Oral Manifestations and Complications in Childhood Acute Myeloid Leukemia
Abstract
1. Introduction
2. Acute Myeloid Leukemia
- (1)
- AML with recurrent cytogenetic translocations;
- (2)
- AML with myelodysplasia-related changes;
- (3)
- Therapy-related myeloid neoplasms;
- (4)
- AML not otherwise specified (NOS);
- (5)
- Myeloid sarcoma;
- (6)
- Myeloid proliferations related to Down syndrome [19].
3. Oral Manifestations
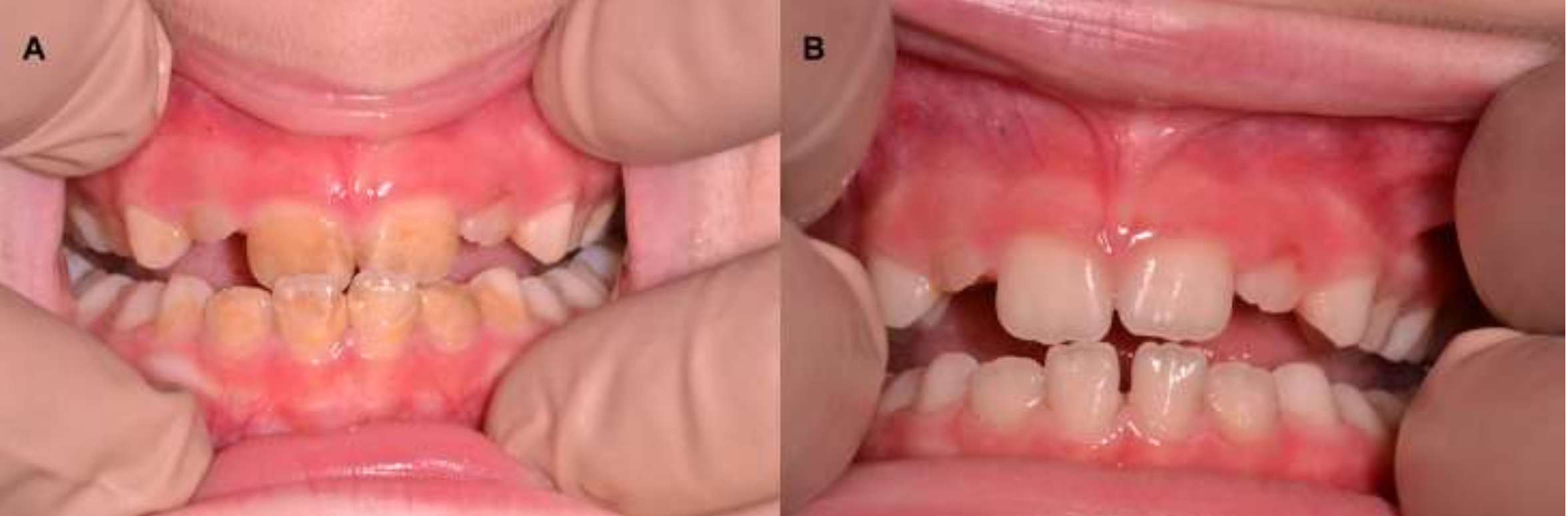
3.1. Gingival Alterations
3.2. Oral Hygiene and Risk of Infections
4. Treatment
The Role of Dentists in Diagnosis and Treatment
5. Long-Term Adverse Effects
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernandes, K.S.; Gallottini, M.; Castro, T.; Amato, M.F.; Lago, J.S.; Silva, P.H.B. Gingival leukemic infiltration as the first manifestation of acute myeloid leukemia. Spec. Care Dent. 2018, 38, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Reenesh, M.; Munishwar, S.; Rath, S.K. Generalised Leukaemic Gingival Enlargement: A Case Report. J. Oral Maxillofac. Res. 2012, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Lupi, S.M.; Baena, A.R.Y.; Cervino, G.; Todaro, C.; Rizzo, S. Long-Term Effects of Acute Myeloid Leukemia Treatment on the Oral System in a Pediatric Patient. Open Dent. J. 2018, 12, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Chowdhri, K.; Tandon, S.; Lamba, A.K.; Faraz, F. Leukemic gingival enlargement: A case report and review of literature. J. Oral Maxillofac. Surg. Med. Pathol. JOMFP 2018, 22, S77–S81. [Google Scholar] [CrossRef]
- Adisen, M.Z.; Yilmaz, S.; Misirlioǧlu, M. Diagnosis of acute myeloid leukemia in a dental hospital; report of a case with severe gingival hypertrophy. Niger. J. Clin. Pr. 2015, 18, 573. [Google Scholar] [CrossRef]
- Sepúlveda, E.; Brethauer, U.; Fernández, E.; Cortés, G.; Mardones, C. Oral manifestations as first clinical sign of acute myeloid leukemia: Report of a case. Pediatr. Dent. 2012, 34, 418–421. [Google Scholar]
- Tsutsumi, I.; Kunisawa, S.; Yoshida, C.; Seki, M.; Komeno, T.; Fushimi, K.; Morita, S.; Imanaka, Y. Impact of oral voriconazole during chemotherapy for acute myeloid leukemia and myelodysplastic syndrome: A Japanese nationwide retrospective cohort study. Int. J. Clin. Oncol. 2019, 24, 1449–1458. [Google Scholar] [CrossRef]
- Shankarapillai, R.; Nair, M.A.; George, R.; Walsh, L.J. Periodontal and gingival parameters in young adults with acute myeloid leukaemia in Kerala, South India. Oral Health Prev. Dent. 2010, 8, 395–400. [Google Scholar]
- Taga, T.; Tomizawa, D.; Takahashi, H.; Adachi, S. Acute myeloid leukemia in children: Current status and future directions. Pediatr. Int. 2016, 58, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Mester, A.; Irimie, A.; Oprita, L.; Dima, D.; Petrushev, B.; Lucaciu, P.O.; Câmpian, R.-S.; Tanase, A. Oral manifestations in stem cell transplantation for acute myeloid leukemia. Med. Hypotheses 2018, 121, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.S.; Faria, F.; Falcão, L.M. Bilateral facial palsy and acute myeloid leukemia: An unusual association. Acta Med. Port. 2012, 25, 250–253. [Google Scholar] [PubMed]
