Optimization of the Enrichment of Circulating Tumor Cells for Downstream Phenotypic Analysis in Patients with Non-Small Cell Lung Cancer Treated with Anti-PD-1 Immunotherapy
Abstract
1. Introduction
2. Results
2.1. Optimization of Cell Harvest on Glass Slides for Downstream Immunofluorescence Analysis
2.2. Comparison of Different CTC-Enrichment Methods for Downstream Immunofluorescence Analysis
2.3. Detection and Characterization of CTCs Enriched by Different Approaches in Patients with NSCLC
2.3.1. Frequency of CTC Detection Following Enrichment Using Different Approaches
2.3.2. Phenotype of CTCs Enriched Using Different Approaches
2.3.3. Investigation of Immune Checkpoints on CTCs Enriched by Different Approaches
2.4. Clinical Relevance of CTCs and Distinct CTC Subsets among Patients with Metastatic NSCLC Treated with Anti-PD-1 Inhibitors
2.4.1. Patients
2.4.2. Correlation of CTCs with Clinicopathological Parameters and Response to Anti-PD-1 Inhibitors
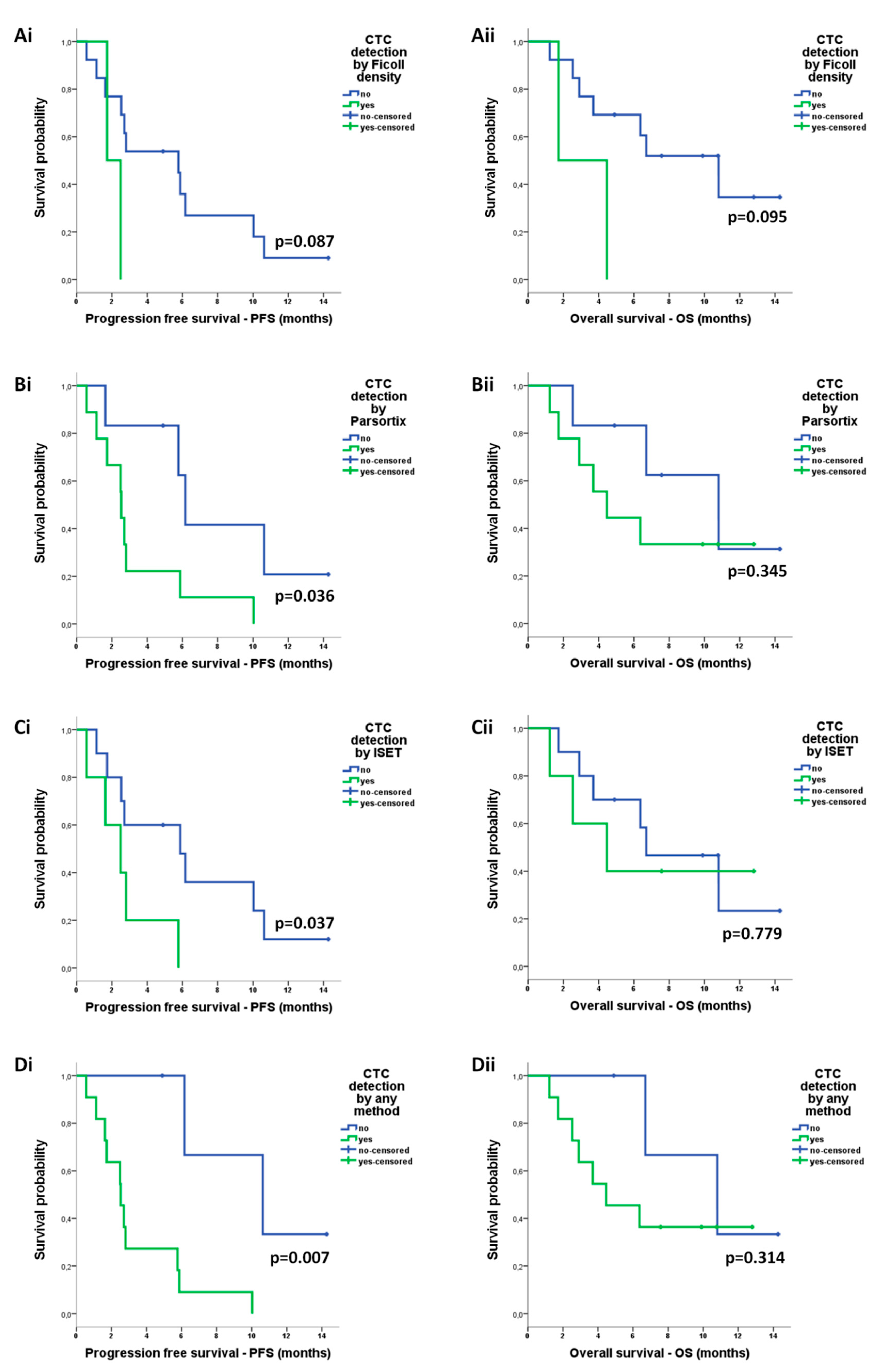
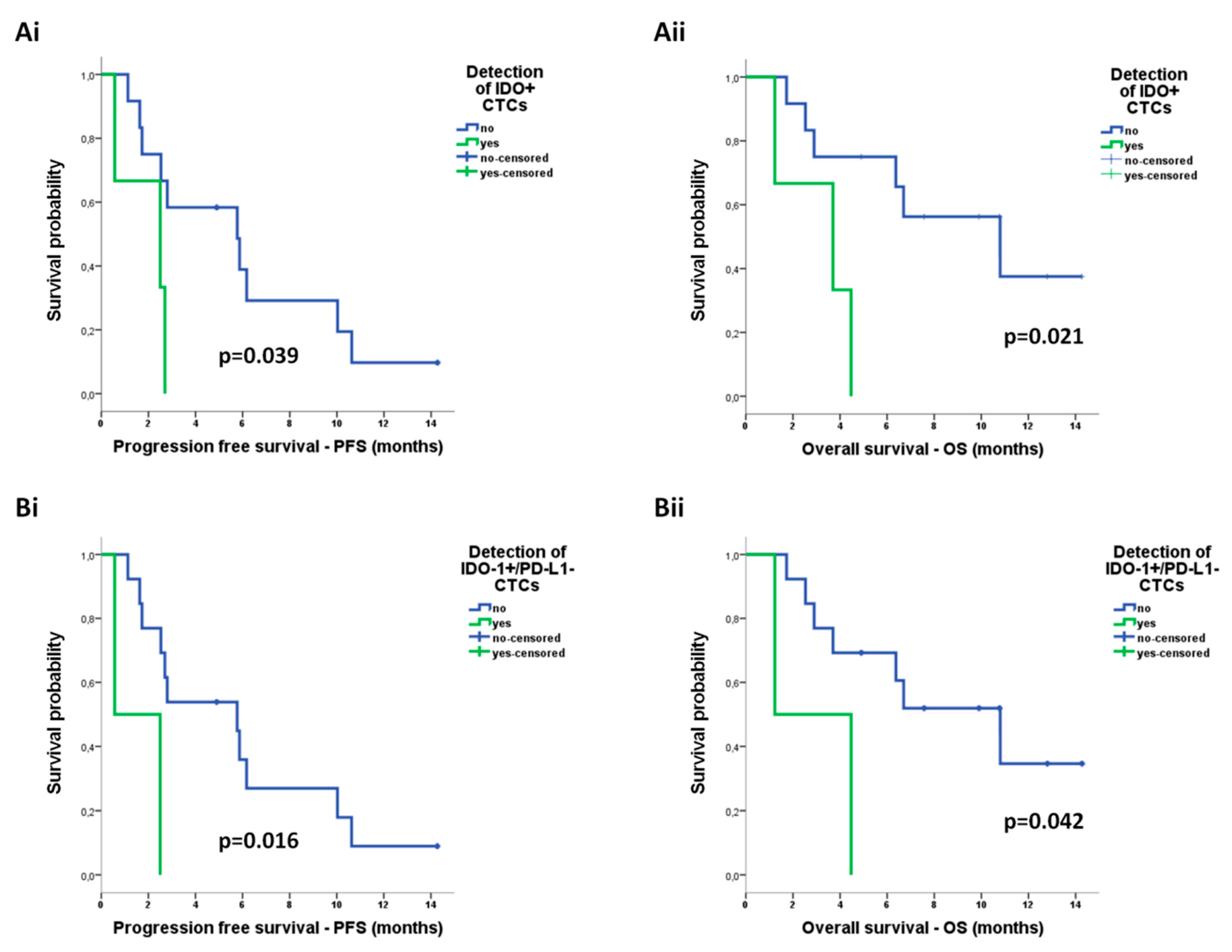
2.4.3. Correlation of CTCs with Survival Measures
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Cell Lines
4.2.1. Cell Culture
4.2.2. Cell Labeling Using CellTracker™ Dye
4.3. Spiking Experiments
4.4. Optimization of Cell Harvest on Glass Slides for Downstream Immunofluorescence Analysis
4.5. CTC-Enrichment Methodologies
4.5.1. Ficoll Density Gradient Centrifugation
4.5.2. Red Blood Cell Lysis—Erythrolysis
4.5.3. Magnetic Separation Using CD45 Dynabeads
4.5.4. ISET Filtration
4.5.5. Parsortix Separation
4.6. Immunofluorescence Approaches for CTC Detection and Characterization
4.6.1. CK/EpCAM/CD45 Staining
4.6.2. CK/IDO/PD-L1 Staining
4.7. Enrichment, Detection, and Phenotypic Characterization of CTCs from Patients’ Samples
4.8. Image Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Cancer.Net. Available online: https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/ (accessed on 11 June 2020).
- Castellanos, E.H.; Horn, L. Immunotherapy in Lung Cancer. Cancer Treat Res. 2016, 170, 203–223. [Google Scholar] [PubMed]
- Mok, T.S.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V.; Laktionov, K.P.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Restifo, N.P.; Smyth, M.J.; Snyder, A. Acquired resistance to immunotherapy and future challenges. Nat. Rev. Cancer 2016, 16, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Prelaj, A.; Tay, R.; Ferrara, R.; Chaput, N.; Besse, B.; Califano, R. Predictive biomarkers of response for immune checkpoint inhibitors in non-small-cell lung cancer. Eur. J. Cancer 2018, 106, 144–159. [Google Scholar] [CrossRef]
- Uyttenhove, C.; Pilotte, L.; Théate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; Eynde, B.J.V.D. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef]
- Holmgaard, R.B.; Zamarin, D.; Li, Y.; Gasmi, B.; Munn, D.H.; Allison, J.P.; Merghoub, T.; Wolchok, J.D. Tumor-Expressed IDO Recruits and Activates MDSCs in a Treg-Dependent Manner. Cell Rep. 2015, 13, 412–424. [Google Scholar] [CrossRef]
