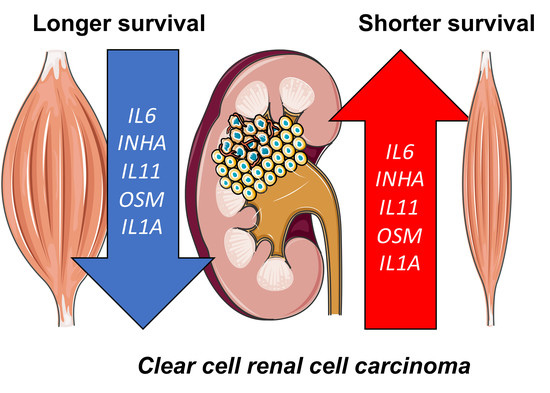
The Combination of Low Skeletal Muscle Mass and High Tumor Interleukin-6 Associates with Decreased Survival in Clear Cell Renal Cell Carcinoma
Abstract
1. Introduction
2. Results
2.1. Patient Clinical Characteristics
2.2. Body Composition
2.3. Characteristics Associated with Survival
2.4. Body Composition Versus Tumor Gene Expression
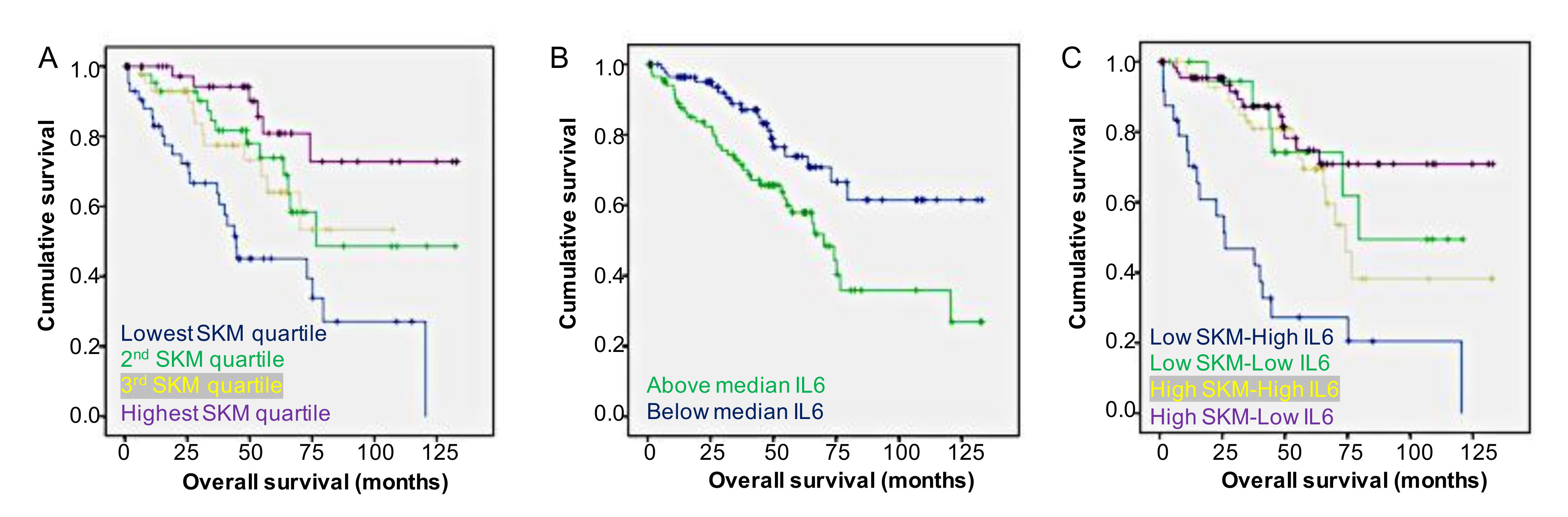
2.5. Body Composition and Tumor Gene Expression Versus Survival
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Chow, W.-H.; Devesa, S.S.; Warren, J.L.; Fraumeni, J.J.F. Rising Incidence of Renal Cell Cancer in the United States. JAMA 1999, 281, 1628–1631. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.K.; Hong, S.K.; Lee, S.; Kwak, C.; Oh, J.J.; Jeong, C.W.; Kim, Y.J.; Kang, S.H.; Hong, S.-H.; Byun, S.-S. Prognostic Value of Body Mass Index According to Histologic Subtype in Nonmetastatic Renal Cell Carcinoma: A Large Cohort Analysis. Clin. Genitourin. Cancer 2015, 13, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Feng, C.; Song, N.; Fang, Z.; Xia, G.; Jiang, H.-W.; Hua, L.-X.; Ding, Q. Paraneoplastic symptoms: Cachexia, polycythemia, and hypercalcemia are, respectively, related to vascular endothelial growth factor (VEGF) expression in renal clear cell carcinoma. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 1820–1825. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Song, N.; Feng, C.; Xia, G.; Jiang, H.-W.; Hua, L.-X.; Ding, Q. Is There an Association between Advanced Stage of Renal Cell Carcinoma and Paraneoplastic Syndrome? Med. Princ. Pract. 2012, 21, 370–374. [Google Scholar] [CrossRef]
- Kim, H.L.; Belldegrun, A.S.; Freitas, D.G.; Bui, M.H.; Han, K.-R.; Dorey, F.J.; Figlin, R.A. Paraneoplastic Signs and Symptoms of Renal Cell Carcinoma: Implications for Prognosis. J. Urol. 2003, 170, 1742–1746. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Han, K.-R.; Zisman, A.; Figlin, R.A.; Belldegrun, A.S. Cachexia-Like Symptoms Predict a Worse Prognosis in Localized T1 Renal Cell Carcinoma. J. Urol. 2004, 171, 1810–1813. [Google Scholar] [CrossRef]
- Baracos, V. Pitfalls in defining and quantifying cachexia. J. Cachexia Sarcopenia Muscle 2011, 2, 71–73. [Google Scholar] [CrossRef]
- Von Haehling, S.; Anker, S.D. Cachexia as a major underestimated and unmet medical need: facts and numbers. J. Cachex-Sarcopenia Muscle 2010, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Blum, D.; Stene, G.B.; Solheim, T.S.; Fayers, P.; Hjermstad, M.J.; Baracos, V.E.; Fearon, K.; Strasser, F.; Kassa, S. Validation Fo the Consensus-Definition for Cancer Cachexia and Evaluation of a Classification Model—a Study Based on Data from an International Multicentre Project (Epcrc-Csa). Ann. Oncol. 