8th Edition Tumor, Node, and Metastasis T-Stage Prognosis Discrepancies: Solid Component Diameter Predicts Prognosis Better than Invasive Component Diameter
Abstract
1. Introduction
2. Result
3. Discussion
4. Materials and Method
4.1. Patients Selection
4.2. Pathological Evaluation
4.3. Radiological Evaluation
4.4. Patient Follow-up
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rami-Porta, R.; Bolejack, V.; Crowley, J.; Ball, D.; Kim, J.; Lyons, G.; Rice, T.; Suzuki, K.; Thomas, C.F.; Travis, W.D.; et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2015, 10, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Bankier, A.A.; Beasley, M.B.; Flieder, U.B.; Goo, J.M.; MacMahon, H.; Naidich, D.P.; Powell, C.A.; Prokop, M.; Yatabe, Y.; et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. 2016, 11, 1204–1223. [Google Scholar] [CrossRef] [PubMed]
- Urer, H.N.; Ahiskali, R.; Arda, N.; Batur, S.; Cinel, L.; Dekan, G.; Fener, N.; Firat, P.; Geleff, S.; Oz, B.; et al. Interobserver agreement among histological patterns and diagnosis in lung adenocarcinomas. Turk. J. Pathol. 2013, 30, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Boland, J.M.; Froemming, A.T.; Wampfler, J.A.; Maldonado, F.; Peikert, T.D.; Hyland, C.; Andrade, M.D.; Aubry, M.C.; Yang, P.; Yi, E.S. Adenocarcinoma in situ, minimally invasive adenocarcinoma, and invasive pulmonary adenocarcinoma—Analysis of interobserver agreement, survival, radiographic characteristics, and gross pathology in 296 nodules. Hum. Pathol. 2016, 51, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Burke, A.; Marx, A.; Nicholson, A.G. WHO classification of tumours of the lung, pleura, thymus and heart. Int. Agency Res. Cancer 2015. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Aokage, K.; Miyoshi, T.; Ishii, G.; Kusumoto, M.; Nomura, S.; Katsumata, S.; Sekihara, K.; Hishida, T.; Tsuboi, M. Clinical and Pathological Staging Validation in the Eighth Edition of the TNM Classification for Lung Cancer: Correlation between Solid Size on Thin-Section Computed Tomography and Invasive Size in Pathological Findings in the New T Classification. J. Thorac. Oncol. 2017, 12, 1403–1412. [Google Scholar] [CrossRef]
- Jung, H.S.; Lee, J.G.; Lee, C.Y.; Kim, D.J.; Chung, K.Y. Validation of the T descrior in the new 8th TNM Classification for non-small cell lung cancer. J. Thorac. Dis. 2018, 10, 162–167. [Google Scholar] [CrossRef]
- Hwang, E.J.; Park, C.M.; Ryu, Y.; Lee, S.M.; Kim, Y.T.; Kim, Y.W.; Goo, J.M. Pulmonary adenocarcinomas appearing as part-solid ground-glass nodules: Is measuring solid component size a better prognostic indicator? Eur. Radiol. 2014, 25, 558–567. [Google Scholar] [CrossRef]