- Walter, R.B.; Estey, E.H. Acute Myeloid Leukemia. Emerg. Cancer Ther. 2011, 2, 219–237. [Google Scholar] [CrossRef]
- Muramatsu, H. Genetic predisposition to pediatric myeloid malignancies. Rinsho Ketsueki 2016, 57, 730–735. [Google Scholar] [PubMed]
- Savage, S.A.; Walsh, M.F. Myelodysplastic Syndrome, Acute Myeloid Leukemia, and Cancer Surveillance in Fanconi Anemia. Hematol. Clin. N. Am. 2018, 32, 657–668. [Google Scholar] [CrossRef]
- Callea, M.; Bellacchio, E.; Fattori, F.; Bertini, E.; Callea, F.; Cammarata-Scalisi, F. Acute myeloid leukemia in a 3 years old child with cleidocranial dysplasia. Leuk. Lymphoma 2015, 57, 2189–2191. [Google Scholar] [CrossRef]
- Schnerch, D.; Lausch, E.; Becker, H.; Felthaus, J.; Pfeifer, D.; Mundlos, S.; Engelhardt, M.; Schwabe, M.; Wäsch, R. Up-regulation of RUNX2 in acute myeloid leukemia in a patient with an inherent RUNX2 haploinsufficiency and cleidocranial dysplasia. Leuk. Lymphoma 2014, 55, 1930–1932. [Google Scholar] [CrossRef]
- Callea, M.; Fattori, F.; Bertini, E.; Cammarata-Scalisi, F.; Callea, F.; Bellacchio, E. Blood malignancies presenting with mutations at equivalent residues in RUNX1–2 suggest a common leukemogenic pathway. Leuk. Lymphoma 2017, 58, 2002–2004. [Google Scholar] [CrossRef]
- Thesleff, I. The genetic basis of tooth development and dental defects. Am. J. Med. Genet. Part A 2006, 140, 2530–2535. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Zwaan, C.M.; Kolb, E.A.; Reinhardt, D.; Abrahamsson, J.; Adachi, S.; Aplenc, R.; De Bont, E.S.; De Moerloose, B.; Dworzak, M.; Gibson, B.E.; et al. Collaborative Efforts Driving Progress in Pediatric Acute Myeloid Leukemia. J. Clin. Oncol. 2015, 33, 2949–2962. [Google Scholar] [CrossRef]
- Im, H.J. Current treatment for pediatric acute myeloid leukemia. Blood Res. 2018, 53, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Gowda, T.M.; Thomas, R.; Shanmukhappa, S.M.; Agarwal, G.; Mehta, D.S. Gingival enlargement as an early diagnostic indicator in therapy-related acute myeloid leukemia: A rare case report and review of literature. J. Indian Soc. Periodontol. 2013, 17, 248–252. [Google Scholar] [CrossRef] [PubMed]
- George, N.; Santhosh, V.C.; Kumar, H.; Gopal, S. Gingival enlargement in myelodysplastic syndrome. J. Indian Soc. Periodontol. 2015, 19, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Gulati, R.; Ratre, M.S.; Khetarpal, S.; Parihar, A. Regular oral screening and vigilance: Can it be a potential lifesaver? J. Indian Soc. Periodontol. 2018, 22, 171–173. [Google Scholar] [CrossRef]
