First-In-Class CD13-Targeted Tissue Factor tTF-NGR in Patients with Recurrent or Refractory Malignant Tumors: Results of a Phase I Dose-Escalation Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Good Manufacturing Practice (GMP) Production of tTF-NGR
2.2. Study Design and Patients
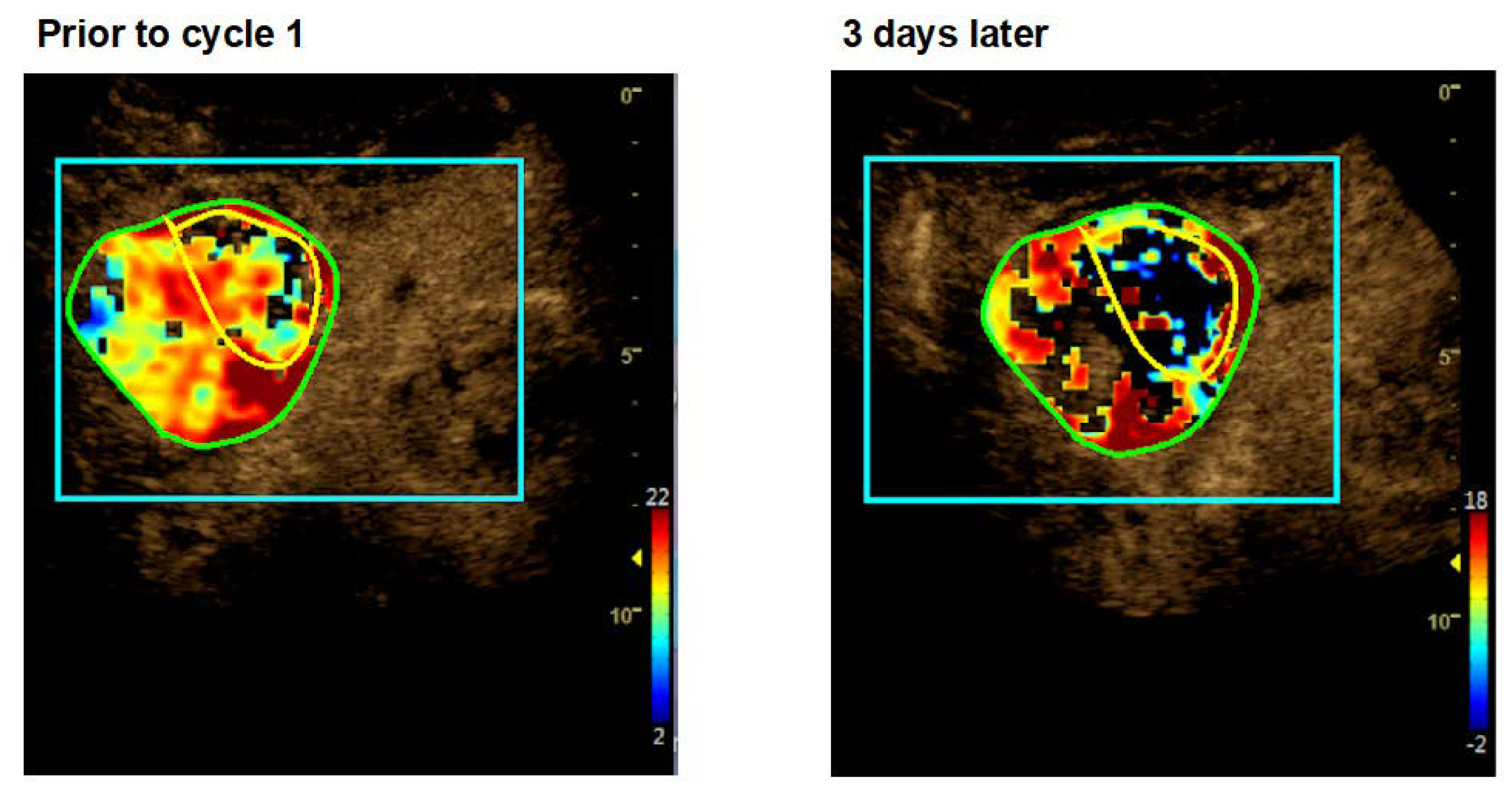
2.3. Dynamic Contrast-Enhanced Ultrasound (CEUS)
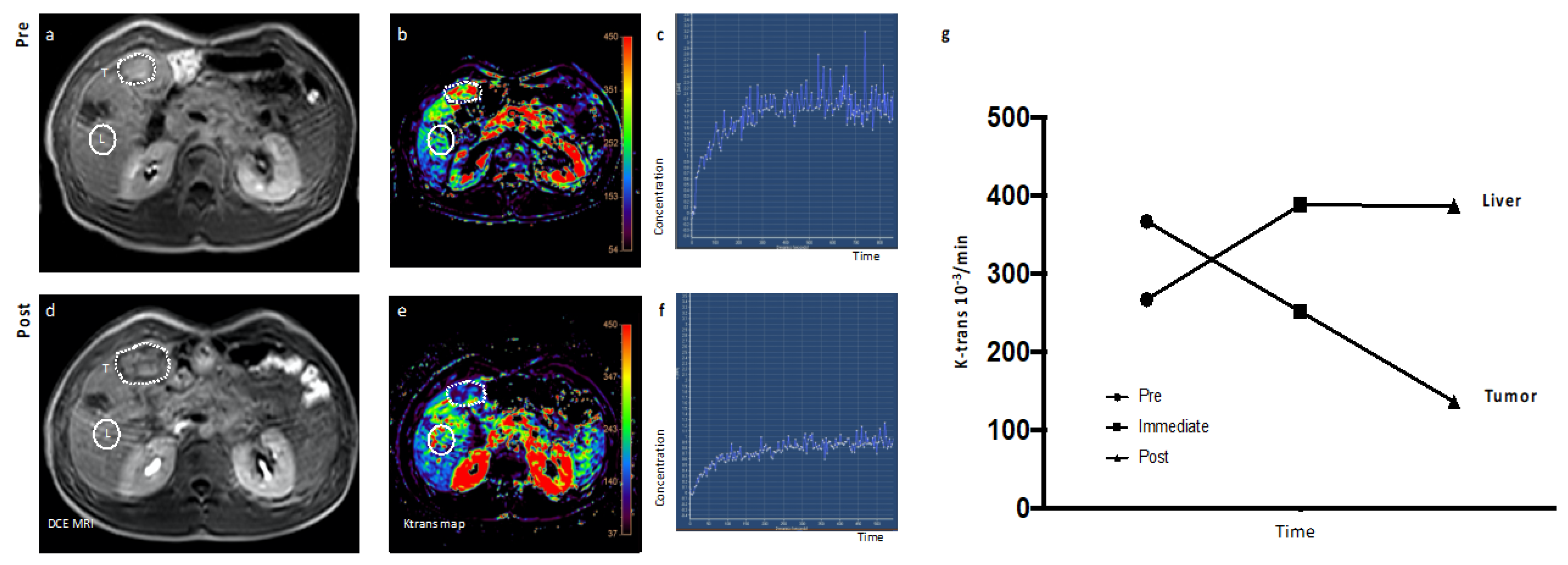
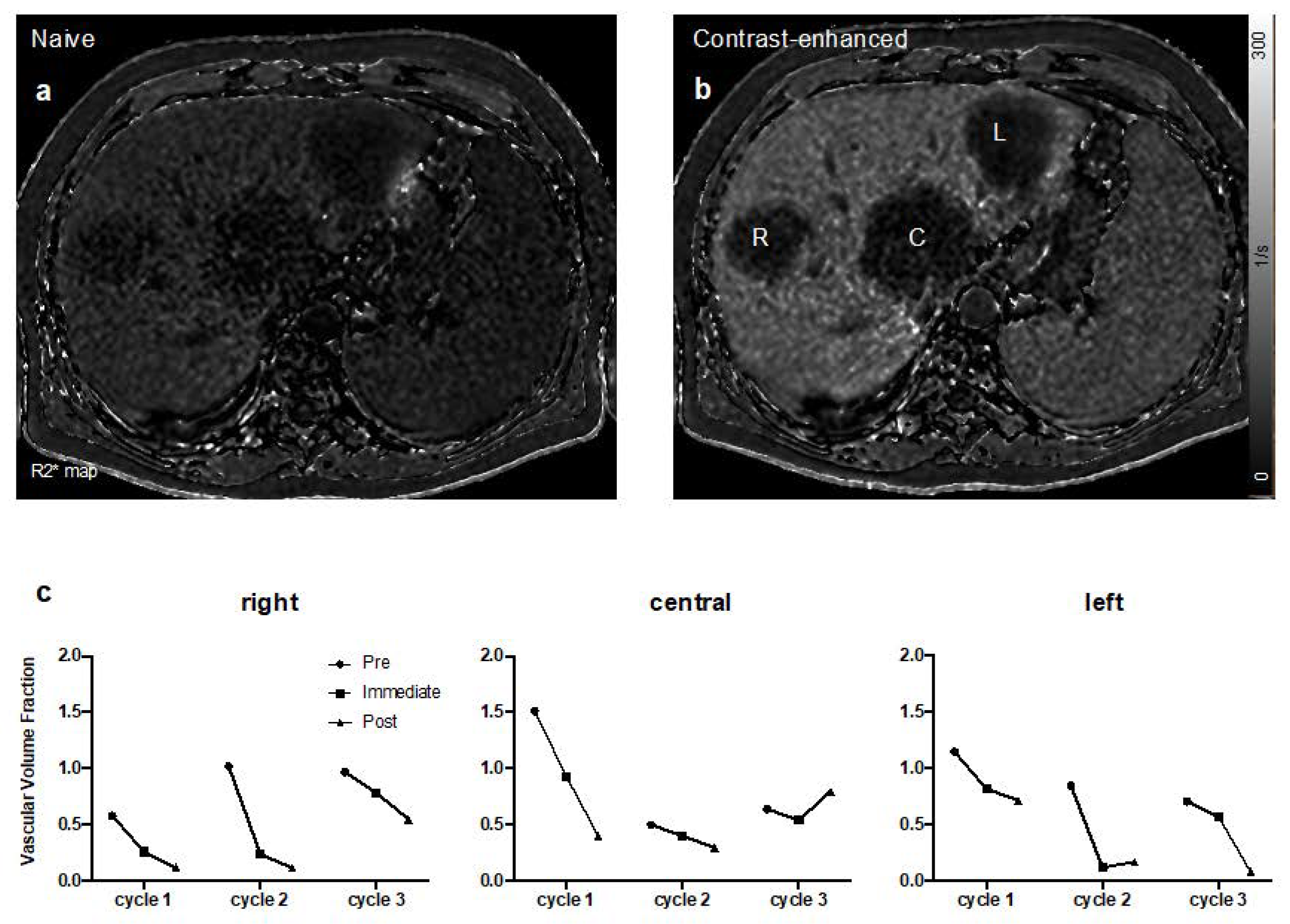
2.4. Dynamic Contrast-Enhanced (DCE) Magnetic Resonance Imaging (MRI)
2.5. Pharmacokinetic Determination of tTF-NGR in Human Plasma
- AUC0–t last = extrapolated area from time zero to the last quantifiable plasma concentration
- Kel = elimination rate constant
- t1/2 = alpha (distribution phase), terminal (elimination phase) half-life
2.6. Human Anti-Fusion Protein Antibody (HAFA) Determination
3. Results
3.1. Patients Demographics and Disease Characteristics
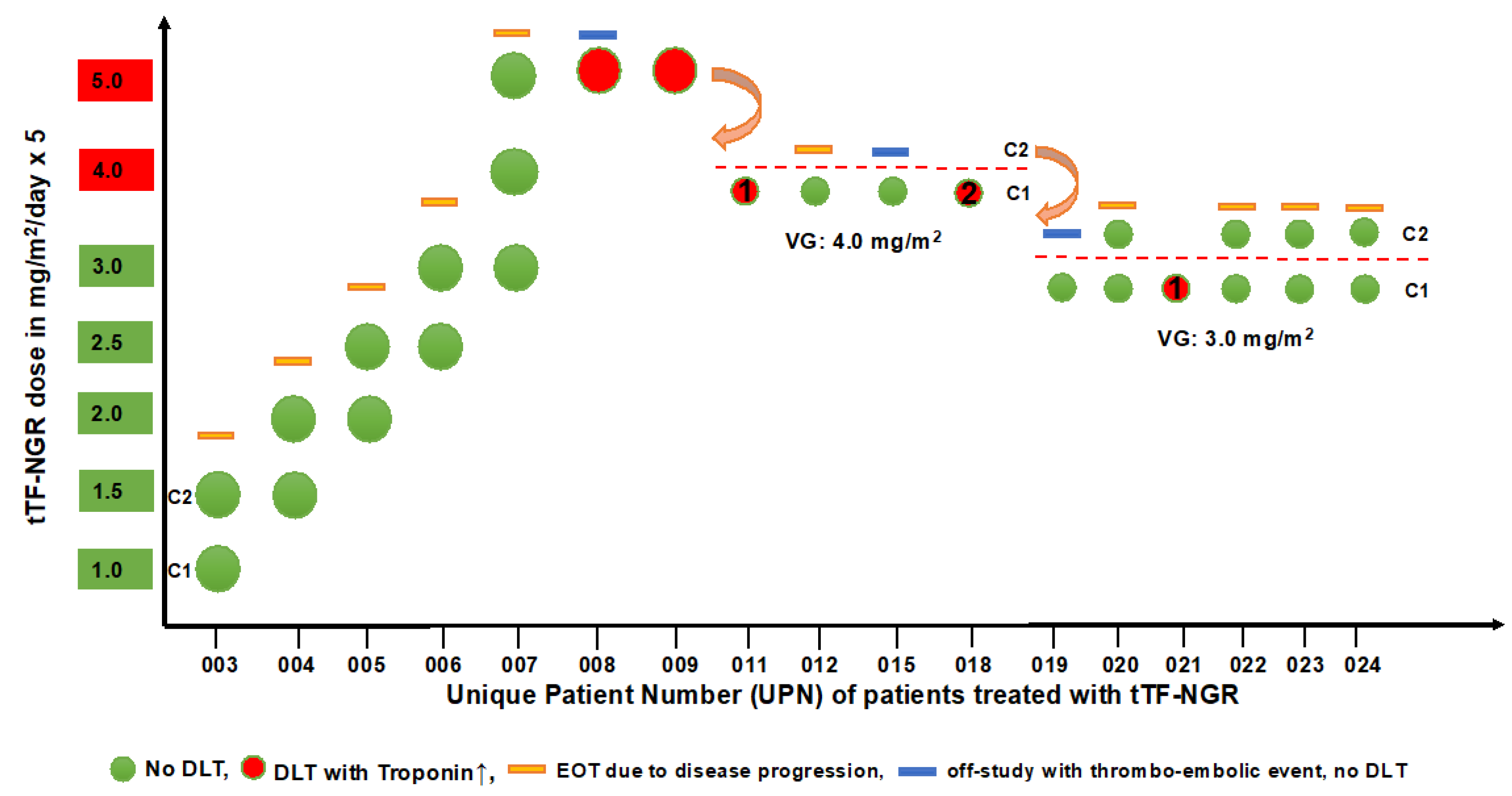
3.2. Safety and Tolerability
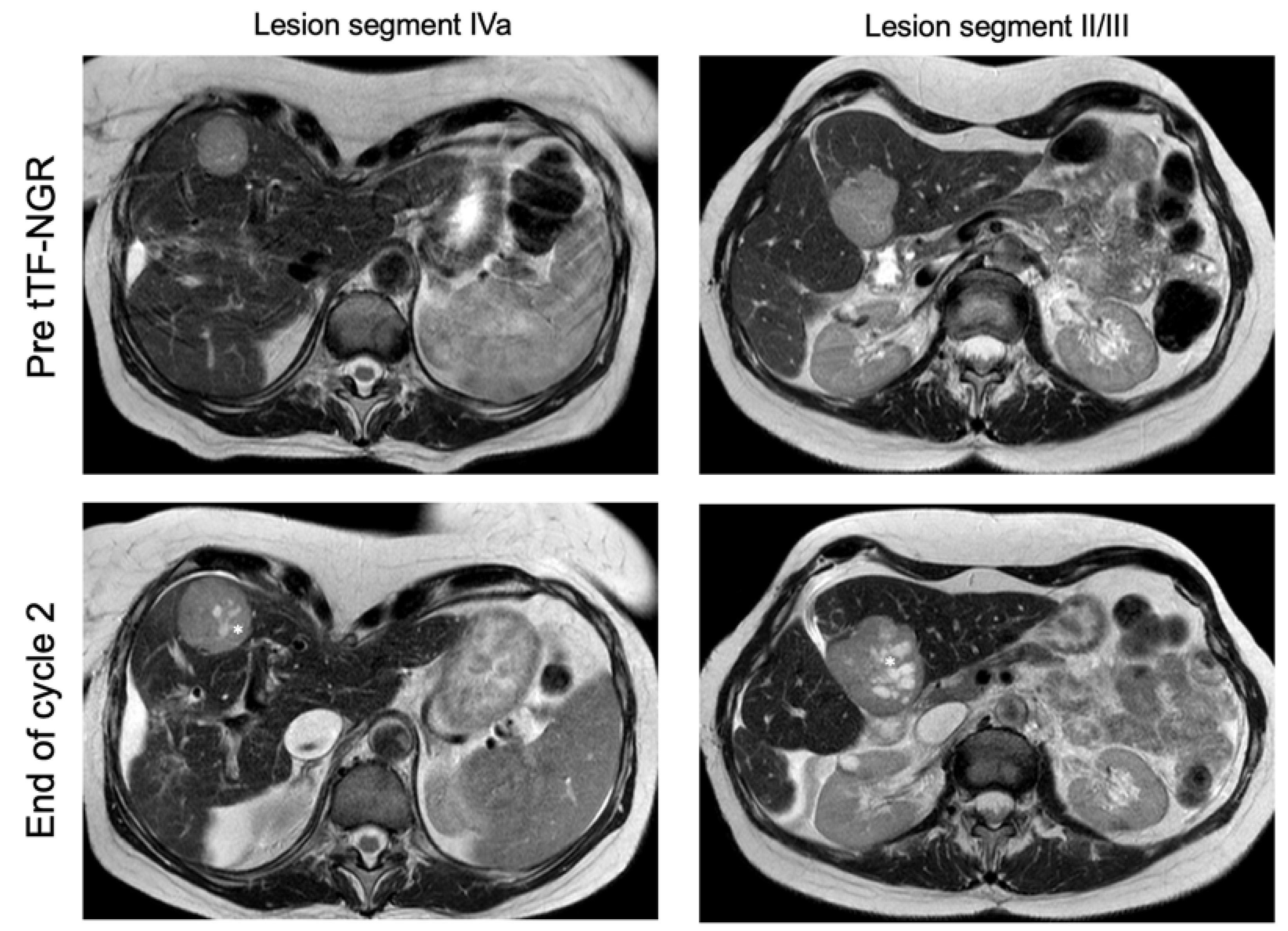
3.3. Antitumor Activity
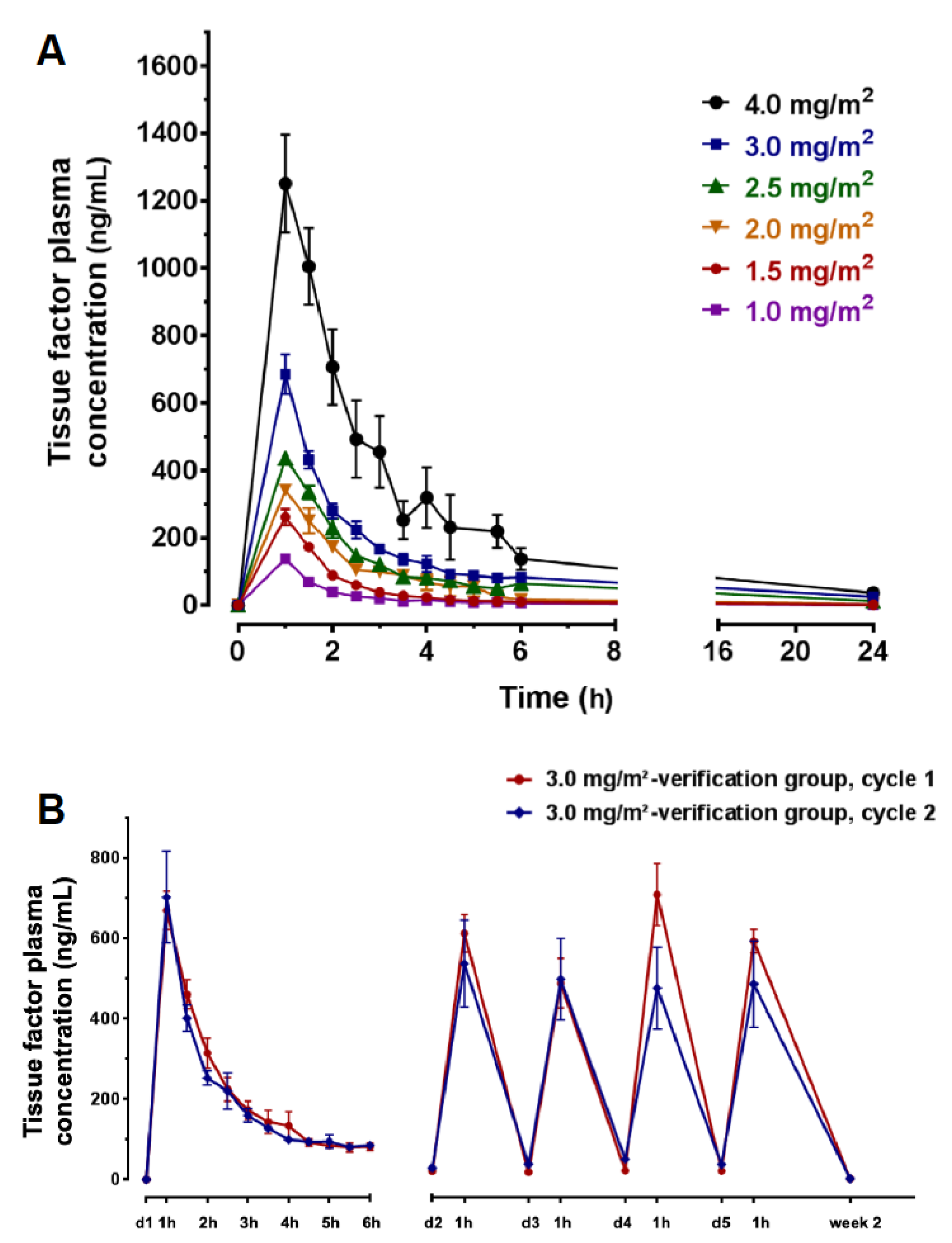
3.4. Pharmacokinetics (PK)
3.5. Human Anti-Fusion Protein (tTF-NGR) Antibodies (HAFA)
3.6. Single-Parameter Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Denekamp, J. Endothelial cell proliferation as a novel approach to targeting tumor therapy. Br. J. Cancer 1982, 45, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Molema, G.; King, S.; Watkins, L.; Edgington, T.S.; Thorpe, P.E. Tumor infarction in mice by antibody-directed targeting of tissue factor to tumor vasculature. Science 