Absence of HTATIP2 Expression in A549 Lung Adenocarcinoma Cells Promotes Tumor Plasticity in Response to Hypoxic Stress
Abstract
1. Introduction
2. Results
2.1. Knockdown of HTATIP2 in A549 Cells Affected Cell Migration but Had Little Impact on Invasion and Response to Sorafenib Treatment in Vitro under Normoxic and Hypoxic Conditions
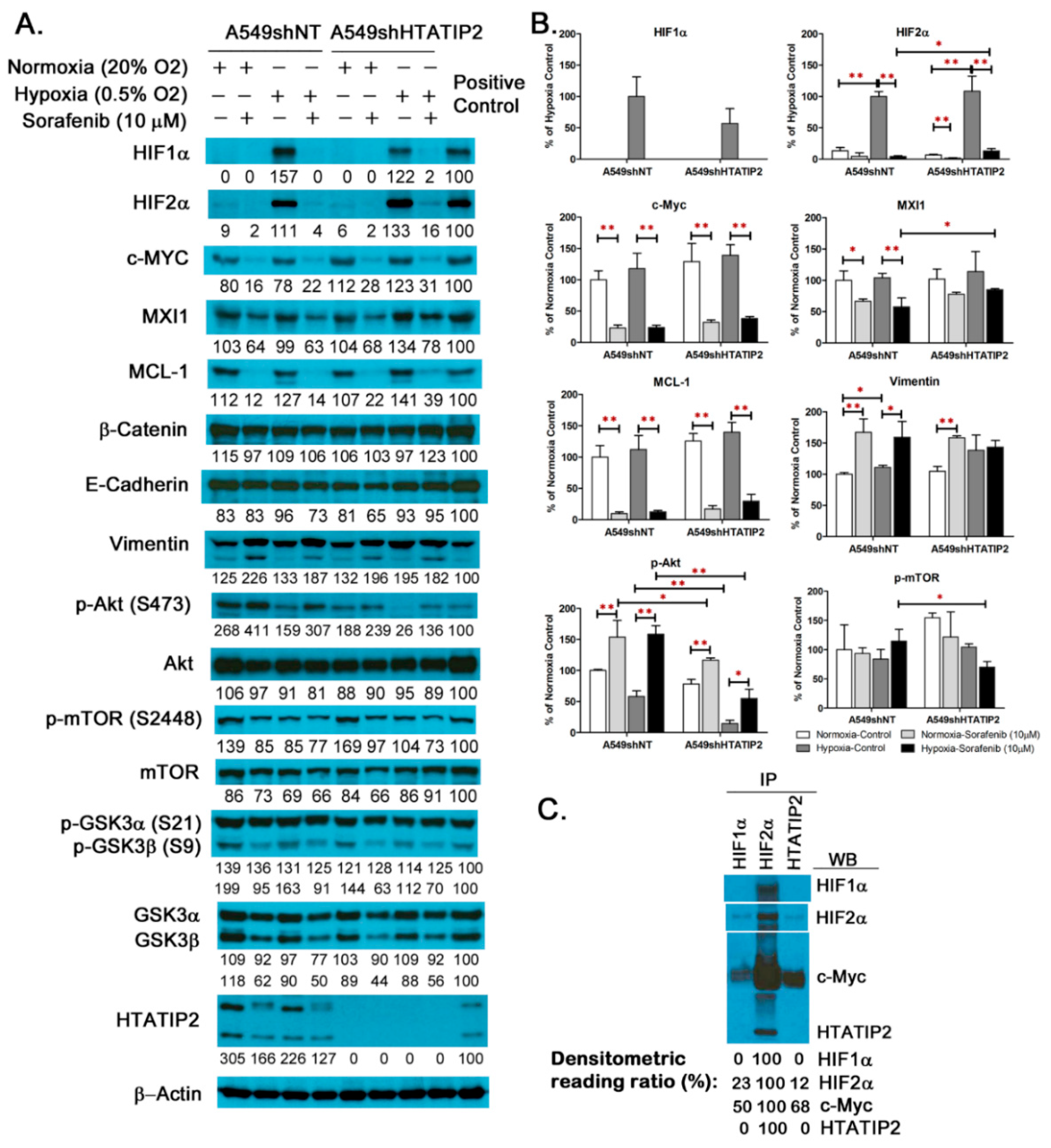
2.2. Examination of Molecular Changes in Response to Hypoxic Stress and Sorafenib Treatment in Cultured A549shNT and A549shHTATIP2 Cells
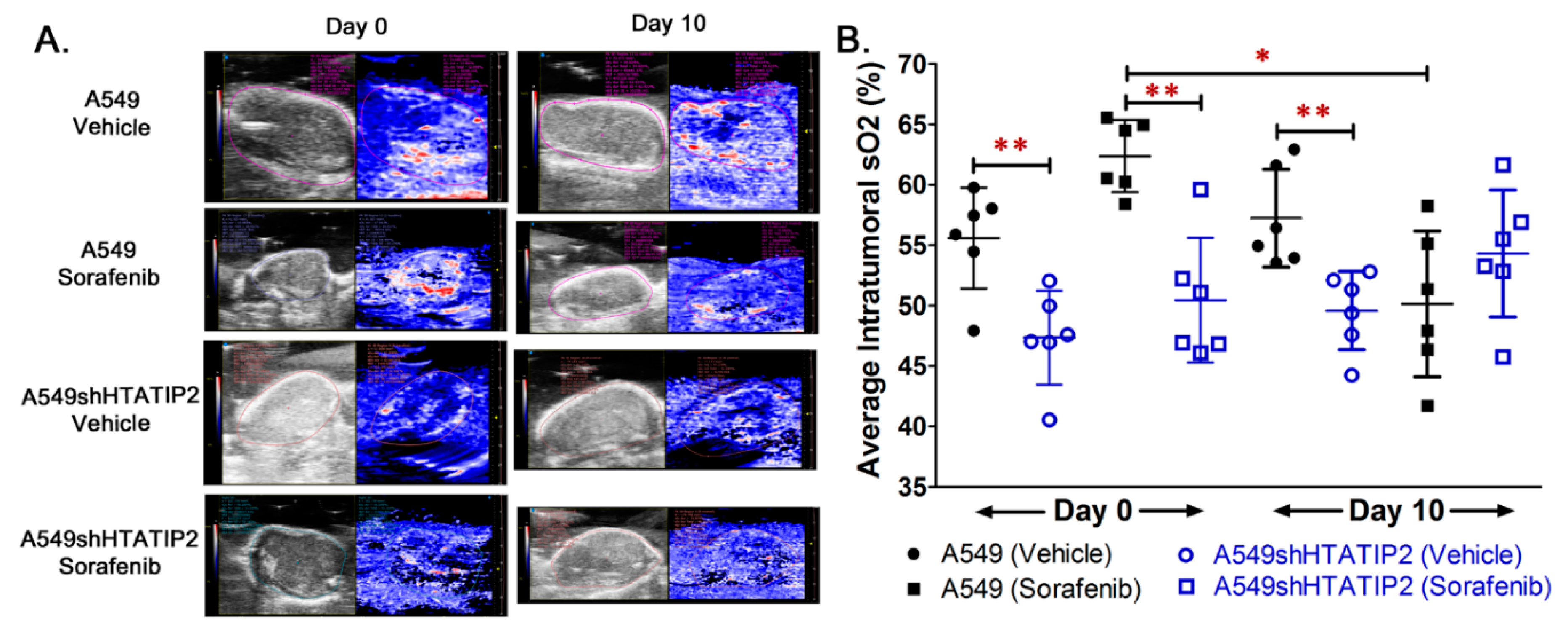
2.3. Absence of HTATIP2 Expression Promoted Tumor Growth, Decreased Tumor Oxygenation and Reduced Sensitivity to Sorafenib Treatment without Affecting Sorafenib Tumor Distribution
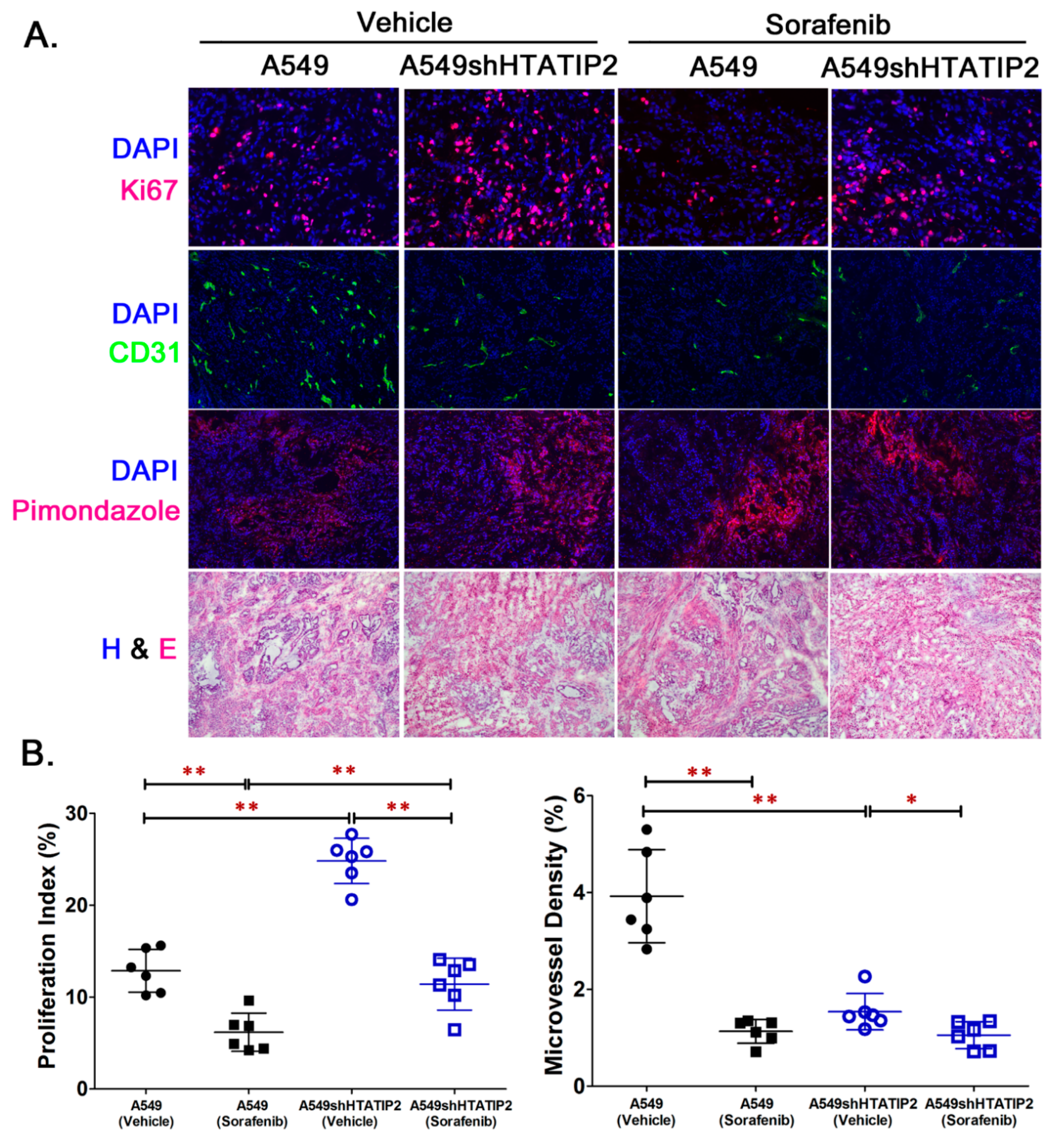
2.4. Reduced HTATIP2 Expression Promoted Cell Proliferation In Vivo Despite of Stalled Tumor Angiogenesis
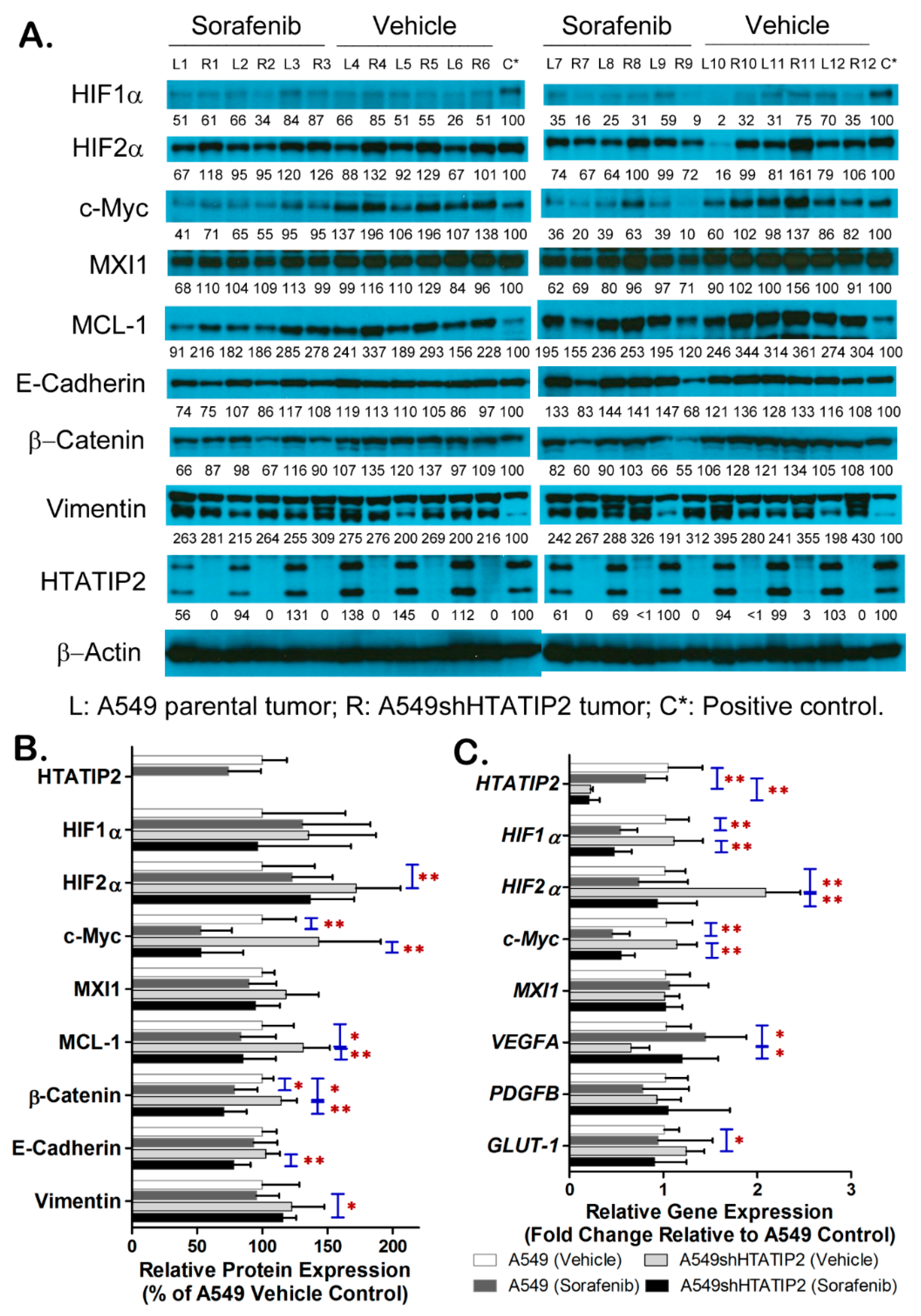
2.5. Absent Expression of HTATIP2 Resulted in Upregulated HIF2α Expression, Enhanced β-Catenin/c-Myc/MCL-1 Signaling and Elicited EMT in Response to Sorafenib Treatment
2.6. Metabolomic Profiling of A549 and A549shHTATIP2 Xenografts
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Cell Lines, Cell Culture and Stable Short Hairpin RNA (shRNA) Knockdown
4.4. Hypoxia
4.5. Wound-Healing Assay
4.6. Cell Invasion Assay
4.7. Co-Immunoprecipitation
4.8. In Vitro Cytotoxicity Assay
4.9. In Vivo Study Protocol
4.10. Measurement of Intratumoral Oxygen Saturation Using Real-Time In Vivo Photoacoustic Imaging
4.11. Immunofluorescence Staining
4.12. Semi-Quantitative Western Blot Analysis
4.13. Quantitative Real-Time Polymerase Chain Reaction (PCR)
4.14. Determination of Sorafenib Concentrations in Plasma and Tumor Tissue Homogenates using High Performance Liquid Chromatography
4.15. Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) Based Targeted Metabolomics
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Dengler, V.L.; Galbraith, M.; Espinosa, J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; McKnight, S.L.; Russell, D.W. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997, 11, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Wiesener, M.S.; Jurgensen, J.S.; Rosenberger, C.; Scholze, C.K.; Horstrup, J.H.; Warnecke, C.; Mandriota, S.; Bechmann, I.; Frei, U.A.; Pugh, C.W.; et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 271–273. [Google Scholar] [CrossRef]
- Hu, C.J.; Sataur, A.; Wang, L.; Chen, H.; Simon, M.C. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol. Biol. Cell 2007, 18, 4528–4542. [Google Scholar] [CrossRef]
