ImmunoPET in Multiple Myeloma—What? So What? Now What?
Abstract
1. Introduction
2. What?
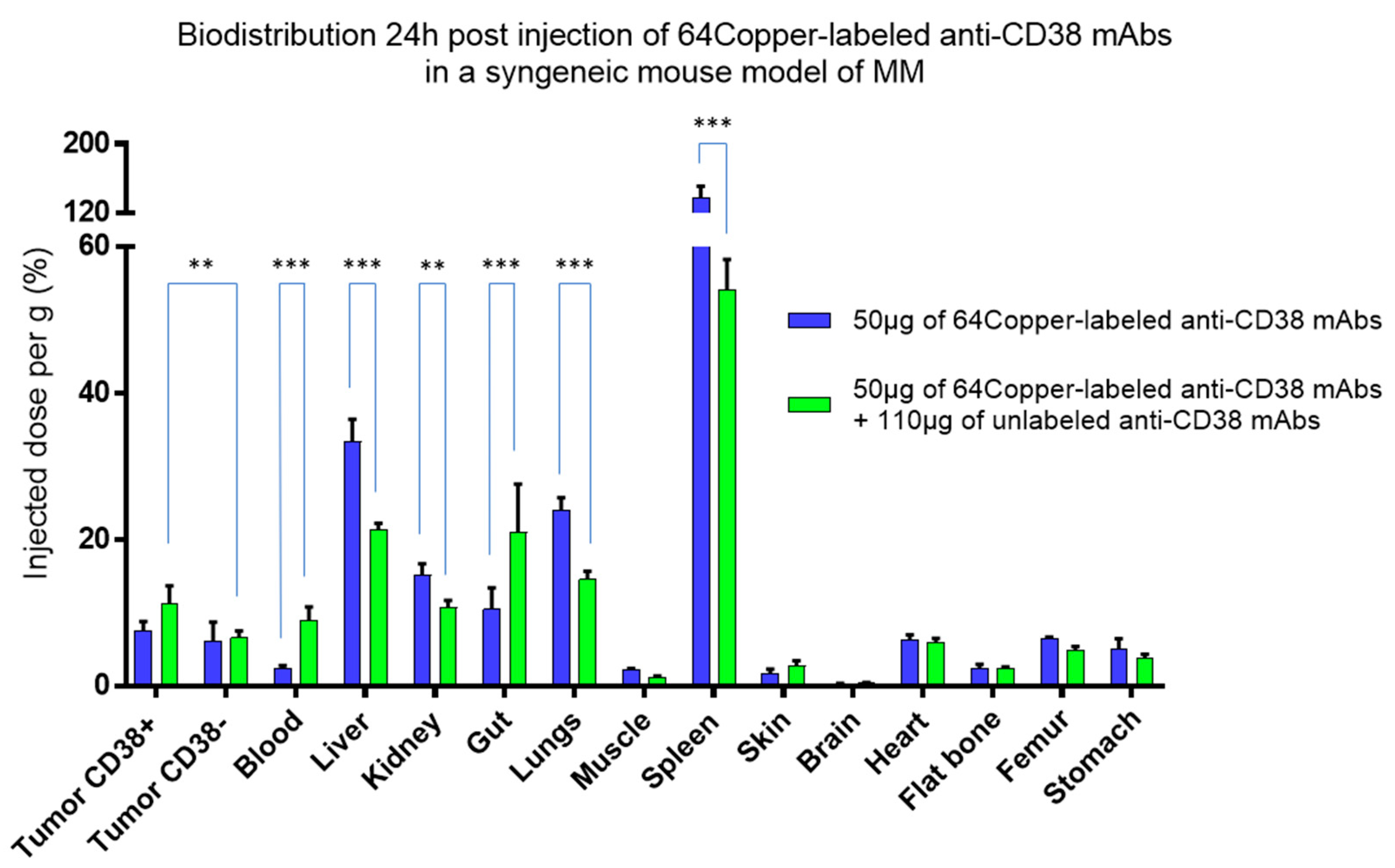
3. So What?
4. Now What?
5. What’s More?
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
References
- Röllig, C.; Knop, S.; Bornhäuser, M. Multiple myeloma. Lancet 2015, 385, 2197–2208. [Google Scholar] [CrossRef]
- Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Bladé, J.; Merlini, G.; Mateos, M.-V.; Rajkumar, S.V.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Nandakumar, B.; Binder, M.; Dispenzieri, A.; Kapoor, P.; Buadi, F.; Gertz, M.A.; Lacy, M.; Dingli, D.; Hwa, L.; Leung, N.; et al. Continued improvement in survival in multiple myeloma (MM) including high-risk patients. J. Clin. Oncol. 2019, 37, 8039. [Google Scholar] [CrossRef]
- Blimark, C.H.; Turesson, I.; Genell, A.; Ahlberg, L.; Björkstrand, B.; Carlson, K.; Forsberg, K.; Juliusson, G.; Linder, O.; Mellqvist, U.-H.; et al. Outcome and survival of myeloma patients diagnosed 2008–2015. Real-world data on 4904 patients from the Swedish Myeloma Registry. Haematology 2017, 103, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Branagan, A.; Lei, M.; Lou, U.; Raje, N. Current Treatment Strategies for Multiple Myeloma. JCO Oncol. Pr. 2020, 16, 5–14. [Google Scholar] [CrossRef]
- Bailly, C.; Cléry, P.-F.; Faivre-Chauvet, A.; Bourgeois, M.; Guérard, F.; Haddad, F.; Barbet, J.; Chérel, M.; Kraeber-Bodere, F.; Carlier, T.; et al. Immuno-PET for Clinical Theranostic Approaches. Int. J. Mol. Sci. 2016, 18, 57. [Google Scholar] [CrossRef]
- Rolfe, G.; Freshwater, D.; Jasper, M. Critical Reflection for Nursing and the Helping Professions: A User’s Guide; Palgrave: London, UK, 2001; ISBN 978-0-333-77795-4. [Google Scholar]
- Giuliani, N.; Malavasi, F. Editorial: Immunotherapy in Multiple Myeloma. Front. Immunol. 2019, 10, 1945. [Google Scholar] [CrossRef]
- Touzeau, C.; Moreau, P.; Dumontet, C. Monoclonal antibody therapy in multiple myeloma. Leukemia 2017, 31, 1039–1047. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Komarnicki, M.; Suzuki, K.; et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. New Engl. J. Med. 2016, 375, 1319–1331. [Google Scholar] [CrossRef]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Mun, Y.C.; Mateos, M.V.; et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef]
- Zamagni, E.; Tacchetti, P.; Pantani, L.; Cavo, M. Anti-CD38 and anti-SLAMF7: The future of myeloma immunotherapy. Expert Rev. Hematol. 2018, 11, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Durie, B.; Palumbo, A.; San-Miguel, J. Monoclonal antibodies in the treatment of multiple myeloma: Current status and future perspectives. Leukemia 2015, 30, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Plesner, T.; Krejcik, J. Daratumumab for the Treatment of Multiple Myeloma. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Afifi, S.; Michael, A.; Lesokhin, A. Immunotherapy. Ann. Pharmacother. 2016, 50, 555–568. [Google Scholar] [CrossRef]
- Van De Donk, N.W.C.J.; Richardson, P.G.; Malavasi, F. CD38 antibodies in multiple myeloma: Back to the future. Blood 2018, 131, 13–29. [Google Scholar] [CrossRef]
- Dostalek, M.; Gardner, I.; Gurbaxani, B.M.; Rose, R.H.; Chetty, M. Pharmacokinetics, Pharmacodynamics and Physiologically-Based Pharmacokinetic Modelling of Monoclonal Antibodies. Clin. Pharm. 2013, 52, 83–124. [Google Scholar] [CrossRef]
- Keizer, R.J.; Huitema, A.D.R.; Schellens, J.H.M.; Beijnen, J.H. Clinical Pharmacokinetics of Therapeutic Monoclonal Antibodies. Clin. Pharm. 2010, 49, 493–507. [Google Scholar] [CrossRef]