1997, 275, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, R.; Koivunen, E.; Kain, R.; Lahdenranta, J.; Sakamoto, M.; Stryhn, A.; Ashmun, R.A.; Shapiro, L.H.; Arap, W.; Ruoshlahti, E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000, 60, 722–727. [Google Scholar]
- Curnis, F.; Arrigoni, G.; Sacchi, A.; Fischetti, L.; Arap, W.; Pasqualini, R.; Corti, A. Differential binding of drugs containing the NGR motif to CD13 isoforms in tumor vessels, epithelia, and myeloid cells. Cancer Res. 2002, 62, 867–874. [Google Scholar]
- Wickström, M.; Larsson, R.; Nygren, P.; Gullbo, J. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 2011, 102, 501–508. [Google Scholar] [CrossRef]
- Guzman-Rojas, L.; Rangel, R.; Salameh, A.; Edwards, J.K.; Dondossola, E.; Kim, Y.G.; Saghatelian, A.; Giordano, R.J.; Kolonin, M.G.; Staquicini, F.I.; et al. Cooperative effects of aminopeptidase N (CD13) expressed by nonmalignant and cancer cells within the tumor microenvironment. Proc. Natl. Acad. Sci. USA 2012, 109, 1637–1642. [Google Scholar] [CrossRef]
- Tokuhara, T.; Hattori, N.; Ishida, H.; Hirai, T.; Higashiyama, M.; Kodama, K.; Miyake, M. Clinical significance of aminopeptidase N in non-small cell lung cancer. Clin. Cancer Res. 2006, 12, 3971–3978. [Google Scholar] [CrossRef]
- Ikeda, N.; Nakajima, Y.; Tokuhara, T.; Hattori, N.; Sho, M.; Kanehiro, H.; Miyake, M. Clinical significance of aminopeptidase N/CD13 expression in human pancreatic carcinoma. Clin. Cancer Res. 2003, 9, 1503–1508. [Google Scholar]
