Liver Tumor Burden in Pancreatic Neuroendocrine Tumors: CT Features and Texture Analysis in the Prediction of Tumor Grade and 18F-FDG Uptake
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Imaging Technique and Evaluation
2.3. Texture Analysis
2.4. Pathological and PET-CT Evaluation
2.5. Statistical Analysis
3. Results
3.1. Pattern and Qualitative Descriptors
3.2. Tumor Grade and Ki67
3.2.1. Qualitative Descriptors
3.2.2. Histogram-Derived Parameters
3.3. 18F-FDG Standardized Uptake Value
3.3.1. Qualitative Descriptors
3.3.2. Histogram-Derived Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choe, J.; Kim, K.W.; Kim, H.J.; Kim, D.W.; Kim, K.P.; Hong, S.; Ryu, J.; Tirumani, S.H.; Krajewski, K.; Ramaiya, N. What Is New in the 2017 World Health Organization Classification and 8th American Joint Committee on Cancer Staging System for Pancreatic Neuroendocrine Neoplasms ? Korean J. Radiol. 2019, 20, 5–17. [Google Scholar] [CrossRef]
- Basturk, O.; Yang, Z.; Tang, L.H.; Hruban, R.H.; Adsay, V.; McCall, C.M.; Krasinskas, A.M.; Jang, K.-T.; Frankel, W.L.; Balci, S.; et al. The High-grade (WHO G3) Pancreatic Neuroendocrine Tumor Category Is Morphologically and Biologically Heterogenous and Includes Both Well Differentiated and Poorly Differentiated Neoplasms. Am. J. Surg. Pathol. 2015, 39, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Casadei, R.; Taffurelli, G.; D’Ambra, M.; Monari, F.; Campana, D.; Tomassetti, P.; Santini, D.; Minni, F. WHO 2010 classification of pancreatic endocrine tumors. Is the new always better than the old? Pancreatology 2014, 14, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Pape, U.F.; Berndt, U.; Müller-Nordhorn, J.; Böhmig, M.; Roll, S.; Koch, M.; Willich, S.N.; Wiedenmann, B. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr. Relat. Cancer 2008, 15, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Panzuto, F.; Merola, E.; Pavel, M.E.; Rinke, A.; Kump, P.; Partelli, S.; Rinzivillo, M.; Rodriguez-Laval, V.; Pape, U.F.; Lipp, R.; et al. Stage IV Gastro-Entero-Pancreatic Neuroendocrine Neoplasms: A Risk Score to Predict Clinical Outcome. Oncologist 2017, 22, 409–415. [Google Scholar] [CrossRef]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef]