2014, 25, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Fearon, K.C. Cachexia: Prevalence and impact in medicine. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Choi, H.Y.; Lee, H.M. Influence of Body Mass Index, Smoking, and Blood Pressure on Survival of Patients with Surgically-Treated, Low Stage Renal Cell Carcinoma: A 14-Year Retrospective Cohort Study. J. Korean Med. Sci. 2013, 28, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Shoshtari, K.Z.; Caracciolo, J.T.; Fishman, M.; Poch, M.A.; Pow-Sang, J.; Sexton, W.J.; Spiess, P.E. Sarcopenia as a predictor of overall survival after cytoreductive nephrectomy for metastatic renal cell carcinoma. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 339.e17–339.e23. [Google Scholar] [CrossRef] [PubMed]
- Haferkamp, A.; Pritsch, M.; Bedke, J.; Wagener, N.; Pfitzenmaier, J.; Buse, S.; Hohenfellner, M. The Infuence of Body Mass Index on the Long-Term Survival of Patients with Renal Cell Carcinoma after Tumour Nephrectomy. BJU Int. 2008, 101, 1243–1246. [Google Scholar] [CrossRef]
- Morgan, T.M.; Tang, D.; Stratton, K.L.; Barocas, D.A.; Anderson, C.B.; Gregg, J.R.; Chang, S.S.; Cookson, M.S.; Herrell, S.B.; Smith, J.A., Jr.; et al. Preoperative Nutrtional Status Is an Important Predictor of Survival in Patients Undergoing Surgery for Renal Cell Carcinoma. Eur. J. Urol. 2011, 59, 923–928. [Google Scholar] [CrossRef]
- Antoun, S.; Lanoy, E.; Iacovelli, R.; Albiges-Sauvin, L.; Loriot, Y.; Merad-Taoufik, M.; Fizazi, K.; Di Palma, M.; Baracos, V.; Escudier, B. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer 2013, 119, 3377–3384. [Google Scholar] [CrossRef]
- Corcoran, A.; Kaffenberger, S.; Clark, P.E.; Walton, J.; Handorf, E.; Piotrowski, Z.; Tomaszewski, J.J.; Ginzburg, S.; Mehrazin, R.; Plimack, E.; et al. Hypoalbuminaemia is associated with mortality in patients undergoing cytoreductive nephrectomy. BJU Int. 2014, 116, 351–357. [Google Scholar] [CrossRef]
- Antoun, S.; Baracos, V.; Birdsell, L.; Escudier, B.; Sawyer, M.B. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann. Oncol. 2010, 21, 1594–1598. [Google Scholar] [CrossRef]
- Zimmers, T.A.; Fishel, M.; Bonetto, A. STAT3 in the systemic inflammation of cancer cachexia. Semin. Cell Dev. Biol. 2016, 54, 28–41. [Google Scholar] [CrossRef]
- Tuomisto, A.; Mäkinen, M.J.; Väyrynen, J.P. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J. Gastroenterol. 2019, 25, 4383–4404. [Google Scholar] [CrossRef]
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer cachexia, mechanism and treatment. World J. Gastrointest. Oncol. 2015, 7, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; López-Soriano, F.J. The role of cytokines in cancer cachexia. Med. Res. Rev. 1999, 19, 223–248. [Google Scholar] [CrossRef]