- Hashida, H.; Takabayashi, A.; Kanai, M.; Adachi, M.; Kondo, K.; Kohno, N.; Yamaoka, Y.; Miyake, M. Aminopeptidase N is involved in cell motility and angiogenesis: Its clinical significance in human colon cancer. Gastroenterology 2002, 122, 376–386. [Google Scholar] [CrossRef]
- Schmidt, L.H.; Brand, C.; Stucke-Ring, J.; Schliemann, C.; Kessler, T.; Harrach, S.; Mohr, M.; Görlich, D.; Marra, A.; Hillejan, L.; et al. Potential therapeutic impact of CD13 expression in non-small cell lung cancer. PLoS ONE 2017, 12, e0177146. [Google Scholar] [CrossRef] [PubMed]
- Surowiak, P.; Drag, M.; Materna, V.; Suchocki, S.; Grzywa, R.; Spaczyński, M.; Dietel, M.; Oleksyszyn, J.; Zabel, M.; Lage, H. Expression of aminopeptidase N/CD13 in human ovarian cancers. Int. J. Gynecol. Cancer 2006, 16, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Kessler, T.; Schwöppe, C.; Liersch, R.; Schliemann, C.; Hintelmann, H.; Bieker, R.; Berdel, W.E.; Mesters, R.M. Generation of fusion proteins for selective occlusion of tumor vessels. Curr. Drug Discov. Technol. 2008, 5, 1–8. [Google Scholar] [PubMed]
- Bieker, R.; Kessler, T.; Schwöppe, C.; Padró, T.; Persigehl, T.; Bremer, C.; Dreischalück, J.; Kolkmeyer, A.; Heindel, W.; Mesters, R.M.; et al. Infarction of tumor vessels by NGR-peptide-directed targeting of tissue factor: Experimental results and first-in man experience. Blood 2009, 113, 5019–5027. [Google Scholar] [CrossRef]
- Schwöppe, C.; Kessler, T.; Persigehl, T.; Liersch, R.; Hintelmann, H.; Dreischalück, J.; Ring, J.; Bremer, C.; Heindel, W.; Mesters, R.M.; et al. Tissue-factor fusion proteins induce occlusion of tumor vessels. Thromb. Res. 2010, 125 (Suppl. 2), S143–S150. [Google Scholar] [CrossRef]
