Blood Flow Measurements Enable Optimization of Light Delivery for Personalized Photodynamic Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tumor Models/PDT
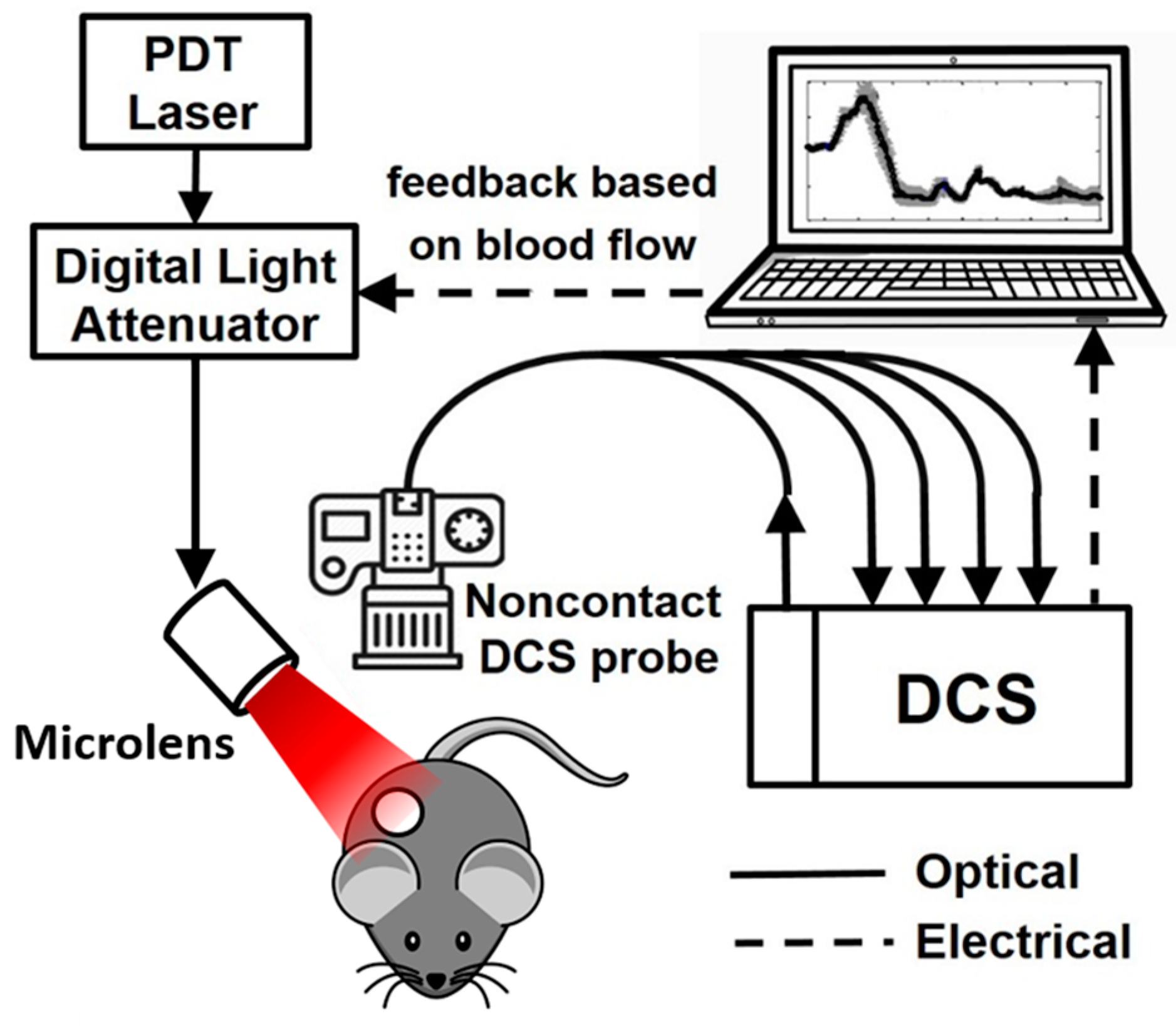
2.2. Diffuse Correlation Spectroscopy
2.2.1. DCS Instrumentation
2.2.2. Tumor Blood Flow Monitoring
2.3. Illumination Schemes
- 150 mWcm−2-continuous: Continuous illumination at high irradiance of 150 mWcm−2 for 15 min.
- 25 mWcm−2-continuous: Continuous illumination at low irradiance of 25 mWcm−2 for 90 min.
- 150 mWcm−2-fractionated: 150 mWcm−2 in equal intermittent intervals of 30 s light-on and 30 s light-off for a total of 30 min.
- Blood-flow-informed-irradiance (BFI-Irrad): Continuous illumination was initially 150 mWcm−2, but illumination was cyclically decreased to 25 mWcm−2 and returned to 150 mWcm−2 in response to the blood flow monitoring parameters. Treatment time was adjusted to deliver a total fluence of 135 Jcm−2 (between 15 and 90 min).