- Holmquist-Mengelbier, L.; Fredlund, E.; Lofstedt, T.; Noguera, R.; Navarro, S.; Nilsson, H.; Pietras, A.; Vallon-Christersson, J.; Borg, A.; Gradin, K.; et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell 2006, 10, 413–423. [Google Scholar] [CrossRef]
- Stiehl, D.P.; Bordoli, M.R.; Abreu-Rodriguez, I.; Wollenick, K.; Schraml, P.; Gradin, K.; Poellinger, L.; Kristiansen, G.; Wenger, R.H. Non-canonical HIF-2alpha function drives autonomous breast cancer cell growth via an AREG-EGFR/ErbB4 autocrine loop. Oncogene 2012, 31, 2283–2297. [Google Scholar] [CrossRef]
- Koh, M.Y.; Lemos, R., Jr.; Liu, X.; Powis, G. The hypoxia-associated factor switches cells from HIF-1alpha- to HIF-2alpha-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res. 2011, 71, 4015–4027. [Google Scholar] [CrossRef]
- Hu, C.J.; Wang, L.Y.; Chodosh, L.A.; Keith, B.; Simon, M.C. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol. Cell. Biol. 2003, 23, 9361–9374. [Google Scholar] [CrossRef]
- Downes, N.L.; Laham-Karam, N.; Kaikkonen, M.U.; Yla-Herttuala, S. Differential but Complementary HIF1alpha and HIF2alpha Transcriptional Regulation. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 1735–1745. [Google Scholar] [CrossRef]
- Keith, B.; Johnson, R.S.; Simon, M.C. HIF1alpha and HIF2alpha: Sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 2011, 12, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lv, L.; Ouyang, X.; Zhang, S.; Fang, J.; Cai, L.; Li, D. Association of TIP30 expression and prognosis of hepatocellular carcinoma in patients with HBV infection. Cancer Med. 2016, 5, 2180–2189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.; Zhang, Y.; Cao, S.; Chen, X.; Lu, Y.; Jin, H.; Sun, S.; Chen, B.; Liu, J.; Ding, J.; et al. Reduction of TIP30 correlates with poor prognosis of gastric cancer patients and its restoration drastically inhibits tumor growth and metastasis. Int. J. Cancer. J. Int. Cancer 2009, 124, 713–721. [Google Scholar] [CrossRef]
- Guo, S.; Jing, W.; Hu, X.; Zhou, X.; Liu, L.; Zhu, M.; Yin, F.; Chen, R.; Zhao, J.; Guo, Y. Decreased TIP30 expression predicts poor prognosis in pancreatic cancer patients. Int. J. Cancer. J. Int. Cancer 2014, 134, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ni, H.; Ma, Y.; Dong, L.; Dai, J.; Zhao, F.; Yan, X.; Lu, B.; Xu, H.; Guo, Y. TIP30/CC3 expression in breast carcinoma: Relation to metastasis, clinicopathologic parameters, and P53 expression. Hum. Pathol. 2007, 38, 293–298. [Google Scholar] [CrossRef]
- Chen, X.; Cao, X.; Dong, W.; Luo, S.; Suo, Z.; Jin, Y. Expression of TIP30 tumor suppressor gene is down-regulated in human colorectal carcinoma. Dig. Dis. Sci. 2010, 55, 2219–2226. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, F.; Liu, F.; Liu, X.; Huang, N.; Cai, X.; Sun, Y.; Li, A.; Luo, R. Overexpression of TIP30 inhibits the growth and invasion of glioma cells. Mol. Med. Rep. 2016, 13, 605–612. [Google Scholar] [CrossRef]