- Schwöppe, C.; Zerbst, C.; Fröhlich, M.; Schliemann, C.; Kessler, T.; Liersch, R.; Overkamp, L.; Holtmeier, R.; Stypmann, J.; Dreiling, A.; et al. Anticancer therapy by tumor vessel infarction with polyethylene glycol conjugated retargeted tissue factor. J. Med. Chem. 2013, 56, 2337–2347. [Google Scholar] [CrossRef]
- Persigehl, T.; Ring, J.; Bremer, C.; Heindel, W.; Holtmeier, R.; Stypmann, J.; Claesener, M.; Hermann, S.; Schäfers, M.; Zerbst, C.; et al. Non-invasive monitoring of tumor-vessel infarction by retargeted truncated tissue factor tTF-NGR using multi-modal imaging. Angiogenesis 2014, 17, 235–246. [Google Scholar] [CrossRef]
- Brand, C.; Fröhlich, M.; Ring, J.; Schliemann, C.; Kessler, T.; Mantke, V.; König, S.; Lücke, M.; Mesters, R.M.; Berdel, W.E.; et al. Tumor Growth Inhibition via Occlusion of Tumor Vasculature Induced by N-Terminally PEGylated Retargeted Tissue Factor tTF-NGR. Mol. Pharm. 2015, 12, 3749–3758. [Google Scholar] [CrossRef]
- Stucke-Ring, J.; Ronnacker, J.; Brand, C.; Höltke, C.; Schliemann, C.; Kessler, T.; Schmidt, L.H.; Harrach, S.; Mantke, V.; Hintelmann, H.; et al. Combinatorial effects of doxorubicin and retargeted tissue factor by intratumoral entrapment of doxorubicin and proapoptotic increase of tumor vascular infarction. Oncotarget 2016, 7, 82458–82472. [Google Scholar] [CrossRef][Green Version]
