Interleukin-1β and Cancer
Abstract
1. Introduction
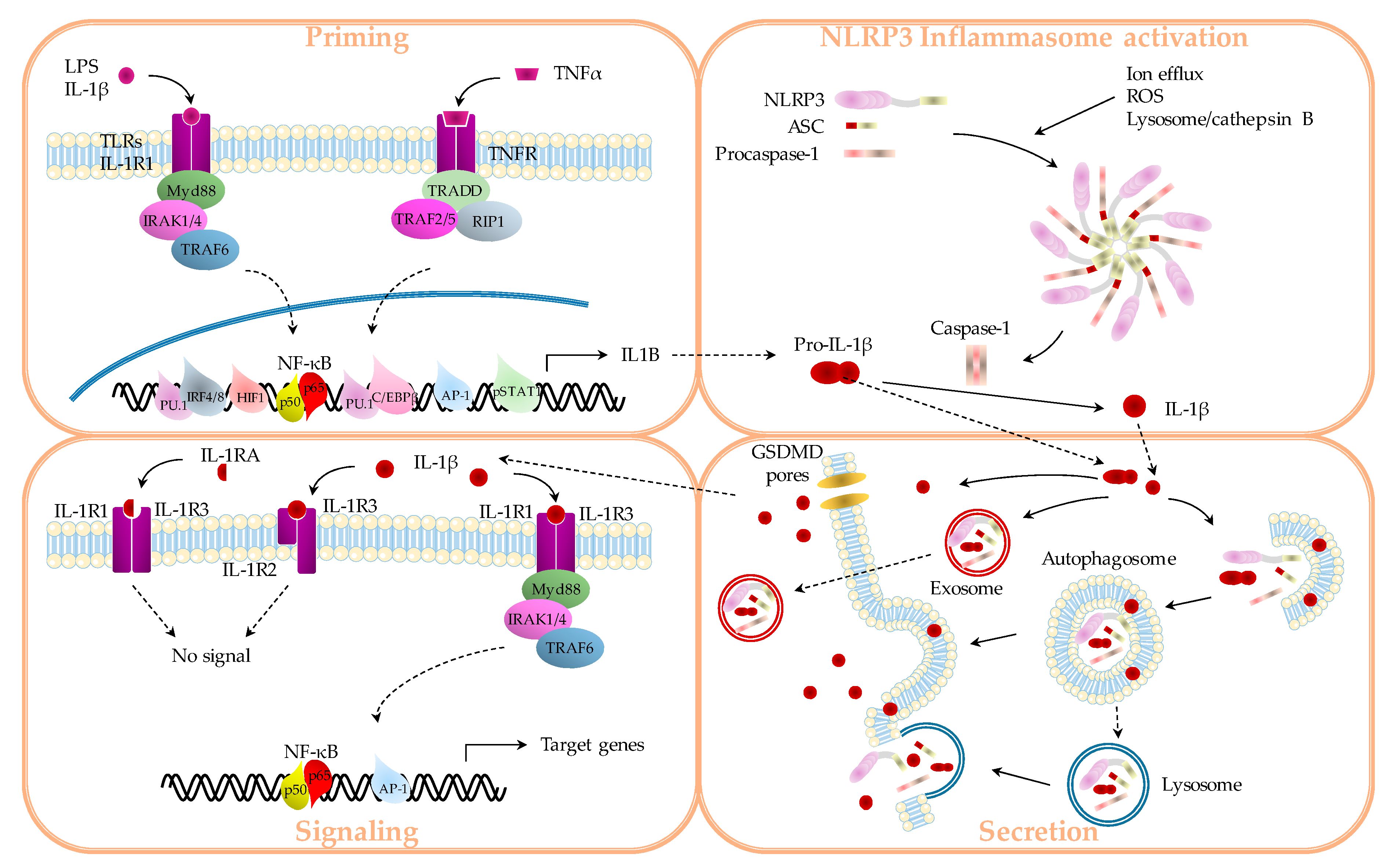
1.1. IL-1β Production
1.1.1. “Priming”
1.1.2. Inflammasomes
1.1.3. Secretion
1.2. IL-1β Signaling
2. IL-1β as a Cancer Marker?
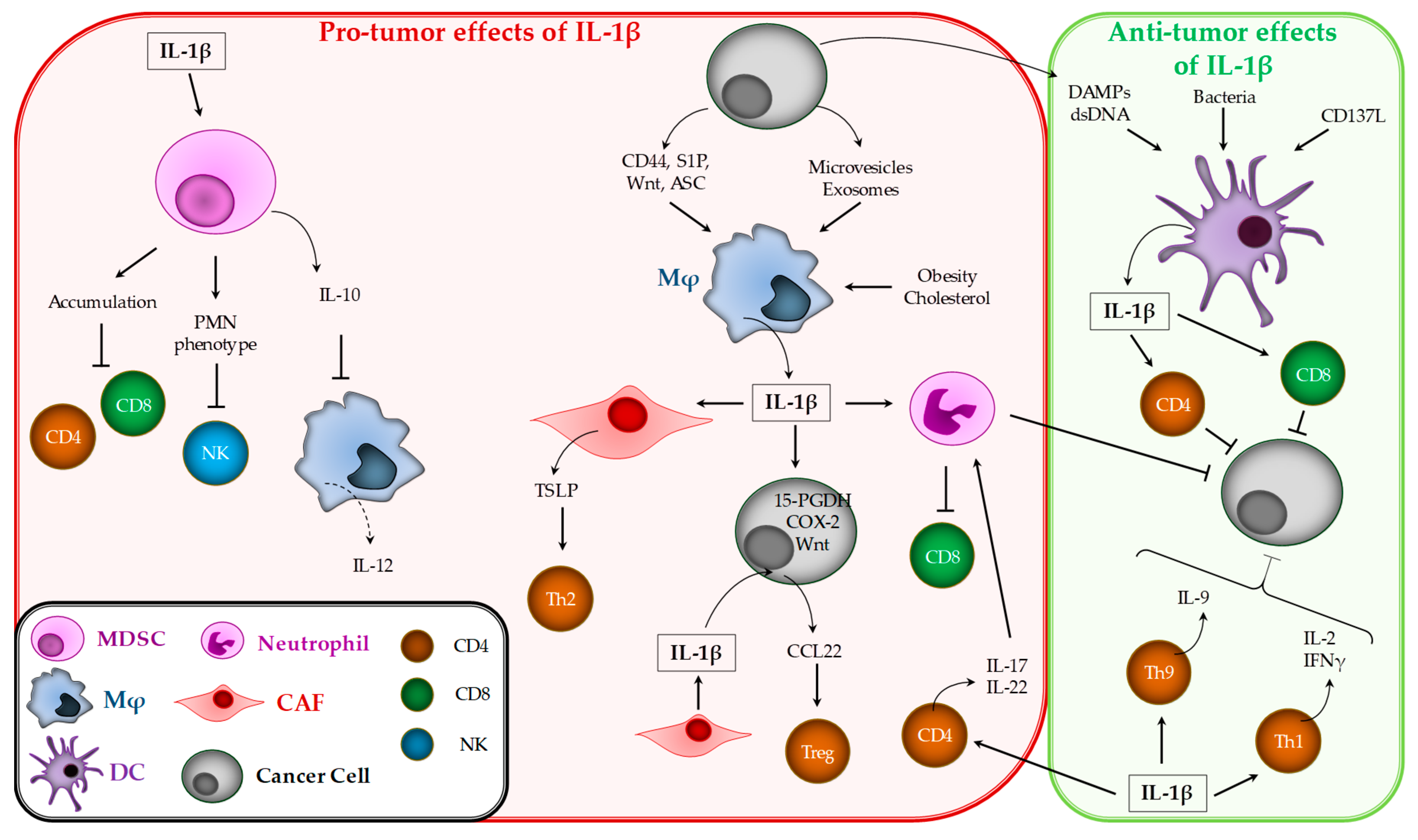
3. Pro-and Anti-Tumor Effects of IL-1β
3.1. IL-1β Effects on Cancer Occurrence
3.1.1. Skin Cancers
3.1.2. Colon Cancer
3.1.3. Lung Cancer
3.1.4. Breast Cancer
3.1.5. Gastric Cancer
3.1.6. Oral Cancers
3.1.7. Pancreatic Cancer
3.1.8. Ovarian Cancer
3.1.9. Prostate Cancer
3.1.10. Mutational Status
3.2. IL-1β Effects on Tumor Immune Response
3.2.1. Myeloid-Derived Suppressor Cells (MDSCs)
3.2.2. Macrophages
3.2.3. Dendritic Cells
3.2.4. Neutrophils
3.2.5. T Lymphocytes
3.3. Effects of IL-1β on Angiogenesis
3.4. Effects of IL-1β on Cancer Metastasis
3.5. Pro- and Anti-Tumor Effects of IL-1β during Cancer Treatment
4. Therapeutic Perspectives
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1 (IL-1) Processing Pathway. Sci. Signal. 2010, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Carmi, Y.; Voronov, E.; Apte, R.N. Interleukin-1α. Semin. Immunol. 2013, 25, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta—A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef]
- Kaler, P.; Augenlicht, L.; Klampfer, L. Macrophage-derived IL-1β stimulates Wnt signaling and growth of colon cancer cells: A crosstalk interrupted by vitamin D3. Oncogene 2009, 28, 3892–3902. [Google Scholar] [CrossRef]
- Afonina, I.S.; Müller, C.; Martin, S.J.; Beyaert, R. Proteolytic Processing of Interleukin-1 Family Cytokines: Variations on a Common Theme. Immunity 2015, 42, 991–1004. [Google Scholar] [CrossRef]
- Davis, B.K.; Wen, H.; Ting, J.P.-Y. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 2011, 29, 707–735. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Chevriaux, A.; Pilot, T.; Derangère, V.; Simonin, H.; Martine, P.; Chalmin, F.; Ghiringhelli, F.; Rébé, C. Cathepsin B Is Required for NLRP3 Inflammasome Activation in Macrophages, Through NLRP3 Interaction. Front. Cell Dev. Boil. 2020, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Sitia, R.; Rubartelli, A. The unconventional secretion of IL-1β: Handling a dangerous weapon to optimize inflammatory responses. Semin. Cell Dev. Boil. 2018, 83, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Zhang, M.; Kenny, S.J.; Ge, L.; Xu, K.; Schekman, R. Translocation of interleukin-1β into a vesicle intermediate in autophagy-mediated secretion. eLife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Franchi, L.; Nunez, G.; Dubyak, G.R. Nonclassical IL-1β Secretion Stimulated by P2X7 Receptors Is Dependent on Inflammasome Activation and Correlated with Exosome Release in Murine Macrophages. J. Immunol. 2007, 179, 1913–1925. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, M.; Stanley, A.C.; Chen, K.W.; Brown, D.; Bezbradica, J.S.; von Pein, J.B.; Holley, C.L.; Boucher, D.; Shakespear, M.; Kapetanovic, R.; et al. Interleukin-1β Maturation Triggers Its Relocation to the Plasma Membrane for Gasdermin-D-Dependent and -Independent Secretion. Cell Rep. 2018, 24, 1425–1433. [Google Scholar] [CrossRef]
- Evavold, C.L.; Ruan, J.; Tan, Y.; Xia, S.; Wu, H.; Kagan, J.C. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 2018, 48, 35–44.e6. [Google Scholar] [CrossRef]
- Sborgi, L.; Rühl, S.; Mulvihill, E.; Pipercevic, J.; Heilig, R.; Stahlberg, H.; Farady, C.J.; Müller, D.J.; Brož, P.; Hiller, S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016, 35, 1766–1778. [Google Scholar] [CrossRef]
- Lachmann, H.J.; Lowe, P.; Felix, S.D.; Rordorf, C.; Leslie, K.; Madhoo, S.; Wittkowski, H.; Bek, S.; Hartmann, N.; Bosset, S.; et al. In vivo regulation of interleukin 1β in patients with cryopyrin-associated periodic syndromes. J. Exp. Med. 2009, 206, 1029–1036. [Google Scholar] [CrossRef]
- Cullen, S.P.; Kearney, C.J.; Clancy, D.M.; Martin, S.J. Diverse Activators of the NLRP3 Inflammasome Promote IL-1β Secretion by Triggering Necrosis. Cell Rep. 2015, 11, 1535–1548. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R.; Kulkarni, O.P.; Rupanagudi, K.V.; Migliorini, A.; Darisipudi, M.N.; Vilaysane, A.; Muruve, D.; Shi, Y.; Munro, F.; Liapis, H.; et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J. Clin. Investig. 2012, 123, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Pelegrin, P.; Barroso-Gutierrez, C.; Surprenant, A. P2X7 Receptor Differentially Couples to Distinct Release Pathways for IL-1β in Mouse Macrophage. J. Immunol. 2008, 180, 7147–7157. [Google Scholar] [CrossRef]
- Garlanda, C.; Anders, H.-J.; Mantovani, A. TIR8/SIGIRR: An IL-1R/TLR family member with regulatory functions in inflammation and T cell polarization. Trends Immunol. 2009, 30, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Loiarro, M.; Ruggiero, V.; Sette, C. Targeting TLR/IL-1R Signalling in Human Diseases. Mediat. Inflamm. 2010, 1–12. [Google Scholar] [CrossRef]
- Chen, L.-C.; Wang, L.-J.; Tsang, N.-M.; Ojcius, D.M.; Chen, C.-C.; Ouyang, C.-N.; Hsueh, C.; Liang, Y.; Chang, K.-P.; Chen, C.-C.; et al. Tumour inflammasome-derived IL-1β recruits neutrophils and improves local recurrence-free survival in EBV-induced nasopharyngeal carcinoma. EMBO Mol. Med. 2012, 4, 1276–1293. [Google Scholar] [CrossRef]
- McLoed, A.G.; Sherrill, T.P.; Cheng, N.-S.; Han, W.; Saxon, J.A.; Gleaves, L.A.; Wu, P.; Polosukhin, V.V.; Karin, M.; Yull, F.E.; et al. Neutrophil-Derived IL-1β Impairs the Efficacy of NF-κB Inhibitors against Lung Cancer. Cell Rep. 2016, 16, 120–132. [Google Scholar] [CrossRef]
- Mitsunaga, S.; Ikeda, M.; Shimizu, S.; Ohno, I.; Furuse, J.; Inagaki, M.; Higashi, S.; Kato, H.; Terao, K.; Ochiai, A. Serum levels of IL-6 and IL-1β can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br. J. Cancer 2013, 108, 2063–2069. [Google Scholar] [CrossRef]
- Kim, J.-W.; Koh, Y.; Kim, N.-W.; Ahn, Y.-O.; Kim, T.M.; Han, S.-W.; Oh, -Y.; Lee, S.-H.; Im, S.-A.; Kim, T.-Y.; et al. Clinical Implications of VEGF, TGF-beta1, and IL-1beta in Patients with Advanced Non-small Cell Lung Cancer. Cancer Res. Treat. 2013, 45, 325–333. [Google Scholar] [CrossRef]
- Martínez-Reza, I.; Díaz, L.; Barrera, D.; Segovia-Mendoza, M.; Pedraza-Sánchez, S.; Soca-Chafre, G.; Larrea, F.; García-Becerra, R. Calcitriol Inhibits the Proliferation of Triple-Negative Breast Cancer Cells through a Mechanism Involving the Proinflammatory Cytokines IL-1βand TNF-α. J. Immunol. Res. 2019, 1–11. [Google Scholar] [CrossRef]
- Matamoros, J.A.; da Silva, M.I.F.; de Moura, P.M.M.F.; Leitão, M.D.C.G.; Coimbra, E.C. Reduced Expression of IL-1β and IL-18 Proinflammatory Interleukins Increases the Risk of Developing Cervical Cancer. Asian Pac. J. Cancer Prev. 2019, 20, 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, J.-K.; Jeon, H.-Y.; Ham, S.W.; Kim, H. CD133 Regulates IL-1β Signaling and Neutrophil Recruitment in Glioblastoma. Mol. Cells 2017, 40, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Braicu, I.; Mustea, A.; Toliat, M.R.; Pirvulescu, C.; Könsgen, D.; Sun, P.; Nürnberg, P.; Lichtenegger, W.; Sehouli, J. Polymorphism of IL-1α, IL-1β and IL-10 in patients with advanced ovarian cancer: Results of a prospective study with 147 patients. Gynecol. Oncol. 2007, 104, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Hefler, L.A.; Ludwig, E.; Lebrecht, A.; Zeillinger, R.; Tong-Cacsire, D.; Koelbl, H.; Leodolter, S.; Tempfer, C.B. Polymorphisms of the interleukin-1 gene cluster and ovarian cancer. J. Soc. Gynecol. Investig. 2002, 9, 386–390. [Google Scholar] [CrossRef]
- Ben-Ahmed, A.; Zidi, S.; Sghaier, I.; Ghazouani, E.; Mezlini, A.; Almawi, W.; Loueslati, B.Y. Common variants in IL-1RN, IL-1β and TNF-α and the risk of ovarian cancer: A case control study. Central Eur. J. Immunol. 2017, 42, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Zienolddiny, S.; Ryberg, D.; Ms, V.M.; Skaug, V.; Canzian, F.; Haugen, A. Polymorphisms of the interleukin-1? gene are associated with increased risk of non-small cell lung cancer. Int. J. Cancer 2004, 109, 353–356. [Google Scholar] [CrossRef]