- Shtivelman, E. A link between metastasis and resistance to apoptosis of variant small cell lung carcinoma. Oncogene 1997, 14, 2167–2173. [Google Scholar] [CrossRef]
- Tong, X.; Li, K.; Luo, Z.; Lu, B.; Liu, X.; Wang, T.; Pang, M.; Liang, B.; Tan, M.; Wu, M.; et al. Decreased TIP30 expression promotes tumor metastasis in lung cancer. Am. J. Pathol. 2009, 174, 1931–1939. [Google Scholar] [CrossRef]
- Xiao, H.; Palhan, V.; Yang, Y.; Roeder, R.G. TIP30 has an intrinsic kinase activity required for up-regulation of a subset of apoptotic genes. EMBO J. 2000, 19, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, X.; Wang, P.; Zhang, H.W.; Zhang, B.H.; Wu, M.C. TIP30 regulates apoptosis-related genes in its apoptotic signal transduction pathway. World J. Gastroenterol. 2005, 11, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, J.; Lu, B.; Dong, L.; Wang, H.; Bi, C.; Wu, G.; Guo, H.; Wu, M.; Guo, Y. TIP30 induces apoptosis under oxidative stress through stabilization of p53 messenger RNA in human hepatocellular carcinoma. Cancer Res. 2008, 68, 4133–4141. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ju, S.K.; Lee, T.Y.; Huh, S.H.; Han, K.H. TIP30 directly binds p53 tumor suppressor protein in vitro. Mol. Cells 2012, 34, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.; Shtivelman, E. CC3/TIP30 regulates metabolic adaptation of tumor cells to glucose limitation. Cell Cycle 2010, 9, 4941–4953. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yin, F.; Yang, L.; Chen, S.; Chen, R.; Zhou, X.; Jing, W.; Fan, X.; Jia, R.; Wang, H.; et al. Contribution of TIP30 to chemoresistance in laryngeal carcinoma. Cell Death Dis. 2014, 5, e1468. [Google Scholar] [CrossRef]
- Wilhelm, S.; Carter, C.; Lynch, M.; Lowinger, T.; Dumas, J.; Smith, R.A.; Schwartz, B.; Simantov, R.; Kelley, S. Discovery and development of sorafenib: A multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 2006, 5, 835–844. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, H.C.; Wang, W.Q.; Zhang, Q.B.; Zhuang, P.Y.; Xiong, Y.Q.; Zhu, X.D.; Xu, H.X.; Kong, L.Q.; Wu, W.Z.; et al. Sorafenib down-regulates expression of HTATIP2 to promote invasiveness and metastasis of orthotopic hepatocellular carcinoma tumors in mice. Gastroenterology 2012, 143, 1641–1649. [Google Scholar] [CrossRef]
- Lu, L.; Sun, H.C.; Zhang, W.; Chai, Z.T.; Zhu, X.D.; Kong, L.Q.; Wang, W.Q.; Zhang, K.Z.; Zhang, Y.Y.; Zhang, Q.B.; et al. Aspirin minimized the pro-metastasis effect of sorafenib and improved survival by up-regulating HTATIP2 in hepatocellular carcinoma. PLoS ONE 2013, 8, e65023. [Google Scholar] [CrossRef]