- Belizário, J.; Fontes-Oliveira, C.C.; Borges, J.P.; Kashiabara, J.A.; Vannier, E. Skeletal muscle wasting and renewal: A pivotal role of myokine IL-6. SpringerPlus 2016, 5, 619. [Google Scholar] [CrossRef] [PubMed]
- Onesti, J.K.; Guttridge, D.C. Inflammation Based Regulation of Cancer Cachexia. BioMed Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Narsale, A.; Carson, J.A. Role of interleukin-6 in cachexia: Therapeutic implications. Curr. Opin. Support. Palliat. Care 2014, 8, 321–327. [Google Scholar] [CrossRef]
- McDonald, J.; McMillan, D.C.; Laird, B.J. Targeting IL-1α in cancer cachexia. Curr. Opin. Support. Palliat. Care 2018, 12, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.E.; Risbridger, G.P.; Gold, E. The therapeutic potential of blocking the activin signalling pathway. Cytokine Growth Factor Rev. 2013, 24, 477–484. [Google Scholar] [CrossRef]
- Guttridge, D.C. A TGF-β pathway associated with cancer cachexia. Nat. Med. 2015, 21, 1248–1249. [Google Scholar] [CrossRef][Green Version]
- Dosquet, C.; Schaetz, A.; Faucher, C.; Lepage, E.; Wautier, J.-L.; Richard, F.; Cabane, J. Tumour necrosis factor-α, interleukin-1β and interleukin-6 in patients with renal cell carcinoma. Eur. J. Cancer 1994, 30, 162–167. [Google Scholar] [CrossRef]
- Blay, J.Y.; Negrier, S.; Combaret, V.; Attali, S.; Goillot, E.; Merrouche, Y.; Mercatello, A.; Ravault, A.; Tourani, J.M.; Moskovtchenko, J.F. Serum level of interleukin 6 as a prognosis factor in metastatic renal cell carcinoma. Cancer Res. 1992, 52, 3317–3322. [Google Scholar]
- Blay, J.-Y.; Rossi, J.-F.; Wijdenes, J.; Menetrier-Caux, C.; Schemann, S.; Negrier, S.; Phillip, T.; Favrot, M. Role of Interleukin-6 in the Paraneoplastic Inflammatory Syndrome Associated with Renal-Cell Carcinoma. Int. J. Cancer 1997, 72, 424–430. [Google Scholar] [CrossRef]
- Fukushima, H.; Nakanishi, Y.; Kataoka, M.; Tobisu, K.-I.; Koga, F. Prognostic Significance of Sarcopenia in Patients with Metastatic Renal Cell Carcinoma. J. Urol. 2016, 195, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Waalkes, S.; Merseburger, A.S.; Kramer, M.W.; Herrmann, T.R.W.; Wegener, G.; Rustemeier, J.; Hofmann, R.; Schrader, M.; Kuczyk, M.A.; Schrader, A.J. Obesity is associated with improved survival in patients with organ-confined clear-cell kidney cancer. Cancer Causes Control. 2010, 21, 1905–1910. [Google Scholar] [CrossRef] [PubMed]
- Steffens, S.; Grunwald, V.; Ringe, K.I.; Seidel, C.; Eggers, H.; Schrader, M.; Wacker, F.; Kuczyk, M.A.; Schrader, A.J. Does Obesity Influence the Prognosis of Metastatic Renal Cell Carcinoma in Patientstreated with Vascular Endothelial Growth Factor-Targeted Therapy? Oncologist 2011, 16, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.-H.; Dong, L.M.; Devesa, S.S. Epidemiology and risk factors for kidney cancer. Nat. Rev. Urol. 2010, 7, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Lowrance, W.T.; Thompson, R.H.; Yee, D.S.; Kaag, M.; Donat, S.; Russo, P. Obesity is associated with a higher risk of clear-cell renal cell carcinoma than with other histologies. BJU Int. 2009, 105, 16–20. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef]