- Blood-flow-informed-fractionated (BFI-Frac): Fractionated illumination was initiated at 150 mWcm−2, but illumination was intermittently discontinued (light-off, 0 mWcm−2) in response to blood flow monitoring. Treatment time was adjusted to deliver a total fluence of 135 Jcm−2, which was reached within 90 min in the current investigations. Note, this guidance platform requires a maximum treatment time to be established irrespective of whether or not a total fluence of 135 Jcm−2 is achieved because the light can remain “off” for extended periods of time if blood flow recovery is slow.
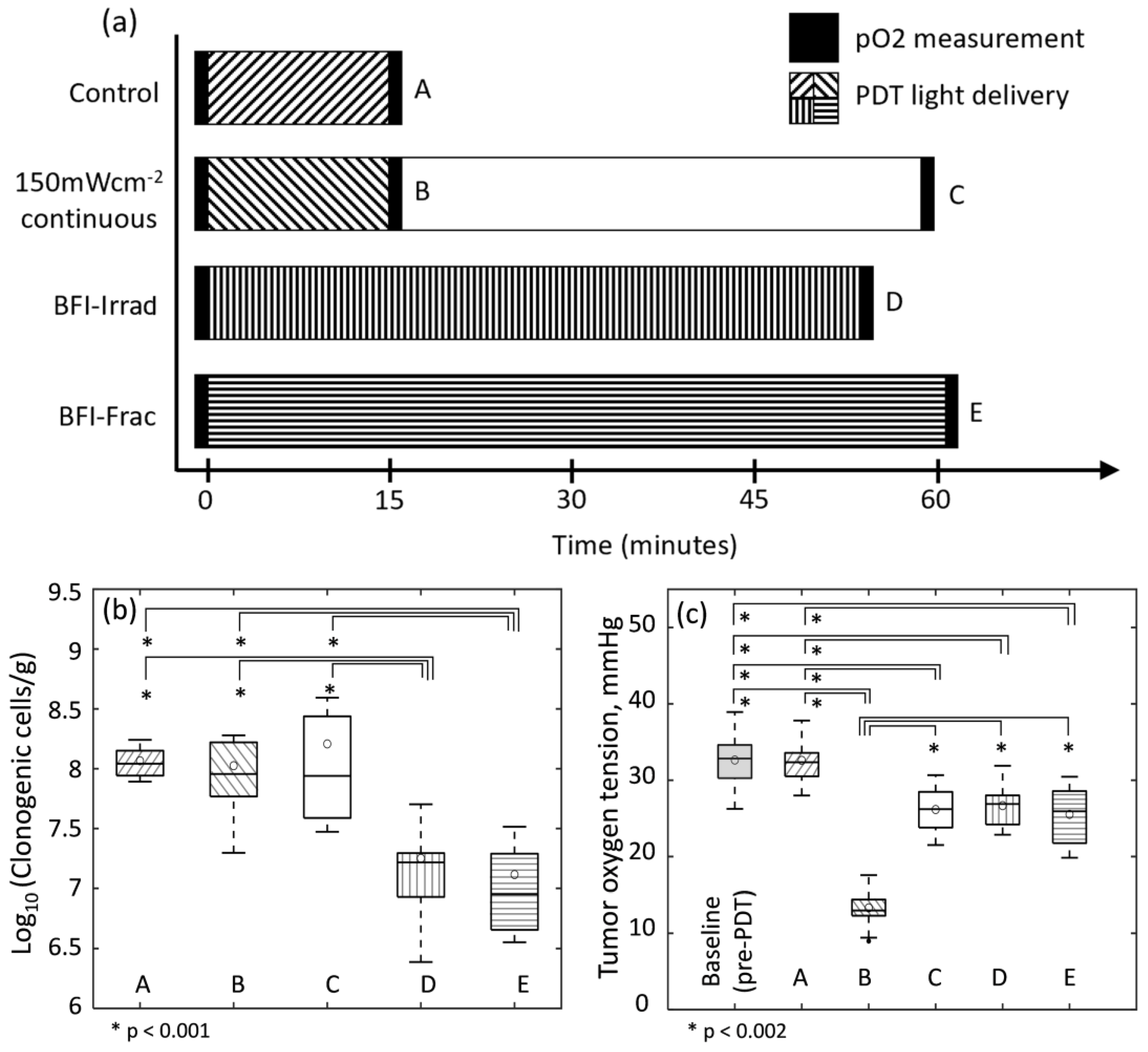
2.4. In Vivo/In Vitro Clonogenic Assay
2.5. Tumor Oxygenation Measurements
2.6. Statistical Analysis
3. Results
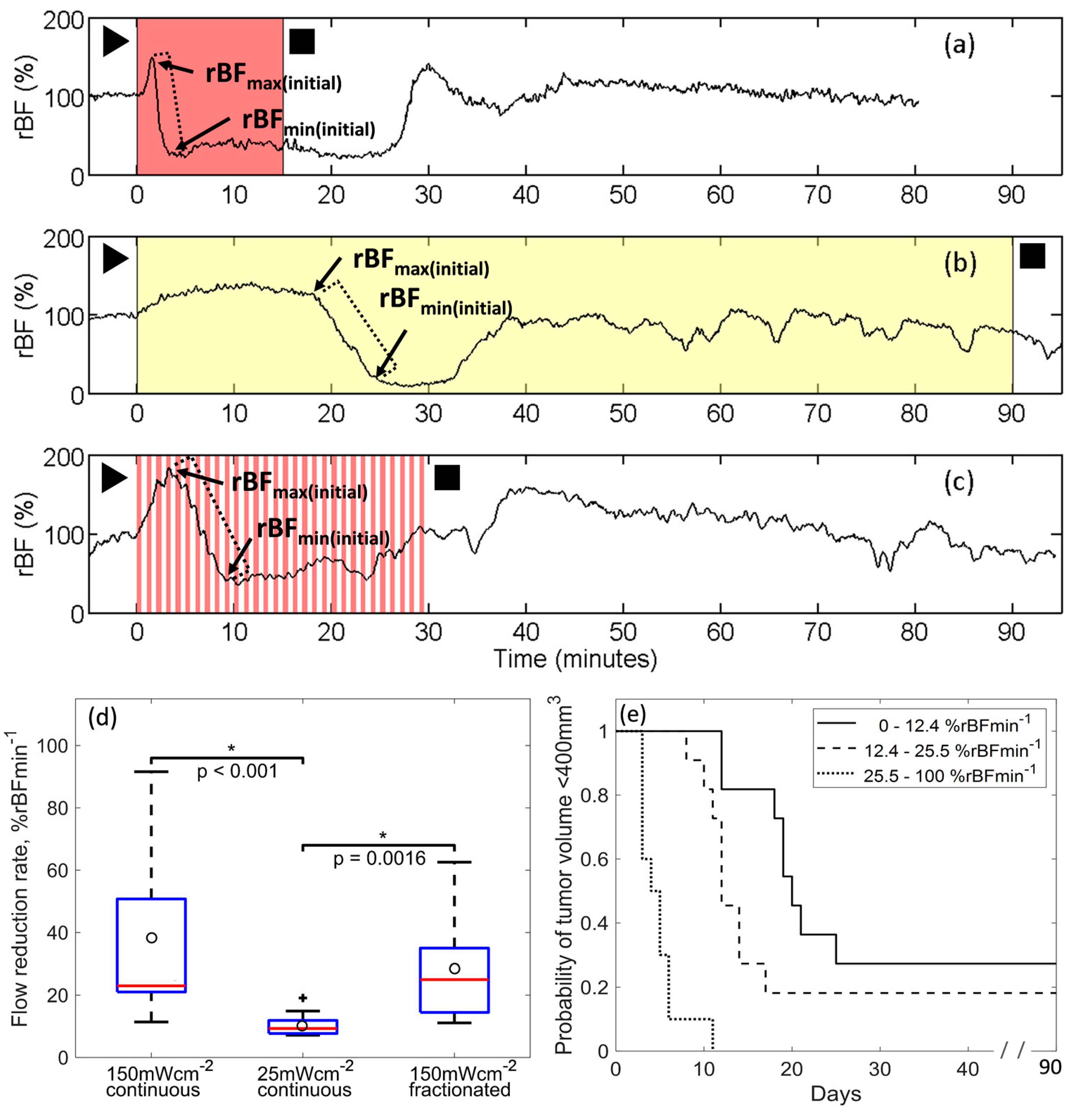
3.1. Irradiance Alters Tumor Blood Flow During PDT
3.2. BFI-PDT Alters Flow Dynamics during Illumination Compared to Standard PDT
3.3. BFI-PDT Decreases Blood Flow Reduction Rate during Light Delivery, While Shortening Treatment Time
3.4. BFI-PDT Alters Mechanisms of PDT Effect on Vascular Damage
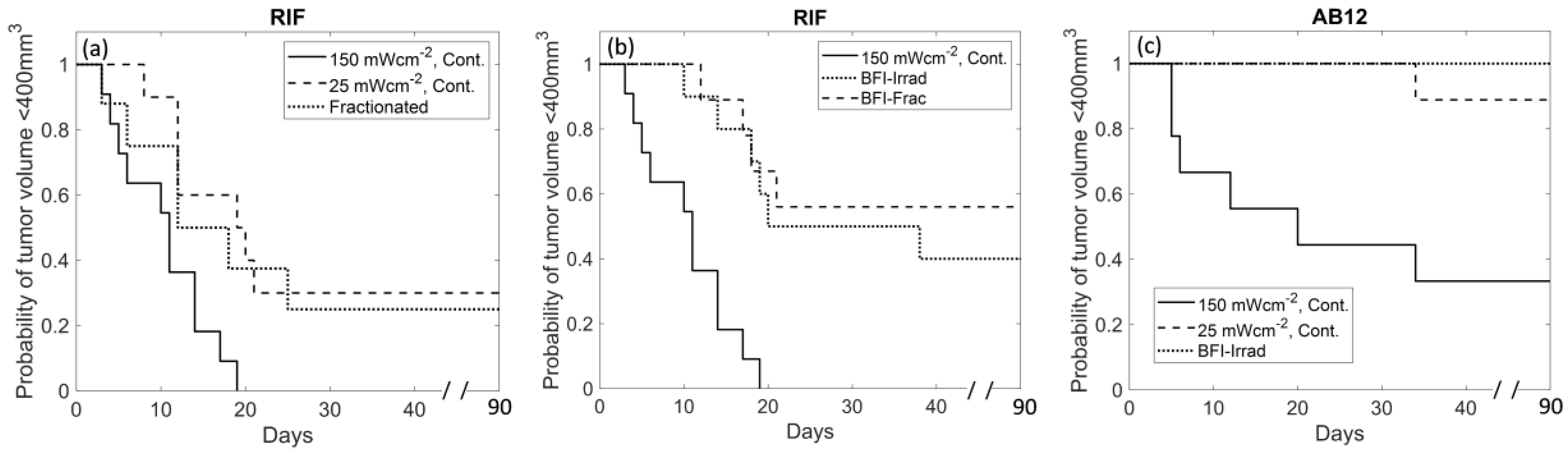
3.5. BFI-PDT Improves Therapeutic Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Thong, P.; Lee, K.; Toh, H.J.; Dong, J.; Tee, C.S.; Low, K.P.; Chang, P.H.; Bhuvaneswari, R.; Tan, N.C.; Soo, K.C. Early assessment of tumor response to photodynamic therapy using combined diffuse optical and diffuse correlation spectroscopy to predict treatment outcome. Oncotarget 2017, 8, 19902–19913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mroz, P.; Hashmi, J.T.; Huang, Y.Y.; Lange, N.; Hamblin, M.R. Stimulation of anti-tumor immunity by photodynamic therapy. Expert Rev. Clin. Immunol. 2011, 7, 75–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fingar, V.H.; Wieman, T.J.; Wiehle, S.A.; Cerrito, P.B. The role of microvascular damage in photodynamic therapy: The effect of treatment on vessel constriction, permeability, and leukocyte adhesion. Cancer Res. 1992, 52, 4914–4921. [Google Scholar]
- Busch, T.M. Local physiological changes during photodynamic therapy. Lasers Surg. Med. 2006, 38, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Khurana, M.; Moriyama, E.H.; Mariampillai, A.; Wilson, B.C. Intravital high-resolution optical imaging of individual vessel response to photodynamic treatment. J. Biomed. Opt. 2008, 13, 040502. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Moriyama, L.T.; Bagnato, V.S. Photodynamic therapy induced vascular damage: An overview of experimental PDT. Laser Phys. Lett. 2013, 10, 023001. [Google Scholar] [CrossRef]