- Frilling, A.; Modlin, I.M.; Kidd, M.; Russell, C.; Breitenstein, S.; Salem, R.; Kwekkeboom, D.; Lau, W.Y.; Klersy, C.; Vilgrain, V.; et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014, 15, e8–e21. [Google Scholar] [CrossRef]
- Panzuto, F.; Merola, E.; Rinzivillo, M.; Partelli, S.; Campana, D.; Iannicelli, E.; Pilozzi, E.; Mercantini, P.; Rossi, L.M.; Capurso, G.; et al. Metastatic Pattern Is an Independent Factor Affecting Clinical Outcome. Pancreas 2014, 43, 212–218. [Google Scholar] [CrossRef]
- Bertani, E.; Falconi, M.; Grana, C.; Botteri, E.; Chiappa, A.; Misitano, P.; Spada, F.; Ravizza, D.; Bazolli, B.; Fazio, N. Small intestinal neuroendocrine tumors with liver metastases and resection of the primary: Prognostic factors for decision making. Int. J. Surg. 2015, 20, 58–64. [Google Scholar] [CrossRef]
- Durante, C.; Boukheris, H.; Dromain, C.; Duvillard, P.; Leboulleux, S.; Elias, D.; de Baere, T.; Malka, D.; Lumbroso, J.; Guigay, J.; et al. Prognostic factors influencing survival from metastatic (stage IV) gastroenteropancreatic well-differentiated endocrine carcinoma. Endocr. Relat. Cancer 2009, 16, 585–597. [Google Scholar] [CrossRef]
- Palazzo, M.; Lombard-Bohas, C.; Cadiot, G.; Matysiak-Budnik, T.; Rebours, V.; Vullierme, M.P.; Couvelard, A.; Hentic, O.; Ruszniewski, P. Ki67 proliferation index, hepatic tumor load, and pretreatment tumor growth predict the antitumoral efficacy of lanreotide in patients with malignant digestive neuroendocrine tumors. Eur. J. Gastroenterol. Hepatol. 2013, 25, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Frilling, A.; Li, J.; Malamutmann, E.; Schmid, K.W.; Bockisch, A.; Broelsch, C.E. Treatment of liver metastases from neuroendocrine tumours in relation to the extent of hepatic disease. Br. J. Surg. 2009, 96, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Rinke, A.; Müller, H.H.; Schade-Brittinger, C.; Klose, K.J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.F.; Bläker, M.; et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID study group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Schernthaner, R.; Ardon, R.; Chapiro, J.; Zhao, Y.; Sohn, J.H.; Fleckenstein, F.; Lin, M.; Geschwind, J.