- Aokage, K.; Miyoshi, T.; Ishii, G.; Kusumoto, M.; Nomura, S.; Katsumata, S.; Sekihara, K.; Tane, K.; Tsuboi, M. Influence of Ground Glass Opacity and the Corresponding Pathological Findings on Survival in Patients with Clinical Stage I Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 533–542. [Google Scholar] [CrossRef]
- Hattori, A.; Hirayama, S.; Matsunaga, T.; Hayashi, T.; Takamochi, K.; Oh, S.; Suzuki, K. Distinct Clinicopathologic Characteristics and Prognosis Based on the Presence of Ground Glass Opacity Component in Clinical Stage IA Lung Adenocarcinoma. J. Thorac. Oncol. 2019, 14, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Tsutani, Y.; Miyata, Y.; Nakayama, H.; Okumura, S.; Adachi, S.; Yoshimura, M.; Okada, M. Prognostic significance of using solid versus whole tumor size on high-resolution computed tomography for predicting pathologic malignant grade of tumors in clinical stage IA lung adenocarcinoma: A multicenter study. J. Thorac. Cardiovasc. Surg. 2012, 143, 607–612. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Su, H.; Dai, C.; She, Y.; Ren, Y.; Zhang, L.; Xie, H.; Xie, D.; Jiang, G.; Chen, C. Which T descriptor is more predictive of recurrence after sublobar resection: Whole tumour size versus solid component size? Eur. J. Cardio-Thorac. Surg. 2018, 54, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, M.; Kusumoto, M.; Johkoh, T.; Noguchi, M.; Minami, Y.; Sakai, F.; Asamura, H.; Tomiyama, N.; Awai, K.; Minami, M.; et al. Radiologic–Pathologic Correlation of Solid Portions on Thin-section CT Images in Lung Adenocarcinoma: A Multicenter Study. Clin. Lung Cancer 2018, 19, e303–e312. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.; Uruga, H.; Bozkurtlar, E.; Chung, J.; Hariri, L.P.; Minami, Y.; Wang, H.; Yoshizawa, A.; Muzikansky, A.; Moreira, A.L.; et al. Problems in the reproducibility of classification of small lung adenocarcinoma: An international interobserver study. Histopathology 2019, 75, 649–659. [Google Scholar] [CrossRef]
- Pignon, J.-P.; Tribodet, H.; Scagliotti, G.V.; Douillard, J.-Y.; Shepherd, F.A.; Stephens, R.J.; Dunant, A.; Torri, V.; Rosell, R.; Seymour, L.; et al. Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group. J. Clin. Oncol. 2008, 26, 3552–3559. [Google Scholar] [CrossRef]
- Hamada, C.; Tanaka, F.; Ohta, M.; Fujimura, S.; Kodama, K.; Imaizumi, M.; Wada, H. Meta-Analysis of Postoperative Adjuvant Chemotherapy With Tegafur-Uracil in Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2005, 23, 4999–5006. [Google Scholar] [CrossRef]