- Bhat, I.A.; Naykoo, N.A.; Qasim, I.; Ganie, F.A.; Yousuf, Q.; Bhat, B.A.; Rasool, R.; Aziz, S.; Shah, Z.A. Association of interleukin 1 beta (IL-1β) polymorphism with mRNA expression and risk of non small cell lung cancer. Meta Gene 2014, 2, 123–133. [Google Scholar] [CrossRef] [PubMed]
- El-Omar, E.M.; Carrington, M.; Chow, W.-H.; McColl, K.E.L.; Bream, J.H.; Young, H.A.; Herrera, J.; Lissowska, J.; Yuan, C.-C.; Rothman, N.; et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 2000, 404, 398–402. [Google Scholar] [CrossRef]
- Machado, J.C.; Pharoah, P.; Sousa, S.; Carvalho, R.; Oliveira, C.; Figueiredo, C.; Amorim, A.; Seruca, R.; Caldas, C.; Carneiro, F.; et al. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology 2001, 121, 823–829. [Google Scholar] [CrossRef]
- Zeng, Z.-R.; Hu, P.-J.; Pang, R.-P.; Chen, M.-H.; Ng, M.; Sung, J.J.Y. Association of interleukin 1B gene polymorphism and gastric cancers in high and low prevalence regions in China. Gut 2003, 52, 1684–1689. [Google Scholar] [CrossRef]
- Furuta, T.; El-Omar, E.; Xiao, F.; Shirai, N.; Takashima, M.; Sugimurra, H. Interleukin 1β polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology 2002, 123, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.C. Interleukin-1 and Interleukin-1 Receptor Antagonist Gene Polymorphisms and Gastric Cancer: A Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1674–1687. [Google Scholar] [CrossRef] [PubMed]
- Drici, A.E.-M.; Moulessehoul, S.; Tifrit, A.; Diaf, M.; Turki, D.K.; Bachir, M.; Tou, A. Effect of IL-1β and IL-1RN polymorphisms in carcinogenesis of the gastric mucosa in patients infected with Helicobacter pylori in Algeria. Libyan J. Med. 2016, 11, 31576. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Luo, M.-X.; Zhou, X.; Lv, Y.; Su, G. Correlation Between Interleukin-1β-511 C/T Polymorphism and Gastric Cancer in Chinese Populations: A Meta-Analysis. Med Sci. Monit. 2016, 22, 1742–1750. [Google Scholar] [CrossRef]
- He, B.-S.; Pan, Y.-Q.; Xu, Y.-F.; Zhu, C.; Qu, L.-L.; Wang, S. Polymorphisms in Interleukin-1B (IL-1B) and Interleukin 1 Receptor Antagonist (IL-1RN) Genes Associate with Gastric Cancer Risk in the Chinese Population. Dig. Dis. Sci. 2011, 56, 2017–2023. [Google Scholar] [CrossRef]
- Chang, Y.-W.; Jang, J.; Kim, N.-H.; Lee, J.W.; Lee, H.J.; Jung, W.W.; Dong, S.-H.; Kim, H.-J.; Kim, B.-H.; Lee, J.-I.; et al. Interleukin-1B (IL-1B) polymorphisms and gastric mucosal levels of IL-1? Cytokine in Korean patients with gastric cancer. Int. J. Cancer 2005, 114, 465–471. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, G.G. A meta-analysis of the association between CTLA-4 +49 A/G, −318 C/T, and IL-1 polymorphisms and susceptibility to cervical cancer. Neoplasma 2014, 61, 481–490. [Google Scholar] [CrossRef]
- Qian, N.; Chen, X.; Han, S.; Qiang, F.; Jin, G.; Zhou, X.; Dong, J.; Wang, X.; Shen, H.; Hu, Z. Circulating IL-1β levels, polymorphisms of IL-1B, and risk of cervical cancer in Chinese women. J. Cancer Res. Clin. Oncol. 2009, 136, 709–716. [Google Scholar] [CrossRef]
- Wang, H.; Hua, M.; Wang, S.; Yu, J.; Chen, C.; Zhao, X.; Zhang, C.; Zhong, C.; Wang, R.; He, N.; et al. Genetic polymorphisms of IL-18 rs1946518 and IL-1β rs16944 are associated with prognosis and survival of acute myeloid leukemia. Inflamm. Res. 2016, 66, 249–258. [Google Scholar] [CrossRef]
- Zhang, A.; Yu, J.; Yan, S.; Zhao, X.; Chen, C.; Zhou, Y.; Zhao, X.; Hua, M.; Wang, R.; Zhang, C.; et al. The genetic polymorphism and expression profiles of NLRP3 inflammasome in patients with chronic myeloid leukemia. Hum. Immunol. 2018, 79, 57–62. [Google Scholar] [CrossRef]
- Akisik, E.; Dalay, N. Functional polymorphism of thymidylate synthase, but not of theCOMT andIL-1B genes, is associated with breast cancer. J. Clin. Lab. Anal. 2007, 21, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Eras, N.; Daloglu, F.T.; Çolak, T.; Guler, M.; Akbas, E. The Correlation between IL-1β-C31T Gene Polymorphism and Susceptibility to Breast Cancer. J. Breast Cancer 2019, 22, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Tak, K.H.; Yu, G.I.; Lee, M.-Y.; Shin, D. Association Between Polymorphisms of Interleukin 1 Family Genes and Hepatocellular Carcinoma. Med. Sci. Monit. 2018, 24, 3488–3495. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liang, X.; Meng, C.; Shao, Z.; Gao, Y.; Wu, Q.; Liu, J.; Wang, H.; Yang, S. Genetic polymorphisms of interleukin-1 beta and osteosarcoma risk. Int. Orthop. 2014, 38, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Wang, S.; Zhang, Y.; Wu, D.; Zhang, C.; Gao, Y.; Liu, X.; Wang, W.; Zhang, S. IL1 genes polymorphism and the risk of renal cell carcinoma in Chinese Han population. Oncotarget 2017, 8, 56021–56029. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-F.; Lu, M.S.; Chen, P.-T.; Chen, W.-C.; Lin, P.-Y.; Lee, K.-D. Role of interleukin 1 beta in esophageal squamous cell carcinoma. J. Mol. Med. 2011, 90, 89–100. [Google Scholar] [CrossRef]
- Kai, H.; Kitadai, Y.; Kodama, M.; Cho, S.; Kuroda, T.; Ito, M.; Tanaka, S.; Ohmoto, Y.; Chayama, K. Involvement of proinflammatory cytokines IL-1beta and IL-6 in progression of human gastric carcinoma. Anticancer. Res. 2005, 25, 709–713. [Google Scholar]
- Deans, D.A.C.; Wigmore, S.J.; Gilmour, H.; Paterson-Brown, S.; Ross, J.A.; Fearon, K.C.H. Elevated tumour interleukin-1β is associated with systemic inflammation: A marker of reduced survival in gastro-oesophageal cancer. Br. J. Cancer 2006, 95, 1568–1575. [Google Scholar] [CrossRef]
- Al-Tahhan, M.A.; Etewa, R.L.; el Behery, M.M. Association between circulating interleukin-1 beta (IL-1β) levels and IL-1β C–511T polymorphism with cervical cancer risk in Egyptian women. Mol. Cell. Biochem. 2011, 353, 159–165. [Google Scholar] [CrossRef]
- El-Omar, E.; Carrington, M.; Chow, W.-H.; McColl, K.E.L.; Bream, J.H.; Young, H.A.; Herrera, J.; Lissowska, J.; Yuan, C.-C.; Rothman, N.; et al. Correction: The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature 2001, 412, 99. [Google Scholar] [CrossRef]
- Fox, J.G.; Wang, T.C. Inflammation, atrophy, and gastric cancer. J. Clin. Investig. 2007, 117, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Lind, H.; Haugen, A.; Zienolddiny, S. Differential binding of proteins to the IL1B −31 T/C polymorphism in lung epithelial cells. Cytokine 2007, 38, 43–48. [Google Scholar] [CrossRef]
- Grimm, C.; Kantelhardt, E.J.; Heinze, G.; Polterauer, S.; Zeillinger, R.; Kölbl, H.; Reinthaller, A.; Hefler, L. The prognostic value of four interleukin-1 gene polymorphisms in caucasian women with breast cancer—A multicenter study. BMC Cancer 2009, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-A.; Ki, C.-S.; Kim, H.-J.; Sohn, K.-M.; Kim, J.-W.; Kang, W.K.; Rhee, J.C.; Song, S.Y.; Sohn, T.S. Novel interleukin 1β polymorphism increased the risk of gastric cancer in a Korean population. J. Gastroenterol. 2004, 39, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.L.; Mamaï, O.; Sborgi, L.; Boussofara, L.; Hopkins, R.; Robinson, K.; Szeverényi, I.; Takeichi, T.; Balaji, R.; Lau, A.; et al. Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation. Cell 2016, 167, 187–202. [Google Scholar] [CrossRef]
- Okamoto, M.; Liu, W.; Luo, Y.; Tanaka, A.; Cai, X.; Norris, D.A.; Dinarello, C.A.; Fujita, M. Constitutively Active Inflammasome in Human Melanoma Cells Mediating Autoinflammation via Caspase-1 Processing and Secretion of Interleukin-1β. J. Boil. Chem. 2009, 285, 6477–6488. [Google Scholar] [CrossRef]
- Voronov, E.; Shouval, D.S.; Krelin, Y.; Cagnano, E.; Benharroch, D.; Iwakura, Y.; Dinarello, C.A.; Apte, R.N. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 2645–2650. [Google Scholar] [CrossRef]
- Krelin, Y.; Voronov, E.; Dotan, S.; Elkabets, M.; Reich, E.; Fogel, M.; Huszar, M.; Iwakura, Y.; Segal, S.; Dinarello, C.A.; et al. Interleukin-1β–Driven Inflammation Promotes the Development and Invasiveness of Chemical Carcinogen–Induced Tumors. Cancer Res. 2007, 67, 1062–1071. [Google Scholar] [CrossRef]
- Chien, C.-H.; Lee, M.-J.; Liou, H.-C.; Liou, H.-H.; Fu, W.-M. Local Immunosuppressive Microenvironment Enhances Migration of Melanoma Cells to Lungs in DJ-1 Knockout Mice. PLoS ONE 2015, 10, e0115827. [Google Scholar] [CrossRef]