-F.; Duran, R. Imaging Biomarkers of Tumor Response in Neuroendocrine Liver Metastases Treated with Transarterial Chemoembolization: Can Enhancing Tumor Burden of the Whole Liver Help Predict Patient Survival? Radiology 2016, 283, 883–894. [Google Scholar] [CrossRef]
- Bahri, H.; Laurence, L.; Edeline, J.; Leghzali, H.; Devillers, A.; Raoul, J.-L.; Cuggia, M.; Mesbah, H.; Clement, B.; Boucher, E.; et al. High prognostic value of 18F-FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: a long-term evaluation. J. Nucl. Med. 2014, 55, 1786–1790. [Google Scholar] [CrossRef]
- Ezziddin, S.; Adler, L.; Sabet, A.; Poppel, T.D.; Grabellus, F.; Yuce, A.; Fischer, H.-P.; Simon, B.; Holler, T.; Biersack, H.-J.; et al. Prognostic Stratification of Metastatic Gastroenteropancreatic Neuroendocrine Neoplasms by 18F-FDG PET: Feasibility of a Metabolic Grading System. J. Nucl. Med. 2014, 55, 1260–1266. [Google Scholar] [CrossRef]
- Kubota, K.; Okasaki, M.; Minamimoto, R.; Miyata, Y.; Morooka, M.; Nakajima, K.; Sato, T. Lesion-based analysis of (18)F-FDG uptake and (111)In-Pentetreotide uptake by neuroendocrine tumors. Ann. Nucl. Med. 2014, 28, 1004–1010. [Google Scholar] [CrossRef]
- Davnall, F.; Yip, C.S.P.; Ljungqvist, G.; Selmi, M.; Ng, F.; Sanghera, B.; Ganeshan, B.; Miles, K.A.; Cook, G.J.; Goh, V. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 2012, 3, 573–589. [Google Scholar] [CrossRef]
- De Robertis, R.; Beleù, A.; Cardobi, N.; Frigerio, I.; Ortolani, S.; Gobbo, S.; Maris, B.; Melisi, D.; Montemezzi, S.; D’Onofrio, M. Correlation of MR features and histogram-derived parameters with aggressiveness and outcomes after resection in pancreatic ductal adenocarcinoma. Abdom. Radiol. (New York) 2020. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, X.; Ouyang, H.; Sun, F.; Zhang, H.; Zhou, C. Quantified ADC histogram analysis: A new method for differentiating mass-forming focal pancreatitis from pancreatic cancer. Acta Radiol. 2014, 55, 785–792. [Google Scholar] [CrossRef]