- Wang, W.Q.; Liu, L.; Xu, H.X.; Sun, H.C.; Wu, C.T.; Zhu, X.D.; Zhang, W.; Xu, J.; Liu, C.; Long, J.; et al. The combination of HTATIP2 expression and microvessel density predicts converse survival of hepatocellular carcinoma with or without sorafenib. Oncotarget 2014, 5, 3895–3906. [Google Scholar] [CrossRef]
- You, A.; Cao, M.; Guo, Z.; Zuo, B.; Gao, J.; Zhou, H.; Li, H.; Cui, Y.; Fang, F.; Zhang, W.; et al. Metformin sensitizes sorafenib to inhibit postoperative recurrence and metastasis of hepatocellular carcinoma in orthotopic mouse models. J. Hematol. Oncol. 2016, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Guo, X.; Yang, L. Metformin Enhances the Effect of Regorafenib and Inhibits Recurrence and Metastasis of Hepatic Carcinoma After Liver Resection via Regulating Expression of Hypoxia Inducible Factors 2alpha (HIF-2alpha) and 30 kDa HIV Tat-Interacting Protein (TIP30). Med. Sci. Monit. 2018, 24, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Gordan, J.D.; Simon, M.C. Hypoxia-inducible factors: Central regulators of the tumor phenotype. Curr. Opin. Genet. Dev. 2007, 17, 71–77. [Google Scholar] [CrossRef]
- Chen, K.F.; Chen, H.L.; Tai, W.T.; Feng, W.C.; Hsu, C.H.; Chen, P.J.; Cheng, A.L. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J. Pharmacol. Exp. Ther. 2011, 337, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Corn, P.G. Reactivation of p53 function with a demethylating agent. Cancer Biol. Ther. 2006, 5, 1161–1162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Graves, E.E.; Vilalta, M.; Cecic, I.K.; Erler, J.T.; Tran, P.T.; Felsher, D.; Sayles, L.; Sweet-Cordero, A.; Le, Q.T.; Giaccia, A.J. Hypoxia in models of lung cancer: Implications for targeted therapeutics. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 4843–4852. [Google Scholar] [CrossRef]
- Masson, N.; Ratcliffe, P.J. Hypoxia signaling pathways in cancer metabolism: The importance of co-selecting interconnected physiological pathways. Cancer Metab. 2014, 2, 3. [Google Scholar] [CrossRef]
- Yang, M.; Vousden, K.H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 2016, 16, 650–662. [Google Scholar] [CrossRef]
- Combs, J.A.; DeNicola, G.M. The Non-Essential Amino Acid Cysteine Becomes Essential for Tumor Proliferation and Survival. Cancers 2019, 11, 678. [Google Scholar] [CrossRef]
- Melone, M.A.B.; Valentino, A.; Margarucci, S.; Galderisi, U.; Giordano, A.; Peluso, G. The carnitine system and cancer metabolic plasticity. Cell Death Dis. 2018, 9, 228. [Google Scholar] [CrossRef]
- Ito, M.; Jiang, C.; Krumm, K.; Zhang, X.; Pecha, J.; Zhao, J.; Guo, Y.; Roeder, R.G.; Xiao, H. TIP30 deficiency increases susceptibility to tumorigenesis. Cancer Res. 2003, 63, 8763–8767. [Google Scholar] [PubMed]