- Rohrbach, D.J.; Tracy, E.C.; Walker, J.; Baumann, H.; Sunar, U. Blood flow dynamics during local photoreaction in a head and neck tumor model. Front. Phys. 2015, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Durduran, T.; Zhou, C.; Zhu, T.C.; Finlay, J.C.; Busch, T.M.; Malkowicz, S.B.; Hahn, S.M.; Yodh, A.G. Real-time in situ monitoring of human prostate photodynamic therapy with diffuse light. Photochem. Photobiol. 2006, 82, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.L.; Paquette, A.D.; Keymel, K.R.; Henderson, B.W.; Sunar, U. Monitoring blood flow responses during topical ALA-PDT. Biomed. Opt. Express 2010, 2, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Busch, T.M.; Xing, X.; Yu, G.; Yodh, A.; Wileyto, E.P.; Wang, H.W.; Durduran, T.; Zhu, T.C.; Wang, K.K. Fluence rate-dependent intratumor heterogeneity in physiologic and cytotoxic responses to Photofrin photodynamic therapy. Photochem. Photobiol. Sci. 2009, 8, 1683–1693. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Durduran, T.; Zhou, C.; Wang, H.W.; Putt, M.E.; Saunders, H.M.; Sehgal, C.M.; Glatstein, E.; Yodh, A.G.; Busch, T.M. Noninvasive monitoring of murine tumor blood flow during and after photodynamic therapy provides early assessment of therapeutic efficacy. Clin. Cancer Res. 2005, 11, 3543–3552. [Google Scholar] [CrossRef] [Green Version]
- Busch, T.M.; Wileyto, E.P.; Emanuele, M.J.; Del Piero, F.; Marconato, L.; Glatstein, E.; Koch, C.J. Photodynamic Therapy Creates Fluence Rate-dependent Gradients in the Intratumoral Spatial Distribution of Oxygen. Cancer Res. 2002, 62, 7273. [Google Scholar] [PubMed]
- Henderson, B.W.; Busch, T.M.; Snyder, J.W. Fluence rate as a modulator of PDT mechanisms. Lasers Surg. Med. 2006, 38, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Angell-Petersen, E.; Spetalen, S.; Madsen, S.J.; Sun, C.-H.; Peng, Q.; Carper, S.W.; Sioud, M.; Hirschberg, H. Influence of light fluence rate on the effects of photodynamic therapy in an orthotopic rat glioma model. J. Neurosurg. 2006, 104, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, I.; Anbil, S.; Alagic, N.; Celli, J.; Zheng, L.Z.; Palanisami, A.; Glidden, M.D.; Pogue, B.W.; Hasan, T. PDT dose parameters impact tumoricidal durability and cell death pathways in a 3D ovarian cancer model. Photochem. Photobiol. 2013, 89, 942–952. [Google Scholar] [CrossRef]
- Foster, T.H.; Hartley, D.F.; Nichols, M.G.; Hilf, R. Fluence rate effects in photodynamic therapy of multicell tumor spheroids. Cancer Res. 1993, 53, 1249–1254. [Google Scholar]