- Available online: https://www.haigan.gr.jp/guideline/2019/1/2/190102040100.html (accessed on 15 May 2020).
- Sawabata, N.; Miyaoka, E.; Asamura, H.; Nakanishi, Y.; Eguchi, K.; Mori, M.; Nomori, H.; Fujii, Y.; Okumura, M.; Yokoi, K. Japanese Lung Cancer Registry Study of 11,663 Surgical Cases in 2004: Demographic and Prognosis Changes Over Decade. J. Thorac. Oncol. 2011, 6, 1229–1235. [Google Scholar] [CrossRef]
- Noguchi, M.; Minami, Y.; Iijima, T.; Matsuno, Y. Reproducibility of the diagnosis of small adenocarcinoma of the lung and usefulness of an educational program for the diagnostic criteria. Pathol. Int. 2005, 55, 8–13. [Google Scholar] [CrossRef]
- Suzuki, K.; Yokose, T.; Yoshida, J.; Nishimura, M.; Takahashi, K.; Nagai, K.; Nishiwaki, Y. Prognostic significance of the size of central fibrosis in peripheral adenocarcinoma of the lung. Ann. Thorac. Surg. 2000, 69, 893–897. [Google Scholar] [CrossRef]
- Wittekind, C.; Compton, C.C.; Brierley, J.; Lee, A.; Eycken, E.V. TNM Supplement: A Commentary on Uniform Use, 4th ed.; Wiley-Blackwell: Oxford, UK, 2012. [Google Scholar]
- Anderson, K.R.; Onken, A.; Heidinger, B.; Chen, Y.; Bankier, A.A.; Vanderlaan, P.A. Pathologic T Descriptor of Nonmucinous Lung Adenocarcinomas Now Based on Invasive Tumor Size. Am. J. Clin. Pathol. 2018, 150, 499–506. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | n |
|---|---|
| Histological types | |
| Adenocarcinoma | 125 (71%) |
| Squamous cell carcinoma | 34 (19%) |
| Large cell neuroendocrine carcinoma | 7 (4%) |
| Others | 11 (6%) |
| Median follow up (days) | 624 (range, 97–1207) |
| Median time from CT scan to surgery (days) | 6 (range, 1–60) |
| Number of diagnostic pathologists | 11 people |
| Overall tumor size (T) | 22 mm (range, 6–94) |
| Solid component size (Ts) | 20 mm (range, 0–94) |
| Pathological tumor size (P) | 25 mm (range, 8–98) |
| Pathological invasive size (Pi) | 18 mm (range, 0–98) |
| P minus T | −1 mm (−28 to +41) |
| Pi minus Ts | −1.4 mm (−24 to +97) |
| −5 mm < Pi minus Ts < +5 mm | 99 (56%) |
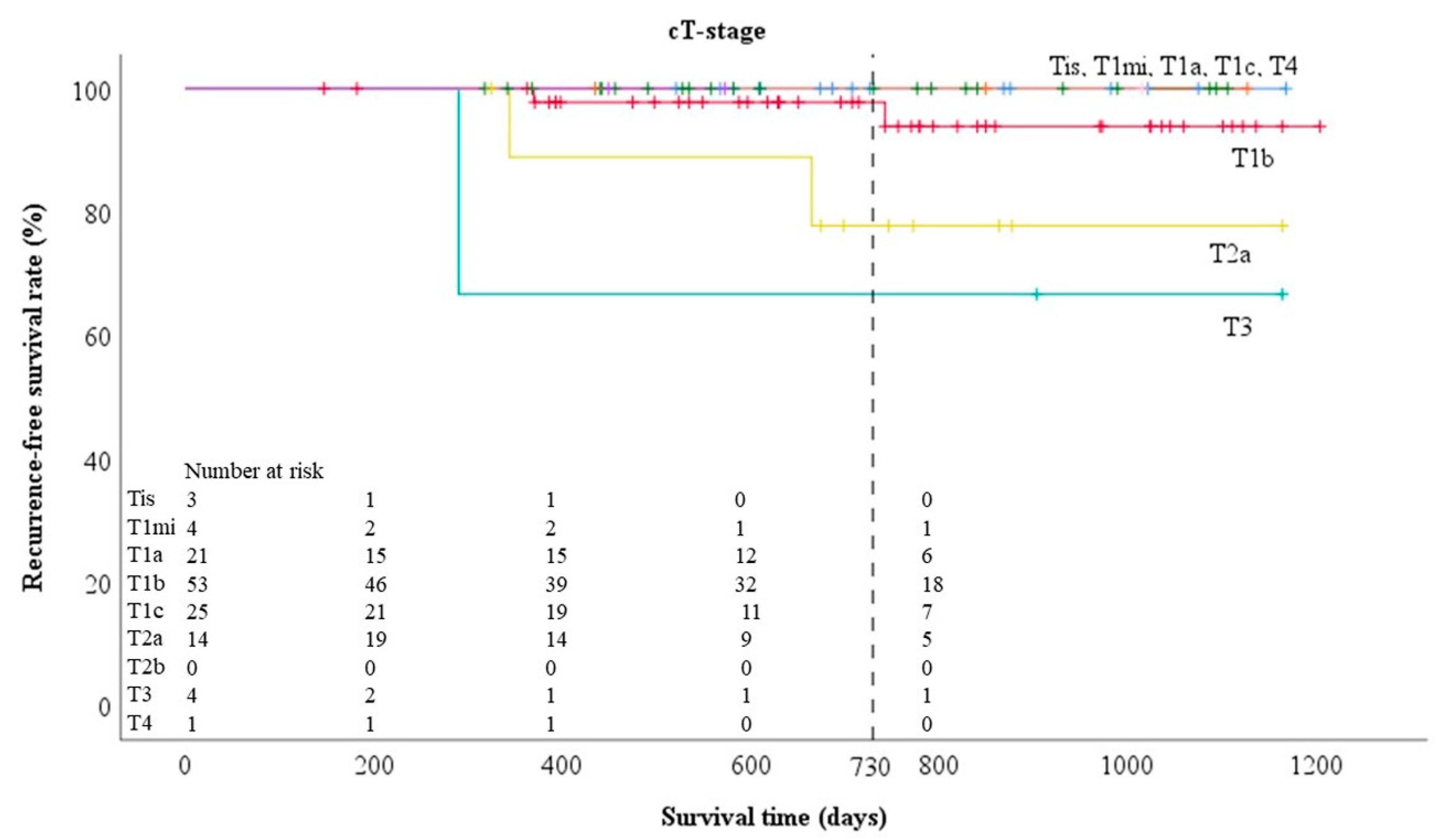
| T-Stage | Clinical T-Stage (People) | Pathological T-Stage (People) |
|---|---|---|
| Tis | 3 (2%) | 4 (2%) |
| T1mi | 4 (2%) | 20 (11%) |
| T1a | 23 (13%) | 22 (13%) |
| T1b | 72 (41%) | 51 (29%) |
| T1c | 38 (21%) | 25 (14%) |
| T2a | 21 (12%) | 31 (18%) |
| T2b | 1 (1%) | 6 (3%) |
| T3 | 9 (5%) | 14 (8%) |
| T4 | 6 (3%) | 4 (2%) |
| Clinical T-Stage | Pathological T-Stage | ||
|---|---|---|---|
| cT-stage | n | Changed from cT-stage to pT-stage | n |