- Katt, M.E.; Placone, A.L.; Wong, A.D.; Xu, Z.S.; Searson, P.C. In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform. Front. Bioeng. Biotechnol. 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- NicAmhlaoibh, R.; Shtivelman, E. Metastasis suppressor CC3 inhibits angiogenic properties of tumor cells in vitro. Oncogene 2001, 20, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Manalo, D.J.; Rowan, A.; Lavoie, T.; Natarajan, L.; Kelly, B.D.; Ye, S.Q.; Garcia, J.G.; Semenza, G.L. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 2005, 105, 659–669. [Google Scholar] [CrossRef]
- Gong, H.; Rehman, J.; Tang, H.; Wary, K.; Mittal, M.; Chaturvedi, P.; Zhao, Y.Y.; Komarova, Y.A.; Vogel, S.M.; Malik, A.B. HIF2alpha signaling inhibits adherens junctional disruption in acute lung injury. J. Clin. Investig. 2015, 125, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Duda, D.G.; Sahani, D.V.; Jain, R.K. HCC and angiogenesis: Possible targets and future directions. Nat. Rev. Clin. Oncol. 2011, 8, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.G.; Mellberg, S.; Claesson-Welsh, L.; Bader, J.S.; Popel, A.S. Analysis of VEGF—A regulated gene expression in endothelial cells to identify genes linked to angiogenesis. PLoS ONE 2011, 6, e24887. [Google Scholar] [CrossRef] [PubMed]
- Falcon, B.L.; Chintharlapalli, S.; Uhlik, M.T.; Pytowski, B. Antagonist antibodies to vascular endothelial growth factor receptor 2 (VEGFR-2) as anti-angiogenic agents. Pharm. Ther. 2016, 164, 204–225. [Google Scholar] [CrossRef]
- Raval, R.R.; Lau, K.W.; Tran, M.G.; Sowter, H.M.; Mandriota, S.J.; Li, J.L.; Pugh, C.W.; Maxwell, P.H.; Harris, A.L.; Ratcliffe, P.J. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol. Cell. Biol. 2005, 25, 5675–5686. [Google Scholar] [CrossRef]
- Gordan, J.D.; Bertout, J.A.; Hu, C.J.; Diehl, J.A.; Simon, M.C. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell 2007, 11, 335–347. [Google Scholar] [CrossRef]
- Koshiji, M.; Kageyama, Y.; Pete, E.A.; Horikawa, I.; Barrett, J.C.; Huang, L.E. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004, 23, 1949–1956. [Google Scholar] [CrossRef]
- Cazzalini, O.; Scovassi, A.I.; Savio, M.; Stivala, L.A.; Prosperi, E. Multiple roles of the cell cycle inhibitor p21(CDKN1A) in the DNA damage response. Mutat. Res. 2010, 704, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, P.; Fukuda, R.; Kumar, G.; Krishnamachary, B.; Zeller, K.I.; Dang, C.V.; Semenza, G.L. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 2007, 11, 407–420. [Google Scholar] [CrossRef]