- Guo, H.W.; Lin, L.T.; Chen, P.H.; Ho, M.H.; Huang, W.T.; Lee, Y.J.; Chiou, S.H.; Hsieh, Y.S.; Dong, C.Y.; Wang, H.W. Low-fluence rate, long duration photodynamic therapy in glioma mouse model using organic light emitting diode (OLED). Photodiagnosis Photodyn. Ther. 2015, 12, 504–510. [Google Scholar] [CrossRef]
- Seshadri, M.; Bellnier, D.A.; Vaughan, L.A.; Spernyak, J.A.; Mazurchuk, R.; Foster, T.H.; Henderson, B.W. Light delivery over extended time periods enhances the effectiveness of photodynamic therapy. Clin. Cancer Res. 2008, 14, 2796–2805. [Google Scholar] [CrossRef] [Green Version]
- Busch, T.M.; Wang, H.W.; Wileyto, E.P.; Yu, G.; Bunte, R.M. Increasing damage to tumor blood vessels during motexafin lutetium-PDT through use of low fluence rate. Radiat. Res. 2010, 174, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Sitnik, T.M.; Hampton, J.A.; Henderson, B.W. Reduction of tumour oxygenation during and after photodynamic therapy in vivo: Effects of fluence rate. Br. J. Cancer 1998, 77, 1386–1394. [Google Scholar] [CrossRef]
- Iinuma, S.; Schomacker, K.T.; Wagnieres, G.; Rajadhyaksha, M.; Bamberg, M.; Momma, T.; Hasan, T. In vivo fluence rate and fractionation effects on tumor response and photobleaching: Photodynamic therapy with two photosensitizers in an orthotopic rat tumor model. Cancer Res. 1999, 59, 6164–6170. [Google Scholar] [PubMed]
- de Bruijn, H.S.; Brooks, S.; van der Ploeg-van den Heuvel, A.; Ten Hagen, T.L.; de Haas, E.R.; Robinson, D.J. Light Fractionation Significantly Increases the Efficacy of Photodynamic Therapy Using BF-200 ALA in Normal Mouse Skin. PLoS ONE 2016, 11, e0148850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Bruijn, H.S.; van der Veen, N.; Robinson, D.J.; Star, W.M. Improvement of systemic 5-aminolevulinic acid-based photodynamic therapy in vivo using light fractionation with a 75-min interval. Cancer Res. 1999, 59, 901–904. [Google Scholar] [PubMed]
- de Haas, E.R.; Kruijt, B.; Sterenborg, H.J.; Martino Neumann, H.A.; Robinson, D.J. Fractionated illumination significantly improves the response of superficial basal cell carcinoma to aminolevulinic acid photodynamic therapy. J. Investig. Dermatol. 2006, 126, 2679–2686. [Google Scholar] [CrossRef] [Green Version]
- de Vijlder, H.C.; Sterenborg, H.J.; Neumann, H.A.; Robinson, D.J.; de Haas, E.R. Light fractionation significantly improves the response of superficial basal cell carcinoma to aminolaevulinic acid photodynamic therapy: Five-year follow-up of a randomized, prospective trial. Acta Derm. Venereol. 2012, 92, 641–647. [Google Scholar] [CrossRef] [Green Version]
- van der Veen, N.; Hebeda, K.M.; de Bruijn, H.S.; Star, W.M. Photodynamic effectiveness and vasoconstriction in hairless mouse skin after topical 5-aminolevulinic acid and single- or two-fold illumination. Photochem. Photobiol. 1999, 70, 921–929. [Google Scholar] [CrossRef]