- Mohamed-Ali, V.; Goodrick, S.; Rawesh, A.; Katz, D.R.; Miles, J.M.; Yudkin, J.S.; Klein, S.; Coppack, S. Subcutaneous Adipose Tissue Releases Interleukin-6, But Not Tumor Necrosis Factor-α, In Vivo. J. Clin. Endocrinol. Metab. 1997, 82, 4196–4200. [Google Scholar] [CrossRef]
- Fain, J.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the Release of Adipokines by Adipose Tissue, Adipose Tissue Matrix, and Adipocytes from Visceral and Subcutaneous Abdominal Adipose Tissues of Obese Humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef]
- Fried, S.; Bunkin, D.; Greenberg, A. Omental and Subcutaneous Adipose Tissue of Obese Subjects Release Interleukin-6: Depot Difference Ad Regulation by Glucocorticoid. J. Clin. Endocrinol. Metab. 1998, 83, 847–850. [Google Scholar]
- Hsu, C.-P.; Chung, Y.-C. Influence of interleukin-6 on the invasiveness of human colorectal carcinoma. Anticancer Res. 2007, 26, 4607–4614. [Google Scholar]
- Chung, Y.-C.; Chang, Y.-F. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J. Surg. Oncol. 2003, 83, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, A.; Aydoğdu, T.; Kunzevitzky, N.; Guttridge, D.C.; Khuri, S.; Koniaris, L.G.; Zimmers, T.A. STAT3 Activation in Skeletal Muscle Links Muscle Wasting and the Acute Phase Response in Cancer Cachexia. PLoS ONE 2011, 6, e22538. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, A.; Aydogdu, T.; Jin, X.; Zhang, Z.; Zhan, R.; Puzis, L.; Koniaris, L.G.; Zimmers, T.A. Jak/Stat3 Pathway Inhibition Blocks Skeletal Muscle Wasting Downstrea of Il-6 and in Experimental Cancer Cachexia. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E410–E421. [Google Scholar] [CrossRef] [PubMed]
- Kümmell, S.B.; Frey, E. Range of Movement in Ray I of Manus and Pes and the Prehensility of the Autopodia in the Early Permian to Late Cretaceous Non-Anomodont Synapsida. PLoS ONE 2014, 9, e113911. [Google Scholar] [CrossRef]
- Clark, K.; Vendt, B.; Smith, K.; Freymann, J.; Kirby, J.; Koppel, P.; Moore, S.; Phillips, S.; Maffitt, D.; Pringle, M.; et al. The Cancer Imaging Archive (TCIA): Maintaining and Operating a Public Information Repository. J. Digit. Imaging 2013, 26, 1045–1057. [Google Scholar] [CrossRef]
- Radiology Data from The Cancer Genome Atlas Kidney Renal Clear Cell Carcinoma [TCGA-KIRC] Collection. The Cancer Imaging Archive. Available online: http://doi.org/10.7937/K9/TCIA.2016.V6PBVTDR (accessed on 1 June 2020).