- Ahamadi, M.; Freshwater, T.; Prohn, M.; Li, C.H.; De Alwis, D.P.; De Greef, R.; Elassaiss-Schaap, J.; Kondic, A.; Stone, J.A. Model-Based Characterization of the Pharmacokinetics of Pembrolizumab: A Humanized Anti–PD-1 Monoclonal Antibody in Advanced Solid Tumors. CPT Pharmacomet. Syst. Pharmacol. 2016, 6, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, G.; Wang, X.; Agrawal, S.; Gupta, M.; Roy, A.; Feng, Y. Model-Based Population Pharmacokinetic Analysis of Nivolumab in Patients With Solid Tumors. CPT Pharmacomet. Syst. Pharmacol. 2016, 6, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ogungbenro, K.; Patel, A.; Duncombe, R.; Nuttall, R.; Clark, J.; Lorigan, P.C. Dose Rationalization of Pembrolizumab and Nivolumab Using Pharmacokinetic Modeling and Simulation and Cost Analysis. Clin. Pharmacol. Ther. 2017, 103, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Clemens, P.L.; Yan, X.; Lokhorst, H.M.; Lonial, S.; Losic, N.; Khan, I.; Jansson, R.; Ahmadi, T.; Lantz, K.; Zhou, H.; et al. Pharmacokinetics of Daratumumab Following Intravenous Infusion in Relapsed or Refractory Multiple Myeloma After Prior Proteasome Inhibitor and Immunomodulatory Drug Treatment. Clin. Pharm. 2017, 56, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.S.; Yan, X.; Puchalski, T.; Lonial, S.; Lokhorst, H.M.; Voorhees, P.M.; Plesner, T.; Liu, K.; Khan, I.; Jansson, R.; et al. Clinical Implications of Complex Pharmacokinetics for Daratumumab Dose Regimen in Patients With Relapsed/Refractory Multiple Myeloma. Clin. Pharmacol. Ther. 2017, 101, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Van De Donk, N.W.; Usmani, S.Z. CD38 Antibodies in Multiple Myeloma: Mechanisms of Action and Modes of Resistance. Front. Immunol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Mogollón, P.; Díaz-Tejedor, A.; Algarín, E.; Paíno, T.; Garayoa, M.; Ocio, E.M. Biological Background of Resistance to Current Standards of Care in Multiple Myeloma. Cells 2019, 8, 1432. [Google Scholar] [CrossRef]
- Oliva, S.; Troia, R.; D’Agostino, M.; Boccadoro, M.; Gay, F. Promises and Pitfalls in the Use of PD-1/PD-L1 Inhibitors in Multiple Myeloma. Front. Immunol. 2018, 9, 2749. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Avigan, D. Targeting the PD-1/PD-L1 axis in multiple myeloma: A dream or a reality? Blood 2017, 129, 275–279. [Google Scholar] [CrossRef]
- Tamura, H.; Ishibashi, M.; Yamashita, T.; Tanosaki, S.; Okuyama, N.; Kondo, A.; Hyodo, H.; Shinya, E.; Takahashi, H.; Dong, H.; et al. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia 2012, 27, 464–472. [Google Scholar] [CrossRef]
- Jelinek, T.; Paiva, B.; Hajek, R. Update on PD-1/PD-L1 Inhibitors in Multiple Myeloma. Front. Immunol. 2018, 9, 2431. [Google Scholar] [CrossRef]
- Costa, F.; Das, R.; Bailur, J.K.; Dhodapkar, K.; Dhodapkar, M.V. Checkpoint Inhibition in Myeloma: Opportunities and Challenges. Front. Immunol. 2018, 9, 2204. [Google Scholar] [CrossRef] [PubMed]
- Paiva, B.; Azpilikueta, A.; Puig, N.; Ocio, E.M.; Sharma, R.; Oyajobi, B.O.; Labiano, S.; San-Segundo, L.; Rodriguez, A.; Aires-Mejía, I.; et al. PD-L1/PD-1 presence in the tumor microenvironment and activity of PD-1 blockade in multiple myeloma. Leukemia 2015, 29, 2110–2113. [Google Scholar] [CrossRef] [PubMed]
