Diffusion Kurtosis Imaging—A Superior Approach to Assess Tumor–Stroma Ratio in Pancreatic Ductal Adenocarcinoma
Abstract
:1. Introduction
2. Results
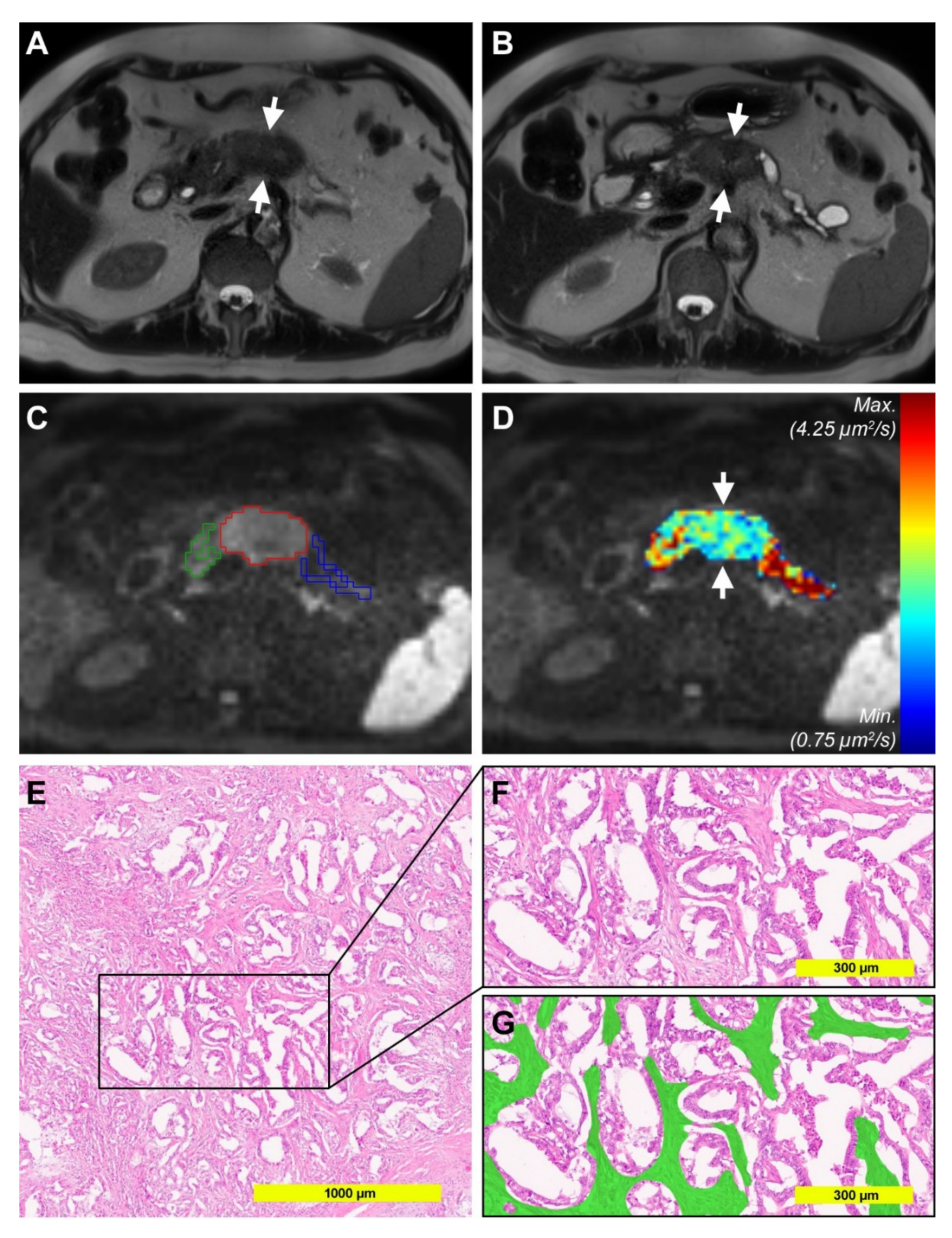
2.1. Diffusion-Weighted Imaging Analysis
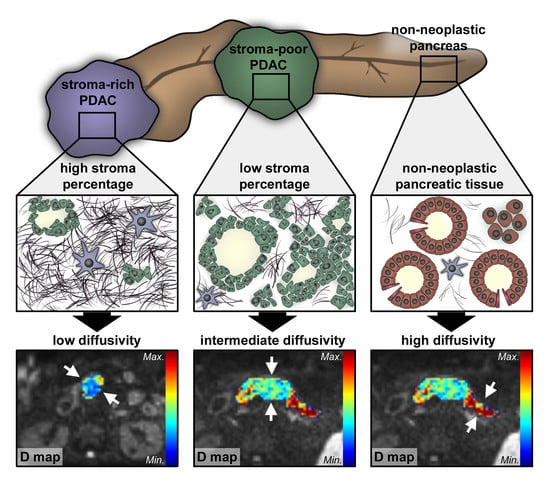
2.2. Histopathological Analysis of Tumor Stroma and Tumor Cell Content
2.3. Correlation of Diffusion-Weighted Imaging Analysis with Histopathological Parameters
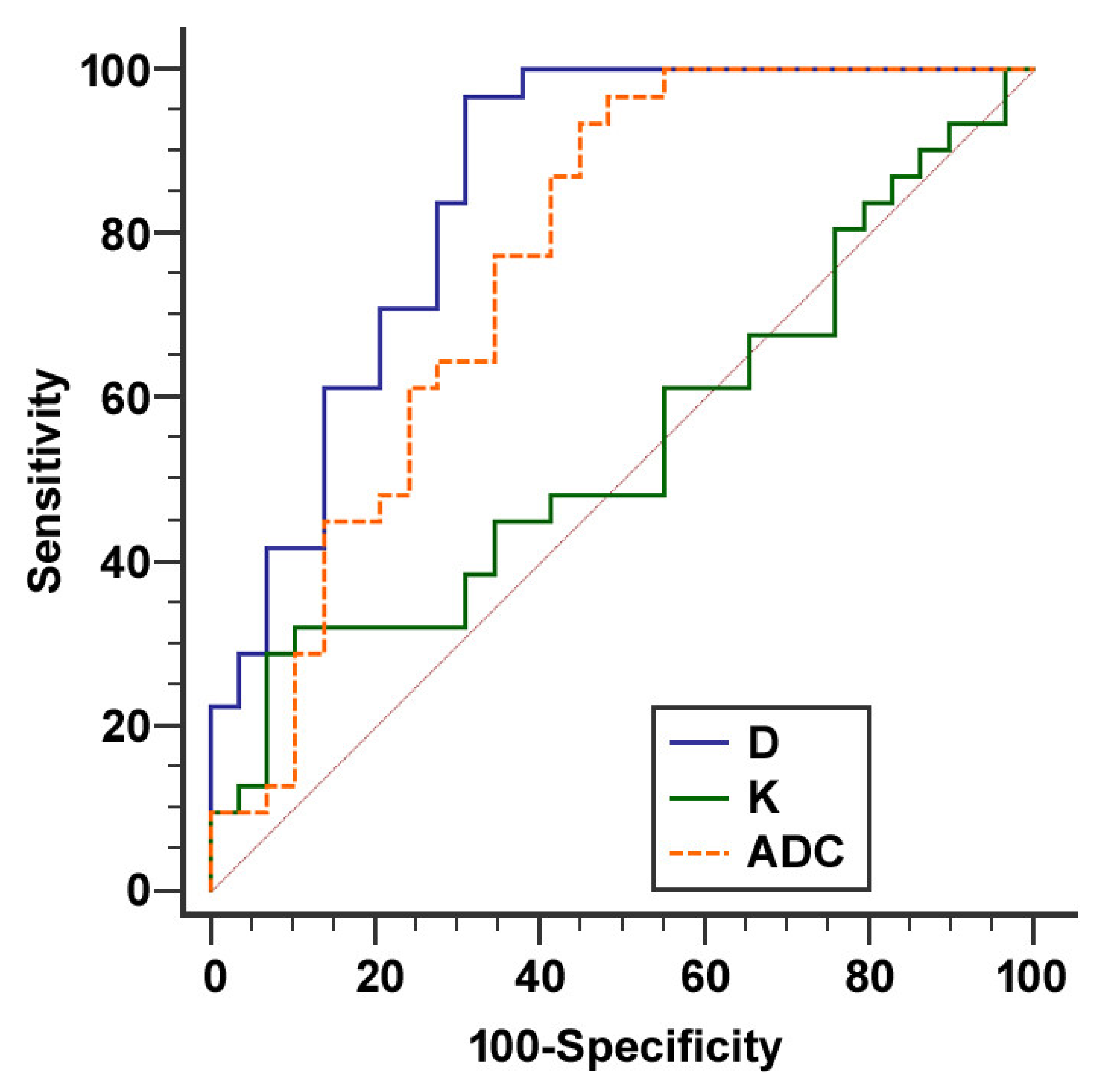
2.4. Comparison of Conventional MRI Features and Diffusion Kurtosis Imaging Analysis
3. Discussion
4. Materials and Methods
4.1. Magnetic Resonance Imaging
4.2. Post Processing of Diffusion-Weighted Magnetic Resonance Imaging Data
4.3. Qualitative assessment of Magnetic Resonance Images
4.4. Histopathological Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Erkan, M.; Adler, G.; Apte, M.V.; Bachem, M.G.; Buchholz, M.; Detlefsen, S.; Esposito, I.; Friess, H.; Gress, T.M.; Habisch, H.-J.; et al. StellaTUM: Current consensus and discussion on pancreatic stellate cell research. Gut 2012, 61, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Radhakrishnan, P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol. Cancer 2019, 18. [Google Scholar] [CrossRef] [PubMed]
- Lafaro, K.J.; Melstrom, L.G. The Paradoxical Web of Pancreatic Cancer Tumor Microenvironment. Am. J. Pathol. 2019, 189, 44–57. [Google Scholar] [CrossRef] [Green Version]
- Weniger, M.; Honselmann, K.C.; Liss, A.S. The Extracellular Matrix and Pancreatic Cancer: A Complex Relationship. Cancers 2018, 10, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzesiak, J.J.; Ho, J.C.; Moossa, A.R.; Bouvet, M. The integrin-extracellular matrix axis in pancreatic cancer. Pancreas 2007, 35, 293–301. [Google Scholar] [CrossRef]