- Cuconati, A.; Mukherjee, C.; Perez, D.; White, E. DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 2003, 17, 2922–2932. [Google Scholar] [CrossRef]
- Clohessy, J.G.; Zhuang, J.; de Boer, J.; Gil-Gomez, G.; Brady, H.J. Mcl-1 interacts with truncated Bid and inhibits its induction of cytochrome c release and its role in receptor-mediated apoptosis. J. Biol. Chem. 2006, 281, 5750–5759. [Google Scholar] [CrossRef] [PubMed]
- Labisso, W.L.; Wirth, M.; Stojanovic, N.; Stauber, R.H.; Schnieke, A.; Schmid, R.M.; Kramer, O.H.; Saur, D.; Schneider, G. MYC directs transcription of MCL1 and eIF4E genes to control sensitivity of gastric cancer cells toward HDAC inhibitors. Cell Cycle 2012, 11, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Borner, M.M.; Brousset, P.; Pfanner-Meyer, B.; Bacchi, M.; Vonlanthen, S.; Hotz, M.A.; Altermatt, H.J.; Schlaifer, D.; Reed, J.C.; Betticher, D.C. Expression of apoptosis regulatory proteins of the Bcl-2 family and p53 in primary resected non-small-cell lung cancer. Br. J. Cancer 1999, 79, 952–958. [Google Scholar] [CrossRef]
- Wesarg, E.; Hoffarth, S.; Wiewrodt, R.; Kroll, M.; Biesterfeld, S.; Huber, C.; Schuler, M. Targeting BCL-2 family proteins to overcome drug resistance in non-small cell lung cancer. Int. J. Cancer. J. Int. Cancer 2007, 121, 2387–2394. [Google Scholar] [CrossRef]
- Allen, T.D.; Zhu, C.Q.; Jones, K.D.; Yanagawa, N.; Tsao, M.S.; Bishop, J.M. Interaction between MYC and MCL1 in the genesis and outcome of non-small-cell lung cancer. Cancer Res. 2011, 71, 2212–2221. [Google Scholar] [CrossRef]
- Shortt, J.; Johnstone, R.W. Oncogenes in cell survival and cell death. Cold Spring Harb. Perspect. Biol. 2012, 4, a009829. [Google Scholar] [CrossRef]
- Cadigan, K.M.; Nusse, R. Wnt signaling: A common theme in animal development. Genes Dev. 1997, 11, 3286–3305. [Google Scholar] [CrossRef] [PubMed]
- He, T.C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYC as a target of the APC pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Tillo, E.; de Barrios, O.; Siles, L.; Cuatrecasas, M.; Castells, A.; Postigo, A. beta-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc. Natl. Acad. Sci. USA 2011, 108, 19204–19209. [Google Scholar] [CrossRef] [PubMed]
- Perciavalle, R.M.; Stewart, D.P.; Koss, B.; Lynch, J.; Milasta, S.; Bathina, M.; Temirov, J.; Cleland, M.M.; Pelletier, S.; Schuetz, J.D.; et al. Anti-apoptotic MCL-1 localizes to the mitochondrial matrix and couples mitochondrial fusion to respiration. Nat. Cell Biol. 2012, 14, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Perez, A.A.; Fujie, S.; Warden, C.; Li, J.; Wang, Y.; Yung, B.; Chen, Y.R.; Liu, X.; Zhang, H.; et al. Wnt modulates MCL1 to control cell survival in triple negative breast cancer. BMC Cancer 2014, 14, 124. [Google Scholar] [CrossRef]