- Lesokhin, A.M.; Ansell, S.M.; Armand, P.; Scott, E.C.; Halwani, A.; Gutierrez, M.; Millenson, M.M.; Cohen, A.D.; Schuster, S.J.; Lebovic, D.; et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J. Clin. Oncol. 2016, 34, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Ribrag, V.; Avigan, D.E.; Green, D.J.; Wise-Draper, T.; Posada, J.G.; Vij, R.; Zhu, Y.; Farooqui, M.Z.H.; Marinello, P.; Siegel, D.S. Phase 1b trial of pembrolizumab monotherapy for relapsed/refractory multiple myeloma: KEYNOTE-013. Br. J. Haematol. 2019, 186, e41–e44. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Orlowski, R.Z.; Ocio, E.M.; Rodríguez-Otero, P.; Reece, N.; Moreau, P.; Munshi, N.; Avigan, D.E.; Siegel, D.S.; Ghori, R.; et al. Pembrolizumab combined with lenalidomide and low-dose dexamethasone for relapsed or refractory multiple myeloma: Phase I KEYNOTE -023 study. Br. J. Haematol. 2019, 186, e117–e121. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Schjesvold, F.; Oriol, A.; Karlin, L.; Cavo, M.; Rifkin, R.M.; Yimer, H.A.; Leblanc, R.; Takezako, N.; McCroskey, R.D.; et al. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): A randomised, open-label, phase 3 trial. Lancet Haematol. 2019, 6, e448–e458. [Google Scholar] [CrossRef]
- Mateos, M.-V.; Blacklock, H.; Schjesvold, F.; Oriol, A.; Simpson, D.; George, A.; Goldschmidt, H.; LaRocca, A.; Chanan-Khan, A.; Sherbenou, D.; et al. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): A randomised, open-label, phase 3 trial. Lancet Haematol. 2019, 6, e459–e469. [Google Scholar] [CrossRef]
- Costello, C. The future of checkpoint inhibition in multiple myeloma? Lancet Haematol. 2019, 6, e439–e440. [Google Scholar] [CrossRef]
- Zelle-Rieser, C.; Thangavadivel, S.; Biedermann, R.; Brunner, A.; Stoitzner, P.; Willenbacher, E.; Greil, R.; Jöhrer, K. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J. Hematol. Oncol. 2016, 9, 116. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Danhof, S.; Strifler, S.; Hose, D.; Kortüm, M.; Bittrich, M.; Hefner, J.; Einsele, H.; Knop, S.; Schreder, M. Clinical and biological characteristics of myeloma patients influence response to elotuzumab combination therapy. J. Cancer Res. Clin. Oncol. 2018, 145, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Jakubowiak, A.; Offidani, M.; Pegourie, B.; De La Rubia, J.; Garderet, L.; Laribi, K.; Bosi, A.; Marasca, R.; Laubach, J.; Mohrbacher, A.; et al. Randomized phase 2 study: Elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM. Blood 2016, 127, 2833–2840. [Google Scholar] [CrossRef] [PubMed]
- Nijhof, I.S.; Casneuf, T.; Van Velzen, J.; Van Kessel, B.; Axel, A.E.; Syed, K.; Groen, R.; Van Duin, M.; Sonneveld, P.; Minnema, M.C.; et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood 2016, 128, 959–970. [Google Scholar] [CrossRef]
- Pick, M.; Vainstein, V.; Goldschmidt, N.; Lavie, D.; Libster, D.; Gural, A.; Grisariu, S.; Avni, B.; Ben-Yehuda, D.; Gatt, M. Daratumumab resistance is frequent in advanced-stage multiple myeloma patients irrespective of CD38 expression and is related to dismal prognosis. Eur. J. Haematol. 2018, 100, 494–501. [Google Scholar] [CrossRef]
- Kitadate, A.; Kobayashi, H.; Abe, Y.; Narita, K.; Miura, D.; Takeuchi, M.; Matsue, K. CD38 Expression Levels on Myeloma Cells and the Frequency of Circulating CD38-Positive Treg Cells Are Associated with the Response to Daratumumab in Multiple Myeloma. Blood 2018, 132, 1883. [Google Scholar] [CrossRef]