- Lynch, M.; Ship, I. Oral manifestations of leukemia: A postdiagnostic study. J. Am. Dent. Assoc. 1967, 75, 1139–1144. [Google Scholar] [CrossRef]
- Dreizen, S.; McCredie, K.B.; Keating, M.J.; Luna, M.A. Malignant gingival and skin “infiltrates” in adult leukemia. Oral Surg. Oral Med. Oral Pathol. 1983, 55, 572–579. [Google Scholar] [CrossRef]
- Ishikawa, S.; Kato, Y.; Kabasawa, T.; Yoshioka, C.; Kitabatake, K.; Yamakawa, M.; Ishizawa, K.; Iino, M. A case of myeloid sarcoma of the mandibular gingiva as extramedullary relapse of acute myeloid leukemia. Oral Maxillofac. Surg. 2019, 24, 121–126. [Google Scholar] [CrossRef]
- Allareddy, V.; Prakasam, S.; Allareddy, V.; I Martinez-Schlurmann, N.; Rampa, S.; Nalliah, R.; Eswaran, S.V.K.; Elangovan, S. Poor Oral Health Linked with Increased Risk of Infectious Complications in Adults with Leukemia. J. Mass. Dent. Soc. 2015, 64, 38–42. [Google Scholar]
- Reynolds, M.A.; Minah, G.E.; Peterson, D.E.; Weikel, D.S.; Williams, L.T.; Overholser, C.D.; DePaola, L.G.; Suzuki, J.B. Periodontal disease and oral microbial successions during myelosuppressive cancer chemotherapy*. J. Clin. Periodontol. 1989, 16, 185–189. [Google Scholar] [CrossRef]
- Djuric, M.; Hillier-Kolarov, V.; Belic, A.; Janković, L. Mucositis prevention by improved dental care in acute leukemia patients. Support. Care Cancer 2005, 14, 137–146. [Google Scholar] [CrossRef]
- Lauritano, D.; Petruzzi, M. Decayed, missing and filled teeth index and dental anomalies in long-term survivors leukaemic children: A prospective controlled study. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e977–e980. [Google Scholar] [CrossRef] [PubMed]
- Zarina, R.S.R.; Nik-Hussein, N.N. Dental abnormalities of a long-term survivor of a childhood hematological malignancy: Literature review and report of a case. J. Clin. Pediatr. Dent. 2005, 29, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fantasia, J.E.; Kaplan, R. Oral Manifestations of Acute Myelomonocytic Leukemia: A Case Report and Review of the Classification of Leukemias. J. Periodontol. 2002, 73, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Demirer, S.; Özdemir, H.; Şencanc, M.; Marakoğlud, I. Gingival Hyperplasia as an Early Diagnostic Oral Manifestation in Acute Monocytic Leukemia: A Case Report. Eur. J. Dent. 2007, 1, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Ellegaard, B.; Bergmann, O.J.; Ellegaard, J. Effect of plaque removal on patients with acute leukemia. J. Oral Pathol. Med. 1989, 18, 54–58. [Google Scholar] [CrossRef] [PubMed]
- McGaw, W.; Belch, A. Oral complications of acute leukemia: Prophylactic impact of a chlorhexidine mouth rinse regimen. Oral Surg. Oral Med. Oral Pathol. 1985, 60, 275–280. [Google Scholar] [CrossRef]
- Fu, R.; Gundrum, J.; Sung, A.H. Health-care utilization and outcomes of patients at high risk of invasive fungal infection. Clin. Outcomes Res. 2018, 10, 371–387. [Google Scholar] [CrossRef]