- Hoffman, D.H.; Ream, J.M.; Hajdu, C.H.; Rosenkrantz, A.B. Utility of whole-lesion ADC histogram metrics for assessing the malignant potential of pancreatic intraductal papillary mucinous neoplasms (IPMNs). Abdom. Radiol. (New York) 2017, 42, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, R.; Maris, B.; Cardobi, N.; Tinazzi Martini, P.; Gobbo, S.; Capelli, P.; Ortolani, S.; Cingarlini, S.; Paiella, S.; Landoni, L.; et al. Can histogram analysis of MR images predict aggressiveness in pancreatic neuroendocrine tumors? Eur. Radiol. 2018, 28, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.A.S.; Rosado, E.; Bali, M.; Metens, T.; Chao, S.-L. Pancreatic neuroendocrine tumors: correlation between histogram analysis of apparent diffusion coefficient maps and tumor grade. Abdom. Imaging 2015, 40, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [PubMed]
- Worhunsky, D.J.; Krampitz, G.W.; Poullos, P.D.; Visser, B.C.; Kunz, P.L.; Fisher, G.A.; Norton, J.A.; Poultsides, G.A. Pancreatic neuroendocrine tumours: Hypoenhancement on arterial phase computed tomography predicts biological aggressiveness. Hpb 2014, 16, 304–311. [Google Scholar] [CrossRef]
- Canellas, R.; Burk, K.S.; Parakh, A.; Sahani, D.V. Prediction of pancreatic neuroendocrine tumor grade based on CT features and texture analysis. Am. J. Roentgenol. 2018, 210, 341–346. [Google Scholar] [CrossRef]
- Zamboni, G.A.; Ambrosetti, M.C.; Zivelonghi, C.; Lombardo, F.; Butturini, G.; Cingarlini, S.; Capelli, P.; Pozzi Mucelli, R. Solid non-functioning endocrine tumors of the pancreas: correlating computed tomography and pathology. Hpb 2017, 19, 986–991. [Google Scholar] [CrossRef]