- Viola, D.; Dona, A.; Gunes, E.G.; Troadec, E.; Wu, X.; Branciamore, S.; McDonald, T.; Ghoda, L.; Streatfield, A.; Sanchez, J.F.; et al. Immune Mediated Mechanisms of Resistance to Daratumumab. Blood 2018, 132, 3201. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, E.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Yan, X.; Clemens, P.L.; Puchalski, T.; Lonial, S.; Lokhorst, H.M.; Orlowski, R.Z.; Losic, N.; Khan, I.; Jansson, R.; Ahmadi, T.; et al. Target-Mediated Drug Disposition of Daratumumab Following Intravenous Infusion in Relapsed or Refractory Multiple Myeloma after Prior Proteasome Inhibitors and Immunomodulatory Drugs: A Population Pharmacokinetic Analysis. Blood 2015, 126, 4222. [Google Scholar] [CrossRef]
- Centanni, M.; Moes, D.J.A.R.; Trocóniz, I.F.; Ciccolini, J.; Van Hasselt, J.G.C. Clinical Pharmacokinetics and Pharmacodynamics of Immune Checkpoint Inhibitors. Clin. Pharm. 2019, 58, 835–857. [Google Scholar] [CrossRef]
- Deng, R.; Jin, F.; Prabhu, S.; Iyer, S. Monoclonal antibodies: What are the pharmacokinetic and pharmacodynamic considerations for drug development? Expert Opin. Drug Metab. Toxicol. 2012, 8, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Deslandes, A. Comparative clinical pharmacokinetics of antibody-drug conjugates in first-in-human Phase 1 studies. mAbs 2014, 6, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Bartelink, I.H.; Jones, E.F.; Shahidi-Latham, S.K.; Lee, P.R.E.; Zheng, Y.; Vicini, P.; van ‘t Veer, L.; Wolf, D.; Iagaru, A.; Kroetz, D.L.; et al. Tumor Drug Penetration Measurements Could Be the Neglected Piece of the Personalized Cancer Treatment Puzzle. Clin. Pharmacol. Ther. 2018, 106, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cerdá, L.; Asín-Prieto, E.; Parra-Guillen, Z.P.; Trocóniz, I.F. The Long Neglected Player: Modeling Tumor Uptake to Guide Optimal Dosing. Clin. Cancer Res. 2018, 24, 3236–3238. [Google Scholar] [CrossRef] [PubMed]
- Rasche, L.; Chavan, S.S.; Stephens, O.W.; Patel, P.H.; Tytarenko, R.; Ashby, C.; Bauer, M.A.; Stein, C.; Deshpande, S.; Wardell, C.; et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat. Commun. 2017, 8, 268. [Google Scholar] [CrossRef]
- Rasche, L.; Kortüm, K.M.; Raab, M.S.; Weinhold, N. The Impact of Tumor Heterogeneity on Diagnostics and Novel Therapeutic Strategies in Multiple Myeloma. Int. J. Mol. Sci. 2019, 20, 1248. [Google Scholar] [CrossRef]
- Xu, X.S.; Dimopoulos, M.A.; Sonneveld, P.; Ho, P.J.; Belch, A.; Leiba, M.; Capra, M.; Gomez, D.; Medvedova, E.; Iida, S.; et al. Pharmacokinetics and Exposure–Response Analyses of Daratumumab in Combination Therapy Regimens for Patients with Multiple Myeloma. Adv. Ther. 2018, 35, 1859–1872. [Google Scholar] [CrossRef]
- Chillemi, A.; Quarona, V.; Zito, A.; Morandi, F.; Marimpietri, D.; Cuccioloni, M.; Robert, O.J.; Mark, C.S.; Bolzoni, M.; Toscani, D.; et al. Generation and Characterization of Microvesicles after Daratumumab Interaction with Myeloma Cells. Blood 2015, 126, 1849. [Google Scholar] [CrossRef]
- Krejcik, J.; Frerichs, K.A.; Nijhof, I.S.; Van Kessel, B.; Van Velzen, J.F.; Bloem, A.C.; Broekmans, M.E.; Zweegman, S.; Van Meerloo, J.; Musters, R.J.; et al. Monocytes and Granulocytes Reduce CD38 Expression Levels on Myeloma Cells in Patients Treated with Daratumumab. Clin. Cancer Res. 2017, 23, 7498–7511. [Google Scholar] [CrossRef]
- Zonder, J.A.; Mohrbacher, A.F.; Singhal, S.; Van Rhee, F.; Bensinger, W.I.; Ding, H.; Fry, J.; Afar, D.E.H.; Singhal, A.K. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood 2012, 120, 552–559. [Google Scholar] [CrossRef]