- Gürgan, C.; Ozcan, M.; Karakuş, Ö.; Zincircioğlu, G.; Arat, M.; Soydan, E.; Topçuoğlu, P.; Gürman, G.; Bostancı, H. Periodontal status and post-transplantation complications following intensive periodontal treatment in patients underwent allogenic hematopoietic stem cell transplantation conditioned with myeloablative regimen. Int. J. Dent. Hyg. 2012, 11, 84–90. [Google Scholar] [CrossRef]
- Grzegorczyk-Jaźwińska, A.; Kozak, I.; Karakulska-Prystupiuk, E.; Rokicka, M.; Ganowicz, E.; Dwilewicz-Trojaczek, J.; Górska, R. Transient oral cavity and skin complications after mucositis preventing therapy (palifermin) in a patient after allogeneic PBSCT. Case history. Adv. Med. Sci. 2006, 51, 66–68. [Google Scholar]
- Pour-Fard-Pachekenari, A.K.; Rahmani, A.; Ghahramanian, A.; Asghari-Jafarabadi, M.; Onyeka, T.C.; Davoodi, A. The effect of an oral care protocol and honey mouthwash on mucositis in acute myeloid leukemia patients undergoing chemotherapy: A single-blind clinical trial. Clin. Oral Investig. 2018, 23, 1811–1821. [Google Scholar] [CrossRef]
- Epstein, J.B.; Raber-Durlacher, J.E.; Huysmans, M.-C.; Schoordijk, M.C.; Cheng, J.E.; Bensadoun, R.-J.; Arany, P.R. Photobiomodulation Therapy Alleviates Tissue Fibroses Associated with Chronic Graft-Versus-Host Disease: Two Case Reports and Putative Anti-Fibrotic Roles of TGF-β. Photomed. Laser Surg. 2018, 36, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Busjan, R.; Hasenkamp, J.; Schmalz, G.; Haak, R.; Trümper, L.; Ziebolz, D. Oral health status in adult patients with newly diagnosed acute leukemia. Clin. Oral Investig. 2017, 22, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Bewersdorf, J.P.; Shallis, R.M.; Wang, R.; Huntington, S.F.; Perreault, S.; Ma, X.; Zeidan, A.M. Healthcare expenses for treatment of acute myeloid leukemia. Expert Rev. Hematol. 2019, 12, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.O.F.; Júnior, A.S.; Noce, C.W.; Ferreira, M.; Maiolino, A.; Torres, S.R. The frequency of oral conditions detected in hematology inpatients. Hematol. Transfus. Cell Ther. 2018, 40, 240–244. [Google Scholar] [CrossRef]
- Dahl, J.E. Immediate and delayed effects of repeated doxorubicin injections on rat incisor mesenchymal cells. Acta Odontol. Scand. 1985, 43, 155–162. [Google Scholar] [CrossRef]
- Lyaruu , D.M.; van Duin, M.A.; Bervoets, T.J.; Bronckers, A.L.; Woltgens, J.H. Daunorubicin-Induced Pathology in the Developing Hamster Molar Tooth Germ in vitro. Cancer Detect. Prev. 1999, 23, 343–350. [Google Scholar] [CrossRef]
- Proc, P.; Szczepanska, J.; Skiba, A.; Zubowska, M.; Fendler, W.; Młynarski, W. Dental Anomalies as Late Adverse Effect among Young Children Treated for Cancer. Cancer Res. Treat. 2016, 48, 658–667. [Google Scholar] [CrossRef]