- Makareeva, E.; Han, S.; Vera, J.C.; Sackett, D.L.; Holmbeck, K.; Phillips, C.L.; Visse, R.; Nagase, H.; Leikin, S. Carcinomas contain a matrix metalloproteinase-resistant isoform of type I collagen exerting selective support to invasion. Cancer Res. 2010, 70, 4366–4374. [Google Scholar] [CrossRef] [Green Version]
- Shields, M.A.; Dangi-Garimella, S.; Redig, A.J.; Munshi, H.G. Biochemical role of the collagen-rich tumour microenvironment in pancreatic cancer progression. Biochem. J. 2012, 441, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Gordillo, C.H.; Sandoval, P.; Muñoz-Hernández, P.; Pascual-Antón, L.; López-Cabrera, M.; Jiménez-Heffernan, J.A. Mesothelial-to-Mesenchymal Transition Contributes to the Generation of Carcinoma-Associated Fibroblasts in Locally Advanced Primary Colorectal Carcinomas. Cancers 2020, 12, 499. [Google Scholar] [CrossRef] [Green Version]
- Nowinska, K.; Jablonska, K.; Pawelczyk, K.; Piotrowska, A.; Partynska, A.; Gomulkiewicz, A.; Ciesielska, U.; Katnik, E.; Grzegrzolka, J.; Glatzel-Plucinska, N.; et al. Expression of Irisin/FNDC5 in Cancer Cells and Stromal Fibroblasts of Non-small Cell Lung Cancer. Cancers 2019, 11, 1538. [Google Scholar] [CrossRef] [Green Version]
- Drev, D.; Harpain, F.; Beer, A.; Stift, A.; Gruber, E.S.; Klimpfinger, M.; Thalhammer, S.; Reti, A.; Kenner, L.; Bergmann, M.; et al. Impact of Fibroblast-Derived SPARC on Invasiveness of Colorectal Cancer Cells. Cancers 2019, 11, 1421. [Google Scholar] [CrossRef] [Green Version]