- Botticelli, A.; Cerbelli, B.; Lionetto, L.; Zizzari, I.; Salati, M.; Pisano, A.; Federica, M.; Simmaco, M.; Nuti, M.; Marchetti, P. Can IDO activity predict primary resistance to anti-PD-1 treatment in NSCLC? J. Transl. Med. 2018, 16, 219. [Google Scholar] [CrossRef]
- Schalper, K.A.; Carvajal-Hausdorf, D.; McLaughlin, J.; Altan, M.; Velcheti, V.; Gaule, P.; Sanmamed, M.F.; Chen, L.; Herbst, R.S.; Rimm, D.L. Differential Expression and Significance of PD-L1, IDO-1, and B7-H4 in Human Lung Cancer. Clin. Cancer Res. 2016, 23, 370–378. [Google Scholar] [CrossRef]
- Volaric, A.; Gentzler, R.; Hall, R.; Mehaffey, J.H.; Stelow, E.B.; Bullock, T.N.; Martin, L.W.; Mills, A.M. Indoleamine-2,3-Dioxygenase in Non-Small Cell Lung Cancer: A Targetable Mechanism of Immune Resistance Frequently Coexpressed with PD-L1. Am. J. Surg. Pathol. 2018, 42, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, D.A.; Chang, A.L.; Dey, M.; Balyasnikova, I.V.; Kim, C.K.; Tobias, A.; Cheng, Y.; Kim, J.W.; Qiao, J.; Zhang, L.; et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin. Cancer Res. 2014, 20, 5290–5301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Kem, M.; Mooradian, M.J.; Eliane, J.-P.; Huynh, T.G.; Iafrate, A.J.; Gainor, J.F.; Mino-Kenudson, M. Differential expression of PD-L1 and IDO1 in association with the immune microenvironment in resected lung adenocarcinomas. Mod. Pathol. 2018, 32, 511–523. [Google Scholar] [CrossRef]
- Munari, E.; Zamboni, G.; Lunardi, G.; Marchionni, L.; Marconi, M.; Sommaggio, M.; Brunelli, M.; Martignoni, G.; Netto, G.J.; Hoque, M.O.; et al. PD-L1 Expression Heterogeneity in Non-Small Cell Lung Cancer: Defining Criteria for Harmonization between Biopsy Specimens and Whole Sections. J. Thorac. Oncol. 2018, 13, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Zaidi, A.H.; Smith, M.A.; Omstead, A.N.; Kosovec, J.E.; Matsui, D.; Martin, S.A.; Dicarlo, C.; Werts, E.D.; Silverman, J.F.; et al. The Dynamic and Transient Immune Microenvironment in Locally Advanced Esophageal Adenocarcinoma Post Chemoradiation. Ann. Surg. 2018, 268, 992–999. [Google Scholar] [CrossRef]
- Saez, E.D.; Azkárate, A.; Meri, M.; Calabuig-Fariñas, S.; Aguilar-Gallarrdo, C.; Blasco, A.; Jantus-Lewintre, E.; Camps, C. New insights in non-small-cell lung cancer: Circulating tumor cells and cell-free DNA. J. Thorac. Dis. 2017, 9, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Gabriel, M.; Heymann, M.-F.; Heymann, D. Circulating Tumor Cells as a Tool for Assessing Tumor Heterogeneity. Theranostics 2019, 9, 4580–4594. [Google Scholar] [CrossRef]
- Michaelidou, K.; Agelaki, S.; Mavridis, K. Molecular markers related to immunosurveillance as predictive and monitoring tools in non-small cell lung cancer: Recent accomplishments and future promises. Expert Rev. Mol. Diagn. 2020, 20, 335–344. [Google Scholar] [CrossRef]
- Lianidou, E.S.; Markou, A.; Strati, A. The Role of CTCs as Tumor Biomarkers. Adv. Exp. Med. Biol. 2015, 867, 341–367. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Rong, Y.; Liu, Q.; Luo, C.-L.; Zhang, Y.; Wang, F. Integrative diagnosis of cancer by combining CTCs and associated peripheral blood cells in liquid biopsy. Clin. Transl. Oncol. 2018, 21, 828–835. [Google Scholar] [CrossRef]