- Kessler, T.; Baumeier, A.; Brand, C.; Grau, M.; Angenendt, L.; Harrach, S.; Stalmann, U.; Schmidt, L.H.; Gosheger, G.; Hardes, J.; et al. Aminopeptidase N (CD13): Expression, prognostic impact, and use as therapeutic target for tissue factor induced tumor vascular infarction in soft tissue sarcoma. Transl. Oncol. 2018, 11, 1271–1282. [Google Scholar] [CrossRef]
- Ehling, J.; Lammers, T.; Kiessling, F. Non-invasive imaging for studying anti-angiogenic therapy effects. Thromb. Haemost. 2013, 109, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Gerwing, M.; Herrmann, K.; Helfen, A.; Schliemann, C.; Berdel, W.E.; Eisenblätter, M.; Wildgruber, M. The beginning of the end for conventional RECIST—Novel therapies require novel imaging approaches. Nat. Rev. Clin. Oncol. 2019, 16, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Auner, H.W.; Tinchon, C.; Linkesch, W.; Tiran, A.; Quehenberger, F.; Link, H.; Sill, H. Prolonged monitoring of troponin T for the detection of anthracycline cardiotoxicity in adults with hematological malignancies. Ann. Hematol. 2003, 82, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Sandri, M.T.; Colombo, A.; Colombo, N.; Boeri, M.; Lamantia, G.; Civelli, M.; Peccatori, F.; Martinelli, G.; Fiorentini, C.; et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004, 109, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- Ky, B.; Putt, M.; Sawaya, H.; French, B.; Januzzi, J.L.; Sebag, I.A.; Plana, J.C.; Cohen, V.; Branchs, J.; Carver, J.R.; et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxans, and trastuzumab. J. Am. Coll. Cardiol. 2014, 63, 809–816. [Google Scholar] [CrossRef]