| Tis | 3 | No change: 33% | Tis: 1 |
| Upstage: 66% | T1a: 1 T1b: 1 | ||
| T1mi | 4 | No change: 75% | T1mi: 3 |
| Upstage: 25% | T1a: 1 | ||
| T1a | 23 | Downstage: 26% | Tis: 2 T1mi: 4 |
| No change: 35% | T1a: 8 | ||
| Upstage: 39% | T1b: 7 T1c: 1 T4: 1 | ||
| T1b | 72 | Downstage: 28% | Tis: 1 T1mi: 12 T1a: 7 |
| No change: 51% | T1b: 37 | ||
| Upstage: 21% | T1c: 4 T2a: 10 (pl) T3: 1 (pm) | ||
| T1c | 38 | Downstage: 24% | T1mi: 1 T1a: 4 T1b: 4 |
| No change: 34% | T1c: 13 | ||
| Upstage: 42% | T2a: 10 (pl: 9) T2b: 2 T3: 4 (pl3: 2, pm: 2) | ||
| T2a | 21 | Downstage: 43% | T1a: 1 T1b: 2 T1c: 6 |
| No change: 38% | T2a: 8 | ||
| Upstage: 19% | T2b: 1 T3: 3 | ||
| T2b | 1 | No change: 100% | T2b: 1 |
| T3 | 9 | Downstage: 45% | T2a: 3 T2b: 1 |
| No change: 45% | T3: 4 | ||
| Upstage: 10% | T4: 1 | ||
| T4 | 6 | Downstage: 67% | T1c: 1 T2b: 1 T3: 2 |
| No change: 33% | T4: 2 | ||
| T-Stage | Clinical T-Stage | Pathological T-Stage |
|---|---|---|
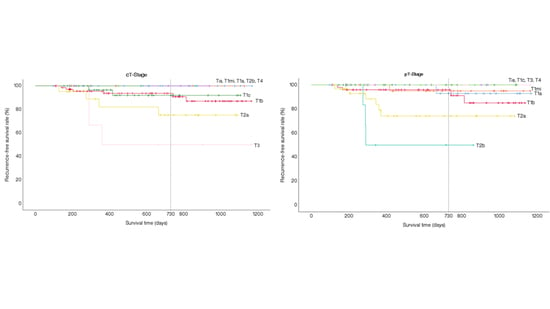
| Tis | 100% | 100% |
| T1mi | 100% | 95.0% |
| T1a | 100% | 92.9% |
| T1b | 93.7% | 95.9% |
| T1c | 92.0% | 100% |
| T2a | 75.2% | 74.1% |
| T2b | 100% | 50% |
| T3 | 50% | 100% |
| T4 | 100% | 100% |
| Characteristics | n |
|---|---|
| Adenocarcinoma | |
| Lepidic | 51 (40%) |
| Acinar | 12 (9%) |
| Papillary | 45 (36%) |
| Micropapillary | 2 (2%) |
| Solid | 2 (2%) |
| Acinar and solid (35% each) | 1 (1%) |
| Variants (Invasive mucinous) | 10 (8%) |
| (Colloid) | 2 (2%) |
| Age | |
| <70 | 69 (55%) |
| ≥70 | 56 (45%) |
| Sex | |
| Female | 66 (53%) |
| Male | 59 (47%) |
| CEA | |
| ≤4.4 | 97 (78%) |
| >4.5 | 28 (22%) |
| Brinkman index | |
| ≤400 | 80 (64%) |
| >400 | 45 (36%) |
| %FEV1.0 | |
| <70% | 119 (95%) |
| ≥70% | 6 (5%) |
| Pleural invasion | |
| Positive | 22 (18%) |
| Negative | 103 (82%) |
| Pulmonary metastasis | |
| Positive | 2 (2%) |
| Negative | 123 (98%) |
| Lymphatic vessel invasion | |
| Positive | 30 (24%) |
| Negative | 95 (76%) |
| Blood vessel invasion | |
| Positive | 44 (35%) |
| Negative | 81 (65%) |
| Spread through alveolar space | |
| Positive | 35 (28%) |
| Negative | 73 (58%) |
| Unknown | 17 (14%) |
| Characteristics | All Histlogical Types | Adenocarcinoma |
|---|---|---|
| Median follow up (days) | 624 (range, 97–1207) | 651 (range, 97–1207) |
| Median time from CT scan to surgery (days) | 6 (range, 1–60) | 6 (range, 1–60) |
| Overall tumor size (T) | 22 mm (range, 6–94) | 22 mm (range, 8–64) |
| Solid component size (Ts) | 20 mm (range, 0–94) | 17 mm (range, 0–64) |