- Kaidi, A.; Williams, A.C.; Paraskeva, C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat. Cell Biol. 2007, 9, 210–217. [Google Scholar] [CrossRef]
- Choi, H.; Chun, Y.S.; Kim, T.Y.; Park, J.W. HIF-2alpha enhances beta-catenin/TCF-driven transcription by interacting with beta-catenin. Cancer Res. 2010, 70, 10101–10111. [Google Scholar] [CrossRef]
- Onder, T.T.; Gupta, P.B.; Mani, S.A.; Yang, J.; Lander, E.S.; Weinberg, R.A. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008, 68, 3645–3654. [Google Scholar] [CrossRef]
- Alvarado, C.G.; Maruyama, S.; Cheng, J.; Ida-Yonemochi, H.; Kobayashi, T.; Yamazaki, M.; Takagi, R.; Saku, T. Nuclear translocation of beta-catenin synchronized with loss of E-cadherin in oral epithelial dysplasia with a characteristic two-phase appearance. Histopathology 2011, 59, 283–291. [Google Scholar] [CrossRef]
- Ghahhari, N.M.; Babashah, S. Interplay between microRNAs and WNT/beta-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer. Eur. J. Cancer 2015, 51, 1638–1649. [Google Scholar] [CrossRef]
- Eales, K.L.; Hollinshead, K.E.; Tennant, D.A. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 2016, 5, e190. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Dominguez, D.; Benitah, S.A. The contributions of cancer cell metabolism to metastasis. Dis. Models Mech. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Nauta, T.D.; van den Broek, M.; Gibbs, S.; van der Pouw-Kraan, T.C.; Oudejans, C.B.; van Hinsbergh, V.W.; Koolwijk, P. Identification of HIF-2alpha-regulated genes that play a role in human microvascular endothelial sprouting during prolonged hypoxia in vitro. Angiogenesis 2017, 20, 39–54. [Google Scholar] [CrossRef]
- Xu, L.; Deng, X. Suppression of cancer cell migration and invasion by protein phosphatase 2A through dephosphorylation of mu- and m-calpains. J. Biol. Chem. 2006, 281, 35567–35575. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Guo, X.; Choksi, R. Activation of Focal Adhesion Kinase and Src Mediates Acquired Sorafenib Resistance in A549 Human Lung Adenocarcinoma Xenografts. J. Pharmacol. Exp. Ther. 2017, 363, 428–443. [Google Scholar] [CrossRef]
- Zhou, Q.; Lv, H.; Mazloom, A.R.; Xu, H.; Ma’ayan, A.; Gallo, J.M. Activation of alternate prosurvival pathways accounts for acquired sunitinib resistance in U87MG glioma xenografts. J. Pharmacol. Exp. Ther. 2012, 343, 509–519. [Google Scholar] [CrossRef]
- Zhou, Q.; Gallo, J.M. Differential effect of sunitinib on the distribution of temozolomide in an orthotopic glioma model. Neuro-Oncology 2009, 11, 301–310. [Google Scholar] [CrossRef]