- Hölttä, P.; Alaluusua, S.; Saarinen-Pihkala, U.M.; Peltola, J.; Hovi, L. Agenesis and microdontia of permanent teeth as late adverse effects after stem cell transplantation in young children. Cancer 2004, 103, 181–190. [Google Scholar] [CrossRef]
- Locatelli, F.; Merli, P.; Pagliara, D.; Pira, G.L.; Falco, M.; Pende, D.; Rondelli, R.; Lucarelli, B.; Brescia, L.P.; Masetti, R.; et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after αβ T-cell and B-cell depletion. Blood 2017, 130, 677–685. [Google Scholar] [CrossRef]
- Pedersen, L.B.; Clausen, N.; Schrøder, H.; Schmidt, M.; Poulsen, S. Microdontia and hypodontia of premolars and permanent molars in childhood cancer survivors after chemotherapy. Int. J. Paediatr. Dent. 2011, 22, 239–243. [Google Scholar] [CrossRef]
- Craig, S.A.; Baker, S.R.; Rodd, H. How do children view other children who have visible enamel defects? Int. J. Paediatr. Dent. 2014, 25, 399–408. [Google Scholar] [CrossRef] [PubMed]

| Findings |
|---|
| Petechiae |
| Spontaneous bleeding |
| Mucosal ulceration |
| Gingival enlargement with or without necrosis |
| Mucosal pallor |
| Enamel discoloration |
| Herpetic opportunistic infections |
| Candidiasis |
| Temporomandibular joint arthritis |
| Osteolytic lesions in the mandible |
| Palatal pigmentation |
| Tooth pain and mobility |
| Hemorrhagic bullae on the tongue |
| Cracked lips |
| Parotid swelling |
| Chin numbness |
| Caries |
| Enamel Malformation | Discoloration |
|---|---|
| Radicular anomalies | Hypoplasia |
| Resorbed or tapered roots | |
| Early apical closure | |
| Delayed dental development | |
| Dental impaction | |
| Dental shape anomalies | Microdontia |
| Macrodontia | |
| Taurodontia | |
| Anomalies in numbers | Hypodontia |
| Supernumerary teeth |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cammarata-Scalisi, F.; Girardi, K.; Strocchio, L.; Merli, P.; Bernardin, A.G.; Galeotti, A.; Magliarditi, F.; Inserra, A.; Callea, M. Oral Manifestations and Complications in Childhood Acute Myeloid Leukemia. Cancers 2020, 12, 1634. https://doi.org/10.3390/cancers12061634
Cammarata-Scalisi F, Girardi K, Strocchio L, Merli P, Bernardin AG, Galeotti A, Magliarditi F, Inserra A, Callea M. Oral Manifestations and Complications in Childhood Acute Myeloid Leukemia. Cancers. 2020; 12(6):1634. https://doi.org/10.3390/cancers12061634
Chicago/Turabian StyleCammarata-Scalisi, Francisco, Katia Girardi, Luisa Strocchio, Pietro Merli, Annelyse Garret Bernardin, Angela Galeotti, Fabio Magliarditi, Alessandro Inserra, and Michele Callea. 2020. "Oral Manifestations and Complications in Childhood Acute Myeloid Leukemia" Cancers 12, no. 6: 1634. https://doi.org/10.3390/cancers12061634
APA StyleCammarata-Scalisi, F., Girardi, K., Strocchio, L., Merli, P., Bernardin, A. G., Galeotti, A., Magliarditi, F., Inserra, A., & Callea, M. (2020). Oral Manifestations and Complications in Childhood Acute Myeloid Leukemia. Cancers, 12(6), 1634. https://doi.org/10.3390/cancers12061634