- Linnebacher, A.; Mayer, P.; Marnet, N.; Bergmann, F.; Herpel, E.; Revia, S.; Yin, L.; Liu, L.; Hackert, T.; Giese, T.; et al. Interleukin 21 Receptor/Ligand Interaction Is Linked to Disease Progression in Pancreatic Cancer. Cells 2019, 8, 1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, P.; Linnebacher, A.; Glennemeier-Marke, H.; Marnet, N.; Bergmann, F.; Hackert, T.; Klauss, M.; Poth, T.; Gaida, M.M. The Microarchitecture of Pancreatic Cancer as Measured by Diffusion-Weighted Magnetic Resonance Imaging Is Altered by T Cells with a Tumor Promoting Th17 Phenotype. Int. J. Mol. Sci. 2020, 21, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, P.; Dinkic, C.; Jesenofsky, R.; Klauss, M.; Schirmacher, P.; Dapunt, U.; Hackert, T.; Uhle, F.; Hänsch, G.M.; Gaida, M.M. Changes in the microarchitecture of the pancreatic cancer stroma are linked to neutrophil-dependent reprogramming of stellate cells and reflected by diffusion-weighted magnetic resonance imaging. Theranostics 2018, 8, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, U.M.; Langhoff, E.; Goni, E.; Costello, E.; Greenhalf, W.; Halloran, C.; Ormanns, S.; Kruger, S.; Boeck, S.; Ribback, S.; et al. Immune Cell and Stromal Signature Associated With Progression-Free Survival of Patients With Resected Pancreatic Ductal Adenocarcinoma. Gastroenterology 2018, 155, 1625–1639. [Google Scholar] [CrossRef] [Green Version]
- Kota, J.; Hancock, J.; Kwon, J.; Korc, M. Pancreatic cancer: Stroma and its current and emerging targeted therapies. Cancer Lett. 2017, 391, 38–49. [Google Scholar] [CrossRef]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef] [Green Version]
- Van Mackelenbergh, M.G.; Stroes, C.I.; Spijker, R.; van Eijck, C.H.; Wilmink, J.W.; Bijlsma, M.F.; van Laarhoven, H.W. Clinical Trials Targeting the Stroma in Pancreatic Cancer: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 588. [Google Scholar] [CrossRef] [Green Version]