- Zhou, Q.; Guo, P.; Wang, X.; Nuthalapati, S.; Gallo, J.M. Preclinical pharmacokinetic and pharmacodynamic evaluation of metronomic and conventional temozolomide dosing regimens. J. Pharmacol. Exp. Ther. 2007, 321, 265–275. [Google Scholar] [CrossRef]
- Bao, X.; Wu, J.; Kim, S.; LoRusso, P.; Li, J. Pharmacometabolomics Reveals Irinotecan Mechanism of Action in Cancer Patients. J. Clin. Pharmacol. 2019, 59, 20–34. [Google Scholar] [CrossRef]
- Van den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef]
- Chen, C.J.; Chou, P.A.; Huang, M.S.; Liu, Y.P. Low TIP30 Protein Expression is Associated with a High Risk of Metastasis and Poor Prognosis for Non-Small-Cell Lung Cancer. J. Clin. Med. 2019, 8, 83. [Google Scholar] [CrossRef] [PubMed]


| Metabolites | Fold Increase a | p-Value |
|---|---|---|
| L-Homocysteine | 5.01 | 0.007 c |
| L-Cysteine | 2.9 | 0.104 |
| Decanoyl-L-Carnitine | 7.91 | 0.18 |
| Lauroyl-L-Carnitine | 2.98 | 0.057 |
| Myristoyl-L-Carnitine | 2.69 | 0.01 |
| Stearoyl-L-Carnitine | 2.18 | 0.062 |
| Octanoyl-L-Carnitine | 2.14 | 0.02 |
| AICA-Riboside | 2.07 | 0.085 |
| Metabolites | Fold Decrease b | p-Value |
| Kynurenic acid (KYNA) | 6.92 | 0.179 |
| Malonyl CoA | 6.70 | 0.145 |
| ADP ribose | 4.50 | 0.126 |
| Coenzyme A | 4.06 | 0.006 |
| Glutathione | 3.16 | 0.003 |
| ATP | 2.81 | 0.109 |
| dGDP | 2.56 | 0.195 |
| NADP | 2.56 | 0.013 |
| ADP | 2.54 | 0.162 |
| Propionyl-CoA | 2.50 | 0.002 |
| Thiamine | 2.43 | 0.006 |
| N-Acetylornithine | 2.33 | 0.001 |
| Acetyl-CoA | 2.30 | 0.034 |
| β-Nicotinamide D-Ribonucleotide | 2.19 | 0.015 |
| Guanosine | 2.12 | 0.007 |
| Ethanolamine | 2.02 | 0.008 |
| Succinyl-CoA | 1.99 | 0.044 |
| Glutamine | 1.94 | 0.005 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Li, J.; Guo, X.; Pan, H.; Zhou, Q. Absence of HTATIP2 Expression in A549 Lung Adenocarcinoma Cells Promotes Tumor Plasticity in Response to Hypoxic Stress. Cancers 2020, 12, 1538. https://doi.org/10.3390/cancers12061538
Li M, Li J, Guo X, Pan H, Zhou Q. Absence of HTATIP2 Expression in A549 Lung Adenocarcinoma Cells Promotes Tumor Plasticity in Response to Hypoxic Stress. Cancers. 2020; 12(6):1538. https://doi.org/10.3390/cancers12061538
Chicago/Turabian StyleLi, Minghua, Jing Li, Xiaofang Guo, Hua Pan, and Qingyu Zhou. 2020. "Absence of HTATIP2 Expression in A549 Lung Adenocarcinoma Cells Promotes Tumor Plasticity in Response to Hypoxic Stress" Cancers 12, no. 6: 1538. https://doi.org/10.3390/cancers12061538
APA StyleLi, M., Li, J., Guo, X., Pan, H., & Zhou, Q. (2020). Absence of HTATIP2 Expression in A549 Lung Adenocarcinoma Cells Promotes Tumor Plasticity in Response to Hypoxic Stress. Cancers, 12(6), 1538. https://doi.org/10.3390/cancers12061538