- Gorges, T.; Penkalla, N.; Schalk, T.; Joosse, S.A.; Riethdorf, S.; Tucholski, J.; Lücke, K.; Wikman, H.; Jackson, S.M.; Brychta, N.; et al. Enumeration and Molecular Characterization of Tumor Cells in Lung Cancer Patients Using a Novel In Vivo Device for Capturing Circulating Tumor Cells. Clin. Cancer Res. 2015, 22, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Farace, F.; Massard, C.; Vimond, N.; Drusch, F.; Jacques, N.; Billiot, F.; Laplanche, A.; Chauchereau, A.; Lacroix, L.; Planchard, D.; et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br. J. Cancer 2011, 105, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Hofman, V.; Ilié, M.; Long, E.; Selva, E.; Bonnetaud, C.; Molina, T.; Venissac, N.; Mouroux, J.; Vielh, P.; Hofman, P. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: Comparison of the efficacy of the CellSearch Assay™ and the isolation by size of epithelial tumor cell method. Int. J. Cancer 2011, 129, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, A.; Wagner, J.; Gorges, T.M.; Taenzer, A.; Uzunoglu, F.G.; Driemel, C.; Stoecklein, N.H.; Knoefel, W.T.; Angenendt, S.; Hauch, S.; et al. Characterization of different CTC subpopulations in non-small cell lung cancer. Sci. Rep. 2016, 6, 28010. [Google Scholar] [CrossRef]
- Krebs, M.G.; Sloane, R.S.; Priest, L.; Lancashire, L.; Hou, J.-M.; Greystoke, A.; Ward, T.; Ferraldeschi, R.; Hughes, A.; Clack, G.; et al. Evaluation and Prognostic Significance of Circulating Tumor Cells in Patients With Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2011, 29, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Allard, W.J. Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas but not in Healthy Subjects or Patients with Nonmalignant Diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef]
- Hou, J.M.; Krebs, M.G.; Lancashire, L.; Sloane, R.; Backen, A.; Swain, R.K.; Priest, L.J.; Greystoke, A.; Zhou, C.; Morris, K.; et al. Dive C: Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 2012, 30, 525–532. [Google Scholar] [CrossRef]
- Krebs, M.G.; Hou, J.M.; Sloane, R.; Lancashire, L.; Priest, L.; Nonaka, D.; Ward, T.H.; Backen, A.; Clack, G.; Hughes, A.; et al. Dive C: Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J. Thorac. Oncol. 2012, 7, 306–315. [Google Scholar] [CrossRef]
- Huang, J.; Wang, K.; Xu, J.; Huang, J.; Zhang, T. Prognostic Significance of Circulating Tumor Cells in Non-Small-Cell Lung Cancer Patients: A Meta-Analysis. PLoS ONE 2013, 8, e78070. [Google Scholar] [CrossRef]
- Huang, H.; Shi, Y.; Huang, J.; Wang, X.; Zhang, R.; Chen, H. Circulating tumor cells as a potential biomarker in diagnosis of lung cancer: A systematic review and meta-analysis. Clin. Respir. J. 2016, 12, 639–645. [Google Scholar] [CrossRef]