| Variable | No. (%) | Male/Female | p Value |
|---|---|---|---|
| Age, mean years (SD) | 59.7 (12.5) | 0.09 | |
| Males | 58.6 (12.6) | ||
| Females | 61.6 (12.1) | ||
| Tumor Grade | |||
| Grade 1 | 0 (0) | 0/0 | |
| Grade 2 | 87 (40) | 48/39 | |
| Grade 3 | 94 (43) | 69/25 | 0.123 |
| Grade 4 | 36 (17) | 22/14 | |
| AJCC Stage | |||
| Stage 1 | 112 (52) | 71/41 | |
| Stage 2 | 18 (8) | 17/1 | |
| Stage 3 | 54 (25) | 34/20 | 0.459 |
| Stage 4 | 33 (15) | 17/16 | |
| Tumor Laterality | |||
| Right | 116 (53) | 78/38 | 0.295 |
| Left | 101 (47) | 61/40 | |
| Variation | Mean SKM, cm2 (SD) | p Value | Mean VAT, cm2 (SD) | p Value | Mean SCAT, cm2 (SD) | p Value |
|---|---|---|---|---|---|---|
| Overall | 155.1 (41.3) | 179.3 (109.9) | 200.8 (93.3) | |||
| Top Quartile | 192.3 (38.1) | <0.001 | 310.0 (85.3) | <0.001 | 326.6 (73.3) | <0.001 |
| Bottom Quartile | 121.9 (28.6) | <0.001 | 65.2 (41.8) | <0.001 | 104.5 (27.0) | <0.001 |
| Age | ||||||
| <59.7 years | 169.3 (41.3) | <0.001 | 177.4 (107.8) | 0.80 | 205.4 (94.5) | 0.49 |
| ≥59.7 years | 140.1 (35.6) | 181.2 (112.5) | 195.5 (92.1) | |||
| AJCC Stage | ||||||
| Stage I | 159.8 (42.3) | <0.001 | 193.6 (109.7) | <0.001 | 212.3 (99.0) | 0.16 |
| Stage II | 181.5 (37.6) | 247.9 (143.4) | 204.4 (75.3) | |||
| Stage III | 149.4 (39.3) | 165.2 (143.4) | 197.3 (93.4) | |||
| Stage IV | 133.7 (31.2) | 116.0 (71.0) | 166.2 (77.1) | |||
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variation | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Overall | ||||
| Age | ||||
| <59.7 years | Ref | 0.004 | Ref | 0.440 |
| ≥59.7 years | 2.1 (1.3–3.6) | 1.3 (0.7–2.6) | ||
| SKM mass | ||||
| Top 3 quartiles | Ref | <0.001 | Ref | 0.081 |
| Lowest quartile | 3.2 (2.0–5.3) | 1.8 (0.9–3.5) | ||
| VAT mass | ||||
| Top 3 quartiles | Ref | 0.03 | Ref | 0.61 |
| Lowest quartile | 1.8 (1.1–3.0) | 1.2 (0.6–2.6) | ||
| SCAT mass | ||||
| Top 3 quartiles | Ref | <0.001 | Ref | 0.027 |
| Lowest quartile | 2.7 (1.6–4.7) | 2.4 (1.1–5.4) | ||
| Tumor Grade | ||||
| Grade 1/2 | Ref | 0.067 | Ref | 0.10 |
| Grade 3/4 | 1.7 (0.96–2.9) | 0.52 (0.2–1.1) | ||
| AJCC Stage | ||||
| Stage 1/2 | Ref | <0.001 | Ref | <0.001 |
| Stage 3/4 | 4.0 (2.4–6.8) | 7.0 (3.3–14.5) | ||
| Laterality | ||||
| Right | Ref | 0.023 | Ref | 0.004 |
| Left | 1.8 (1.1–2.9) | 2.3 (1.3–4.2) | ||
| Sex | ||||
| Male | Ref | 0.123 | Not Included | |
| Female | 1.5 (0.9–2.3) | |||
| Tissue | Gene | Correlation Coefficient | p Value |
|---|---|---|---|
| SKM | INHBB TGFB2 | 0.149 0.150 | 0.029 0.028 |
| VAT | INHBB CCL2 | 1.79 −0.172 | 0.009 0.012 |
| SCAT | CNTF | 0.167 | 0.027 |
| Gene | Correlation Coefficient | Hazard Ratio | p Value |
| IL6 | 0.835 | 2.31 | <0.0001 |
| IL11 | 0.700 | 2.01 | <0.0001 |
| INHA | 0.622 | 1.86 | 0.0001 |
| OSM | 0.585 | 1.80 | 0.0004 |
| IL1A | 0.483 | 1.62 | 0.003 |
| TGFB1 | 0.422 | 1.52 | 0.008 |
| CLCF1 | 0.400 | 1.49 | 0.01 |
| Group | Median OS (Months) | p Value | Hazard Ratio | 95% CI |