- Beckman, R.A.; Von Roemeling, R.; Scott, A.M. Monoclonal antibody dose determination and biodistribution into solid tumors. Ther. Deliv. 2011, 2, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Strik, A.S.; Wang, Y.-M.C.; Ruff, L.E.; Yashar, W.; Messmer, B.T.; Mould, D.R. Individualized Dosing of Therapeutic Monoclonal Antibodies—A Changing Treatment Paradigm? AAPS J. 2018, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.A.; Balthasar, J.P. Understanding Inter-Individual Variability in Monoclonal Antibody Disposition. Antibodies 2019, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Gormley, N.J.; Pazdur, R. Immunotherapy Combinations in Multiple Myeloma—Known Unknowns. N. Engl. J. Med. 2018, 379, 1791–1795. [Google Scholar] [CrossRef]
- Day, D.; Siu, L.L. Approaches to modernize the combination drug development paradigm. Genome Med. 2016, 8, 115. [Google Scholar] [CrossRef]
- Hofmarcher, T.; Lindgren, P.; Wilking, N.; Jonsson, B. The cost of cancer in Europe 2018. Eur. J. Cancer 2020, 129, 41–49. [Google Scholar] [CrossRef]
- Romero, D. To all involved—we have a problem. Nat. Rev. Clin. Oncol. 2018, 15, 397. [Google Scholar] [CrossRef]
- Ma, C.K.K.; Danta, M.; Day, R.; Ma, D.D.F. Dealing with the spiralling price of medicines: Issues and solutions. Intern. Med. J. 2018, 48, 16–24. [Google Scholar] [CrossRef]
- Salas-Vega, S.; Iliopoulos, O.; Mossialos, E. Assessment of Overall Survival, Quality of Life, and Safety Benefits Associated With New Cancer Medicines. JAMA Oncol. 2017, 3, 382. [Google Scholar] [CrossRef]
- Turner, J.H. Theranostic Outcomes in Clinical Practice of Oncology: What, So What, Now What? What’s More. Cancer Biother. Radiopharm 2019, 34, 135–140. [Google Scholar] [CrossRef]
- Sotelo-Rodríguez, D.C.; Ruíz-Patiño, A.; Ricaurte, L.; Arrieta, O.; Zatarain-Barrón, Z.L.; Cardona, A.F.; Trinca, F.; Infante, P.; Dinis, R.; Inácio, M.; et al. Challenges and shifting paradigms in clinical trials in oncology: The case for immunological and targeted therapies. Ecancermedicalscience 2019, 13, 936. [Google Scholar] [CrossRef] [PubMed]
- Hoering, A.; Durie, B.; Wang, H.; Crowley, J. End points and statistical considerations in immuno-oncology trials: Impact on multiple myeloma. Future Oncol. 2017, 13, 1181–1193. [Google Scholar] [CrossRef] [PubMed]
- Gerwing, M.; Herrmann, K.; Helfen, A.; Schliemann, C.; Berdel, W.E.; Eisenblätter, M.; Wildgruber, M. The beginning of the end for conventional RECIST—novel therapies require novel imaging approaches. Nat. Rev. Clin. Oncol. 2019, 16, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Ramaiya, N.H.; Hatabu, H.; Hodi, F.S. Monitoring immune-checkpoint blockade: Response evaluation and biomarker development. Nat. Rev. Clin. Oncol. 2017, 14, 655–668. [Google Scholar] [CrossRef]
- Romano, A.; Palumbo, G.A.; Parrinello, N.L.; Conticello, C.; Martello, M.; Terragna, C. Minimal Residual Disease Assessment Within the Bone Marrow of Multiple Myeloma: A Review of Caveats, Clinical Significance and Future Perspectives. Front. Oncol. 2019, 9, 699. [Google Scholar] [CrossRef]
- Moreau, P.; Zamagni, E. MRD in multiple myeloma: More questions than answers? Blood Cancer J. 2017, 7, 639. [Google Scholar] [CrossRef]
- Kumar, S.K.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.C.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Van Dongen, G.; Visser, G.W.; Hooge, M.N.L.-D.; De Vries, E.G.; Perk, L.R. Immuno-PET: A Navigator in Monoclonal Antibody Development and Applications. Oncology 2007, 12, 1379–1389. [Google Scholar] [CrossRef]
- Guang, M.H.Z.; McCann, A.; Bianchi, G.; Zhang, L.; Dowling, P.; Bazou, D.; O’Gorman, P.; Anderson, K.C. Overcoming multiple myeloma drug resistance in the era of cancer ‘omics’. Leuk. Lymphoma 2017, 59, 542–561. [Google Scholar] [CrossRef]