- Brown, H.K.; Tellez-Gabriel, M.; Cartron, P.-F.; Vallette, F.M.; Heymann, M.-F.; Heymann, D. Characterization of circulating tumor cells as a reflection of the tumor heterogeneity: Myth or reality? Drug Discov. Today 2019, 24, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Pailler, E.; Adam, J.; Barthélémy, A.; Oulhen, M.; Auger, N.; Valent, A.; Borget, I.; Planchard, D.; Taylor, M.; Andre, F.; et al. Detection of Circulating Tumor Cells Harboring a Unique ALK Rearrangement in ALK-Positive Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2013, 31, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Pailler, E.; Auger, N.; Lindsay, C.R.; Vielh, P.; Islas-Morris-Hernandez, A.; Borget, I.; Ngo-Camus, M.; Planchard, D.; Soria, J.-C.; Besse, B.; et al. High level of chromosomal instability in circulating tumor cells of ROS1-rearranged non-small-cell lung cancer. Ann. Oncol. 2015, 26, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Punnoose, E.A.; Atwal, S.; Liu, W.; Raja, R.; Fine, B.; Hughes, B.G.M.; Hicks, R.J.; Hampton, G.M.; Amler, L.C.; Pirzkall, A.; et al. Evaluation of Circulating Tumor Cells and Circulating Tumor DNA in Non-Small Cell Lung Cancer: Association with Clinical Endpoints in a Phase II Clinical Trial of Pertuzumab and Erlotinib. Clin. Cancer Res. 2012, 18, 2391–2401. [Google Scholar] [CrossRef]
- Boral, D.; Vishnoi, M.; Liu, H.N.; Yin, W.; Sprouse, M.L.; Scamardo, A.; Hong, D.S.; Tan, T.Z.; Thiery, J.P.; Chang, J.C.; et al. Molecular characterization of breast cancer CTCs associated with brain metastasis. Nat. Commun. 2017, 8, 196. [Google Scholar] [CrossRef]
- Kallergi, G.; Agelaki, S.; Mavroudis, D.; Nasias, D.; Matikas, A.; Mavroudis, D.; Samonis, G. Expression of truncated human epidermal growth factor receptor 2 on circulating tumor cells of breast cancer patients. Breast Cancer Res. 2015, 17, 113. [Google Scholar] [CrossRef]
- Papadaki, M.A.; Stoupis, G.; Theodoropoulos, P.A.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Circulating Tumor Cells with Stemness and Epithelial-to-Mesenchymal Transition Features Are Chemoresistant and Predictive of Poor Outcome in Metastatic Breast Cancer. Mol. Cancer Ther. 2018, 18, 437–447. [Google Scholar] [CrossRef]
- Papadaki, M.A.; Koutsopoulos, A.V.; Tsoulfas, P.G.; Lagoudaki, E.; Aggouraki, D.; Monastirioti, A.; Koutoulaki, C.; Apostolopoulou, C.A.; Merodoulaki, A.C.; Papadaki, C.; et al. Clinical Relevance of Immune Checkpoints on Circulating Tumor Cells in Breast Cancer. Cancers 2020, 12, 376. [Google Scholar] [CrossRef]
- Kallergi, G.; Mavroudis, D.; Politaki, E.; Mavroudis, D.; Samonis, G.; Agelaki, S. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011, 13, 59. [Google Scholar] [CrossRef]
- Ferreira, M.M.; Ramani, V.C.; Jeffrey, S.S. Circulating tumor cell technologies. Mol. Oncol. 2016, 10, 374–394. [Google Scholar] [CrossRef]
- Chen, L.; Bode, A.M.; Dong, Z. Circulating Tumor Cells: Moving Biological Insights into Detection. Theranostics 2017, 7, 2606–2619. [Google Scholar] [CrossRef] [PubMed]
- Kallergi, G.; Politaki, E.; Alkahtani, S.; Stournaras, C.; Georgoulias, V. Evaluation of Isolation Methods for Circulating Tumor Cells (CTCs). Cell. Physiol. Biochem. 2016, 40, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Veiga, T.; Martínez-Fernández, M.; Abuín, C.; Piñeiro, R.; Cebey-López, V.; Cueva, J.; Palacios, P.; Blanco, C.; Muinelo-Romay, L.; Abalo, A.; et al. CTCs Expression Profiling for Advanced Breast Cancer Monitoring. Cancers 2019, 11, 1941. [Google Scholar] [CrossRef] [PubMed]