- Hackert, T.; Büchler, M.W. Pancreatic Cancer: Advances in Treatment, Results and Limitations. Dig. Dis. 2013, 31, 51–56. [Google Scholar] [CrossRef]
- Nevala-Plagemann, C.; Hidalgo, M.; Garrido-Laguna, I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat. Rev. Clin. Oncol. 2020, 17, 108–123. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Chandana, S.; Babiker, H.M.; Mahadevan, D. Therapeutic trends in pancreatic ductal adenocarcinoma (PDAC). Expert Opin. Investig. Drugs 2019, 28, 161–177. [Google Scholar] [CrossRef]
- Neesse, A.; Bauer, C.A.; Öhlund, D.; Lauth, M.; Buchholz, M.; Michl, P.; Tuveson, D.A.; Gress, T.M. Stromal biology and therapy in pancreatic cancer: Ready for clinical translation? Gut 2019, 68, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Rhim, A.D.; Oberstein, P.E.; Thomas, D.H.; Mirek, E.T.; Palermo, C.F.; Sastra, S.A.; Dekleva, E.N.; Saunders, T.; Becerra, C.P.; Tattersall, I.W.; et al. Stromal Elements Act to Restrain, Rather Than Support, Pancreatic Ductal Adenocarcinoma. Cancer Cell 2014, 25, 735–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hruban, R.H.; Gaida, M.M.; Thompson, E.; Hong, S.; Noë, M.; Brosens, L.A.; Jongepier, M.; Offerhaus, G.J.A.; Wood, L.D. Why is pancreatic cancer so deadly? The pathologist’s view. J. Pathol. 2019, 248, 131–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horowitz, J.M.; Venkatesh, S.K.; Ehman, R.L.; Jhaveri, K.; Kamath, P.; Ohliger, M.A.; Samir, A.E.; Silva, A.C.; Taouli, B.; Torbenson, M.S.; et al. Evaluation of hepatic fibrosis: A review from the society of abdominal radiology disease focus panel. Abdom. Radiol. 2017, 42, 2037–2053. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines (Version 1.2020) Pancreatic Adenocarcinoma. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 28 May 2020).