| Pathological tumor size (P) | 25 mm (range, 8–98) | 22 mm (range, 8–98) |
| Pathological invasive size (Pi) | 18 mm (range, 0–98) | 14 mm (range, 0–98) |
| P minus T | −1 mm (−28 to +41) | 0 mm (−16 to +41) |
| Pi minus Ts | −1.4 mm (−24 to +97) | −1 mm (−32 to +97) |
| −5 mm < Pi minus Ts < +5 mm | 99 (56%) | 65 (52%) |
| Clinical T-Stage | Pathological T-Stage | ||
|---|---|---|---|
| cT-stage | n | Changed from cT-stage to pT-stage | n |
| Tis | 3 | No change: 33% | Tis: 1 |
| Upstage: 66% | T1a: 1 | ||
| T1b: 1 | |||
| T1mi | 4 | No change: 75% | T1mi: 3 |
| Upstage: 25% | T1a: 1 | ||
| T1a | 21 | Downstage: 29% | Tis: 2 |
| T1mi: 4 | |||
| No change: 38% | T1a: 8 | ||
| Upstage: 33% | T1b: 5 | ||
| T1c: 1 | |||
| T4: 1 (size) | |||
| T1b | 53 | Downstage: 36% | Tis: 1 |
| T1mi: 12 | |||
| T1a: 6 | |||
| No change: 41% | T1b: 22 | ||
| Upstage: 23% | T1c: 4 | ||
| T2a: 8 (pl:8) | |||
| T1c | 25 | Downstage: 32% | T1mi: 1 |
| T1a: 3 | |||
| T1b: 4 | |||
| No change: 28% | T1c: 7 | ||
| Upstage: 40% | T2a: 8 (pl:7)) | ||
| T2b: 1 | |||
| T3: 1 (pm) | |||
| T2a | 14 | Downstage: 50% | T1a: 1 |
| T1b: 2 | |||
| T1c: 4 | |||
| No change: 36% | T2a: 5 (pl:1) | ||
| Upstage: 14% | T3: 2 (pl:1, size:1) | ||
| T2b | 0 | - | |
| T3 | 4 | Downstage: 25% | T2a: 1 |
| No change: 50% | T3: 2 | ||
| Upstage: 25% | T4: 1 (size) | ||
| T4 | 1 | No change: 100% | T4: 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funai, K.; Kawase, A.; Mizuno, K.; Koyama, S.; Shiiya, N. 8th Edition Tumor, Node, and Metastasis T-Stage Prognosis Discrepancies: Solid Component Diameter Predicts Prognosis Better than Invasive Component Diameter. Cancers 2020, 12, 1577. https://doi.org/10.3390/cancers12061577
Funai K, Kawase A, Mizuno K, Koyama S, Shiiya N. 8th Edition Tumor, Node, and Metastasis T-Stage Prognosis Discrepancies: Solid Component Diameter Predicts Prognosis Better than Invasive Component Diameter. Cancers. 2020; 12(6):1577. https://doi.org/10.3390/cancers12061577
Chicago/Turabian StyleFunai, Kazuhito, Akikazu Kawase, Kiyomichi Mizuno, Shin Koyama, and Norihiko Shiiya. 2020. "8th Edition Tumor, Node, and Metastasis T-Stage Prognosis Discrepancies: Solid Component Diameter Predicts Prognosis Better than Invasive Component Diameter" Cancers 12, no. 6: 1577. https://doi.org/10.3390/cancers12061577
APA StyleFunai, K., Kawase, A., Mizuno, K., Koyama, S., & Shiiya, N. (2020). 8th Edition Tumor, Node, and Metastasis T-Stage Prognosis Discrepancies: Solid Component Diameter Predicts Prognosis Better than Invasive Component Diameter. Cancers, 12(6), 1577. https://doi.org/10.3390/cancers12061577