- Pogue, B.W.; Hasan, T. A theoretical study of light fractionation and dose-rate effects in photodynamic therapy. Radiat. Res. 1997, 147, 551–559. [Google Scholar] [CrossRef]
- Muller, S.; Walt, H.; Dobler-Girdziunaite, D.; Fiedler, D.; Haller, U. Enhanced photodynamic effects using fractionated laser light. J. Photochem. Photobiol. B 1998, 42, 67–70. [Google Scholar] [CrossRef]
- Estevez, J.P.; Ascencio, M.; Colin, P.; Farine, M.O.; Collinet, P.; Mordon, S. Continuous or fractionated photodynamic therapy? Comparison of three PDT schemes for ovarian peritoneal micrometastasis treatment in a rat model. Photodiagnosis Photodyn. Ther. 2010, 7, 251–257. [Google Scholar] [CrossRef]
- Ascencio, M.; Estevez, J.P.; Delemer, M.; Farine, M.O.; Collinet, P.; Mordon, S. Comparison of continuous and fractionated illumination during hexaminolaevulinate-photodynamic therapy. Photodiagnosis Photodyn. Ther. 2008, 5, 210–216. [Google Scholar] [CrossRef]
- Xiao, Z.; Halls, S.; Dickey, D.; Tulip, J.; Moore, R.B. Fractionated versus standard continuous light delivery in interstitial photodynamic therapy of dunning prostate carcinomas. Clin. Cancer Res. 2007, 13, 7496–7505. [Google Scholar] [CrossRef] [Green Version]
- Durduran, T.; Yodh, A.G. Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. Neuroimage 2014, 85, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Favilla, C.G.; Mesquita, R.C.; Mullen, M.; Durduran, T.; Lu, X.; Kim, M.N.; Minkoff, D.L.; Kasner, S.E.; Greenberg, J.H.; Yodh, A.G.; et al. Optical bedside monitoring of cerebral blood flow in acute ischemic stroke patients during head-of-bed manipulation. Stroke 2014, 45, 1269–1274. [Google Scholar] [CrossRef] [Green Version]
- Busch, D.R.; Rusin, C.G.; Miller-Hance, W.; Kibler, K.; Baker, W.B.; Heinle, J.S.; Fraser, C.D.; Yodh, A.G.; Licht, D.J.; Brady, K.M. Continuous cerebral hemodynamic measurement during deep hypothermic circulatory arrest. Biomed. Opt. Express 2016, 7, 3461–3470. [Google Scholar] [CrossRef]
- Shang, Y.; Gurley, K.; Yu, G. Diffuse Correlation Spectroscopy (DCS) for Assessment of Tissue Blood Flow in Skeletal Muscle: Recent Progress. Anat. Physiol. 2013, 3, 128. [Google Scholar]
- Yu, G.; Floyd, T.F.; Durduran, T.; Zhou, C.; Wang, J.; Detre, J.A.; Yodh, A.G. Validation of diffuse correlation spectroscopy for muscle blood flow with concurrent arterial spin labeled perfusion MRI. Opt. Express 2007, 15, 1064–1075. [Google Scholar] [CrossRef]