- Treadwell, J.R.; Zafar, H.M.; Mitchell, M.D.; Tipton, K.; Teitelbaum, U.; Jue, J. Imaging Tests for the Diagnosis and Staging of Pancreatic Adenocarcinoma: A Meta-Analysis. Pancreas 2016, 45, 789–795. [Google Scholar] [CrossRef] [Green Version]
- Sahani, D.V.; Shah, Z.K.; Catalano, O.A.; Boland, G.W.; Brugge, W.R. Radiology of pancreatic adenocarcinoma: Current status of imaging: Diagnostic imaging: Pancreatic adenocarcinoma. J. Gastroenterol. Hepatol. 2007, 23, 23–33. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.H.; Yu, E.S.; Kim, M.-H.; Kim, J.; Byun, J.H.; Lee, S.S.; Hwang, H.J.; Hwang, J.-Y.; Lee, S.S.; et al. Visually Isoattenuating Pancreatic Adenocarcinoma at Dynamic-Enhanced CT: Frequency, Clinical and Pathologic Characteristics, and Diagnosis at Imaging Examinations. Radiology 2010, 257, 87–96. [Google Scholar] [CrossRef]
- Heyn, C.; Sue-Chue-Lam, D.; Jhaveri, K.; Haider, M.A. MRI of the pancreas: Problem solving tool. J. Magn. Reson. Imaging 2012, 36, 1037–1051. [Google Scholar] [CrossRef]
- Caroli, A.; Schneider, M.; Friedli, I.; Ljimani, A.; De Seigneux, S.; Boor, P.; Gullapudi, L.; Kazmi, I.; Mendichovszky, I.A.; Notohamiprodjo, M.; et al. Diffusion-weighted magnetic resonance imaging to assess diffuse renal pathology: A systematic review and statement paper. Nephrol. Dial. Transplant. 2018, 33, ii29–ii40. [Google Scholar] [CrossRef] [Green Version]
- Koh, D.-M.; Collins, D.J. Diffusion-weighted MRI in the body: Applications and challenges in oncology. AJR Am. J. Roentgenol. 2007, 188, 1622–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenkrantz, A.B.; Padhani, A.R.; Chenevert, T.L.; Koh, D.-M.; De Keyzer, F.; Taouli, B.; Le Bihan, D. Body diffusion kurtosis imaging: Basic principles, applications, and considerations for clinical practice. J. Magn. Reson. Imaging JMRI 2015, 42, 1190–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimaru, D.; Miyati, T.; Suzuki, Y.; Hamada, Y.; Mogi, N.; Funaki, A.; Tabata, A.; Masunaga, A.; Shimada, M.; Tobari, M.; et al. Diffusion kurtosis imaging with the breath-hold technique for staging hepatic fibrosis: A preliminary study. Magn. Reson. Imaging 2018, 47, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Li, Q.; Cheng, Y.; Zhou, L.; Xia, S.; Li, J.; Shen, W. Differentiating mild and substantial hepatic fibrosis from healthy controls: A comparison of diffusion kurtosis imaging and conventional