- Kraeber-Bodere, F.; Bailly, C.; Chérel, M.; Chatal, J.-F. ImmunoPET to help stratify patients for targeted therapies and to improve drug development. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2166–2168. [Google Scholar] [CrossRef]
- Wei, W.; Rosenkrans, Z.T.; Liu, J.; Huang, G.; Luo, Q.-Y.; Cai, W. ImmunoPET: Concept, Design, and Applications. Chem. Rev. 2020, 120, 3787–3851. [Google Scholar] [CrossRef] [PubMed]
- McKnight, B.N.; Viola, N. 89Zr-ImmunoPET companion diagnostics and their impact in clinical drug development. J. Label. Compd. Radiopharm. 2018, 61, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Ghai, A.; Maji, D.; Cho, N.; Chanswangphuwana, C.; Rettig, M.; Shen, D.; DiPersio, J.; Akers, W.; Dehdashti, F.; Achilefu, S.; et al. Preclinical Development of CD38-Targeted [89Zr]Zr-DFO-Daratumumab for Imaging Multiple Myeloma. J. Nucl. Med. 2017, 59, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Caserta, E.; Chea, J.; Minnix, M.; Poku, E.K.; Viola, D.; Vonderfecht, S.; Yazaki, P.; Crow, D.; Khalife, J.; Sanchez, J.F.; et al. Copper 64–labeled daratumumab as a PET/CT imaging tracer for multiple myeloma. Blood 2018, 131, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.; Sobol, N.; O’Donoghue, J.; Burnazi, E.; Staton, K.; Weber, W.; Lyashchenko, S.; Lewis, J.; Landgren, C.O. Preclinical development and First-in-human imaging of 89Zr-Daratumumab for CD38 targeted imaging of myeloma. J. Nucl. Med. 2019, 60, 203. [Google Scholar]
- Pandit-Taskar, N. Functional Imaging Methods for Assessment of Minimal Residual Disease in Multiple Myeloma: Current Status and Novel ImmunoPET Based Methods. Semin. Hematol. 2018, 55, 22–32. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Sobol, N.B.; O’Donoghue, J.A.; Kirov, A.S.; Riedl, C.C.; Min, R.; Smith, E.; Carter, L.M.; Lyashchenko, S.K.; Lewis, J.S.; et al. CD38-targeted Immuno-PET of Multiple Myeloma: From Xenograft Models to First-in-Human Imaging. Radiology 2020, 295, 606–615. [Google Scholar] [CrossRef]
- Bailly, C.; Bodet-Milin, C.; Bourgeois, M.; Gouard, S.; Ansquer, C.; Barbaud, M.; Sébille, J.-C.; Chérel, M.; Kraeber-Bodere, F.; Carlier, T. Exploring Tumor Heterogeneity Using PET Imaging: The Big Picture. Cancers 2019, 11, 1282. [Google Scholar] [CrossRef]
- Bensch, F.; Van Der Veen, E.L.; Hooge, M.N.L.-D.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.J.N.; Van Der Wekken, A.J.; et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef]
- Niemeijer, A.N.; Leung, D.; Huisman, M.C.; Bahce, I.; Hoekstra, O.S.; Van Dongen, G.A.M.S.; Boellaard, R.; Du, S.; Hayes, W.; Smith, R.; et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat. Commun. 2018, 9, 4664. [Google Scholar] [CrossRef]
- Dijkers, E.C.; Munnink, T.H.O.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; De Jong, J.R.; Van Dongen, G.A.; Schröder, C.P.; Hooge, M.N.L.-D.; De Vries, E.G. Biodistribution of 89Zr-trastuzumab and PET Imaging of HER2-Positive Lesions in Patients With Metastatic Breast Cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Muylle, K.; Flamen, P.; Vugts, D.J.; Guiot, T.; Ghanem, G.; Meuleman, N.; Bourgeois, P.; Vanderlinden, B.; Van Dongen, G.A.M.S.; Everaert, H.; et al. Tumour targeting and radiation dose of radioimmunotherapy with (90)Y-rituximab in CD20+ B-cell lymphoma as predicted by (89)Zr-rituximab immuno-PET: Impact of preloading with unlabelled rituximab. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Oordt, C.W.M.-V.D.H.V.; McGeoch, A.; Bergstrom, M.; McSherry, I.; Smith, D.A.; Cleveland, M.; Al-Azzam, W.; Chen, L.; Verheul, H.; Hoekstra, O.S.; et al. Immuno-PET Imaging to Assess Target Engagement: Experience from 89Zr-Anti-HER3 mAb (GSK2849330) in Patients with Solid Tumors. J. Nucl. Med. 2019, 60, 902–909. [Google Scholar] [CrossRef]