- Hille, C.; Joosse, S.A.; Schneegans, S.; Wilken, O.; Janning, M.; Loreth, D.; Müller, V.; Prieske, K.; Banys-Paluchowski, M.; Horst, L.; et al. Pre-Analytical and Analytical Variables of Label-Independent Enrichment and Automated Detection of Circulating Tumor Cells in Cancer Patients. Cancers 2020, 12, 442. [Google Scholar] [CrossRef]
- Xu, L.; Mao, X.; Imrali, A.; Syed, F.; Mutsvangwa, K.; Berney, D.M.; Cathcart, P.; Hines, J.; Shamash, J.; Lu, Y.-J. Optimization and Evaluation of a Novel Size Based Circulating Tumor Cell Isolation System. PLoS ONE 2015, 10, e0138032. [Google Scholar] [CrossRef]
- Gadeberg, O.V.; Rhodes, J.M.; Larsen, S.O. Comparison of smear and cytocentrifugation techniques for estimation of human peripheral blood monocytes. Acta Haematol. 1982, 68, 313–316. [Google Scholar] [CrossRef]
- Papillo, J.L.; Lapen, D. Cell yield. ThinPrep vs. cytocentrifuge. Acta Cytol. 1994, 38, 33–36. [Google Scholar]
- Pezzi, H.M.; Niles, D.J.; Schehr, J.L.; Beebe, D.J.; Lang, J.M. Integration of Magnetic Bead-Based Cell Selection into Complex Isolations. ACS Omega 2018, 3, 3908–3917. [Google Scholar] [CrossRef]
- Laget, S.; Broncy, L.; Hormigos, K.; Dhingra, D.M.; Ben-Mohamed, F.; Capiod, T.; Osteras, M.; Farinelli, L.; Jackson, S.; Paterlini-Brechot, P.; et al. Technical Insights into Highly Sensitive Isolation and Molecular Characterization of Fixed and Live Circulating Tumor Cells for Early Detection of Tumor Invasion. PLoS ONE 2017, 12, e0169427. [Google Scholar] [CrossRef]
- Hvichia, G.E.; Parveen, Z.; Wagner, C.; Janning, M.; Quidde, J.; Stein, A.; Muller, V.; Loges, S.; Neves, R.P.; Stoecklein, N.H.; et al. A novel microfluidic platform for size and deformability based separation and the subsequent molecular characterization of viable circulating tumor cells. Int. J. Cancer 2016, 138, 2894–2904. [Google Scholar] [CrossRef]
- Ilié, M.; Szafer-Glusman, E.; Hofman, V.; Long-Mira, E.; Suttmann, R.; Darbonne, W.; Butori, C.; Lalvée, S.; Fayada, J.; Selva, E.; et al. Expression of MET in circulating tumor cells correlates with expression in tumor tissue from advanced-stage lung cancer patients. Oncotarget 2017, 8, 26112–26121. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.A.; Talasaz, A.H.; Zhang, H.; Coram, M.A.; Reddy, A.; Deng, G.; Telli, M.L.; Advani, R.H.; Carlson, R.W.; Mollick, J.A.; et al. Single Cell Profiling of Circulating Tumor Cells: Transcriptional Heterogeneity and Diversity from Breast Cancer Cell Lines. PLoS ONE 2012, 7, e33788. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.-A.; Koo, G.-B.; Han, H.; Sohn, J.; Choi, W.; Kim, S.I.; Jung, H.-I.; Kim, Y.-S. Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget 2016, 7, 24677–24687. [Google Scholar] [CrossRef] [PubMed]
- Guibert, N.; Delaunay, M.; Lusque, A.; Boubekeur, N.; Rouquette, I.; Clermont, E.; Gouin, S.; Dormoy, I.; Favre, G.; Mazieres, J.; et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer 2018, 120, 108–112. [Google Scholar] [CrossRef]