- Luo, Y.; Dong, Z.; Chen, J.; Chan, T.; Lin, Y.; Chen, M.; Li, Z.-P.; Feng, S.-T. Pancreatic neuroendocrine tumours: correlation between MSCT features and pathological classification. Eur. Radiol. 2014, 24, 2945–2952. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, H.J.; Kim, K.W.; Byun, J.H.; Song, K.B.; Kim, J.H.; Hong, S.-M. Neuroendocrine neoplasms of the pancreas at dynamic enhanced CT: comparison between grade 3 neuroendocrine carcinoma and grade 1/2 neuroendocrine tumour. Eur. Radiol. 2015, 25, 1375–1383. [Google Scholar] [CrossRef]
- Guo, C.; Zhuge, X.; Wang, Z.; Wang, Q.; Sun, K.; Feng, Z.; Chen, X. Textural analysis on contrast-enhanced CT in pancreatic neuroendocrine neoplasms: association with WHO grade. Abdom. Radiol. (New York) 2019, 44, 576–585. [Google Scholar] [CrossRef]
- Denecke, T.; Baur, A.D.J.; Ihm, C.; Steffen, I.G.; Tischer, E.; Arsenic, R.; Pascher, A.; Wiedenmann, B.; Pavel, M. Evaluation of radiological prognostic factors of hepatic metastases in patients with non-functional pancreatic neuroendocrine tumors. Eur. J. Radiol. 2013, 82, e550–e555. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.W.; Kim, J.H.; Yu, M.H.; Park, S.J.; Han, J.K. Pancreatic neuroendocrine tumor: prediction of the tumor grade using CT findings and computerized texture analysis. Acta Radiol. 2018, 59, 383–392. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, M.; Ciaravino, V.; Cardobi, N.; De Robertis, R.; Cingarlini, S.; Landoni, L.; Capelli, P.; Bassi, C.; Scarpa, A. CT Enhancement and 3D Texture Analysis of Pancreatic Neuroendocrine Neoplasms. Sci. Rep. 2019, 9, 2176. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Ganeshan, B.; Kozarski, R.; Miles, K.A.; Goh, V. Assessment of primary colorectal cancer heterogeneity by using whole-tumor texture analysis: contrast-enhanced CT texture as a biomarker of 5-year survival. Radiology 2013, 266, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, A.; Mantovani, W.; Capelli, P.; Beghelli, S.; Boninsegna, L.; Bettini, R.; Panzuto, F.; Pederzoli, P.; Fave, G.D.; Falconi, M. Pancreatic endocrine tumors: Improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod. Pathol. 2010, 23, 824–833. [Google Scholar] [CrossRef]
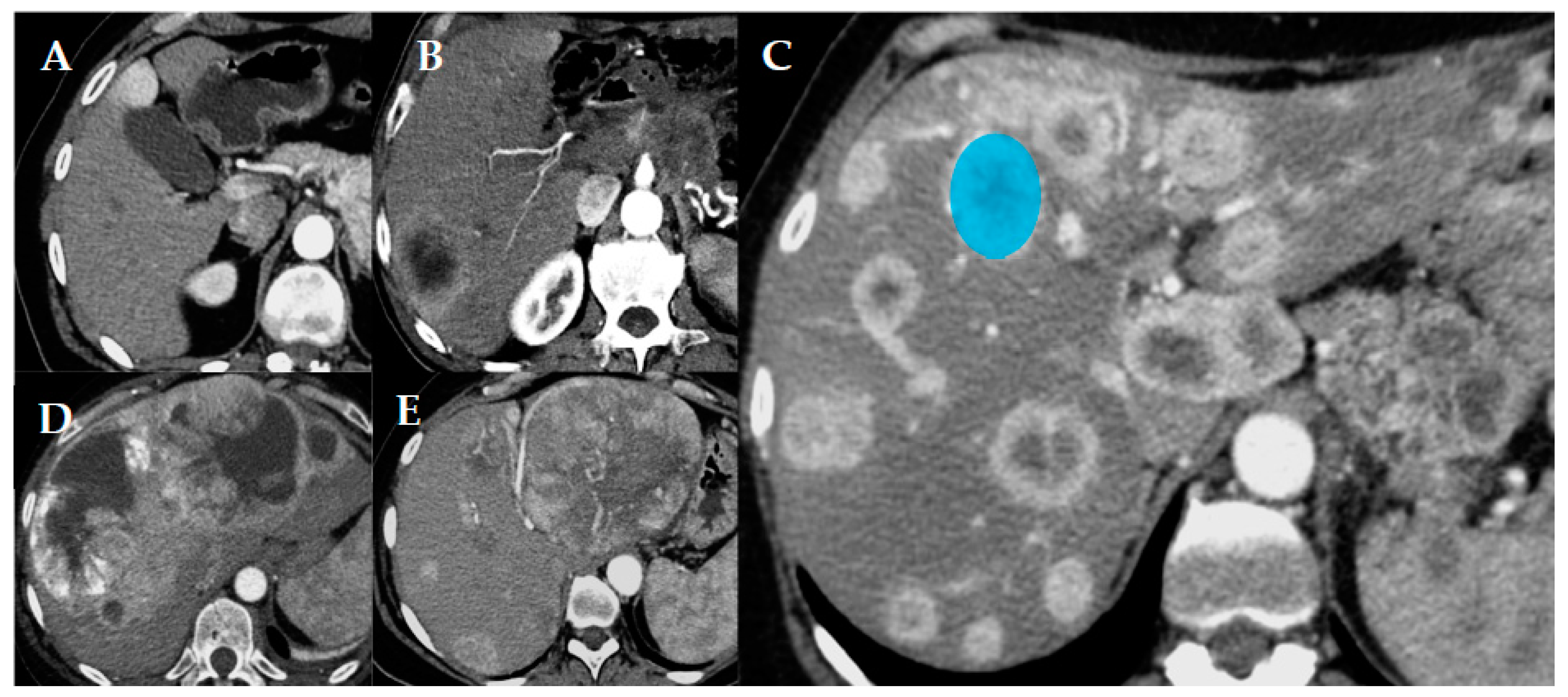

| Parameter (n = 56) | Value (n) | Parameter (n = 56) | Value (n) |