- Dmitrieva-Posocco, O.; Dzutsev, A.; Posocco, D.F.; Hou, V.; Yuan, W.; Thovarai, V.; Mufazalov, I.A.; Gunzer, M.; Shilovskiy, I.; Khaitov, M.R.; et al. Cell-Type-Specific Responses to Interleukin-1 Control Microbial Invasion and Tumor-Elicited Inflammation in Colorectal Cancer. Immunity 2019, 50, 166–180.e7. [Google Scholar] [CrossRef]
- Allen, I.C.; TeKippe, E.M.; Woodford, R.-M.T.; Uronis, J.M.; Holl, E.K.; Rogers, A.B.; Herfarth, H.H.; Jobin, C.; Ting, J.P.-Y. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 2010, 207, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Dupaul-Chicoine, J.; Yeretssian, G.; Doiron, K.; Bergstrom, K.S.; McIntire, C.R.; Leblanc, P.M.; Meunier, C.; Turbide, C.; Gros, P.; Beauchemin, N.; et al. Control of Intestinal Homeostasis, Colitis, and Colitis-Associated Colorectal Cancer by the Inflammatory Caspases. Immunity 2010, 32, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Zaki, H.; Boyd, K.L.; Vogel, P.; Kastan, M.B.; Lamkanfi, M.; Kanneganti, T.-D. The NLRP3 Inflammasome Protects against Loss of Epithelial Integrity and Mortality during Experimental Colitis. Immunity 2010, 32, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Ping, P.H.; Bo, T.F.; Li, L.; Hui, Y.N.; Zhu, H. IL-1β/NF-kb signaling promotes colorectal cancer cell growth through miR-181a/PTEN axis. Arch. Biochem. Biophys. 2016, 604, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Matanic, D.; Beg-Zec, Z.; Stojanović, D.; Matakorić, N.; Flego, V.; Milevoj-Ribic, F. Cytokines in Patients with Lung Cancer. Scand. J. Immunol. 2003, 57, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.-F.; Wu, J.; Xu, S.; Xu, Y.-Y.; Li, D.; Lou, J.; Liu, M.-F. IL-1β-Mediated Repression of microRNA-101 Is Crucial for Inflammation-Promoted Lung Tumorigenesis. Cancer Res. 2014, 74, 4720–4730. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Yuan, R.Q.; Fuchs, A.; Yao, Y.; Joseph, A.; Schwall, R.; Schnitt, S.J.; Guida, A.; Hastings, H.M.; Andres, J.; et al. Expression of interleukin-1beta in human breast carcinoma. Cancer 1997, 80, 421–434. [Google Scholar] [CrossRef]
- Wu, T.; Hong, Y.; Jia, L.; Wu, J.; Xia, J.; Wang, J.; Hu, Q.; Cheng, B. Modulation of IL-1β reprogrammes the tumor microenvironment to interrupt oral carcinogenesis. Sci. Rep. 2016, 6, 20208. [Google Scholar] [CrossRef]
- Snoussi, K.; Strosberg, A.D.; Bouaouina, N.; Ben-Ahmed, S.; Chouchane, L. Genetic variation in pro-inflammatory cytokines (interleukin-1beta, interleukin-1alpha and interleukin-6) associated with the aggressive forms, survival, and relapse prediction of breast carcinoma. Eur. Cytokine Netw. 2005, 16, 253–260. [Google Scholar]
- Oh, K.; Lee, O.-Y.; Park, Y.; Seo, M.W.; Lee, D.-S. IL-1β induces IL-6 production and increases invasiveness and estrogen-independent growth in a TG2-dependent manner in human breast cancer cells. BMC Cancer 2016, 16, 724. [Google Scholar] [CrossRef]
- Reed, J.R.; Leon, R.P.; Hall, M.K.; Schwertfeger, K.L. Interleukin-1beta and fibroblast growth factor receptor 1 cooperate to induce cyclooxygenase-2 during early mammary tumourigenesis. Breast Cancer Res. 2009, 11, R21. [Google Scholar] [CrossRef] [PubMed]
- Kaplanov, I.; Carmi, Y.; Kornetsky, R.; Shemesh, A.; Shurin, G.V.; Shurin, M.R.; Dinarello, C.A.; Voronov, E.; Apte, R.N. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti–PD-1 for tumor abrogation. Proc. Natl. Acad. Sci. USA 2018, 116, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Várady, C.B.S.; Lourenço, A.L.; Mizurini, D.M.; Rondon, A.M.R.; Leal, A.C.; Gonçalves, B.S.; Bou-Habib, D.C.; Medei, E.; Monteiro, R.Q. IL-1β Blockade Attenuates Thrombosis in a Neutrophil Extracellular Trap-Dependent Breast Cancer Model. Front. Immunol. 2019, 10, 2088. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Bhagat, G.; Cui, G.; Takaishi, S.; Kurt-Jones, E.A.; Rickman, B.; Betz, K.S.; Penz-Oesterreicher, M.; Bjorkdahl, O.; Fox, J.G.; et al. Overexpression of Interleukin-1β Induces Gastric Inflammation and Cancer and Mobilizes Myeloid-Derived Suppressor Cells in Mice. Cancer Cell 2008, 14, 408–419. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, L.; Liang, X.; Li, S.; Ma, L.; Zheng, L.; Li, T.; Yu, H.; Chan, H.; Chen, C.; et al. Helicobacter pylori-induced YAP1 nuclear translocation promotes gastric carcinogenesis by enhancing IL-1β expression. Cancer Med. 2019, 8, 3965–3980. [Google Scholar] [CrossRef]
- Yamanaka, N.; Morisaki, T.; Nakashima, H.; Tasaki, A.; Kubo, M.; Kuga, H.; Nakahara, C.; Nakamura, K.; Noshiro, H.; Yao, T.; et al. Interleukin 1 Enhances Invasive Ability of Gastric Carcinoma through Nuclear Factor- B Activation. Clin. Cancer Res. 2004, 10, 1853–1859. [Google Scholar] [CrossRef]
- Guo, T.; Qian, J.; Zhao, Y.-Q.; Li, X.-B.; Zhang, J.-Z. Effects of IL-1? on the proliferation and apoptosis of gastric epithelial cells and acid secretion from isolated rabbit parietal cells. Mol. Med. Rep. 2012, 7, 299–305. [Google Scholar] [CrossRef]
- Brailo, V.; Vucicevic-Boras, V.; Lukac, J.; Biocina-Lukenda, D.; Alajbeg, I.; Milenovic, A.; Balija, M. Salivary and serum interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in patients with leukoplakia and oral cancer. Med. Oral Patol. Oral Cir. Bucal 2011, 17, e10–e15. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chang, J.S.-M.; Syu, S.-H.; Wong, T.S.; Chan, J.Y.-W.; Tang, Y.-C.; Yang, Z.-P.; Yang, W.-C.; Chen, C.-T.; Lu, S.-C.; et al. IL-1β Promotes Malignant Transformation and Tumor Aggressiveness in Oral Cancer. J. Cell. Physiol. 2014, 230, 875–884. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Jiang, H.; Li, Q.; Wang-Gillam, A.; Yu, J.; Head, R.; Liu, J.; Ruzinova, M.B.; Lim, K.-H. Tumor–Stroma IL1β-IRAK4 Feedforward Circuitry Drives Tumor Fibrosis, Chemoresistance, and Poor Prognosis in Pancreatic Cancer. Cancer Res. 2018, 78, 1700–1712. [Google Scholar] [CrossRef]
- Marrache, F.; Tu, S.P.; Bhagat, G.; Pendyala, S.; Österreicher, C.H.; Gordon, S.; Ramanathan, V.; Penz-Österreicher, M.; Betz, K.S.; Song, Z.; et al. Overexpression of Interleukin-1β in the Murine Pancreas Results in Chronic Pancreatitis. Gastroenterology 2008, 135, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Woolery, K.T.; Hoffman, M.S.; Kraft, J.; Nicosia, S.V.; Kumar, A.; Kruk, P.A. Urinary interleukin-1β levels among gynecological patients. J. Ovarian Res. 2014, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Stadlmann, S.; Pollheimer, J.; Moser, P.; Raggi, A.; Amberger, A.; Margreiter, R.; Offner, F.; Mikuz, G.; Dirnhofer, S.; Moch, H. Cytokine-regulated expression of collagenase-2 (MMP-8) is involved in the progression of ovarian cancer. Eur. J. Cancer 2003, 39, 2499–2505. [Google Scholar] [CrossRef] [PubMed]
- Eiro, N.; Bermudez-Fernandez, S.; Fernández-García, B.; Atienza, S.; Beridze, N.; Escaf, S.; Vizoso, F.J. Analysis of the Expression of Interleukins, Interferon β, and Nuclear Factor-κ B in Prostate Cancer and their Relationship With Biochemical Recurrence. J. Immunother. 2014, 37, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Berriguete, G.; Sanchez-Espiridion, B.; Cansino, J.R.; Olmedilla, G.; Martínez-Onsurbe, P.; Sanchez-Chapado, M.; Paniagua, R.; Fraile, B.; Royuela, M. Clinical significance of both tumor and stromal expression of components of the IL-1 and TNF-α signaling pathways in prostate cancer. Cytokine 2013, 64, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Hobisch, A.; Herold, M.; Hittmair, A.; Thurnher, M.; Eder, I.; Cronauer, M.; Rieser, C.; Ramoner, R.; Bartsch, G.; et al. Interleukin 1β mediates the modulatory effects of monocytes on LNCaP human prostate cancer cells. Br. J. Cancer 1998, 78, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Doroszewicz, J.; Gillen, S.; Gomes, I.; Wilhelm, B.; Stief, T.; Aumüller, G. Proliferation of prostate cancer cells and activity of neutral endopeptidase is regulated by bombesin and IL-1β with IL-1β acting as a modulator of cellular differentiation. Prostate 2003, 58, 82–94. [Google Scholar] [CrossRef]
- Kawada, M.; Inoue, H.; Usami, I.; Takamoto, K.; Masuda, T.; Yamazaki, Y.; Ikeda, D. Establishment of a highly tumorigenic LNCaP cell line having inflammatory cytokine resistance. Cancer Lett. 2006, 242, 46–52. [Google Scholar] [CrossRef]