- Bailly, C.; Gouard, S.; Guérard, F.; Chalopin, B.; Carlier, T.; Faivre-Chauvet, A.; Saëc, P.R.-L.; Bourgeois, M.; Chouin, N.; Rbah-Vidal, L.; et al. What is the Best Radionuclide for Immuno-PET of Multiple Myeloma? A Comparison Study Between 89Zr- and 64Cu-Labeled Anti-CD138 in a Preclinical Syngeneic Model. Int. J. Mol. Sci. 2019, 20, 2564. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Chow, A.; Monette, S.; Vivier, D.; Pourat, J.; Edwards, K.J.; Dilling, T.R.; Abdel-Atti, D.; Zeglis, B.M.; Poirier, J.T.; et al. Fc-Mediated Anomalous Biodistribution of Therapeutic Antibodies in Immunodeficient Mouse Models. Cancer Res. 2018, 78, 1820–1832. [Google Scholar] [CrossRef]
- Paton-Hough, J.; Chantry, A.; Lawson, M.A. A review of current murine models of multiple myeloma used to assess the efficacy of therapeutic agents on tumour growth and bone disease. Bone 2015, 77, 57–68. [Google Scholar] [CrossRef]
- Sanderson, R.D.; Yang, Y. Syndecan-1: A dynamic regulator of the myeloma microenvironment. Clin. Exp. Metastasis 2007, 25, 149–159. [Google Scholar] [CrossRef]
- Cavo, M.; Terpos, E.; Nanni, C.; Moreau, P.; Lentzsch, S.; Zweegman, S.; Hillengass, J.; Engelhardt, M.; Usmani, S.Z.; Vesole, D.H.; et al. Role of 18F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: A consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017, 18, e206–e217. [Google Scholar] [CrossRef]
- Green, D.J.; Press, O. Whither radioimmunotherapy: To be or not to be? Cancer Res. 2017, 77, 2191–2196. [Google Scholar] [CrossRef]
- Rizvi, S.N.F.; Visser, O.J.; Vosjan, M.J.W.D.; Van Lingen, A.; Hoekstra, O.S.; Zijlstra, J.M.; Huijgens, P.C.; Van Dongen, G.A.M.S.; Lubberink, M. Biodistribution, radiation dosimetry and scouting of 90Y-ibritumomab tiuxetan therapy in patients with relapsed B-cell non-Hodgkin’s lymphoma using 89Zr-ibritumomab tiuxetan and PET. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 512–520. [Google Scholar] [CrossRef]
- Morschhauser, F.; Radford, J.; Van Hoof, A.; Vitolo, U.; Soubeyran, P.; Tilly, H.; Huijgens, P.C.; Kolstad, A.; D’Amore, F.; Diaz, M.G.; et al. Phase III Trial of Consolidation Therapy With Yttrium-90–Ibritumomab Tiuxetan Compared With No Additional Therapy After First Remission in Advanced Follicular Lymphoma. J. Clin. Oncol. 2008, 26, 5156–5164. [Google Scholar] [CrossRef] [PubMed]
- Witzig, T.E.; Flinn, I.W.; Gordon, L.I.; Emmanouilides, C.; Czuczman, M.S.; Saleh, M.N.; Cripe, L.; Wiseman, G.; Olejnik, T.; Multani, P.S.; et al. Treatment With Ibritumomab Tiuxetan Radioimmunotherapy in Patients With Rituximab-Refractory Follicular Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2002, 20, 3262–3269. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Martínez-Ramirez, M.; Martínez-Caballero, D.; Beneit, P.; Clavel, J.; Figueroa, G.; Verdú, J. Radioinmunoterapia en el linfoma no Hodgkin, posicionamiento, seguridad y eficacia de 90Y-ibritumomab. Experiencia y seguimiento a los 10 años. Revista Española de Medicina Nuclear e Imagen Molecular 2017, 36, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Scholz, C.W.; Pinto, A.; Linkesch, W.; Lindén, O.; Viardot, A.; Keller, U.; Hess, G.; Lastoria, S.; Lerch, K.; Frigeri, F.; et al. 90Yttrium-Ibritumomab-Tiuxetan as First-Line Treatment for Follicular Lymphoma: 30 Months of Follow-Up Data From an International Multicenter Phase II Clinical Trial. J. Clin. Oncol. 2013, 31, 308–313. [Google Scholar] [CrossRef]
