pathol. 2013, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Phansalkar, N.; More, S.; Sabale, A.; Joshi, M. Adaptive local thresholding for detection of nuclei in diversity stained cytology images. In Proceedings of the 2011 International Conference on Communications and Signal Processing, Calicut, India, 10–12 February 2011; pp. 218–220. [Google Scholar]
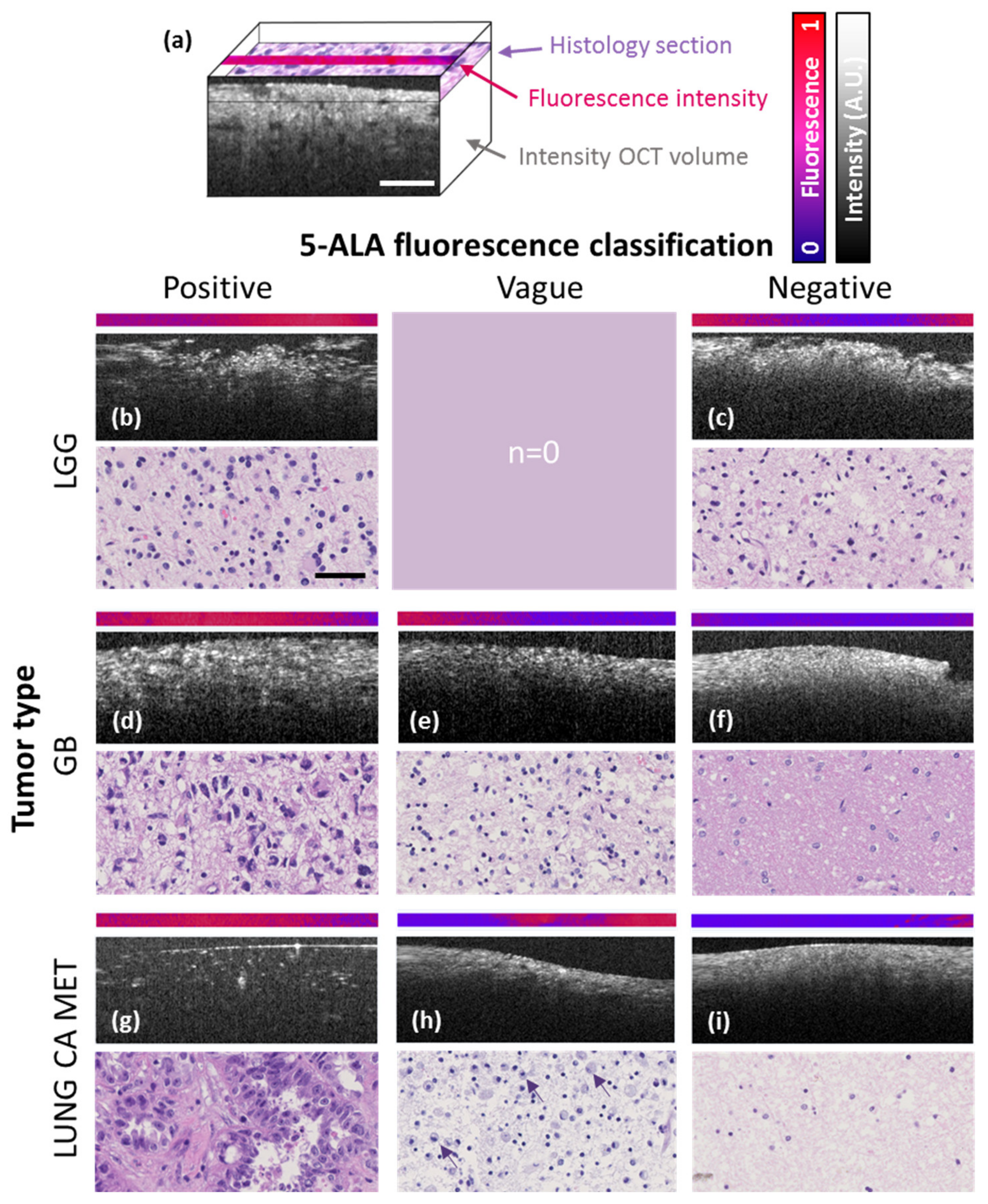
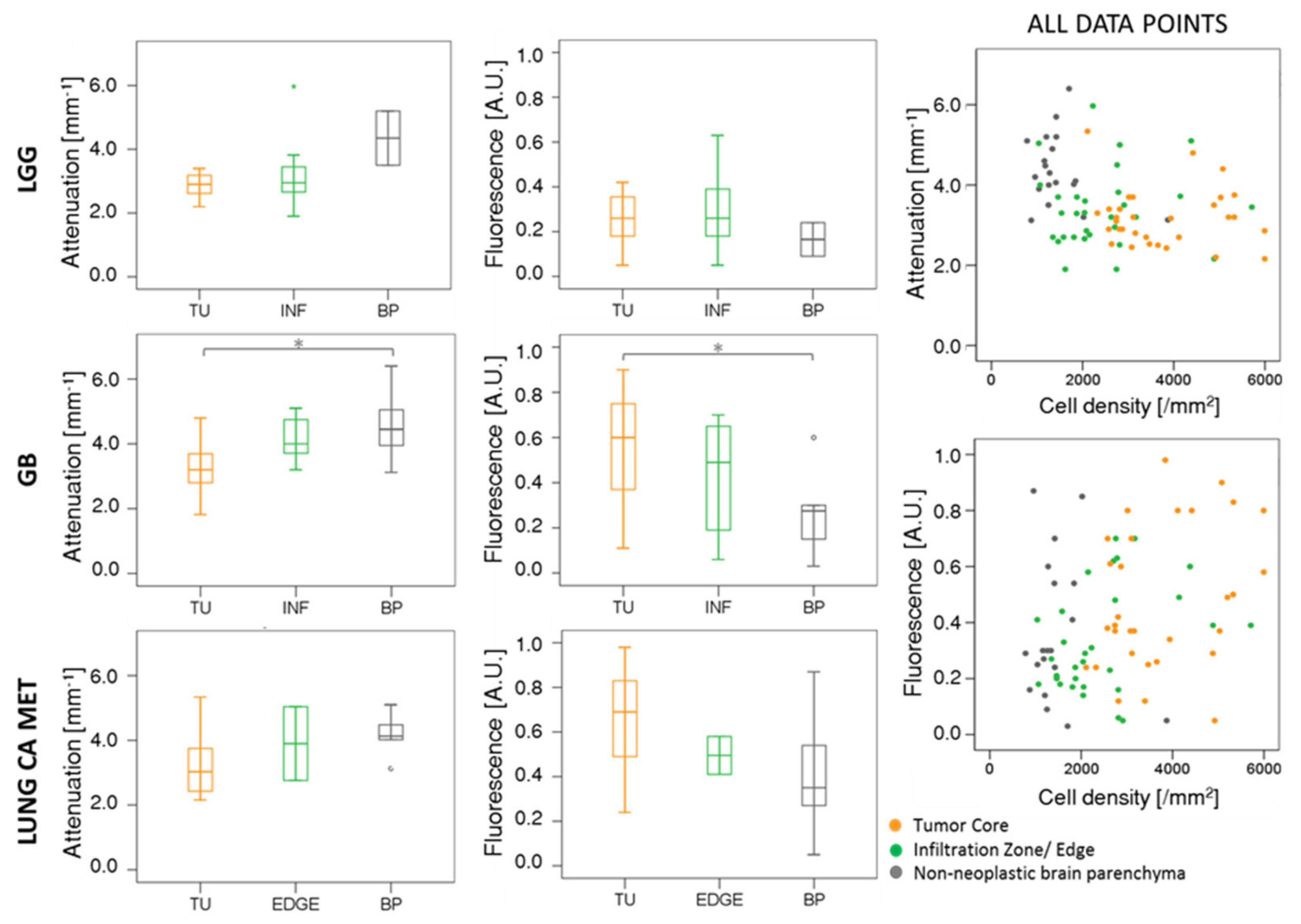
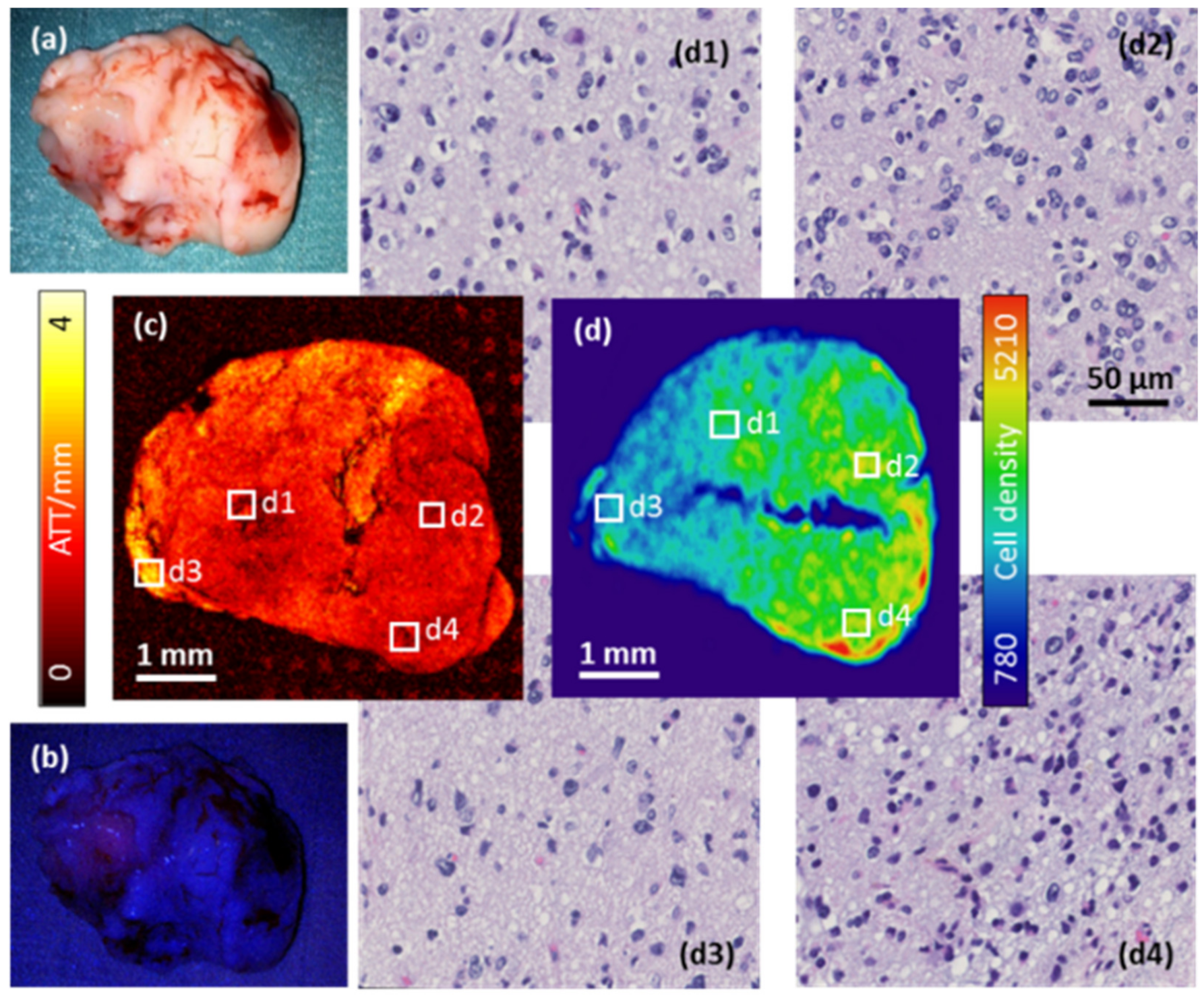
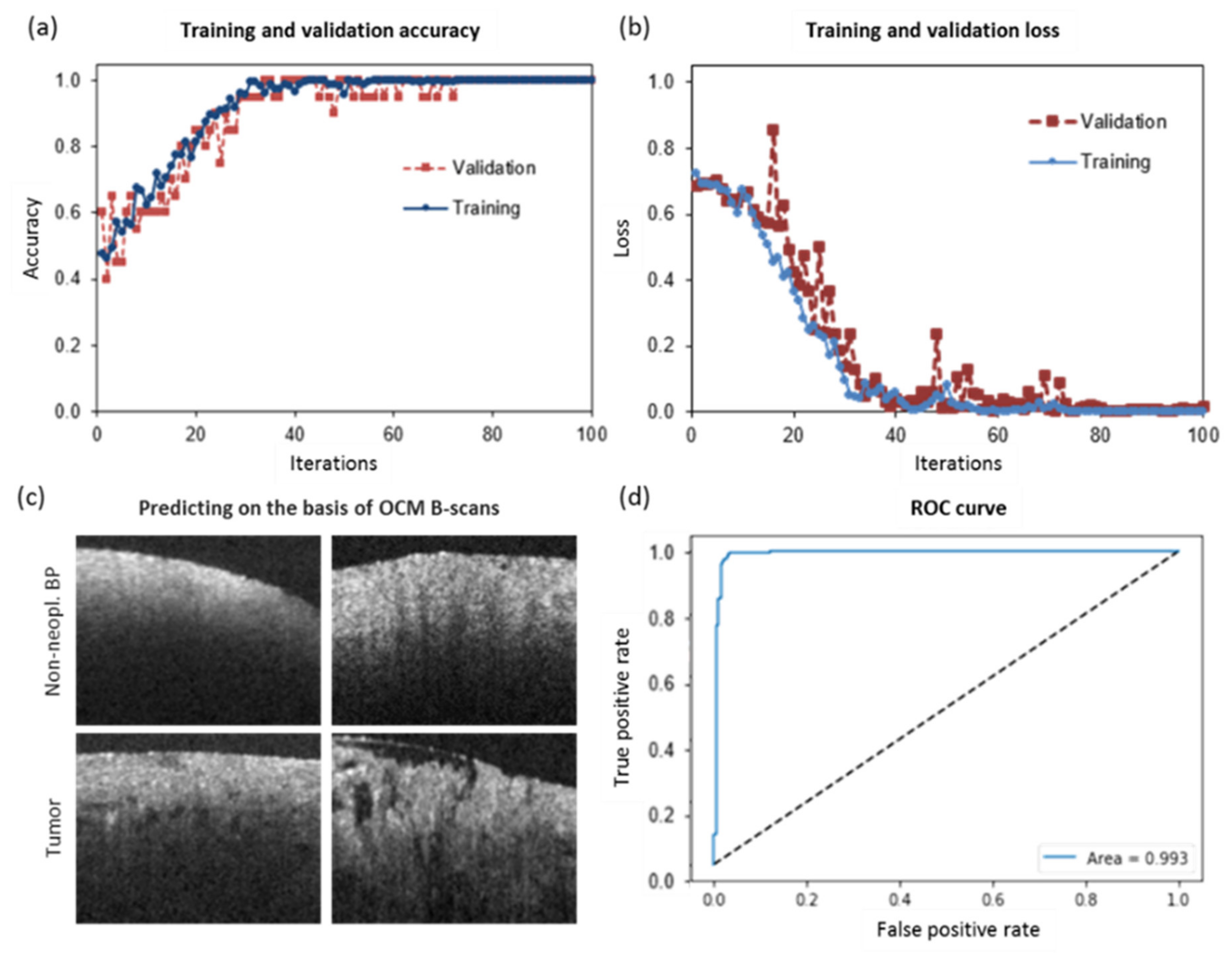