- Kawada, M.; Ishizuka, M.; Takeuchi, T. Enhancement of Antiproliferative Effects of Interleukin-1β and Tumor Necrosis Factor-α on Human Prostate Cancer LNCaP Cells by Coculture with Normal Fibroblasts through Secreted Interleukin-6. Jpn. J. Cancer Res. 1999, 90, 546–554. [Google Scholar] [CrossRef]
- Longoni, N.; Sarti, M.; Albino, D.; Civenni, G.; Malek, A.; Pinton, S.; Mello-Grand, M.; Ostano, P.; D’Ambrosio, G.; Sessa, F.; et al. ETS Transcription Factor ESE1/ELF3 Orchestrates a Positive Feedback Loop That Constitutively Activates NF-?B and Drives Prostate Cancer Progression. Cancer Res. 2013, 73, 4533–4547. [Google Scholar] [CrossRef]
- le Brun, G.; Aubin, P.; Soliman, H.; Ropiquet, F.; Villette, J.-M.; Berthon, P.; Créminon, C.; Cussenot, O.; Fiet, J. Upregulation of endothelin 1 and its precursor by IL-1beta, TNF-alpha, and TGF-beta in the PC3 human prostate cancer cell line. Cytokine 1999, 11, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.D.; Borchers, A.H.; Sundareshan, P.; Bougelet, C.; Berkman, M.R.; Nagle, R.B.; Bowden, G. Interleukin-1β Secreted from Monocytic Cells Induces the Expression of Matrilysin in the Prostatic Cell Line LNCaP. J. Boil. Chem. 1997, 272, 14188–14192. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Jardin, S.E.; Kanchwala, M.; Jacob, J.; Merchant, S.; Meade, R.; Gahnim, N.M.; Nawas, A.F.; Xing, C.; Delk, N.A. Identification of an IL-1-induced gene expression pattern in AR+PCa cells that mimics the molecular phenotype of AR−PCa cells. Prostate 2018, 78, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Patel, V.; Gwede, M.; Morgado, M.; Tomasevich, K.; Fong, E.; Farach-Carson, M.C.; Delk, N.A. IL-1β induces p62/SQSTM1 and represses androgen receptor expression in prostate cancer cells. J. Cell. Biochem. 2014, 115, 2188–2197. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, K.; Shen, F.; Worrede, A.; Liu, Q.; Gong, Y.; Garcia, F.U.; Fatatis, A. Cooperation among heterogeneous prostate cancer cells in the bone metastatic niche. Oncogene 2016, 36, 2846–2856. [Google Scholar] [CrossRef] [PubMed]
- Salman, H.; Bergman, M.; Blumberger, N.; Djaldetti, M.; Bessler, H. Do androgen deprivation drugs affect the immune crosstalk between mononuclear and prostate cancer cells? Biomed. Pharmacother. 2014, 68, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Beaupre, D.M.; Talpaz, M.; Marini, F.C.; Cristiano, R.J.; A Roth, J.; Estrov, Z.; Albitar, M.; Freedman, M.H.; Kurzrock, R. Autocrine interleukin-1beta production in leukemia: Evidence for the involvement of mutated RAS. Cancer Res. 1999, 59, 2971–2980. [Google Scholar]
- Hamarsheh, S.; Osswald, L.; Saller, B.S.; Unger, S.; de Feo, D.; Vinnakota, J.M.; Konantz, M.; Uhl, F.M.; Becker, H.; Lübbert, M.; et al. Oncogenic KrasG12D causes myeloproliferation via NLRP3 inflammasome activation. Nat. Commun. 2020, 11, 1659. [Google Scholar] [CrossRef]
- Takahashi, R.; Macchini, M.; Sunagawa, M.; Jiang, Z.; Tanaka, T.; Valenti, G.; Renz, B.W.; White, R.A.; Hayakawa, Y.; Westphalen, C.B.; et al. Interleukin-1beta-induced pancreatitis promotes pancreatic ductal adenocarcinoma via B lymphocyte-mediated immune suppression. Gut 2020. [Google Scholar] [CrossRef]
- Marazioti, A.; Lilis, I.; Vreka, M.; Apostolopoulou, H.; Kalogeropoulou, A.; Giopanou, I.; Giotopoulou, G.A.; Krontira, A.C.; Iliopoulou, M.; Kanellakis, N.I.; et al. Myeloid-derived interleukin-1β drives oncogenic KRAS-NF-κΒ addiction in malignant pleural effusion. Nat. Commun. 2018, 9, 672. [Google Scholar] [CrossRef]
- Khalili, J.S.; Liu, S.; Rodríguez-Cruz, T.G.; Whittington, M.; Wardell, S.; Liu, C.; Zhang, M.; Cooper, Z.A.; Frederick, D.T.; Li, Y.; et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin. Cancer Res. 2012, 18, 5329–5340. [Google Scholar] [CrossRef] [PubMed]
- Whipple, C.A.; E Brinckerhoff, C. BRAFV600E melanoma cells secrete factors that activate stromal fibroblasts and enhance tumourigenicity. Br. J. Cancer 2014, 111, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, Z.; Bai, X. BRAF V600E and RET/PTC Promote the Activity of Nuclear Factor-κB, Inflammatory Mediators, and Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Study of 50 Patients in Inner Mongolia. Med Sci. Monit. 2018, 24, 6795–6808. [Google Scholar] [CrossRef] [PubMed]
- Hajek, E.; Krebs, F.; Bent, R.; Haas, K.; Bast, A.; Steinmetz, I.; Tuettenberg, A.; Grabbe, S.; Bros, M. BRAF inhibitors stimulate inflammasome activation and interleukin 1 beta production in dendritic cells. Oncotarget 2018, 9, 28294–28308. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lan, X.; Wang, T.; Lu, H.; Cao, M.; Yan, S.; Cui, Y.; Jia, D.; Cai, L.; Xing, Y. Targeting the IL-1β/EHD1/TUBB3 axis overcomes resistance to EGFR-TKI in NSCLC. Oncogene 2019, 39, 1739–1755. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, J.; Wang, Z.-M.; Gu, X.; Fan, Y.; Zhang, W.; Xu, L.; Zhang, J.; Cai, D. NF-kappaB-dependent MicroRNA-425 upregulation promotes gastric cancer cell growth by targeting PTEN upon IL-1β induction. Mol. Cancer 2014, 13, 40. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Chen, S.; Zang, X.; Miao, J. Interleukin-1β/nuclear factor-κB signaling promotes osteosarcoma cell growth through the microRNA-181b/phosphatase and tensin homolog axis. J. Cell. Biochem. 2018, 120, 1763–1772. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, H.; Hao, Y.; Lin, H.; Dong, M.; Ye, J.; Song, L.; Wang, Y.; Li, Q.; Shan, B.; et al. Myeloid PTEN promotes chemotherapy-induced NLRP3-inflammasome activation and antitumour immunity. Nature 2020, 22, 716–727. [Google Scholar] [CrossRef]
- Schauer, I.G.; Zhang, J.; Xing, Z.; Guo, X.; Mercado-Uribe, I.; Sood, A.K.; Huang, P.; Liu, J. Interleukin-1β Promotes Ovarian Tumorigenesis through a p53/NF-κB-Mediated Inflammatory Response in Stromal Fibroblasts. Neoplasia 2013, 15, 409–420. [Google Scholar] [CrossRef]
- Qin, Y.; Ekmekcioglu, S.; Liu, P.; Duncan, L.M.; Lizée, G.; Poindexter, N.; Grimm, E.A. Constitutive aberrant endogenous interleukin-1 facilitates inflammation and growth in human melanoma. Mol. Cancer Res. 2011, 9, 1537–1550. [Google Scholar] [CrossRef]
- Ubertini, V.; Norelli, G.; D’Arcangelo, D.; Gurtner, A.; Cesareo, E.; Baldari, S.; Gentileschi, M.P.; Piaggio, G.; Nisticò, P.; Soddu, S.; et al. Mutant p53 gains new function in promoting inflammatory signals by repression of the secreted interleukin-1 receptor antagonist. Oncogene 2014, 34, 2493–2504. [Google Scholar] [CrossRef] [PubMed]
- Wellenstein, M.D.; Coffelt, S.B.; Duits, D.E.M.; van Miltenburg, M.H.; Slagter, M.; de Rink, I.; Henneman, L.; Kas, S.M.; Prekovic, S.; Hau, C.-S.; et al. Loss of p53 triggers Wnt-dependent systemic inflammation to drive breast cancer metastasis. Nature 2019, 572, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Vikhreva, P.; Petrova, V.; Gokbulut, T.; Pestlikis, I.; Mancini, M.; Di Daniele, N.; Knight, R.A.; Melino, G.; Amelio, I. TAp73 upregulates IL-1β in cancer cells: Potential biomarker in lung and breast cancer? Biochem. Biophys. Res. Commun. 2017, 482, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Woolery, K.T.; Mohamed, M.; Linger, R.J.; Dobrinski, K.P.; Roman, J.; Kruk, P.A. BRCA1 185delAG Mutation Enhances Interleukin-1β Expression in Ovarian Surface Epithelial Cells. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Dutta, S.; Veettil, M.V.; Roy, A.; Ansari, M.A.; Iqbal, J.; Chikoti, L.; Kumar, B.; Johnson, K.E.; Chandran, B. BRCA1 Regulates IFI16 Mediated Nuclear Innate Sensing of Herpes Viral DNA and Subsequent Induction of the Innate Inflammasome and Interferon-β Responses. PLoS Pathog. 2015, 11, e1005030. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Bronte, V.; Chen, S.H.; Colombo, M.P.; Ochoa, A.; Ostrand-Rosenberg, S.; Schreiber, H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007, 67, 425. [Google Scholar] [CrossRef]
- Almand, B.; Clark, J.I.; Nikitina, E.; van Beynen, J.; English, N.R.; Knight, S.C.; Carbone, D.P.; Gabrilovich, D.I. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J. Immunol. 2001, 166, 678–689. [Google Scholar] [CrossRef]