- Sarocchi, M.; Grossi, F.; Arboscello, E.; Bellodi, A.; Genova, C.; Dal Bello, M.G.; Rijavec, E.; Barletta, G.; Rossi, G.; Biello, F.; et al. Serial troponin for detection of nivolumab cardiotoxicity in advanced non-small cell lung cancer patients. Oncologist 2018, 23, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Schmidinger, M.; Zielinski, C.C.; Vogl, U.M.; Bojic, A.; Schukro, C.; Ruhsam, M.; Hejna, M.; Schmidinger, H. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2008, 26, 5204–5212. [Google Scholar] [CrossRef]
- Eggers, K.M.; Lindahl, B. Application of cardiac troponin in cardiovascular diseases other than acute coronary syndrome. Clin. Chem. 2017, 63, 223–225. [Google Scholar] [CrossRef]
- Jaffe, A.S. The 10 commandments of troponin, with special reference to high sensitivity assays. Heart 2011, 97, 940–946. [Google Scholar] [CrossRef]
- Mahajan, V.S.; Jarolim, P. How to interpret elevated cardiac troponin levels. Circulation 2011, 124, 2350–2354. [Google Scholar] [CrossRef]
- Giannitsis, E.; Katus, H.A. Cardiac troponin level elevations not related to acute coronary syndromes. Nat. Rev. Cardiol. 2013, 10, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.V.; Lahdenranta, J.; Giordano, R.; Arap, W.; Pasqualini, R.; Shapiro, L.H. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood 2001, 97, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Gao, B.; Xu, G.; Weng, D.; Xie, M.; Quian, Y. Possible contribution of aminopeptidase N (APN/CD13) to migration and invasion of human osteosarcoma cell lines. Int. J. Oncol. 2014, 45, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.proteinatlas.org/ENSG00000166825-ANPEP/tissue (accessed on 18 May 2020).