|---|---|---|---|
| Age (years) | 57 ± 10 | Ki67 (%) | 10 (5–21) * |
| Sex (males) | 60.7 | SUV 1 | 8 (5.4–12) * |
| Tumor grade (%) 1 2 3 | 5.4 (3) 71.4 (40) 23.3 (13) | Pre-contrast (%) Hypodense Isodense Hyperdense | 92.2 (52) 5.8 (3) 2 (1) |
| Pattern (%) Uninodular Paucinodular Multinodular Confluent multinodular Bulky | 7.1 (4) 17.9 (10) 55.4 (31) 10.7 (6) 8.9 (5) | Arterial phase (%) Hypodense Hyperdense | 25.9 (15) 74.1 (41) |
| Venous phase (%) Hypodense Isodense Hyperdense | 81.5 (46) 7.4 (4) 11.1 (6) | ||
| Number of metastases | 14 (4–43) * | Calcifications (%) | 7.1 (4) |
| Greater metastasis (mm) | 39 (22–65) * | Cystic (%) | 10.7 (6) |
| Sharp margins (%) | 85.7 (48) | Necrosis (%) | 64.3 (36) |
| Sporadic (%) | 98.2 (55) | Functioning (%) | 1.8 (1) |
| Total | Tumor Grade | SUV 1 | |||||
|---|---|---|---|---|---|---|---|
| 1–2 | 3 | p | <4.5 | >4.5 | p | ||
| ArtHUmean | 77 (65–95) | 83 (51–95) | 67 (53–81) | 0.569 | 113 (67–159) | 83 (62–94) | 0.760 |
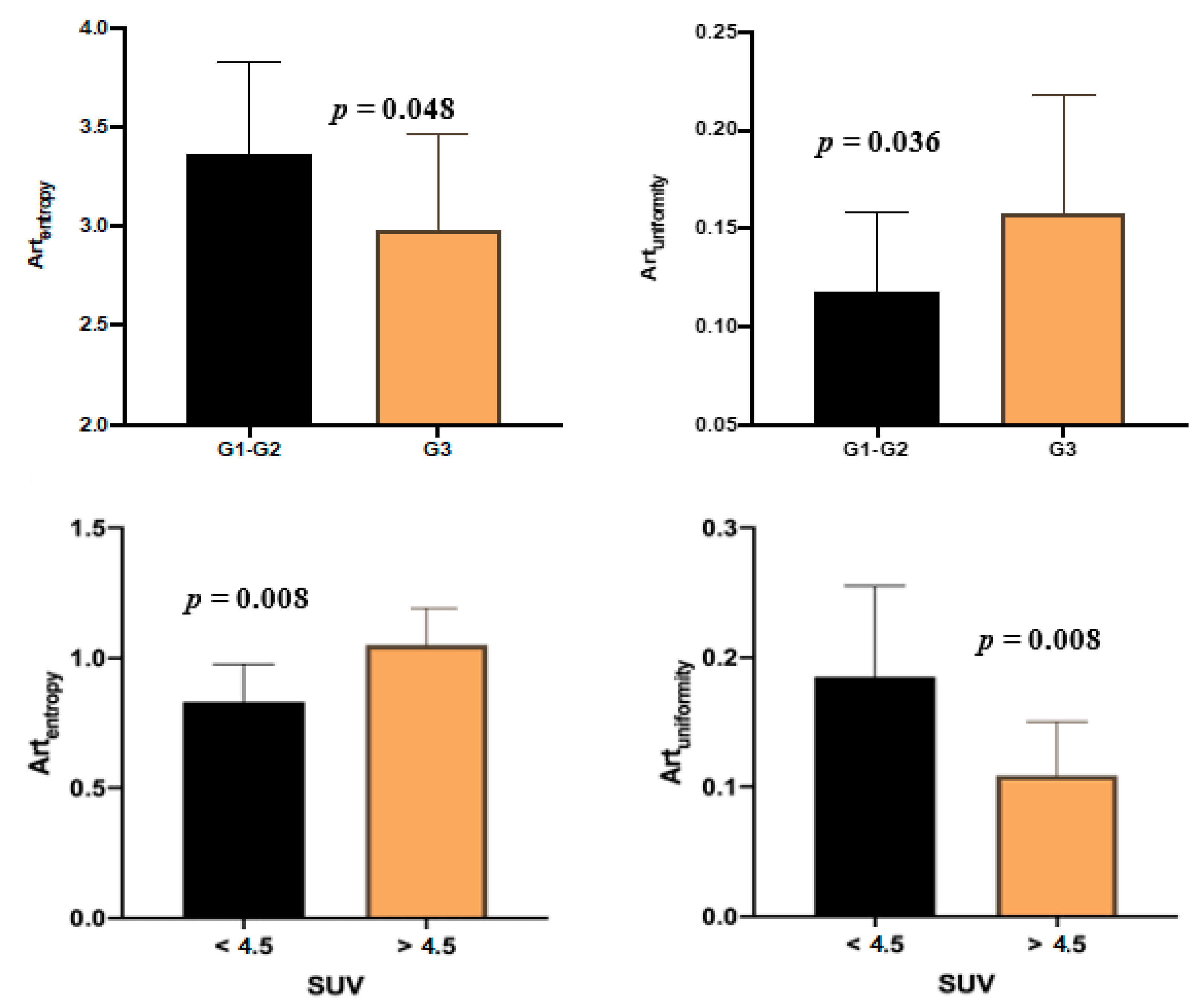
| Artentropy | 3.25 ± 0.49 | 3.37 ± 0.46 | 2.99 ± 0.47 | 0.038 | 2.76 ± 0.49 | 3.49 ± 0.47 | 0.008 |
| Artuniformity | 0.13 ± 0.05 | 0.12 ± 0.04 | 0.16 ± 0.06 | 0.036 | 0.18 ± 0.07 | 0.11 ± 0.04 | 0.008 |
| Artkurtosis | 2.94 (2.66–3.43) | 2.86 (2.54–3.37) | 3.07 (2.72–3.62) | 0.272 | 3.51 (3.07–3.96) | 2.78 (2.48-2.99) | 0.106 |
| Artskewness | −0.04 (−0.18–0.14) | −0.03 (−0.43–0.14) | −0.10 (−0.15–−0.02) | 0.776 | −0.14 (−0.43–0.14) | −0.06 (−0.47–−0.01) | 0.827 |
| VenHUmean | 86 ± 19 | 86 ± 18 | 87 ± 23 | 0.938 | 104 ± 23 | 88 ± 15 | 0.147 |
| Venentropy | 3.15 ± 0.4 | 3.23 ± 0.41 | 2.98 ± 0.33 | 0.172 | 2.98 ± 0.70 | 3.28 ± 0.39 | 0.324 |
| Venuniformity | 0.14 ± 0.04 | 0.13 ± 0.036 | 0.15 ± 0.04 | 0.184 | 0.16 ± 0.07 | 0.13 ± 0.04 | 0.210 |
| Venkurtosis | 3.04 (2.68–3.78) | 3.06 (2.72–3.78) | 3.05 (2.52–3.76) | 0.916 | 3.40 (3.05–3.76) | 2.89 (2.59–3.45) | 1.000 |
| Venskewness | −0.11 ± 0.47 | −0.18 ± 0.45 | 0.03 ± 0.51 | 0.335 | 0.16 ± 0.74 | −0.27 ± 0.29 | 0.423 |
| Artentropy | Artuniformity | |
|---|---|---|