|---|---|---|---|---|
| SKM/IL6 | ||||
| High SKM/Low IL6 | Not Defined a | <0.001 | Reference | |
| High SKM/High IL6 | 79.5 | 1.67 | 0.81–3.45 | |
| Low SKM/Low IL6 | 74.2 | 1.45 | 0.54–3.87 | |
| Low SKM/High IL6 | 26.1 | 5.95 | 2.86–12.38 | |
| SKM/INHA | ||||
| High SKM/Low INHA | Not Defined a | <0.001 | Reference | |
| High SKM/High INHA | 70.2 | 1.82 | 0.88–3.77 | |
| Low SKM/Low INHA | Not Defined a | 3.17 | 1.24–8.10 | |
| Low SKM/High INHA | 40.00 | 4.71 | 2.24–9.87 | |
| SKM/IL11 | ||||
| High SKM/Low IL11 | Not Defined a | <0.001 | Reference | |
| High SKM/High IL11 | Not Defined a | 1.39 | 0.68–2.84 | |
| Low SKM/Low IL11 | 73.0 | 2.54 | 1.08–5.97 | |
| Low SKM/High IL11 | 40.0 | 4.71 | 2.24–9.94 | |
| SKM/OSM | ||||
| High SKM/Low OSM | Not Defined a | <0.001 | Reference | |
| High SKM/High OSM | Not Defined a | 0.83 | 0.41–1.67 | |
| Low SKM/Low OSM | 79.5 | 1.45 | 0.57–3.71 | |
| Low SKM/High OSM | 37.8 | 4.10 | 2.08–8.06 | |
| SKM/IL1A | ||||
| High SKM/Low IL1A | Not Defined a | 0.001 | Reference | |
| High SKM/High IL1A | Not Defined a | 0.93 | 0.47–1.87 | |
| Low SKM/Low IL1A | 75.2 | 2.40 | 0.99–5.85 | |
| Low SKM/High IL1A | 43.9 | 2.84 | 1.42–5.68 | |
| SKM/TGFB1 | ||||
| High SKM/Low TGFB1 | Not Defined a | <0.001 | Reference | |
| High SKM/High TGFB1 | Not Defined a | 1.31 | 0.65–2.66 | |
| Low SKM/Low TGFB1 | 41.0 | 3.95 | 1.74–8.93 | |
| Low SKM/High TGFB1 | 44.6 | 3.15 | 1.50–6.60 | |
| SKM/CLCF1 | ||||
| High SKM/Low CLCF1 | Not Defined a | <0.001 | Reference | |
| High SKM/High CLCF1 | Not Defined a | 0.53 | 0.26–1.09 | |
| Low SKM/Low CLCF1 | 75.2 | 1.44 | 0.65–3.18 | |
| Low SKM/High CLCF1 | 26.1 | 3.44 | 1.74–6.82 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kays, J.K.; Koniaris, L.G.; Cooper, C.A.; Pili, R.; Jiang, G.; Liu, Y.; Zimmers, T.A. The Combination of Low Skeletal Muscle Mass and High Tumor Interleukin-6 Associates with Decreased Survival in Clear Cell Renal Cell Carcinoma. Cancers 2020, 12, 1605. https://doi.org/10.3390/cancers12061605
Kays JK, Koniaris LG, Cooper CA, Pili R, Jiang G, Liu Y, Zimmers TA. The Combination of Low Skeletal Muscle Mass and High Tumor Interleukin-6 Associates with Decreased Survival in Clear Cell Renal Cell Carcinoma. Cancers. 2020; 12(6):1605. https://doi.org/10.3390/cancers12061605
Chicago/Turabian StyleKays, Joshua K., Leonidas G. Koniaris, Caleb A. Cooper, Roberto Pili, Guanglong Jiang, Yunlong Liu, and Teresa A. Zimmers. 2020. "The Combination of Low Skeletal Muscle Mass and High Tumor Interleukin-6 Associates with Decreased Survival in Clear Cell Renal Cell Carcinoma" Cancers 12, no. 6: 1605. https://doi.org/10.3390/cancers12061605
APA StyleKays, J. K., Koniaris, L. G., Cooper, C. A., Pili, R., Jiang, G., Liu, Y., & Zimmers, T. A. (2020). The Combination of Low Skeletal Muscle Mass and High Tumor Interleukin-6 Associates with Decreased Survival in Clear Cell Renal Cell Carcinoma. Cancers, 12(6), 1605. https://doi.org/10.3390/cancers12061605