- Faintuch, B.L.; Oliveira, E.A.; Targino, R.C.; Moro, A.M. Radiolabeled NGR phage display peptide sequence for tumor targeting. Appl. Radiat. Isot. 2014, 86, 41–45. [Google Scholar] [CrossRef]
- Kim, D.-W.; Kim, W.H.; Kim, M.H.; Kim, C.G. Synthesis and evaluation of novel Tc-99m labeled NGR-containig hexapeptides as tumor imaging agents. J. Label. Compd. Radiopharm. 2015, 58, 30–35. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Zong, S.; Wang, J.; Conti, P.S.; Chen, K. MicroPET imaging of CD13 expression using a 64Cu-labeled dimeric NGR peptide based on sacophagine cage. Mol. Pharm. 2014, 11, 3938–3946. [Google Scholar] [CrossRef]
- Mate, G.; Kertesz, I.; Enyedi, K.N.; Mezö, G.; Angyal, J.; Vasas, N.; Kis, A.; Szabo, E.; Emri, M.; Biro, T.; et al. In vivo imaging of aminopeptidase N (CD13) receptors in experimental renal tumors using the novel radiotracer 68Ga-NOTA-c(NGR). Eur. J. Pharm. Sci. 2015, 69, 61–71. [Google Scholar] [CrossRef]
- Schmidt, L.H.; Stucke-Ring, J.; Brand, C.; Schliemann, C.; Harrach, S.; Muley, T.; Herpel, E.; Kessler, T.; Mohr, M.; Görlich, D.; et al. CD13 as target for tissue factor induced tumor vascular infarction in small cell lung cancer. Lung Cancer 2017, 113, 121–127. [Google Scholar] [CrossRef]
- Corti, A.; Curnis, F.; Rossoni, G.; Marcucci, F.; Gregorc, V. Peptide-mediated targeting of cytokines to tumor vasculature: The NGR-hTNF example. BioDrugs 2013, 27, 591–603. [Google Scholar] [CrossRef]
- Gregorc, V.; Zucali, P.A.; Santoro, A.; Ceresoli, G.L.; Citterio, G.; de Pas, T.M.; Zilembo, N.; de Vincenzo, F.; Simonelli, M.; Rossoni, G.; et al. Phase II study of asparagine-glycine-arginine-human tumor necrosis factor alpha, a selective vascular targeting agent, in previously treated patients with malignant pleural mesothelioma. J. Clin. Oncol. 2010, 28, 2604–2611. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Calimeri, T.; Conte, G.M.; Cattaneo, D.; Fallanca, F.; Ponzoni, M.; Scarano, E.; Curnis, F.; Nonis, A.; Lopedote, P.; et al. R-CHOP preceded by blood-brain barrier permeabilization with engineered tumor necrosis factor-alpha in primary CNS lymphoma. Blood 2019, 134, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Bauvois, B.; Dauzonne, D. Aminopeptidase-N/CD13 (EC 3.4.11.2) inhibitors: Chemistry, biological evaluations, and therapeutic prospects. Med. Res. Rev. 2006, 26, 88–130. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, Y.; Genka, K.; Koike, T.; Kato, H.; Watanabe, Y.; Mori, T.; Iioka, S.; Sakuma, A.; Ohta, M. Randomized double-blind placebo controlled trial of bestatin in patients with resected stage I squamous-cell cancer. J. Natl. Cancer Inst. 2003, 95, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Rondon, A.M.R.; Kroone, C.; Kapteijn, M.Y.; Versteeg, H.H.; Buijs, J.T. Role of tissue factor in tumor progression and cancer-associated thrombosis. Semin. Thromb. Hemost. 2019, 45, 396–412. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Connolly, G.C. Assessing risk of venous thromboembolism in the patient with cancer. J. Clin. Oncol. 2009, 27, 4839–4847. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kamphuisen, P.W.; Meyer, G.; Bauersachs, R.; Janas, M.S.; Jarner, M.F.; Lee, A.Y.Y. Tissue factor as a predictor of recurrent venous thromboembolism in malignancy: Biomarker analyses of the CATCH trial. J. Clin. Oncol. 2016, 35, 1078–1085. [Google Scholar] [CrossRef]
- Graf, C.; Wilgenbus, P.; Pagel, S.; Pott, J.; Marini, F.; Reyda, S.; Kitano, M.; Macher-Göppinger, S.; Weiler, H.; Ruf, W. Myeloid cell-synthesized coagulation factor X dampens antitumor immunity. Sci. Immunol. 2019, 4, eaaw8405. [Google Scholar] [CrossRef]

| UPN | Gender (m/f) | Age (year) | Diagnosis | Previous Tx | tTF-NGRcycles | tTF-NGR Dose (mg/m2) | DLT Related to IMP | Imaging (I), Outcome (O), and QOL (Q) |
|---|---|---|---|---|---|---|---|---|
| 003 | m | 22 | metastatic non-seminomatous germ cell tumor | PEI, HD-PEI, surgery, TI, TIP, HD-CE, Gem/Ox, Radiation, Nivolumab | 2 | cycle 1: 1.0 cycle 2: 1.5 | good tolerability, no DLT | I: hemorrhagic/necrotic areas in liver lesions upon therapy (MRI) O: RECIST PD; Q: n.c. |
| 004 | f | 29 | hepatocellular carcinoma + malignant ascites, multifocal | surgery, Sorafenib, Regorafenib, TACE | 2 | cycle 1: 1.5 cycle 2: 2.0 | good tolerability, no DLT | I: hypointense areas in liver lesions upon therapy (sonography) O: RECIST PD; Q: n.c. |
| 005 | m | 63 | small cell lung cancer (SCLC), extensive disease, metastatic | radiochemo CE, CNS-PCI, CE, Topotecan, ACO | 2 | cycle 1: 2.0 cycle 2: 2.5 | good tolerability, no DLT | O: FUO unrelated to IMP; RECIST PD Q: n.c. |
| 006 | m | 46 | rectal adeno, metastatic | neoadj. radiochemo, surgery + HIPEC, FOLFOX, liver surgery, radiation, FOLFIRI + Bev, SIRT, FOLFOX + Bev, TAS-102 (Trifluridin/Tipiracil) | 2 | cycle 1: 2.5 cycle 2: 3.0 | good tolerability, no DLT | O: RECIST PD Q: n.c. |
| 007 | m | 58 | colon adeno, metastaticRAS-mutated | surgery, FOLFOX + Bev, FOLFIRI + Bev,Ramucirumab,TAS-102 | 3 | cycle 1: 3.0 cycle 2: 4.0 cycle 3: 5.0 | good tolerability, no DLT | O: RECIST PD Q: n.c. |