diffusion-weighted imaging. Acta Radiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lu, Y.; Yang, B.; Zhang, C.; Li, J.; Liu, H.; Wang, H.; Wang, D. Diffusion Metrics for Staging Pancreatic Fibrosis and Correlating With Epithelial-Mesenchymal Transition Markers in a Chronic Pancreatitis Rat Model at 11.7T MRI. J. Magn. Reson. Imaging JMRI 2020, 52, 197–206. [Google Scholar] [CrossRef]
- Türkbey, B.; Aras, Ö.; Karabulut, N.; Turgut, A.T.; Akpinar, E.; Alibek, S.; Pang, Y.; Ertürk, Ş.M.; El Khouli, R.H.; Bluemke, D.A.; et al. Diffusion-weighted MRI for detecting and monitoring cancer: A review of current applications in body imaging. Diagn. Interv. Radiol. Ank. Turk. 2012, 18, 46–59. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Tomita, H.; Osada, S.; Watanabe, H.; Imai, H.; Sasaki, Y.; Goshima, S.; Kondo, H.; Kanematsu, M.; Hara, A.; et al. Significance of histopathological evaluation of pancreatic fibrosis to predict postoperative course after pancreatic surgery. Anticancer Res. 2015, 35, 1749–1756. [Google Scholar]
- Muraoka, N.; Uematsu, H.; Kimura, H.; Imamura, Y.; Fujiwara, Y.; Murakami, M.; Yamaguchi, A.; Itoh, H. Apparent diffusion coefficient in pancreatic cancer: Characterization and histopathological correlations. J. Magn. Reson. Imaging JMRI 2008, 27, 1302–1308. [Google Scholar] [CrossRef]
- Sheng, R.F.; Wang, H.Q.; Yang, L.; Jin, K.P.; Xie, Y.H.; Chen, C.Z.; Zeng, M.S. Diffusion kurtosis imaging and diffusion-weighted imaging in assessment of liver fibrosis stage and necroinflammatory activity. Abdom. Radiol. 2017, 42, 1176–1182. [Google Scholar] [CrossRef]
- Hectors, S.J.; Semaan, S.; Song, C.; Lewis, S.; Haines, G.K.; Tewari, A.; Rastinehad, A.R.; Taouli, B. Advanced Diffusion-weighted Imaging Modeling for Prostate Cancer Characterization: Correlation with Quantitative Histopathologic Tumor Tissue Composition—A Hypothesis-generating Study. Radiology 2018, 286, 918–928. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Li, N.; Zhao, W.; Ren, J.; Wei, M.; Yang, Y.; Wang, Y.; Fu, X.; Zhang, Z.; Larson, A.C.; et al. Apparent Diffusion Coefficient and Dynamic Contrast-Enhanced Magnetic Resonance Imaging in Pancreatic Cancer: Characteristics and Correlation with Histopathologic Parameters. J. Comput. Assist. Tomogr. 