- Díaz-Montero, C.M.; Salem, M.L.; Nishimura, M.I.; Garrett-Mayer, E.; Cole, D.J.; Montero, A.J. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 2008, 58, 49–59. [Google Scholar] [CrossRef]
- Nagaraj, S.; Gupta, K.; Pisarev, V.; Kinarsky, L.; Sherman, S.; Kang, L.; Herber, D.L.; Schneck, J.; Gabrilovich, D.I. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 2007, 13, 828–835. [Google Scholar] [CrossRef]
- Bunt, S.K.; Yang, L.; Sinha, P.; Clements, V.K.; Leips, J.; Ostrand-Rosenberg, S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007, 67, 10019–10026. [Google Scholar] [CrossRef]
- Song, X.; Krelin, Y.; Dvorkin, T.; Bjorkdahl, O.; Segal, S.; Dinarello, C.A.; Voronov, E.; Apte, R.N. CD11b+/Gr-1+ Immature Myeloid Cells Mediate Suppression of T Cells in Mice Bearing Tumors of IL-1β-Secreting Cells. J. Immunol. 2005, 175, 8200–8208. [Google Scholar] [CrossRef] [PubMed]
- Bunt, S.K.; Sinha, P.; Clements, V.K.; Leips, J.; Ostrand-Rosenberg, S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J. Immunol. 2006, 176, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Elkabets, M.; Ribeiro, V.S.G.; Dinarello, C.A.; Ostrand-Rosenberg, S.; Di Santo, J.P.; Apte, R.N.; Vosshenrich, C. IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur. J. Immunol. 2010, 40, 3347–3357. [Google Scholar] [CrossRef] [PubMed]
- Bunt, S.K.; Clements, V.K.; Hanson, E.M.; Sinha, P.; Ostrand-Rosenberg, S. Inflammation enhances myeloid-derived suppressor cell crosstalk by signaling through Toll-like receptor 4. J. Leukoc. Boil. 2009, 85, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Martinez, F.O. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453. [Google Scholar] [CrossRef]
- Rey-Giraud, F.; Hafner, M.; Ries, C.H. In Vitro Generation of Monocyte-Derived Macrophages under Serum-Free Conditions Improves Their Tumor Promoting Functions. PLoS ONE 2012, 7, e42656. [Google Scholar] [CrossRef]
- Li, S.; Wang, W.; Zhang, N.; Ma, T.; Zhao, C. IL-1β mediates MCP-1 induction by Wnt5a in gastric cancer cells. BMC Cancer 2014, 14, 480. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, J.; Zhang, Q.; Zhang, J.; Lou, Y.; Yang, J.; Chen, Y.; Wei, T.; Zhang, J.; Fu, Q.; et al. Tumour cell-derived debris and IgG synergistically promote metastasis of pancreatic cancer by inducing inflammation via tumour-associated macrophages. Br. J. Cancer 2019, 121, 786–795. [Google Scholar] [CrossRef]
- Weichand, B.; Popp, R.; Dziumbla, S.; Mora, J.; Strack, E.; Elwakeel, E.; Frank, A.-C.; Scholich, K.; Pierre, S.; Syed, S.N.; et al. S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1β. J. Exp. Med. 2017, 214, 2695–2713. [Google Scholar] [CrossRef]
- Watari, K.; Shibata, T.; Kawahara, A.; Sata, K.-I.; Nabeshima, H.; Shinoda, A.; Abe, H.; Azuma, K.; Murakami, Y.; Izumi, H.; et al. Tumor-Derived Interleukin-1 Promotes Lymphangiogenesis and Lymph Node Metastasis through M2-Type Macrophages. PLoS ONE 2014, 9, e99568. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Kim, D.-H.; Lim, J.M.; Lee, J.W.; Jeong, S.J.; Kim, K.P.; Dong, Z. Breast cancer cell-derived soluble CD44 promotes tumor progression by triggering macrophage IL-1β production. Cancer Res. 2020, 80, 1342–1356. [Google Scholar] [CrossRef]
- Chen, J.; Sun, W.; Zhang, H.; Ma, J.; Xu, P.; Yu, Y.; Fang, H.; Zhou, L.; Lv, J.; Xie, J.; et al. Macrophages reprogrammed by lung cancer microparticles promote tumor development via release of IL-1beta. Cell Mol. Immunol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Linton, S.S.; Abraham, T.; Liao, J.; Clawson, G.A.; Butler, P.J.; Fox, T.; Kester, M.; Matters, G.L. Tumor-promoting effects of pancreatic cancer cell exosomes on THP-1-derived macrophages. PLoS ONE 2018, 13, e0206759. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Komohara, Y.; Bu, L.; Tsukamoto, M.; Itoyama, R.; Miyake, K.; Uchihara, T.; Ogata, Y.; Nakagawa, S.; Okabe, H.; et al. Downregulation of 15-hydroxyprostaglandin dehydrogenase by interleukin-1β from activated macrophages leads to poor prognosis in pancreatic cancer. Cancer Sci. 2018, 109, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Falcone, D.J.; Subbaramaiah, K.; Dannenberg, A.J. Macrophages induce COX-2 expression in breast cancer cells: Role of IL-1β autoamplification. Carcinogenesis 2011, 32, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Kaler, P.; Godasi, B.N.; Augenlicht, L.; Klampfer, L. The NF-κB/AKT-dependent Induction of Wnt Signaling in Colon Cancer Cells by Macrophages and IL-1β. Cancer Microenviron. 2009, 2, 69–80. [Google Scholar] [CrossRef]
- Brunetto, E.; de Monte, L.; Balzano, G.; Camisa, B.; Laino, V.; Riba, M.; Heltai, S.; Bianchi, M.; Bordignon, C.; Falconi, M.; et al. The IL-1/IL-1 receptor axis and tumor cell released inflammasome adaptor ASC are key regulators of TSLP secretion by cancer associated fibroblasts in pancreatic cancer. J. Immunother. Cancer 2019, 7, 45. [Google Scholar] [CrossRef]
- Ohashi, K.; Wang, Z.; Yang, Y.M.; Billet, S.; Tu, W.; Pimienta, M.; Cassel, S.L.; Pandol, S.J.; Lu, S.C.; Sutterwala, F.S.; et al. NOD-like receptor C4 Inflammasome Regulates the Growth of Colon Cancer Liver Metastasis in NAFLD. Hepatology 2019, 70, 1582–1599. [Google Scholar] [CrossRef]
- Du, Q.; Wang, Q.; Fan, H.; Wang, J.; Liu, X.; Wang, H.; Wang, Y.; Hu, R. Dietary cholesterol promotes AOM-induced colorectal cancer through activating the NLRP3 inflammasome. Biochem. Pharmacol. 2016, 105, 42–54. [Google Scholar] [CrossRef]
- Arendt, L.M.; McCready, J.; Keller, P.J.; Baker, D.D.; Naber, S.P.; Seewaldt, V.; Kuperwasser, C. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res. 2013, 73, 6080–6093. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Lagoudas, G.K.; Zhao, C.; Bullman, S.; Bhutkar, A.; Hu, B.; Ameh, S.; Sandel, D.; Liang, X.S.; Mazzilli, S.; et al. Commensal Microbiota Promote Lung Cancer Development via γδ T Cells. Cell 2019, 176, 998–1013. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.; Inaba, K.; Steinman, R.M. Dendritic cells are the principal cells in mouse spleen bearing immunogenic fragments of foreign proteins. J. Exp. Med. 1990, 172, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M. The Dendritic Cell System and its Role in Immunogenicity. Annu. Rev. Immunol. 1991, 9, 271–296. [Google Scholar] [CrossRef] [PubMed]
- Wieckowski, E.; Chatta, G.S.; Mailliard, R.M.; Gooding, W.; Palucka, K.; Banchereau, J.; Kalinski, P. Type-1 polarized dendritic cells loaded with apoptotic prostate cancer cells are potent inducers of CD8+ T cells against prostate cancer cells and defined prostate cancer-specific epitopes. Prostate 2010, 71, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.C.; Thomas, R.; Nielsen, L.K. Generation and Maturation of Dendritic Cells for Clinical Application Under Serum-Free Conditions. J. Immunother. 2005, 28, 599–609. [Google Scholar] [CrossRef]
- Fang, H.; Ang, B.; Xu, X.; Huang, X.; Wu, Y.; Sun, Y.; Wang, W.; Li, N.; Cao, X.; Wan, T. TLR4 is essential for dendritic cell activation and anti-tumor T-cell response enhancement by DAMPs released from chemically stressed cancer cells. Cell. Mol. Immunol. 2013, 11, 150–159. [Google Scholar] [CrossRef]
- Koo, J.E.; Shin, S.W.; Um, S.H.; Lee, J.Y. X-shaped DNA potentiates therapeutic efficacy in colitis-associated colon cancer through dual activation of TLR9 and inflammasomes. Mol. Cancer 2015, 14, 104. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, Z.; Jiang, S.; Chen, J.; Zhan, Y.; Chen, J. Secreting-lux/pT-ClyA engineered bacteria suppresses tumor growth via interleukin-1β in two pathways. AMB Express 2019, 9, 189–212. [Google Scholar] [CrossRef]
- Kim, J.-E.; Phan, T.X.; Nguyen, V.H.; Dinh-Vu, H.-V.; Zheng, J.H.; Yun, M.; Park, S.-G.; Hong, Y.; Choy, H.E.; Szardenings, M.; et al. Salmonella typhimurium Suppresses Tumor Growth via the Pro-Inflammatory Cytokine Interleukin-1β. Theranostics 2015, 5, 1328–1342. [Google Scholar] [CrossRef]