| 008 | m | 45 | synovial sarcoma, metastatic | surgery, Doxo/Ifo, Trabectedin, Gem/Doc, Doxo + Fibromun (study), Pazopanib, Bendamustin, Vandetanib, HD-Ifo | 1 | Cycle 1: 5.0 (3 days) | DLT, related to IMP, Troponin T hs, grade 3; DVT, related to IMP, grade 2 | O: DLT (Trop) and DVT reversible; RECIST PD Q: n.c. |
| 009 | m | 56 | lung squamous, metastatic | radiochemo, Cisplatin/Vinorelbin, Nivolumab, Docetaxel, Gemcitabine, Afatinib, | 1 | cycle 1: 5.0 (1 day) | DLT, related to IMP, Troponin T hs, grade 3 | O: DLT (Trop) reversible Q: n.c. |
| 011 | m | 74 | colon adeno, metastatic | surgery, FOLFOX + Bev, FOLF + Bev + anti-PDL-1 (study), FOLFIRI + Cetuximab, FOLFOX + Cetuximab | 1 | cycle 1: 4.0 (2 days) | DLT, related to IMP,Troponin T hs, grade 3 | O: DLT (Trop) reversible; RECIST PD Q: n.c. |
| 012 | m | 26 | extragonadal germ cell tumor, metastatic | PEI, surgery, TI, TIP, HD-CE, radiation, Gem/Ox/Paclitaxel | 1 | cycle 1: 4.0 | good tolerability, no DLT | O: only 1 cycle since fast PD Q: n.c. |
| 015 | m | 63 | medullary thyroid cancer | surgery, Vandetanib, Cabozantinib | 1(com-pleted) | cycle 1: 4.0 cycle 2: 4.0 (1 day) | cycle 1: good tolerability, no DLT; cycle 2: catheter-associated DVT related to IMP, grade 2 | O: catheter-associated DVT reversible; RECIST PD Q: decreased in cycle 2, reversible |
| 018 | f | 54 | undifferentiated teratoma, then squamous carcinoma, CUP, metastatic | surgery, PEB, PEI, Carboplatin/Paclitaxel | 1 | cycle 1: 4.0 (3 days) | DLT, related to IMP, Troponin T hs, grade 3 | O: DLT (Trop) reversible; RECIST PD Q: n.c. |
| 019 | f | 41 | angiosarcoma of the heart, metastatic | surgery, radiochemo, Paclitaxel, Doxo/Ifo, Pazopanib, radiation, Gem/Doc, Gem, Trabectedin, SIRT | 1 | cycle 1: 3.0 cycle 2: 3.0 (1 day) | cycle 1: good tolerability; cycle 2: TIA, related to IMP, grade 2 | O: TIA reversible; RECIST PD Q: n.c. |
| 020 | m | 19 | desmoplastic small/round cell tumor, metastatic | VIDE, VAI, Temodal/Irinitecan, | 2 | cycle 1: 3.0 cycle 2: 3.0 | good tolerability, no DLT | I: hypointense areas in liver lesions upon therapy (sonography) O: RECIST PD Q: improved under therapy |
| 021 | m | 63 | synovial sarcoma, metastatic | surgery, radiation, Doxo/Ifo, Trabectedin, Gem/Doc, | 1 | cycle 1: 3.0 (1 day) | DLT, related to IMP, Troponin T hs, grade 3 | O: DLT (Trop) reversible; RECIST PD Q: n.c. |
| 022 | f | 56 | hepatocellular carcinoma, metastatic | TACE, SIRT, Sorafenib (toxicity), Nivolumab (toxicity) | 2 | cycle 1: 3.0 cycle 2: 3.0 | good tolerability | I: hemorrhagic/necrotic areas in liver lesions upon therapy (MRI) O: RECIST PD; Q: n.c. |
| 023 | m | 60 | colon adeno, metastatic | FOLFIRI-OX + Panitumumab, 5-FU + Panitumumab, radiotherapy, FOLFIRI + Bevacizumab, Trifluridin/Tipiracil, Irinotecan + Cetuximab | 2 | cycle 1: 3.0 cycle 2: 3.0 | good tolerability | O: RECIST PD Q: n.c. |
| 024 | m | 24 | undifferentiated pleomorphic sarcoma, metastatic | EURAMOS protocol (Cisplatin, Doxo, HD-MTX and surgery), adjuvant Mifamurtid, Ifo + Etoposide, surgery of metastasis, radiotherapy, Gem/Doc, Nivolumab, Pazopanib | cycle 1: 3.0 cycle 2: 3.0 | good tolerability | O: RECIST PD Q: improved under therapy |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schliemann, C.; Gerwing, M.; Heinzow, H.; Harrach, S.; Schwöppe, C.; Wildgruber, M.; Hansmeier, A.A.; Angenendt, L.; Berdel, A.F.; Stalmann, U.; et al. First-In-Class CD13-Targeted Tissue Factor tTF-NGR in Patients with Recurrent or Refractory Malignant Tumors: Results of a Phase I Dose-Escalation Study. Cancers 2020, 12, 1488. https://doi.org/10.3390/cancers12061488
Schliemann C, Gerwing M, Heinzow H, Harrach S, Schwöppe C, Wildgruber M, Hansmeier AA, Angenendt L, Berdel AF, Stalmann U, et al. First-In-Class CD13-Targeted Tissue Factor tTF-NGR in Patients with Recurrent or Refractory Malignant Tumors: Results of a Phase I Dose-Escalation Study. Cancers. 2020; 12(6):1488. https://doi.org/10.3390/cancers12061488
Chicago/Turabian StyleSchliemann, Christoph, Mirjam Gerwing, Hauke Heinzow, Saliha Harrach, Christian Schwöppe, Moritz Wildgruber, Anna A. Hansmeier, Linus Angenendt, Andrew F. Berdel, Ursula Stalmann, and et al. 2020. "First-In-Class CD13-Targeted Tissue Factor tTF-NGR in Patients with Recurrent or Refractory Malignant Tumors: Results of a Phase I Dose-Escalation Study" Cancers 12, no. 6: 1488. https://doi.org/10.3390/cancers12061488
APA StyleSchliemann, C., Gerwing, M., Heinzow, H., Harrach, S., Schwöppe, C., Wildgruber, M., Hansmeier, A. A., Angenendt, L., Berdel, A. F., Stalmann, U., Berning, B., Kratz-Albers, K., Middelberg-Bisping, K., Wiebe, S., Albring, J., Wilms, C., Hartmann, W., Wardelmann, E., Krähling, T., ... Berdel, W. E. (2020). First-In-Class CD13-Targeted Tissue Factor tTF-NGR in Patients with Recurrent or Refractory Malignant Tumors: Results of a Phase I Dose-Escalation Study. Cancers, 12(6), 1488. https://doi.org/10.3390/cancers12061488