- Chudziak, J.; Burt, D.J.; Mohan, S.; Rothwell, D.G.; Antonello, J.; Dalby, S.; Ayub, M.; Priest, L.; Carter, L.; Krebs, M.G.; et al. Clinical evaluation of a novel microfluidic device for epitope-independent enrichment of circulating tumour cells in patients with small cell lung cancer. Analyst 2016, 141, 669–678. [Google Scholar] [CrossRef]
- Politaki, E.; Agelaki, S.; Apostolaki, S.; Hatzidaki, D.; Strati, A.; Koinis, F.; Perraki, M.; Saloustrou, G.; Stoupis, G.; Kallergi, G.; et al. A Comparison of Three Methods for the Detection of Circulating Tumor Cells in Patients with Early and Metastatic Breast Cancer. Cell. Physiol. Biochem. 2017, 44, 594–606. [Google Scholar] [CrossRef]
- Nicolazzo, C.; Raimondi, C.; Mancini, M.; Caponnetto, S.; Gradilone, A.; Gandini, O.; Mastromartino, M.; del Bene, G.; Prete, A.A.; Longo, F.; et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci. Rep. 2016, 6, 31726. [Google Scholar] [CrossRef]
- Punyadeera, C.; Kapeleris, J.; Cooper, C.; Warkiani, M.E.; O’Byrne, K.J.; Punyadeera, C. Phenotypic Characterization of Circulating Lung Cancer Cells for Clinically Actionable Targets. Cancers 2019, 11, 380. [Google Scholar] [CrossRef]
- Ilié, M.; Szafer-Glusman, E.; Hofman, V.; Chamorey, E.; Lalvée, S.; Selva, E.; Leroy, S.; Marquette, C.-H.; Kowanetz, M.; Hedge, P.; et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann. Oncol. 2018, 29, 193–199. [Google Scholar] [CrossRef]
- Manjunath, Y.; Upparahalli, S.V.; Avella, D.; Deroche, C.B.; Kimchi, E.; Staveley-O’Carroll, K.; Smith, C.J.; Li, G.; Kaifi, J.T. PD-L1 Expression with Epithelial Mesenchymal Transition of Circulating Tumor Cells Is Associated with Poor Survival in Curatively Resected Non-Small Cell Lung Cancer. Cancers 2019, 11, 806. [Google Scholar] [CrossRef]
- Chen, Y.L.; Huang, W.C.; Lin, F.M.; Hsieh, H.B.; Hsieh, C.H.; Hsieh, R.K.; Chen, K.W.; Yen, M.H.; Lee, J.; Su, S.; et al. Novel circulating tumor cell-based blood test for the assessment of PD-L1 protein expression in treatment-naive, newly diagnosed patients with non-small cell lung cancer. Cancer Immunol. Immunother. 2019, 68, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Janning, M.; Kobus, F.; Babayan, A.; Wikman, H.; Velthaus, J.L.; Bergmann, S.; Schatz, S.; Falk, M.; Berger, L.A.; Bottcher, L.M.; et al. Determination of PD-L1 Expression in Circulating Tumor Cells of NSCLC Patients and Correlation with Response to PD-1/PD-L1 Inhibitors. Cancers 2019, 11, 835. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.R.; Villalobos, P.; Zhang, J.; Behrens, C.; Mino, B.; Swisher, S.; Sepesi, B.; Weissferdt, A.; Kalhor, N.; Heymach, J.V.; et al. Immunohistochemical and Image Analysis-Based Study Shows That Several Immune Checkpoints are Co-expressed in Non–Small Cell Lung Carcinoma Tumors. J. Thorac. Oncol. 2018, 13, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Leonardi, G.C.; Puccetti, P.; Fallarino, F.; Bianconi, V.; Sahebkar, A.; Baglivo, S.; Chiari, R.; Pirro, M. Targeting indoleamine-2,3-dioxygenase in cancer: Scientific rationale and clinical evidence. Pharmacol. Ther. 2019, 196, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Pailler, E.; Oulhen, M.; Billiot, F.; Galland, A.; Auger, N.; Faugeroux, V.; Laplace-Builhe, C.; Besse, B.; Loriot, Y.; Ngo-Camus, M.; et al. Method for semi-automated microscopy of filtration-enriched circulating tumor cells. BMC Cancer 2016, 16, 477. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Robinson, P.S.; Wagner, C.; O’Shannessy, D.J. The Parsortix™ Cell Separation System—A versatile liquid biopsy platform. Cytom. Part A 2018, 93, 1234–1239. [Google Scholar] [CrossRef]