- Segovia, M.; Russo, S.; Jeldres, M.; Mahmoud, Y.; Perez, V.; Duhalde, M.; Charnet, P.; Rousset, M.; Victoria, S.; Veigas, F.; et al. Targeting TMEM176B Enhances Antitumor Immunity and Augments the Efficacy of Immune Checkpoint Blockers by Unleashing Inflammasome Activation. Cancer Cell 2019, 35, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Saxena, S.; Awaji, M.; Singh, R.K. Wu Tumor-Associated Neutrophils in Cancer: Going Pro. Cancers 2019, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Wang, Y.; Han, G.-C.; Wang, R.; Xiao, H.; Li, X.-Y.; Hou, C.-M.; Ma, Y.-F.; Sheng, D.-S.; Shen, B.-F.; et al. Complement activation promotes colitis-associated carcinogenesis through activating intestinal IL-1β/IL-17A axis. Mucosal Immunol. 2015, 8, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Shiku, H. Importance of CD4+ helper T-cells in antitumor immunity. Int. J. Hematol. 2003, 77, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Marrogi, A.J.; Munshi, A.; Merogi, A.J.; Ohadike, Y.; El-Habashi, A.; Marrogi, O.L.; Freeman, S.M. Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. Int. J. Cancer 1997, 74, 492–501. [Google Scholar] [CrossRef]
- Galon, J.; Coleno-Costes, A.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Zinzindohoué, F.; Bruneval, P.; Cugnenc, P.-H.; et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Hiraoka, K.; Miyamoto, M.; Cho, Y.; Suzuoki, M.; Oshikiri, T.; Nakakubo, Y.; Itoh, T.; Ohbuchi, T.; Kondo, S.; Katoh, H. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br. J. Cancer 2006, 94, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Tüting, T.; Storkus, W.J.; Lotze, M.T. Gene-based strategies for the immunotherapy of cancer. J. Mol. Med. 1997, 75, 478–491. [Google Scholar] [CrossRef]
- Ashok, A.; Keener, R.A.; Rubenstein, M.; Stookey, S.; Bajpai, S.; Hicks, J.; Alme, A.K.; Drake, C.G.; Zheng, Q.; Trabzonlu, L.; et al. Consequences of interleukin 1β-triggered chronic inflammation in the mouse prostate gland: Altered architecture associated with prolonged CD4+infiltration mimics human proliferative inflammatory atrophy. Prostate 2019, 79, 732–745. [Google Scholar] [CrossRef]
- North, R.J.; Neubauer, R.H.; Huang, J.J.; Newton, R.C.; E Loveless, S. Interleukin 1-induced, T cell-mediated regression of immunogenic murine tumors. Requirement for an adequate level of already acquired host concomitant immunity. J. Exp. Med. 1988, 168, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Haabeth, O.A.W.; Lorvik, K.B.; Yagita, H.; Bogen, B.; Corthay, A. Interleukin-1 is required for cancer eradication mediated by tumor-specific Th1 cells. OncoImmunology 2015, 5, e1039763. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Chang, S.H.; Martinez, G.J.; Yang, X.O.; Nurieva, R.; Kang, H.S.; Ma, L.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Critical Regulation of Early Th17 Cell Differentiation by Interleukin-1 Signaling. Immunity 2009, 30, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Troutman, T.D.; Edukulla, R.; Pasare, C. Priming Microenvironments Dictate Cytokine Requirements for T Helper 17 Cell Lineage Commitment. Immunity 2011, 35, 1010–1022. [Google Scholar] [CrossRef]
- Miyahara, Y.; Odunsi, K.; Chen, W.; Peng, G.; Matsuzaki, J.; Wang, R.-F. Generation, and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc. Natl. Acad. Sci. USA 2008, 105, 15505–15510. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chang, C.; Kuo, Y.; Fang, W.; Kao, H.; Tsai, S.; Wu, L.-W. Cancer-associated fibroblast-derived interleukin-1β activates protumor C-C motif chemokine ligand 22 signaling in head and neck cancer. Cancer Sci. 2019, 110, 2783–2793. [Google Scholar] [CrossRef] [PubMed]
- Vegran, F.; Berger, H.; Boidot, R.; Mignot, G.; Bruchard, M.; Dosset, M.; Chalmin, F.; Rébé, C.; Derangere, V.; Ryffel, B.; et al. The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat. Immunol. 2014, 15, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.-S.; Verstegen, N.; Ciampricotti, M.; Hawinkels, L.J.; Jonkers, J.; et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef]
- Dharmadhikari, B.; Nickles, E.; Harfuddin, Z.; Ishak, N.D.B.; Zeng, Q.; Bertoletti, A.; Schwarz, H. CD137L dendritic cells induce potent response against cancer-associated viruses and polarize human CD8+ T cells to Tc1 phenotype. Cancer Immunol. Immunother. 2018, 67, 893–905. [Google Scholar] [CrossRef]
- Ben-Sasson, S.Z.; Hogg, A.; Hu-Li, J.; Wingfield, P.; Chen, X.; Crank, M.; Caucheteux, S.; Ratner-Hurevich, M.; Berzofsky, J.A.; Nir-Paz, R.; et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J. Exp. Med. 2013, 210, 491–502. [Google Scholar] [CrossRef]
- Lee, P.-H.; Yamamoto, T.N.; Gurusamy, D.; Sukumar, M.; Yu, Z.; Hu-Li, J.; Kawabe, T.; Gangaplara, A.; Kishton, R.J.; Henning, A.N.; et al. Host conditioning with IL-1β improves the antitumor function of adoptively transferred T cells. J. Exp. Med. 2019, 216, 2619–2634. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Carmi, Y.; Apte, R.N. The role IL-1 in tumor-mediated angiogenesis. Front. Physiol. 2014, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Vimalraj, S.; Pavani, K.; Ramesh, N.; Sumantran, V.N. Intussusceptive angiogenesis as a key therapeutic target for cancer therapy. Life Sci 2020, 252, 117670. [Google Scholar] [CrossRef] [PubMed]
- Shchors, K.; Shchors, E.; Rostker, F.; Lawlor, E.R.; Brown-Swigart, L.; Evan, G.I. The Myc-dependent angiogenic switch in tumors is mediated by interleukin 1β. Genome Res. 2006, 20, 2527–2538. [Google Scholar] [CrossRef]
- Carmi, Y.; Voronov, E.; Dotan, S.; Lahat, N.; Rahat, M.A.; Fogel, M.; Huszar, M.; White, M.R.; Dinarello, C.A.; Apte, R.N. The Role of Macrophage-Derived IL-1 in Induction and Maintenance of Angiogenesis. J. Immunol. 2009, 183, 4705–4714. [Google Scholar] [CrossRef] [PubMed]
- Bar, D.; Apte, R.N.; Voronov, E.; Dinarello, C.A.; Cohen, S. A continuous delivery system of IL-1 receptor antagonist reduces angiogenesis and inhibits tumor development. FASEB J. 2003, 18, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Tanaka, M.; Miki, M.; Usui, K.; Suzuki, T.; Maemondo, M.; Hong, X.; Tazawa, R.; Kikuchi, T.; Matsushima, K.; et al. Proinflammatory Cytokine IL-1β Promotes Tumor Growth of Lewis Lung Carcinoma by Induction of Angiogenic Factors: In Vivo Analysis of Tumor-Stromal Interaction. J. Immunol. 2002, 169, 469–475. [Google Scholar] [CrossRef]
- Nakao, S.; Kuwano, T.; Tsutsumi-Miyahara, C.; Ueda, S.-I.; Kimura, Y.N.; Hamano, S.; Sonoda, K.-H.; Saijo, Y.; Nukiwa, T.; Strieter, R.M.; et al. Infiltration of COX-2–expressing macrophages is a prerequisite for IL-1β–induced neovascularization and tumor growth. J. Clin. Investig. 2005, 115, 2979–2991. [Google Scholar] [CrossRef]
- Carmi, Y.; Dotan, S.; Rider, P.; Kaplanov, I.; White, M.R.; Baron, R.; Abutbul, S.; Huszar, M.; Dinarello, C.A.; Apte, R.N.; et al. The Role of IL-1β in the Early Tumor Cell–Induced Angiogenic Response. J. Immunol. 2013, 190, 3500–3509. [Google Scholar] [CrossRef]
- Kolb, R.; Kluz, P.; Tan, Z.W.; Borcherding, N.; Bormann, N.; Vishwakarma, A.; Balcziak, L.; Zhu, P.; Davies, B.S.; Gourronc, F.; et al. Obesity-associated inflammation promotes angiogenesis and breast cancer via angiopoietin-like 4. Oncogene 2018, 38, 2351–2363. [Google Scholar] [CrossRef]
- Kolb, R.; Phan, L.; Borcherding, N.; Liu, Y.; Yuan, F.; Janowski, A.M.; Xie, Q.; Markan, K.R.; Li, W.; Potthoff, M.J.; et al. Obesity-associated NLRC4 inflammasome activation drives breast cancer progression. Nat. Commun. 2016, 7, 13007. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.K.B.A.; Shimizu, A.; Ogita, H.; Amin, A. The Pivotal Roles of the Epithelial Membrane Protein Family in Cancer Invasiveness and Metastasis. Cancers 2019, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Giavazzi, R.; Garofalo, A.; Bani, M.R.; Abbate, M.; Ghezzi, P.; Boraschi, D.; Mantovani, A.; Dejana, E. Interleukin 1-induced augmentation of experimental metastases from a human melanoma in nude mice. Cancer Res. 1990, 50, 4771–4775. [Google Scholar] [PubMed]
- Vidal-Vanaclocha, F.; Amézaga, C.; Asumendi, A.; Kaplanski, G.; A Dinarello, C. Interleukin-1 receptor blockade reduces the number and size of murine B16 melanoma hepatic metastases. Cancer Res. 1994, 54, 2667–2672. [Google Scholar] [PubMed]