- Hohloch, K. Radioimmunotherapy of lymphoma: An underestimated therapy option. Lancet Haematol. 2017, 4, e6–e7. [Google Scholar] [CrossRef]
- Illidge, T. Radioimmunotherapy of Lymphoma: A Treatment Approach Ahead of Its Time or Past Its Sell-By Date? J. Clin. Oncol. 2010, 28, 2944–2946. [Google Scholar] [CrossRef] [PubMed]
- Dadachova, E. Cancer Therapy with Alpha-Emitters Labeled Peptides. Semin. Nucl. Med. 2010, 40, 204–208. [Google Scholar] [CrossRef]
- Fichou, N.; Gouard, S.; Maurel, C.; Barbet, J.; Ferrer, L.; Morgenstern, A.; Bruchertseifer, F.; Faivre-Chauvet, A.; Bigot-Corbel, E.; Davodeau, F.; et al. Single-Dose Anti-CD138 Radioimmunotherapy: Bismuth-213 is More Efficient than Lutetium-177 for Treatment of Multiple Myeloma in a Preclinical Model. Front. Med. 2015, 2, 553. [Google Scholar] [CrossRef]
- Gouard, S.; Chalopin, B.; Saï-Maurel, C.; Guérard, F.; Navarro, L.; Gestin, J.-F.; Chouin, N.; Haddad, F.; Alliot, C.; Kraeber-Bodéré, F.; et al. Efficacy of Astatine-211 Radioimmunotherapy of Multiple Myeloma Using an Anti-mCD138 Monoclonal Antibody in a Syngeneic Murine Model. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, S166–S167. [Google Scholar]
- Gouard, S.; Pallardy, A.; Gaschet, J.; Faivre-Chauvet, A.; Bruchertseifer, F.; Morgenstern, A.; Maurel, C.; Matous, E.; Kraeber-Bodere, F.; Davodeau, F.; et al. Comparative analysis of multiple myeloma treatment by CD138 antigen targeting with bismuth-213 and Melphalan chemotherapy. Nucl. Med. Boil. 2014, 41, e30–e35. [Google Scholar] [CrossRef]
- Chérel, M.; Gouard, S.; Gaschet, J.; Saï-Maurel, C.; Bruchertseifer, F.; Morgenstern, A.; Bourgeois, M.; Gestin, J.-F.; Kraeber-Bodere, F.; Barbet, J.; et al. 213Bi Radioimmunotherapy with an Anti-mCD138 Monoclonal Antibody in a Murine Model of Multiple Myeloma. J. Nucl. Med. 2013, 54, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Gouard, S.; Lacombe, M.; Saec, P.R.-L.; Chalopin, B.; Bourgeois, M.; Chouin, N.; Tripier, R.; Halime, Z.; Haddad, F.; et al. Comparison of Immuno-PET of CD138 and PET imaging with 64CuCl2 and 18F-FDG in a preclinical syngeneic model of multiple myeloma. Oncotarget 2018, 9, 9061–9072. [Google Scholar] [CrossRef] [PubMed][Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, C.; Chalopin, B.; Gouard, S.; Carlier, T.; Remaud-Le Saëc, P.; Marionneau-Lambot, S.; Moreau, P.; Touzeau, C.; Kraeber-Bodere, F.; Bodet-Milin, C.; et al. ImmunoPET in Multiple Myeloma—What? So What? Now What? Cancers 2020, 12, 1467. https://doi.org/10.3390/cancers12061467
Bailly C, Chalopin B, Gouard S, Carlier T, Remaud-Le Saëc P, Marionneau-Lambot S, Moreau P, Touzeau C, Kraeber-Bodere F, Bodet-Milin C, et al. ImmunoPET in Multiple Myeloma—What? So What? Now What? Cancers. 2020; 12(6):1467. https://doi.org/10.3390/cancers12061467
Chicago/Turabian StyleBailly, Clément, Benjamin Chalopin, Sébastien Gouard, Thomas Carlier, Patricia Remaud-Le Saëc, Séverine Marionneau-Lambot, Philippe Moreau, Cyrille Touzeau, Françoise Kraeber-Bodere, Caroline Bodet-Milin, and et al. 2020. "ImmunoPET in Multiple Myeloma—What? So What? Now What?" Cancers 12, no. 6: 1467. https://doi.org/10.3390/cancers12061467
APA StyleBailly, C., Chalopin, B., Gouard, S., Carlier, T., Remaud-Le Saëc, P., Marionneau-Lambot, S., Moreau, P., Touzeau, C., Kraeber-Bodere, F., Bodet-Milin, C., & Chérel, M. (2020). ImmunoPET in Multiple Myeloma—What? So What? Now What? Cancers, 12(6), 1467. https://doi.org/10.3390/cancers12061467