- Polioudaki, H.; Agelaki, S.; Chiotaki, R.; Politaki, E.; Mavroudis, D.; Matikas, A.; Georgoulias, V.; Theodoropoulos, P.A. Variable expression levels of keratin and vimentin reveal differential EMT status of circulating tumor cells and correlation with clinical characteristics and outcome of patients with metastatic breast cancer. BMC Cancer 2015, 15, 399. [Google Scholar] [CrossRef]

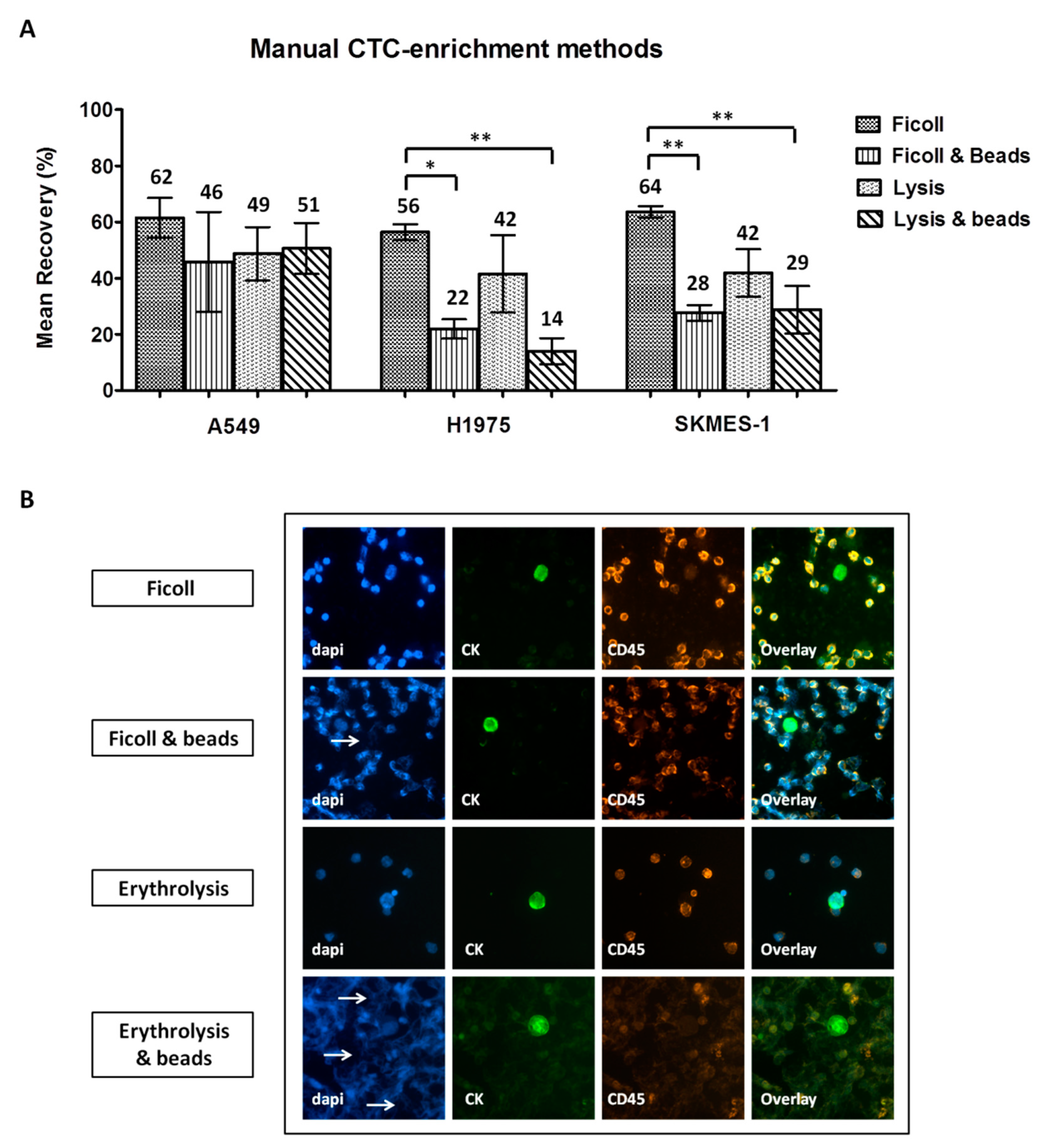
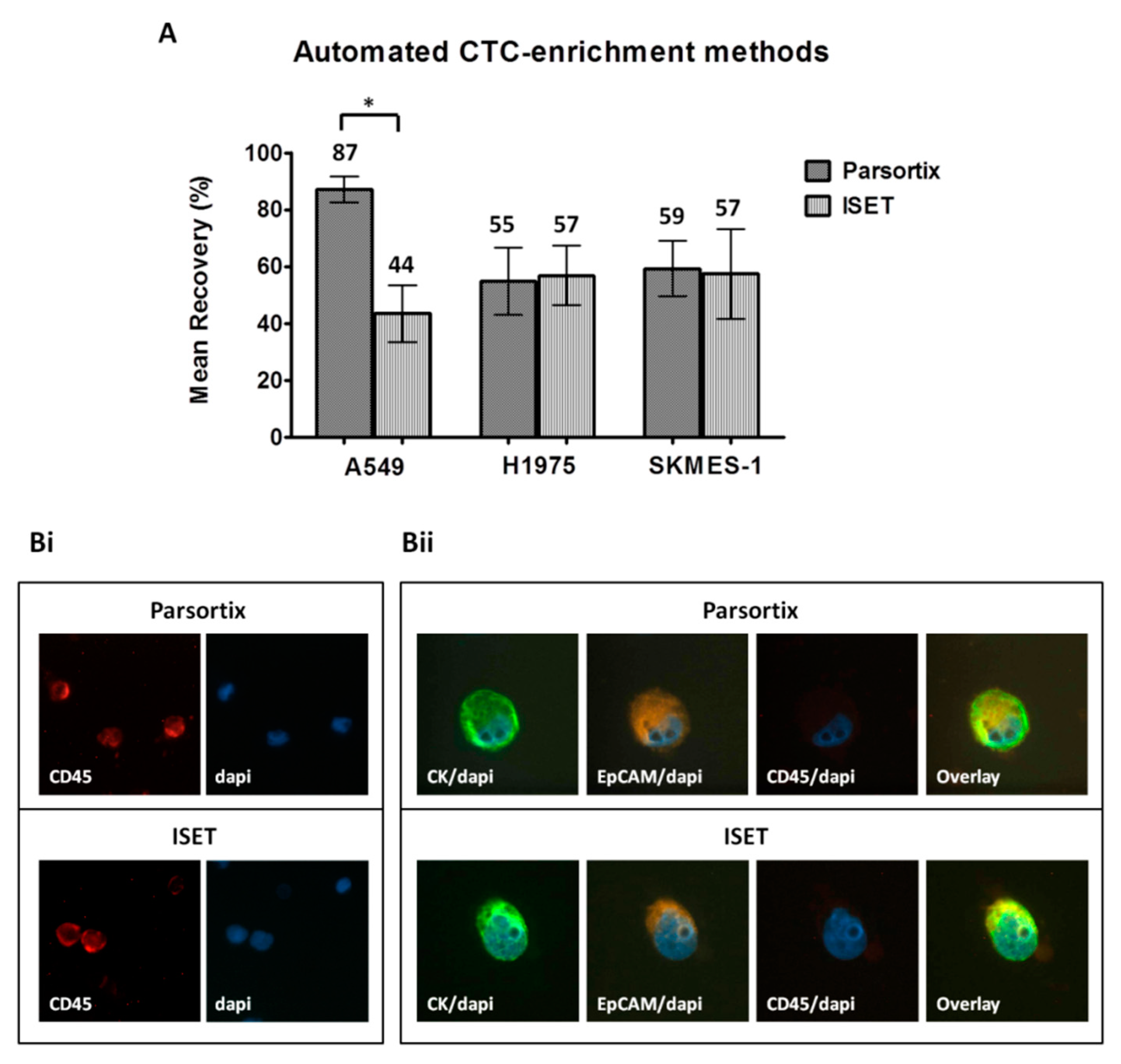
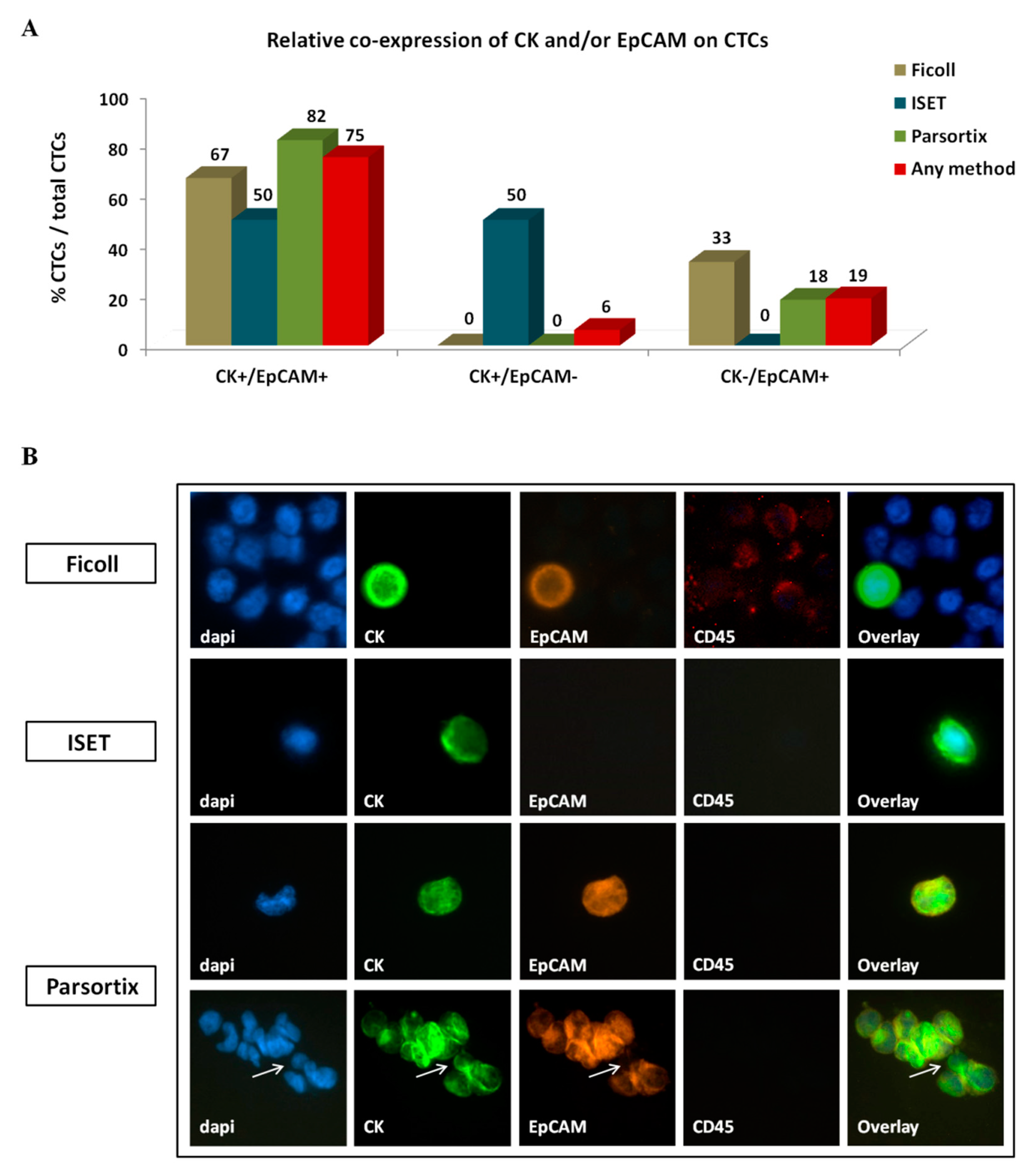
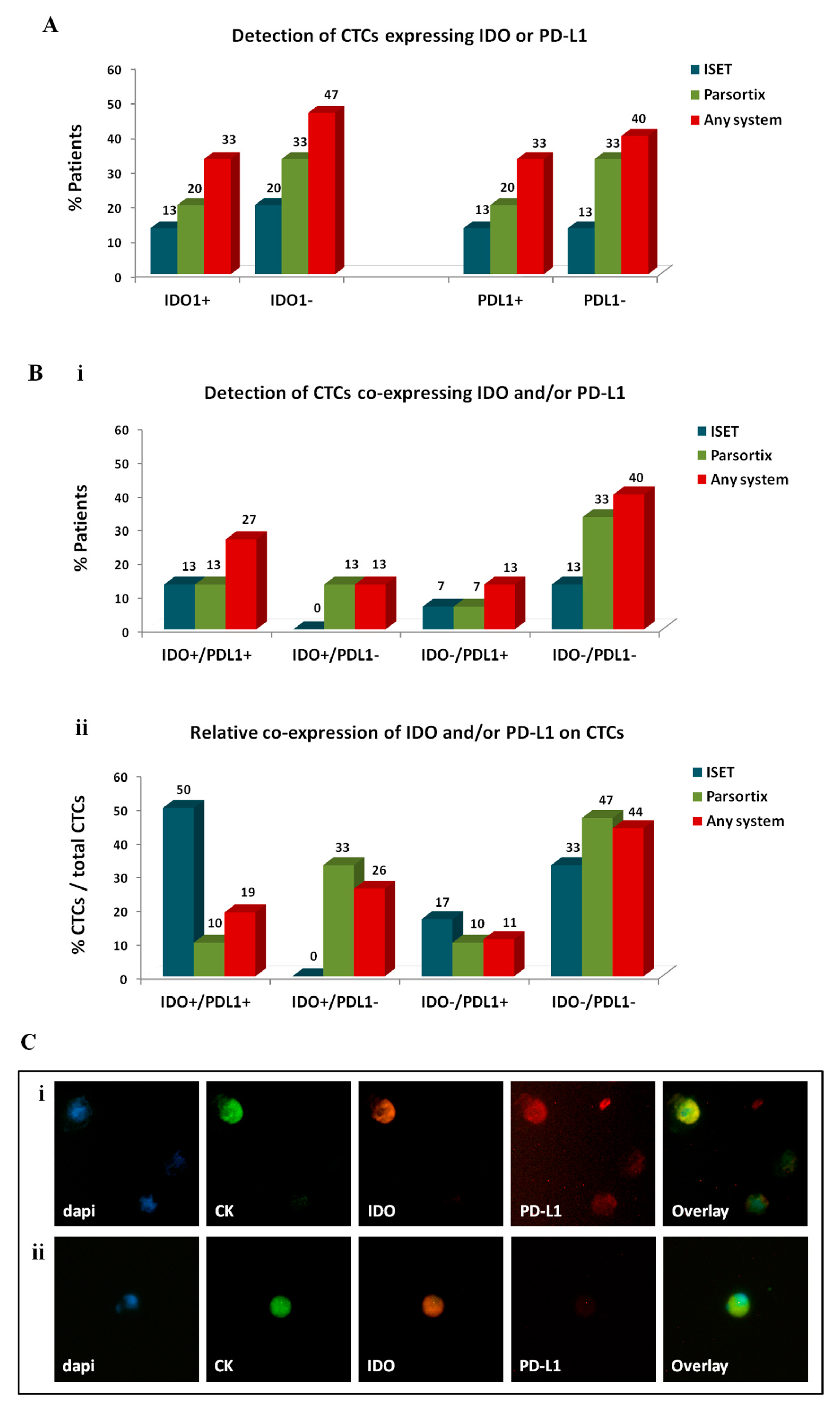
| Enrichment Method | CTC Detection a | Friedman Exact Test (Mean Ranks) | ||
|---|---|---|---|---|
| Positive Patients no (%) | Total CTCs no | No. of CTCs/Patient Mean (range) | ||
| Ficoll | 2 (13) | 3 | 0.2 (0–2) | 1.67 |
| ISET | 5 (33) | 8 | 0.53 (0–3) | 1.87 |
| Parsortix | 9 (60) | 32 | 2.13 (0–12) | 2.47 |
| Any method | 11 (73) | 43 | 2.87 (0–12) | p = 0.010 * |
| Patients | n (%) | Patients | n (%) |
|---|---|---|---|
| Age, years | Metastatic sites | ||
| median (range) | 70 (61–82) | Lung | 12 (80) |
| Gender | Liver | 4 (26.7) | |
| Male | 13 (86.7) | Brain | 2 (13.3) |
| Female | 2 (13.3) | Bones | 5 (33.3) |
| Smoking status | Adrenal gland | 2 (13.3) | |
| Ex-smoker | 4 (26.7) | Lymph nodes | 10 (66.7) |
| Current smoker | 11 (73.3) | NLR | |
| Performance status (ECOG) | <3 | 3 (20) | |
| ≥3 | 12 (80) | ||
| 0–1 | 9 (60) | Line of treatment | |
| 2 | 6 (40) | 2nd | 10 (66.7) |
| Histology subtype | 3rd | 5 (33.3) | |
| Squamous | 10 (66.7) | Best response to immunotherapy | |
| Non-squamous | 5 (33.3) | Partial response | 3 (20) |
| No. of organs affected | Stable disease | 3 (20) | |
| 1–2 | 10 (66.7) | Progressive disease | 8 (53.3) |
| ≥3 | 5 (33.3) | Non-evaluable a | 1 (6.7) |
| Best Response | CTC-Positive Patients, n (%) | |||
|---|---|---|---|---|
| Ficoll | ISET | Parsortix | Any Method | |
| PR | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| SD | 0 (0) | 1 (33.3) | 1 (33.3) | 2 (66.7) |
| PD | 2 (25) | 3 (37.5) | 7 (87.5) | 8 (100) |
| p-value | 1.000 | 0.748 | 0.016* | 0.006 * |
| Univariate Cox-Regression Analysis | Progression Free Survival (PFS) | Overall Survival (OS) | ||
|---|---|---|---|---|
| Covariates | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Age (<70 vs ≥70 years) | 1.745 (0.518–5.880) | 0.369 | 3.282 (0.750–14.360) | 0.115 |
| Gender (male vs female) | 2.329 (0.297–18.293) | 0.421 | 1.293 (0.159–10.550) | 0.810 |
| Smoking status (current vs ex-smoker) | 1.279 (0.379–4.318) | 0.691 | 0.747 (0.186–3.004) | 0.682 |
| Performance status (2 vs 0–1) | 1.022 (0.329–3.174) | 0.970 | 1.875 (0.502–7.011) | 0.350 |