- Cochran, J.M.; Chung, S.H.; Leproux, A.; Baker, W.B.; Busch, D.R.; DeMichele, A.M.; Tchou, J.; Tromberg, B.J.; Yodh, A.G. Longitudinal optical monitoring of blood flow in breast tumors during neoadjuvant chemotherapy. Phys. Med. Biol. 2017, 62, 4637–4653. [Google Scholar] [CrossRef]
- Dong, L.; Kudrimoti, M.; Cheng, R.; Shang, Y.; Johnson, E.L.; Stevens, S.D.; Shelton, B.J.; Yu, G. Noninvasive diffuse optical monitoring of head and neck tumor blood flow and oxygenation during radiation delivery. Biomed. Opt. Express 2012, 3, 259–272. [Google Scholar] [CrossRef] [Green Version]
- Mesquita, R.C.; Han, S.W.; Miller, J.; Schenkel, S.S.; Pole, A.; Esipova, T.V.; Vinogradov, S.A.; Putt, M.E.; Yodh, A.G.; Busch, T.M. Tumor blood flow differs between mouse strains: Consequences for vasoresponse to photodynamic therapy. PLoS ONE 2012, 7, e37322. [Google Scholar] [CrossRef] [Green Version]
- Ong, Y.H.; Dimofte, A.; Kim, M.M.; Finlay, J.C.; Sheng, T.; Singhal, S.; Cengel, K.A.; Yodh, A.G.; Busch, T.M.; Zhu, T.C. Reactive Oxygen Species Explicit Dosimetry for Photofrin-mediated Pleural Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 340–348. [Google Scholar] [CrossRef]
- Li, L.B.; Luo, R.C. Effect of drug-light interval on the mode of action of Photofrin photodynamic therapy in a mouse tumor model. Lasers Med. Sci. 2009, 24, 597–603. [Google Scholar] [CrossRef]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef]
- Hsieh, Y.J.; Yu, J.S.; Lyu, P.C. Characterization of photodynamic therapy responses elicited in A431 cells containing intracellular organelle-localized photofrin. J. Cell. Biochem. 2010, 111, 821–833. [Google Scholar] [CrossRef]
- Shafirstein, G.; Bellnier, D.A.; Oakley, E.; Hamilton, S.; Habitzruther, M.; Tworek, L.; Hutson, A.; Spernyak, J.A.; Sexton, S.; Curtin, L.; et al. Irradiance controls photodynamic efficacy and tissue heating in experimental tumours: Implication for interstitial PDT of locally advanced cancer. Br. J. Cancer 2018, 119, 1191–1199. [Google Scholar] [CrossRef]
- Buckley, E.M.; Parthasarathy, A.B.; Grant, P.E.; Yodh, A.G.; Franceschini, M.A. Diffuse correlation spectroscopy for measurement of cerebral blood flow: Future prospects. Neurophotonics 2014, 1, 011009. [Google Scholar] [CrossRef] [Green Version]
- Durduran, T.; Choe, R.; Baker, W.B.; Yodh, A.G. Diffuse optics for tissue monitoring and tomography. Rep. Prog. Phys. 2010, 73, 076701. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-W.; Rickter, E.; Yuan, M.; Wileyto, E.P.; Glatstein, E.; Yodh, A.; Busch*, T.M. Effect of Photosensitizer Dose on Fluence Rate Responses to Photodynamic Therapy. Photochem. Photobiol. 2007, 83, 1040–1048. [Google Scholar] [CrossRef]