2016, 40, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Kartalis, N.; Manikis, G.C.; Loizou, L.; Albiin, N.; Zöllner, F.G.; Del Chiaro, M.; Marias, K.; Papanikolaou, N. Diffusion-weighted MR imaging of pancreatic cancer: A comparison of mono-exponential, bi-exponential and non-Gaussian kurtosis models. Eur. J. Radiol. Open 2016, 3, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.-D.; Wu, C.-J.; Bao, M.-L.; Li, H.; Yan, X.; Liu, X.-S.; Shi, H.-B. New RESOLVE-Based Diffusional Kurtosis Imaging in MRI-Visible Prostate Cancer: Effect of Reduced b Value on Image Quality and Diagnostic Effectiveness. Am. J. Roentgenol. 2016, 207, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Nnewihe, A.N.; Grafendorfer, T.; Daniel, B.L.; Calderon, P.; Alley, M.T.; Robb, F.; Hargreaves, B.A. Custom-fitted 16-channel bilateral breast coil for bidirectional parallel imaging. Magn. Reson. Med. 2011, 66, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueira, L.; Brandão, S.; Matos, E.; Nunes, R.G.; Loureiro, J.; Ramos, I.; Ferreira, H.A. Application of the diffusion kurtosis model for the study of breast lesions. Eur. Radiol. 2014, 24, 1197–1203. [Google Scholar] [CrossRef]
- Roethke, M.C.; Kuder, T.A.; Kuru, T.H.; Fenchel, M.; Hadaschik, B.A.; Laun, F.B.; Schlemmer, H.-P.; Stieltjes, B. Evaluation of Diffusion Kurtosis Imaging Versus Standard Diffusion Imaging for Detection and Grading of Peripheral Zone Prostate Cancer. Invest. Radiol. 2015, 50, 483–489. [Google Scholar] [CrossRef]
- Hayano, K.; Miura, F.; Wada, K.; Suzuki, K.; Takeshita, K.; Amano, H.; Toyota, N.; Sano, K.; Asano, T. Diffusion-weighted MR imaging of pancreatic cancer and inflammation: Prognostic significance of pancreatic inflammation in pancreatic cancer patients. Pancreatology 2016, 16, 121–126. [Google Scholar] [CrossRef]
- Watanabe, H.; Kanematsu, M.; Tanaka, K.; Osada, S.; Tomita, H.; Hara, A.; Goshima, S.; Kondo, H.; Kawada, H.; Noda, Y.; et al. Fibrosis and Postoperative Fistula of the Pancreas: Correlation with MR Imaging Findings—Preliminary Results. Radiology 2014, 270, 791–799. [Google Scholar] [CrossRef]
- Barral, M.; Taouli, B.; Guiu, B.; Koh, D.-M.; Luciani, A.; Manfredi, R.; Vilgrain, V.; Hoeffel, C.; Kanematsu, M.; Soyer, P. Diffusion-weighted MR imaging of the pancreas: Current status and recommendations. Radiology 2015, 274, 45–63. [Google Scholar] [CrossRef] [Green Version]