| Histology (squamous vs adenocarcinoma) | 2.364 (0.646–8.655) | 0.194 | 2.800 (0.566–13.843) | 0.207 |
| Metastatic sites (yes vs no) | ||||
| Lung | 1.716 (0.369–7.969) | 0.491 | 3.433 (0.391–30.173) | 0.266 |
| Liver | 1.928 (0.562–6.614) | 0.297 | 1.375 (0.342–5.524) | 0.654 |
| Brain | 0.429 (0.055–3.373) | 0.421 | 0.773(0.095–6.309) | 0.810 |
| Bones | 1.295 (0.414–4.051) | 0.656 | 2.720 (0.726–10.188) | 0.138 |
| Adrenal gland | 0.022 (0.000–5.625) | 0.177 | 0.340 (0.037–3.094) | 0.338 |
| Lymph nodes | 1.588 (0.485–5.199) | 0.445 | 1.567 (0.382–6.434) | 0.533 |
| No of organs affected (≥3 vs <3) | 1.330 (0.425–4.160) | 0.624 | 3.063 (0.820–14.443) | 0.096 |
| Neutrophil–lymphocyte ratio (≥3 vs <3) | 4.400 (0.562–34.432) | 0.158 | 2.607 (0.323–21.021) | 0.368 |
| Line of treatment (2nd vs 3rd) | 2.089 (0.567–17.697) | 0.268 | 4.367 (0.544–35.044) | 0.165 |
| CTC detection (yes vs. no) | ||||
| Ficoll-positive | 4.237 (0.700–25.653) | 0.116 | 4.351 (0.787–24.066) | 0.092 |
| ISET-positive | 3.746 (0.994–14.117) | 0.051 | 1.220 (0.304–4.892) | 0.779 |
| Parsortix-positive | 3.819 (1.005–14.515) | 0.049* | 1.946 (0.478–7.921) | 0.353 |
| Positive by any method | 10.733 (1.330–86.579) | 0.026 * | 2.249(0.448–11.288) | 0.325 |
| CTC subsets detected by Parsortix (yes vs. no) | ||||
| IDO + CTCs | 4.305 (0.951–19.492) | 0.058 | 5.462 (1.088–27.417) | 0.039 * |
| IDO+/PD-L1− CTCs | 6.782 (1.118–41.121) | 0.037 * | 4.964 (0.900–27.376) | 0.066 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadaki, M.A.; Sotiriou, A.I.; Vasilopoulou, C.; Filika, M.; Aggouraki, D.; Tsoulfas, P.G.; Apostolopoulou, C.A.; Rounis, K.; Mavroudis, D.; Agelaki, S. Optimization of the Enrichment of Circulating Tumor Cells for Downstream Phenotypic Analysis in Patients with Non-Small Cell Lung Cancer Treated with Anti-PD-1 Immunotherapy. Cancers 2020, 12, 1556. https://doi.org/10.3390/cancers12061556
Papadaki MA, Sotiriou AI, Vasilopoulou C, Filika M, Aggouraki D, Tsoulfas PG, Apostolopoulou CA, Rounis K, Mavroudis D, Agelaki S. Optimization of the Enrichment of Circulating Tumor Cells for Downstream Phenotypic Analysis in Patients with Non-Small Cell Lung Cancer Treated with Anti-PD-1 Immunotherapy. Cancers. 2020; 12(6):1556. https://doi.org/10.3390/cancers12061556
Chicago/Turabian StylePapadaki, Maria A, Afroditi I Sotiriou, Christina Vasilopoulou, Maria Filika, Despoina Aggouraki, Panormitis G Tsoulfas, Christina A Apostolopoulou, Konstantinos Rounis, Dimitrios Mavroudis, and Sofia Agelaki. 2020. "Optimization of the Enrichment of Circulating Tumor Cells for Downstream Phenotypic Analysis in Patients with Non-Small Cell Lung Cancer Treated with Anti-PD-1 Immunotherapy" Cancers 12, no. 6: 1556. https://doi.org/10.3390/cancers12061556
APA StylePapadaki, M. A., Sotiriou, A. I., Vasilopoulou, C., Filika, M., Aggouraki, D., Tsoulfas, P. G., Apostolopoulou, C. A., Rounis, K., Mavroudis, D., & Agelaki, S. (2020). Optimization of the Enrichment of Circulating Tumor Cells for Downstream Phenotypic Analysis in Patients with Non-Small Cell Lung Cancer Treated with Anti-PD-1 Immunotherapy. Cancers, 12(6), 1556. https://doi.org/10.3390/cancers12061556