| Tumor grade 1–2 | 3.37 ± 0.46 | 0.12 ± 0.04 |
| Tumor grade 3 | 2.99 ± 0.47 | 0.16 ± 0.06 |
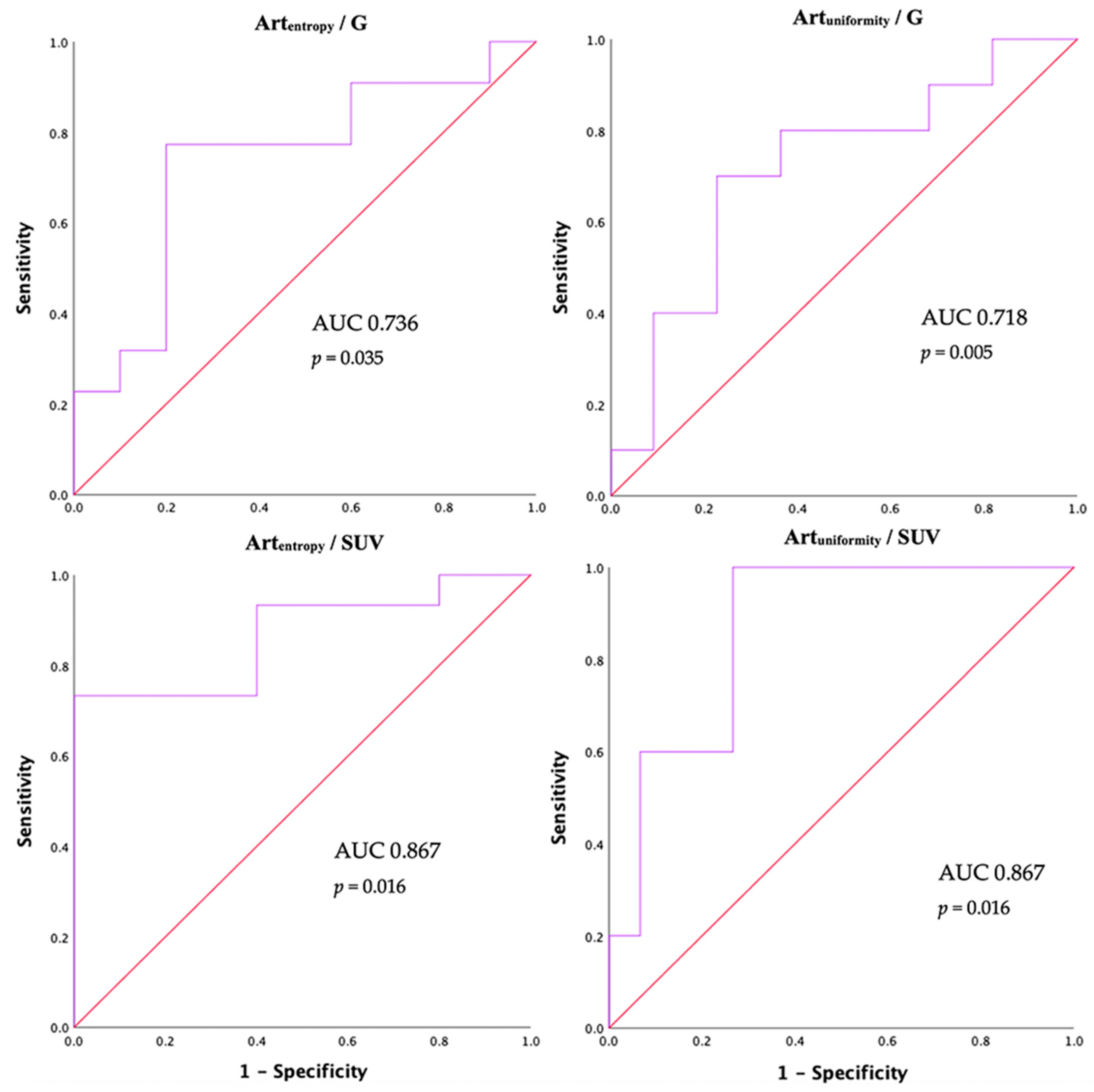
| AUC (95% CI) * | 0.736 (0.545–0.928) | 0.718 (0.522–0.914) |
| Cut-off | 3.16 | 0.12 |
| Sensitivity (%) | 77.3 | 80 |
| Specificity (%) | 80 | 64 |
| Artentropy | Artuniformity | |
|---|---|---|
| SUV 1 <4.5 | 2.76 ± 0.49 | 0.18 ± 0.07 |
| SUV 1 >4.5 | 3.49 ± 0.47 | 0.11 ± 0.04 |
| AUC (95% CI) * | 0.867 (0.704–1) | 0.867 (0.704–1) |
| Cut-off | 2.68 | 0.12 |
| Sensitivity (%) | 93.3 | 80 |
| Specificity (%) | 60 | 73.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beleù, A.; Rizzo, G.; De Robertis, R.; Drudi, A.; Aluffi, G.; Longo, C.; Sarno, A.; Cingarlini, S.; Capelli, P.; Landoni, L.; et al. Liver Tumor Burden in Pancreatic Neuroendocrine Tumors: CT Features and Texture Analysis in the Prediction of Tumor Grade and 18F-FDG Uptake. Cancers 2020, 12, 1486. https://doi.org/10.3390/cancers12061486
Beleù A, Rizzo G, De Robertis R, Drudi A, Aluffi G, Longo C, Sarno A, Cingarlini S, Capelli P, Landoni L, et al. Liver Tumor Burden in Pancreatic Neuroendocrine Tumors: CT Features and Texture Analysis in the Prediction of Tumor Grade and 18F-FDG Uptake. Cancers. 2020; 12(6):1486. https://doi.org/10.3390/cancers12061486
Chicago/Turabian StyleBeleù, Alessandro, Giulio Rizzo, Riccardo De Robertis, Alessandro Drudi, Gregorio Aluffi, Chiara Longo, Alessandro Sarno, Sara Cingarlini, Paola Capelli, Luca Landoni, and et al. 2020. "Liver Tumor Burden in Pancreatic Neuroendocrine Tumors: CT Features and Texture Analysis in the Prediction of Tumor Grade and 18F-FDG Uptake" Cancers 12, no. 6: 1486. https://doi.org/10.3390/cancers12061486
APA StyleBeleù, A., Rizzo, G., De Robertis, R., Drudi, A., Aluffi, G., Longo, C., Sarno, A., Cingarlini, S., Capelli, P., Landoni, L., Scarpa, A., Bassi, C., & D’Onofrio, M. (2020). Liver Tumor Burden in Pancreatic Neuroendocrine Tumors: CT Features and Texture Analysis in the Prediction of Tumor Grade and 18F-FDG Uptake. Cancers, 12(6), 1486. https://doi.org/10.3390/cancers12061486