- Esipova, T.V.; Karagodov, A.; Miller, J.; Wilson, D.F.; Busch, T.M.; Vinogradov, S.A. Two New “Protected” Oxyphors for Biological Oximetry: Properties and Application in Tumor Imaging. Anal. Chem. 2011, 83, 8756–8765. [Google Scholar] [CrossRef] [Green Version]
- Gallagher-Colombo, S.M.; Miller, J.; Cengel, K.A.; Putt, M.E.; Vinogradov, S.A.; Busch, T.M. Erlotinib Pretreatment Improves Photodynamic Therapy of Non–Small Cell Lung Carcinoma Xenografts via Multiple Mechanisms. Cancer Res. 2015, 75, 3118–3126. [Google Scholar] [CrossRef] [Green Version]
- Harrington, D.P.; Fleming, T.R. A Class of Rank Test Procedures for Censored Survival Data. Biometrika 1982, 69, 553–566. [Google Scholar] [CrossRef]
- Maas, A.L.; Carter, S.L.; Wileyto, E.P.; Miller, J.; Yuan, M.; Yu, G.; Durham, A.C.; Busch, T.M. Tumor Vascular Microenvironment Determines Responsiveness to Photodynamic Therapy. Cancer Res. 2012, 72, 2079. [Google Scholar] [CrossRef] [Green Version]
- Engbrecht, B.W.; Menon, C.; Kachur, A.V.; Hahn, S.M.; Fraker, D.L. Photofrin-mediated Photodynamic Therapy Induces Vascular Occlusion and Apoptosis in a Human Sarcoma Xenograft Model. Cancer Res. 1999, 59, 4334. [Google Scholar] [PubMed]
- Sitnik, T.M.; Henderson, B.W. The effect of fluence rate on tumor and normal tissue responses to photodynamic therapy. Photochem. Photobiol. 1998, 67, 462–466. [Google Scholar] [CrossRef]
- Feins, R.H.; Hilf, R.; Ross, H.; Gibson, S.L. Photodynamic therapy for human malignant mesothelioma in the nude mouse. J. Surg. Res. 1990, 49, 311–314. [Google Scholar] [CrossRef]
- Gibson, S.L.; Foster, T.H.; Feins, R.H.; Raubertas, R.F.; Fallon, M.A.; Hilf, R. Effects of photodynamic therapy on xenografts of human mesothelioma and rat mammary carcinoma in nude mice. Br. J. Cancer 1994, 69, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Babilas, P.; Schacht, V.; Liebsch, G.; Wolfbeis, O.S.; Landthaler, M.; Szeimies, R.M.; Abels, C. Effects of light fractionation and different fluence rates on photodynamic therapy with 5-aminolaevulinic acid in vivo. Br. J. Cancer 2003, 88, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Francois, A.; Salvadori, A.; Bressenot, A.; Bezdetnaya, L.; Guillemin, F.; D’Hallewin, M.A. How to avoid local side effects of bladder photodynamic therapy: Impact of the fluence rate. J. Urol. 2013, 190, 731–736. [Google Scholar] [CrossRef]
- Tetard, M.C.; Vermandel, M.; Leroy, H.A.; Leroux, B.; Maurage, C.A.; Lejeune, J.P.; Mordon, S.; Reyns, N. Interstitial 5-ALA photodynamic therapy and glioblastoma: Preclinical model development and preliminary results. Photodiagnosis Photodyn. Ther. 2016, 13, 218–224. [Google Scholar] [CrossRef] [Green Version]