- Klauß, M.; Mayer, P.; Bergmann, F.; Maier-Hein, K.; Hase, J.; Hackert, T.; Kauczor, H.-U.; Grenacher, L.; Stieltjes, B. Correlation of Histological Vessel Characteristics and Diffusion-Weighted Imaging Intravoxel Incoherent Motion-Derived Parameters in Pancreatic Ductal Adenocarcinomas and Pancreatic Neuroendocrine Tumors. Invest. Radiol. 2015, 50, 792–797. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, X.J. Diffusion MRI of cancer: From low to high b-values: High b-Value Diffusion MRI of Cancer. J. Magn. Reson. Imaging 2019, 49, 23–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, J.H.; Helpern, J.A.; Ramani, A.; Lu, H.; Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 2005, 53, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
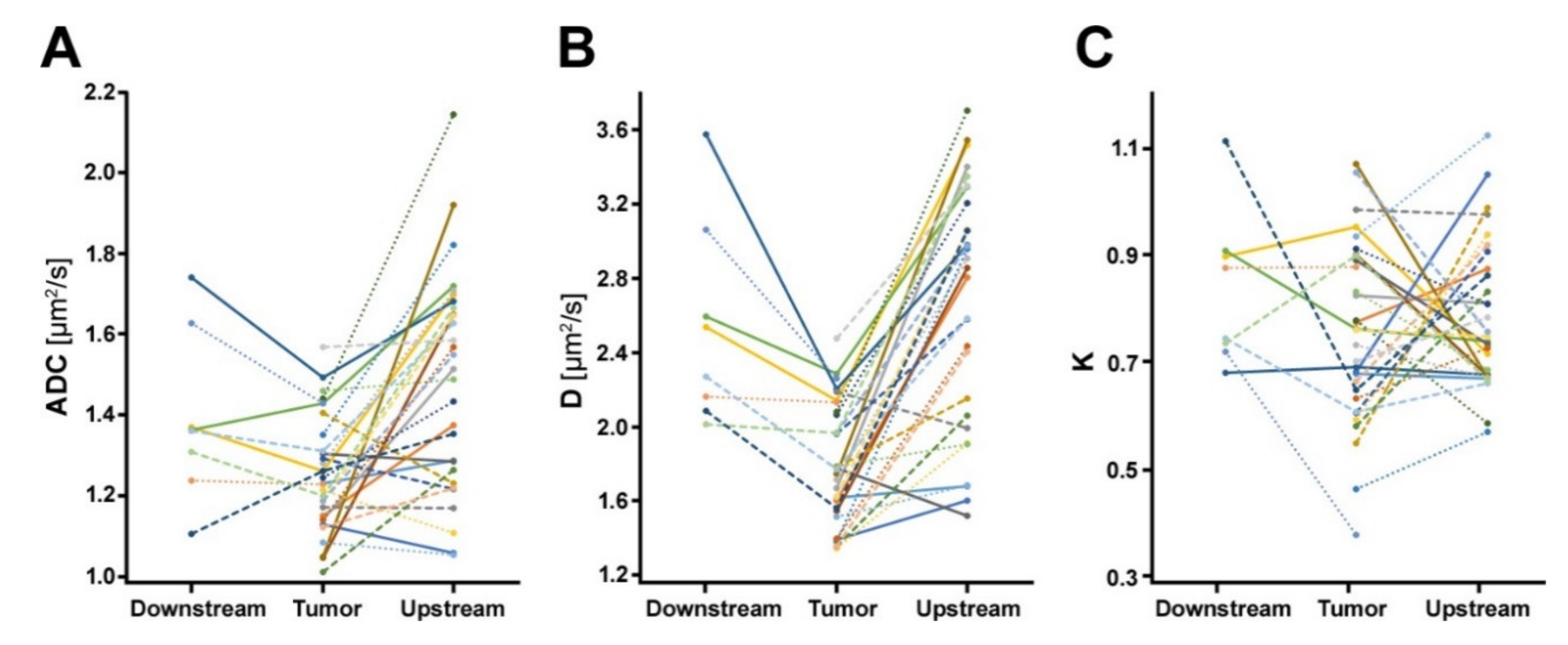



| Tumor (n = 31) | Upstream Parenchyma (n = 29) | Downstream Parenchyma (n = 8) | |
|---|---|---|---|
| ADC [µm2/s] | 1.231 (IQR 1.143 to 1.340) | 1.515 (IQR 1.256 to 1.670) | 1.362 (IQR 1.273 to 1.499) |
| D [µm2/s] | 1.768 (IQR 1.548 to 2.073) | 2.855 (IQR 2.042 to 3.227) | 2.403 (IQR 2.122 to 2.827) |
| K | 0.760 (IQR 0.635 to 0.896) | 0.746 (IQR 0.673 to 0.881) | 0.809 (IQR 0.726 to 0.902) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer, P.; Jiang, Y.; Kuder, T.A.; Bergmann, F.; Khristenko, E.; Steinle, V.; Kaiser, J.; Hackert, T.; Kauczor, H.-U.; Klauß, M.; et al. Diffusion Kurtosis Imaging—A Superior Approach to Assess Tumor–Stroma Ratio in Pancreatic Ductal Adenocarcinoma. Cancers 2020, 12, 1656. https://doi.org/10.3390/cancers12061656
Mayer P, Jiang Y, Kuder TA, Bergmann F, Khristenko E, Steinle V, Kaiser J, Hackert T, Kauczor H-U, Klauß M, et al. Diffusion Kurtosis Imaging—A Superior Approach to Assess Tumor–Stroma Ratio in Pancreatic Ductal Adenocarcinoma. Cancers. 2020; 12(6):1656. https://doi.org/10.3390/cancers12061656
Chicago/Turabian StyleMayer, Philipp, Yixin Jiang, Tristan A. Kuder, Frank Bergmann, Ekaterina Khristenko, Verena Steinle, Jörg Kaiser, Thilo Hackert, Hans-Ulrich Kauczor, Miriam Klauß, and et al. 2020. "Diffusion Kurtosis Imaging—A Superior Approach to Assess Tumor–Stroma Ratio in Pancreatic Ductal Adenocarcinoma" Cancers 12, no. 6: 1656. https://doi.org/10.3390/cancers12061656
APA StyleMayer, P., Jiang, Y., Kuder, T. A., Bergmann, F., Khristenko, E., Steinle, V., Kaiser, J., Hackert, T., Kauczor, H.-U., Klauß, M., & Gaida, M. M. (2020). Diffusion Kurtosis Imaging—A Superior Approach to Assess Tumor–Stroma Ratio in Pancreatic Ductal Adenocarcinoma. Cancers, 12(6), 1656. https://doi.org/10.3390/cancers12061656