- Song, X.; Voronov, E.; Dvorkin, T.; Fima, E.; Cagnano, E.; Benharroch, D.; Shendler, Y.; Bjorkdahl, O.; Segal, S.; Dinarello, C.A.; et al. Differential Effects of IL-1α and IL-1β on Tumorigenicity Patterns and Invasiveness. J. Immunol. 2003, 171, 6448–6456. [Google Scholar] [CrossRef]
- Vidal-Vanaclocha, F.; Fantuzzi, G.; Mendoza, L.; Fuentes, A.M.; Anasagasti, M.J.; Martín, J.; Carrascal, T.; Walsh, P.; Reznikov, L.L.; Kim, S.-H.; et al. IL-18 regulates IL-1beta -dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc. Natl. Acad. Sci. USA 2000, 97, 734–739. [Google Scholar] [CrossRef]
- Guo, B.; Fu, S.; Zhang, J.; Liu, B.; Li, Z. Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Sci. Rep. 2016, 6, 36107. [Google Scholar] [CrossRef]
- Holen, I.; Lefley, D.V.; Francis, S.E.; Rennicks, S.; Bradbury, S.; Coleman, R.E.; Ottewell, P. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget 2016, 7, 75571–75584. [Google Scholar] [CrossRef]
- Liu, Q.; Russell, M.R.; Shahriari, K.; Jernigan, D.; Lioni, M.I.; Garcia, F.U.; Fatatis, A. Interleukin-1 Promotes Skeletal Colonization and Progression of Metastatic Prostate Cancer Cells with Neuroendocrine Features. Cancer Res. 2013, 73, 3297–3305. [Google Scholar] [CrossRef]
- Liao, T.-T.; Yang, M.-H. Revisiting epithelial-mesenchymal transition in cancer metastasis: The connection between epithelial plasticity and stemness. Mol. Oncol. 2017, 11, 792–804. [Google Scholar] [CrossRef]
- Li, R.; Ong, S.L.; Tran, L.M.; Jing, Z.; Liu, B.; Park, S.J.; Huang, Z.L.; Walser, T.C.; Heinrich, E.L.; Lee, G.; et al. Chronic IL-1β-induced inflammation regulates epithelial-to-mesenchymal transition memory phenotypes via epigenetic modifications in non-small cell lung cancer. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Garduño, A.M.; Mendoza-Rodríguez, M.G.; Urrutia-Cabrera, D.; Domínguez-Robles, M.C.; Pérez-Yepez, E.A.; Ayala-Sumuano, J.T.; Meza, I. IL-1β induced methylation of the estrogen receptor ERα gene correlates with EMT and chemoresistance in breast cancer cells. Biochem. Biophys. Res. Commun. 2017, 490, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Rodríguez, M.; Romero, H.A.; Fuentes-Pananá, E.M.; Ayala-Sumuano, J.-T.; Meza, I. IL-1β induces up-regulation of BIRC3, a gene involved in chemoresistance to doxorubicin in breast cancer cells. Cancer Lett. 2017, 390, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Kaler, P.; Galea, V.; Augenlicht, L.; Klampfer, L. Tumor Associated Macrophages Protect Colon Cancer Cells from TRAIL-Induced Apoptosis through IL-1β- Dependent Stabilization of Snail in Tumor Cells. PLoS ONE 2010, 5, e11700. [Google Scholar] [CrossRef] [PubMed]
- Castaño, Z.; Juan, B.P.S.; Spiegel, A.; Pant, A.; de Cristo, M.J.; Laszewski, T.; Ubellacker, J.M.; Janssen, S.R.; Dongre, A.; Reinhardt, F.; et al. IL-1β inflammatory response driven by primary breast cancer prevents metastasis-initiating cell colonization. Nature 2018, 20, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Pappan, L.; Galliher-Beckley, A.; Shi, J. IL-1β promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol. Cancer 2012, 11, 87. [Google Scholar] [CrossRef]
- Li, H.-J.; Reinhardt, F.; Herschman, H.R.; A Weinberg, R. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discov. 2012, 2, 840–855. [Google Scholar] [CrossRef]
- Yu, A.; Wang, Y.; Bian, Y.; Chen, L.; Guo, J.; Shen, W.; Chen, D.; Liu, S.; Sun, X. IL-1β promotes the nuclear translocaiton of S100A4 protein in gastric cancer cells MGC803 and the cell’s stem-like properties through PI3K pathway. J. Cell. Biochem. 2018, 119, 8163–8173. [Google Scholar] [CrossRef]
- Fei, F.; Qu, J.; Zhang, S.; Li, Y.; Zhang, S. S100A4 in cancer progression and metastasis: A systematic review. Oncotarget 2017, 8, 73219–73239. [Google Scholar] [CrossRef]
- Watanabe, T.; Hashimoto, T.; Sugino, T.; Soeda, S.; Nishiyama, H.; Morimura, Y.; Yamada, H.; Goodison, S.; Fujimori, K. Production of IL1-beta by ovarian cancer cells induces mesothelial cell beta1-integrin expression facilitating peritoneal dissemination. J. Ovarian Res. 2012, 5, 7. [Google Scholar] [CrossRef]
- Hübner, M.; Effinger, D.; Wu, T.; Strauß, G.; Pogoda, K.; Kreth, F.-W.; Kreth, S. The IL-1 Antagonist Anakinra Attenuates Glioblastoma Aggressiveness by Dampening Tumor-Associated Inflammation. Cancers 2020, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Lee, J.Y.; Yang, G.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Inhibition of NLRP3 inflammasome in tumor microenvironment leads to suppression of metastatic potential of cancer cells. Sci. Rep. 2019, 9, 12277–12279. [Google Scholar] [CrossRef] [PubMed]
- Storr, S.J.; Safuan, S.; Ahmad, N.; El-Refaee, M.; Jackson, A.M.; Martin, S.G. Macrophage-derived interleukin-1beta promotes human breast cancer cell migration and lymphatic adhesion in vitro. Cancer Immunol. Immunother. 2017, 66, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Ershaid, N.; Sharon, Y.; Doron, H.; Raz, Y.; Shani, O.; Cohen, N.; Monteran, L.; Leider-Trejo, L.; Ben-Shmuel, A.; Yassin, M.; et al. NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat. Commun. 2019, 10, 4375. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.-Y.; Lee, J.-J.; Yeh, C.-Y.; Yang, C.-J.; Kok, S.-H.; Ko, J.-Y.; Tsai, F.-C.; Chia, J.-S. Reciprocal activation of cancer-associated fibroblasts and oral squamous carcinoma cells through CXCL1. Oral Oncol. 2019, 88, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, C.; Hu, G.; Ma, J.; Chen, Y.; Zhang, J.; Huang, Y.; Zheng, J.; Xue, W.; Xu, Y.; et al. Tumor-educated B cells promote renal cancer metastasis via inducing the IL-1β/HIF-2α/Notch1 signals. Cell Death Dis. 2020, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Lan, F.-H.; Wang, X.; Yu, Y.; Ouyang, X.; Zheng, F.; Han, J.; Lin, Y.; Xie, Y.; Xie, F.; et al. IL-1β-induced activation of p38 promotes metastasis in gastric adenocarcinoma via upregulation of AP-1/c-fos, MMP2 and MMP9. Mol. Cancer 2014, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lan, F.-H.; Zheng, Z.; Xie, F.; Wang, L.; Liu, W.; Han, J.; Zheng, F.; Xie, Y.; Huang, Q. Epidermal growth factor (EGF) and interleukin (IL)-1β synergistically promote ERK1/2-mediated invasive breast ductal cancer cell migration and invasion. Mol. Cancer 2012, 11, 79. [Google Scholar] [CrossRef]
- Franco-Barraza, J.; Valdivia-Silva, J.; Zamudio-Meza, H.; Castillo, A.; Garcia-Zepeda, E.A.; Benitez-Bribiesca, L.; Meza, I. Actin Cytoskeleton Participation in the Onset of IL-1β Induction of an Invasive Mesenchymal-like Phenotype in Epithelial MCF-7 Cells. Arch. Med Res. 2010, 41, 170–181. [Google Scholar] [CrossRef]
- Guo, R.; Qin, Y.; Shi, P.; Xie, J.; Chou, M.; Chen, Y. IL-1β promotes proliferation and migration of gallbladder cancer cells via Twist activation. Oncol. Lett. 2016, 12, 4749–4755. [Google Scholar] [CrossRef]
- Pérez-Yépez, E.A.; Ayala-Sumuano, J.-T.; Lezama, R.; Meza, I. A novel β-catenin signaling pathway activated by IL-1β leads to the onset of epithelial–mesenchymal transition in breast cancer cells. Cancer Lett. 2014, 354, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Westbom, C.; Thompson, J.K.; Leggett, A.; MacPherson, M.; Beuschel, S.; Pass, H.I.; Vacek, P.; Shukla, A. Inflammasome Modulation by Chemotherapeutics in Malignant Mesothelioma. PLoS ONE 2015, 10, e0145404. [Google Scholar] [CrossRef] [PubMed]
- Arlt, A.; Vorndamm, J.; Müerköster, S.; Yu, H.; Schmidt, W.E.; Fölsch, U.R.; Schäfer, H. Autocrine production of interleukin 1beta confers constitutive nuclear factor kappaB activity and chemoresistance in pancreatic carcinoma cell lines. Cancer Res. 2002, 62, 910–916. [Google Scholar] [PubMed]
- Müerköster, S.S.; Werbing, V.; Sipos, B.; A Debus, M.; Witt, M.; Großmann, M.; Leisner, D.; Kötteritzsch, J.; Kappes, H.; Klöppel, G.; et al. Drug-induced expression of the cellular adhesion molecule L1CAM confers anti-apoptotic protection and chemoresistance in pancreatic ductal adenocarcinoma cells. Oncogene 2006, 26, 2759–2768. [Google Scholar] [CrossRef] [PubMed]