| Type of PDT | Group | Flow Reduction Rate (%rBFmin−1) Median (IQR) | Treatment Length in mMminutes Median (IQR) | ΔrBF(%) at 1 h after PDT Mean (SD) | |||
|---|---|---|---|---|---|---|---|
| 150 mWcm−2 | 25 mWcm−2 | 0 mWcm−2 | Total | ||||
| Standard | 150 mWcm−2 continuous | 23.0 (21.4, 49.8) | 15 | 0 | 0 | 15 | 24.8 (67.3) |
| 25 mWcm−2 continuous | 9.3 (7.8, 11.6) (p < 0.0011) | 0 | 90 | 0 | 90 | −46.2 (23.7) (p = 0.001 1) | |
| 150 mWcm−2 fractionated | 25.0 (14.9 34,6) (p = 0.0022) | 15 | 0 | 15 | 30 | −32.2 (31.3) (p = 0.018 1) | |
| Blood-flow informed | BFI-Irrad | 5.2 (4.6, 5.4) (p < 0.001 1,3, p = 0.0012) | 8 (7, 9) | 45 (39, 50) | 0 | 53 (48, 57) | −31.4 (19.7) (p = 0.016 1) |
| BFI-Frac | 10.0 (6.7, 10.2) (p < 0.001 1,3) | 15 | 0 | 46 (34, 55) | 61 (49, 70) | −43.6 (29.0) (p = 0.002 1) | |
| Group | Flow Reduction Rate (%rBFmin−1) Median (IQR) | Treatment Length in Minutes Median (IQR) | ΔrBF(%) at 1 h after PDT Mean (SD) | |||
|---|---|---|---|---|---|---|
| 150 mWcm−2 | 25 mWcm−2 | 0 mWcm−2 | Total | |||
| 150 mWcm−2-continuous | 20.7 (15.5, 32.6) | 15 | 0 | 0 | 15 | −10.7 (21.6) |
| 25 mWcm−2-continuous | 13.4 (11.9, 16.8) | 0 | 90 | 0 | 90 | −56.6 (18.6) (p = 0.001 1) |
| BFI-Irrad | 6.0 (3.7, 12.1) (p = 0.004 1, p = 0.04 2) | 11 (9, 14) | 27 (9, 36) | 0 | 37 (23, 45) | −58.5 (15.3) (p = 0.001 1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, Y.H.; Miller, J.; Yuan, M.; Chandra, M.; El Khatib, M.; Vinogradov, S.A.; Putt, M.E.; Zhu, T.C.; Cengel, K.A.; Yodh, A.G.; et al. Blood Flow Measurements Enable Optimization of Light Delivery for Personalized Photodynamic Therapy. Cancers 2020, 12, 1584. https://doi.org/10.3390/cancers12061584
Ong YH, Miller J, Yuan M, Chandra M, El Khatib M, Vinogradov SA, Putt ME, Zhu TC, Cengel KA, Yodh AG, et al. Blood Flow Measurements Enable Optimization of Light Delivery for Personalized Photodynamic Therapy. Cancers. 2020; 12(6):1584. https://doi.org/10.3390/cancers12061584
Chicago/Turabian StyleOng, Yi Hong, Joann Miller, Min Yuan, Malavika Chandra, Mirna El Khatib, Sergei A. Vinogradov, Mary E. Putt, Timothy C. Zhu, Keith A. Cengel, Arjun G. Yodh, and et al. 2020. "Blood Flow Measurements Enable Optimization of Light Delivery for Personalized Photodynamic Therapy" Cancers 12, no. 6: 1584. https://doi.org/10.3390/cancers12061584
APA StyleOng, Y. H., Miller, J., Yuan, M., Chandra, M., El Khatib, M., Vinogradov, S. A., Putt, M. E., Zhu, T. C., Cengel, K. A., Yodh, A. G., & Busch, T. M. (2020). Blood Flow Measurements Enable Optimization of Light Delivery for Personalized Photodynamic Therapy. Cancers, 12(6), 1584. https://doi.org/10.3390/cancers12061584