| Tumor Entity | Compact Tumor Tissue | Infiltration Zone/Edge | Non-Neoplastic Tissue Adjacent to Tumor |
|---|---|---|---|
| Lower-grade glioma | 9 patients, 11 biopsies | 13 patients, 21 biopsies | 2 patients, 2 biopsies |
| Glioblastoma | 14 patients, 15 biopsies | 4 patients, 7 biopsies | 7 patients, 8 biopsies |
| Lung cancer metastasis | 5 patients, 6 biopsies | 2 patients, 2 biopsies | 6 patients, 6 biopsies |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gesperger, J.; Lichtenegger, A.; Roetzer, T.; Salas, M.; Eugui, P.; Harper, D.J.; Merkle, C.W.; Augustin, M.; Kiesel, B.; Mercea, P.A.; et al. Improved Diagnostic Imaging of Brain Tumors by Multimodal Microscopy and Deep Learning. Cancers 2020, 12, 1806. https://doi.org/10.3390/cancers12071806
Gesperger J, Lichtenegger A, Roetzer T, Salas M, Eugui P, Harper DJ, Merkle CW, Augustin M, Kiesel B, Mercea PA, et al. Improved Diagnostic Imaging of Brain Tumors by Multimodal Microscopy and Deep Learning. Cancers. 2020; 12(7):1806. https://doi.org/10.3390/cancers12071806
Chicago/Turabian StyleGesperger, Johanna, Antonia Lichtenegger, Thomas Roetzer, Matthias Salas, Pablo Eugui, Danielle J. Harper, Conrad W. Merkle, Marco Augustin, Barbara Kiesel, Petra A. Mercea, and et al. 2020. "Improved Diagnostic Imaging of Brain Tumors by Multimodal Microscopy and Deep Learning" Cancers 12, no. 7: 1806. https://doi.org/10.3390/cancers12071806
APA StyleGesperger, J., Lichtenegger, A., Roetzer, T., Salas, M., Eugui, P., Harper, D. J., Merkle, C. W., Augustin, M., Kiesel, B., Mercea, P. A., Widhalm, G., Baumann, B., & Woehrer, A. (2020). Improved Diagnostic Imaging of Brain Tumors by Multimodal Microscopy and Deep Learning. Cancers, 12(7), 1806. https://doi.org/10.3390/cancers12071806