- Müerköster, S.; Wegehenkel, K.; Arlt, A.; Witt, M.; Sipos, B.; Kruse, M.-L.; Sebens, T.; Klöppel, G.; Kalthoff, H.; Fölsch, U.R.; et al. Tumor Stroma Interactions Induce Chemoresistance in Pancreatic Ductal Carcinoma Cells Involving Increased Secretion and Paracrine Effects of Nitric Oxide and Interleukin-1. Cancer Res. 2004, 64, 1331–1337. [Google Scholar] [CrossRef]
- Arlt, A.; Vorndamm, J.; Witt, M.; Grohmann, F. Autokrine IL-1β-Sekretion führt zu erhöhter NF-κB-Aktivität und zu Chemoresistenz in Pankreaskarzinomzellen in vivo. Med. Klin. 2004, 99, 185–190. [Google Scholar] [CrossRef]
- Jin, H.; Ko, Y.S.; Kim, H.J. P2Y2R-mediated inflammasome activation is involved in tumor progression in breast cancer cells and in radiotherapy-resistant breast cancer. Int. J. Oncol. 2018, 53, 1953–1966. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Apetoh, L.; Tesniere, A.; Aymeric, L.; Ma, Y.; Ortiz, C.; Vermaelen, K.; Panaretakis, T.; Mignot, G.; Ullrich, E.; et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β–dependent adaptive immunity against tumors. Nat. Med. 2009, 15, 1170–1178. [Google Scholar] [CrossRef]
- Mattarollo, S.; Loi, S.; Duret, H.; Ma, Y.; Zitvogel, L.; Smyth, M.J. Pivotal Role of Innate and Adaptive Immunity in Anthracycline Chemotherapy of Established Tumors. Cancer Res. 2011, 71, 4809–4820. [Google Scholar] [CrossRef]
- Bruchard, M.; Mignot, G.; Derangere, V.; Chalmin, F.; Chevriaux, A.; Vegran, F.; Boireau, W.; Simon, B.; Ryffel, B.; Connat, J.L.; et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat. Med. 2012, 19, 57–64. [Google Scholar] [CrossRef]
- Isambert, N.; Hervieu, A.; Rébé, C.; Hennequin, A.; Borg, C.; Zanetta, S.; Chevriaux, A.; Richard, C.; Derangère, V.; Limagne, E.; et al. Fluorouracil and bevacizumab plus anakinra for patients with metastatic colorectal cancer refractory to standard therapies (IRAFU): A single-arm phase 2 study. OncoImmunology 2018, 7, e1474319-6. [Google Scholar] [CrossRef] [PubMed]
- Martine, P.; Chevriaux, A.; Derangère, V.; Apetoh, L.; Garrido, C.; Ghiringhelli, F.; Rébé, C. HSP70 is a negative regulator of NLRP3 inflammasome activation. Cell Death Dis. 2019, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Martine, P. Heat Shock Proteins and Inflammasomes. Int. J. Mol. Sci. 2019, 20, 4508. [Google Scholar] [CrossRef] [PubMed]
- Pilot, T.; Fratti, A.; Thinselin, C.; Perrichet, A.; Demontoux, L.; Limagne, E.; Derangère, V.; Ilie, A.; Ndiaye, M.; Jacquin, E.; et al. Heat shock and HSP70 regulate 5-FU-mediated caspase-1 activation in myeloid-derived suppressor cells and tumor growth in mice. J. Immunother. Cancer 2020, 8, e000478. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Shim, D.-W.; Hwang, I.; Park, J.-H.; Yu, J.-W. Chemotherapeutic Agent Paclitaxel Mediates Priming of NLRP3 Inflammasome Activation. Front. Immunol. 2019, 10, 1108. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.-Z.; Yang, F.; Li, C.-G.; Xu, L.-H.; He, X.-H.; Mai, F.-Y.; Zeng, C.-Y.; Zhang, C.-C.; Zha, Q.-B.; Ouyang, D.-Y. Paclitaxel Enhances the Innate Immunity by Promoting NLRP3 Inflammasome Activation in Macrophages. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Voloshin, T.; Alishekevitz, D.; Kaneti, L.; Miller, V.; Isakov, E.; Kaplanov, I.; Voronov, E.; Fremder, E.; Benhar, M.; Machluf, M.; et al. Blocking IL1 Pathway Following Paclitaxel Chemotherapy Slightly Inhibits Primary Tumor Growth but Promotes Spontaneous Metastasis. Mol. Cancer Ther. 2015, 14, 1385–1394. [Google Scholar] [CrossRef]
- Waugh, J.; Perry, C.M. Anakinra. BioDrugs 2005, 19, 189–202. [Google Scholar] [CrossRef]
- Lust, J.A.; Lacy, M.Q.; Zeldenrust, S.R.; Dispenzieri, A.; Gertz, M.A.; Witzig, T.E.; Kumar, S.; Hayman, S.R.; Russell, S.J.; Buadi, F.K.; et al. Induction of a Chronic Disease State in Patients With Smoldering or Indolent Multiple Myeloma by Targeting Interleukin 1β-Induced Interleukin 6 Production and the Myeloma Proliferative Component. Mayo Clin. Proc. 2009, 84, 114–122. [Google Scholar] [CrossRef]
- Dubois, E.A.; Rissmann, R.; Cohen, A.F. Rilonacept and canakinumab. Br. J. Clin. Pharmacol. 2011, 71, 639–641. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Everett, B.M.; Ridker, P.M.; Lorenzatti, A.; Krum, H.; Varigos, J.; Siostrzonek, P.; Sinnaeve, P.; Fonseca, F.; et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: Exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1833–1842. [Google Scholar] [CrossRef]
- van Cutsem, E.; Shitara, K.; Deng, W.; Vaury, A.; Tseng, L.; Wang, X.; Millholland, J.; Shilkrut, M.; Mookerjee, B.; Jonasch, E. Gevokizumab, an interleukin-1β (IL-1β) monoclonal antibody (mAb), in metastatic colorectal cancer (mCRC), metastatic gastroesophageal cancer (mGEC) and metastatic renal cell carcinoma (mRCC): “First-in-cancer” phase Ib study. Ann. Oncol. 2019, 30, iv77–iv78. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.; Fernando, R.; Shinde, T.; Gundamaraju, R.; Southam, B.; Sohal, S.S.; Robertson, A.A.B.; Schroder, K.; Kunde, D.; Eri, R. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci. Rep. 2018, 8, 8618. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, M.; Lurati, A.; Vignati, G.; Marrazza, M.G.; Telese, F.; Re, K.; Bellistri, A. Biomarkers, type II collagen, glucosamine, and chondroitin sulfate in osteoarthritis follow-up: The “Magenta osteoarthritis study. ” J. Orthop. Traumatol. 2008, 9, 81–87. [Google Scholar] [CrossRef]
- Wannamaker, W.; Davies, R.; Namchuk, M.; Pollard, J.; Ford, P.; Ku, G.; Decker, C.; Charifson, P.; Weber, P.; Germann, U.A.; et al. (S)-1-((S)-2-{[1-(4-Amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an Orally Available Selective Interleukin (IL)-Converting Enzyme/Caspase-1 Inhibitor, Exhibits Potent Anti-Inflammatory Activities by Inhibiting the Release of IL-1β and IL-18. J. Pharmacol. Exp. Ther. 2007, 321, 509–516. [Google Scholar] [CrossRef]
- Schroder, K.; Sagulenko, V.; Zamoshnikova, A.; Richards, A.A.; Cridland, J.A.; Irvine, K.; Stacey, K.J.; Sweet, M.J. Acute lipopolysaccharide priming boosts inflammasome activation independently of inflammasome sensor induction. Immunobiology 2012, 217, 1325–1329. [Google Scholar] [CrossRef]
- Helal, K.F.; Badr, M.S.; Rafeek, M.E.-S.; Elnagar, W.M.; Lashin, M.E.-B. Can glyburide be advocated over subcutaneous insulin for perinatal outcomes of women with gestational diabetes? A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2020, 301, 19–32. [Google Scholar] [CrossRef]
- Adinolfi, E.; Raffaghello, L.; Giuliani, A.L.; Cavazzini, L.; Capece, M.; Chiozzi, P.; Bianchi, G.; Kroemer, G.; Pistoia, V.; di Virgilio, F. Expression of P2X7 Receptor Increases In Vivo Tumor Growth. Cancer Res. 2012, 72, 2957–2969. [Google Scholar] [CrossRef]

| Heading | Type of Cancer | Pro (+) or Anti (−) Tumor | Ref. |
|---|---|---|---|
| High IL-1β IHC staining | Nasopharyngeal carcinoma | (−) | [26] |
| High IL-1β blood level | NSCLC | (+) | [27,28,29] |
| High IL1B mRNA | Breast cancer | (−) | [30] |
| High IL1B mRNA | Cervical cancer | (−) | [31] |
| High IL1B-signature | Gliomas | (+) | [32] |
| IL1B-511 C > T (rs16944) T allele | Ovarian cancer | (+) or (−) | [33,34,35] |
| Lung cancer | (+) or (−) | [36,37] | |
| Gastric cancer | (+) or (−) | [38,39,40,41,42,43,44,45,46] | |
| Cervical cancer | (+) | [47,48] | |
| Acute myeloid leukemia | (+) | [49] | |
| Chronic myeloid leukemia | (+) | [50] | |
| IL-1β-31 C > T (rs1143627) T allele | Breast cancer | (+) | [51,52] |
| Lung cancer | (+) | [36,37] | |
| Cervical cancer | (+) | [48] | |
| Hepatocellular carcinoma | (+) | [53] | |
| Osteosarcoma | (+) | [54] | |
| IL-1β-31 C > T (rs1143627) C allele | Gastric cancer | (+) | [45] |
| IL-1β-1464 G > C (rs1143623) G allele | Renal cell carcinoma | (+) | [55] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rébé, C.; Ghiringhelli, F. Interleukin-1β and Cancer. Cancers 2020, 12, 1791. https://doi.org/10.3390/cancers12071791
Rébé C, Ghiringhelli F. Interleukin-1β and Cancer. Cancers. 2020; 12(7):1791. https://doi.org/10.3390/cancers12071791
Chicago/Turabian StyleRébé, Cédric, and François Ghiringhelli. 2020. "Interleukin-1β and Cancer" Cancers 12, no. 7: 1791. https://doi.org/10.3390/cancers12071791
APA StyleRébé, C., & Ghiringhelli, F. (2020). Interleukin-1β and Cancer. Cancers, 12(7), 1791. https://doi.org/10.3390/cancers12071791






