Biomarker Panel for the Diagnosis of Pancreatic Ductal Adenocarcinoma
Abstract
1. Introduction
2. Methods
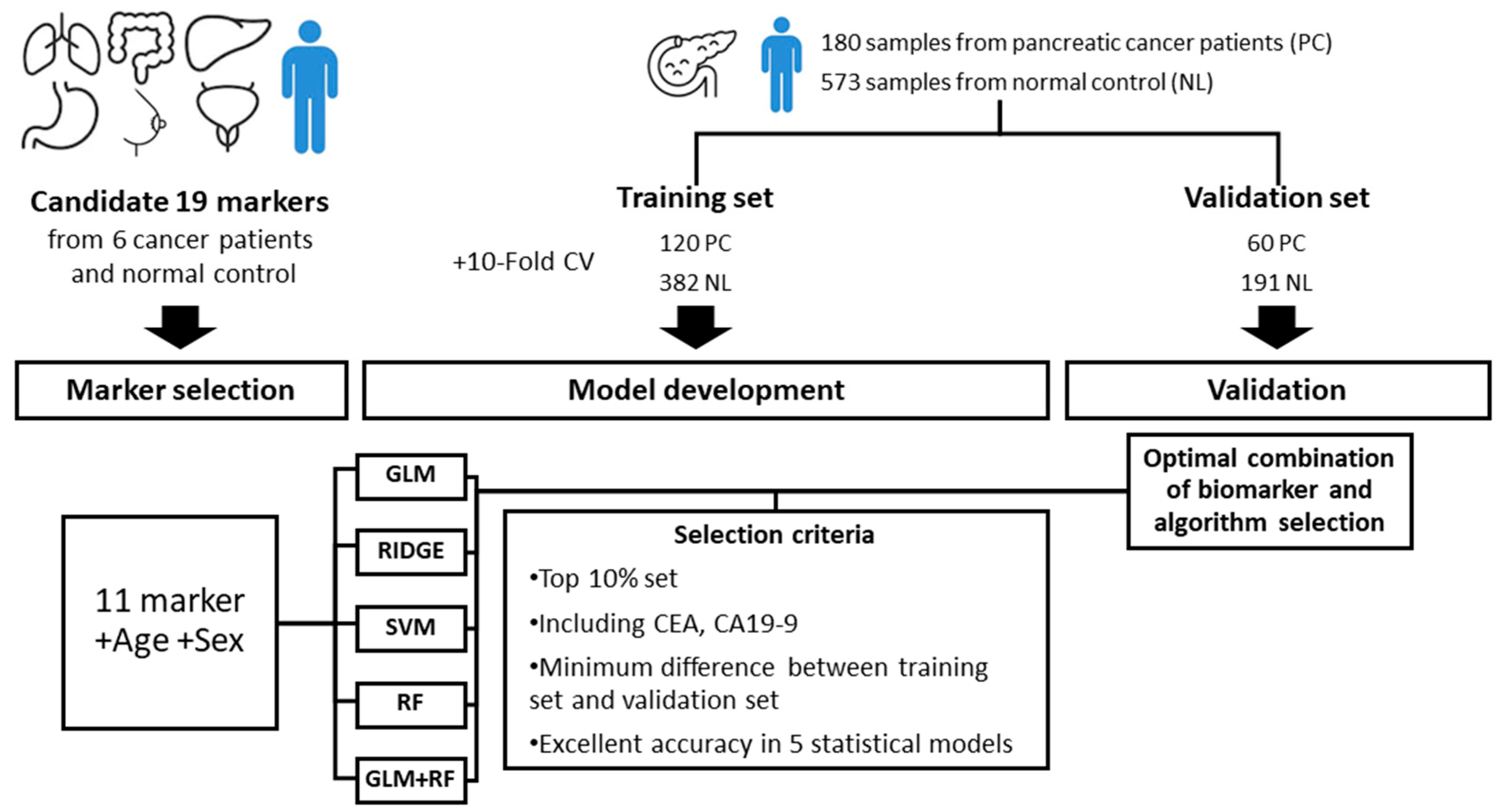
2.1. Patient Samples
2.2. Selection of Candidate Markers
2.3. Data Analysis
3. Results
3.1. Clinical Characteristics
3.2. Biomarker Selection and Model Development
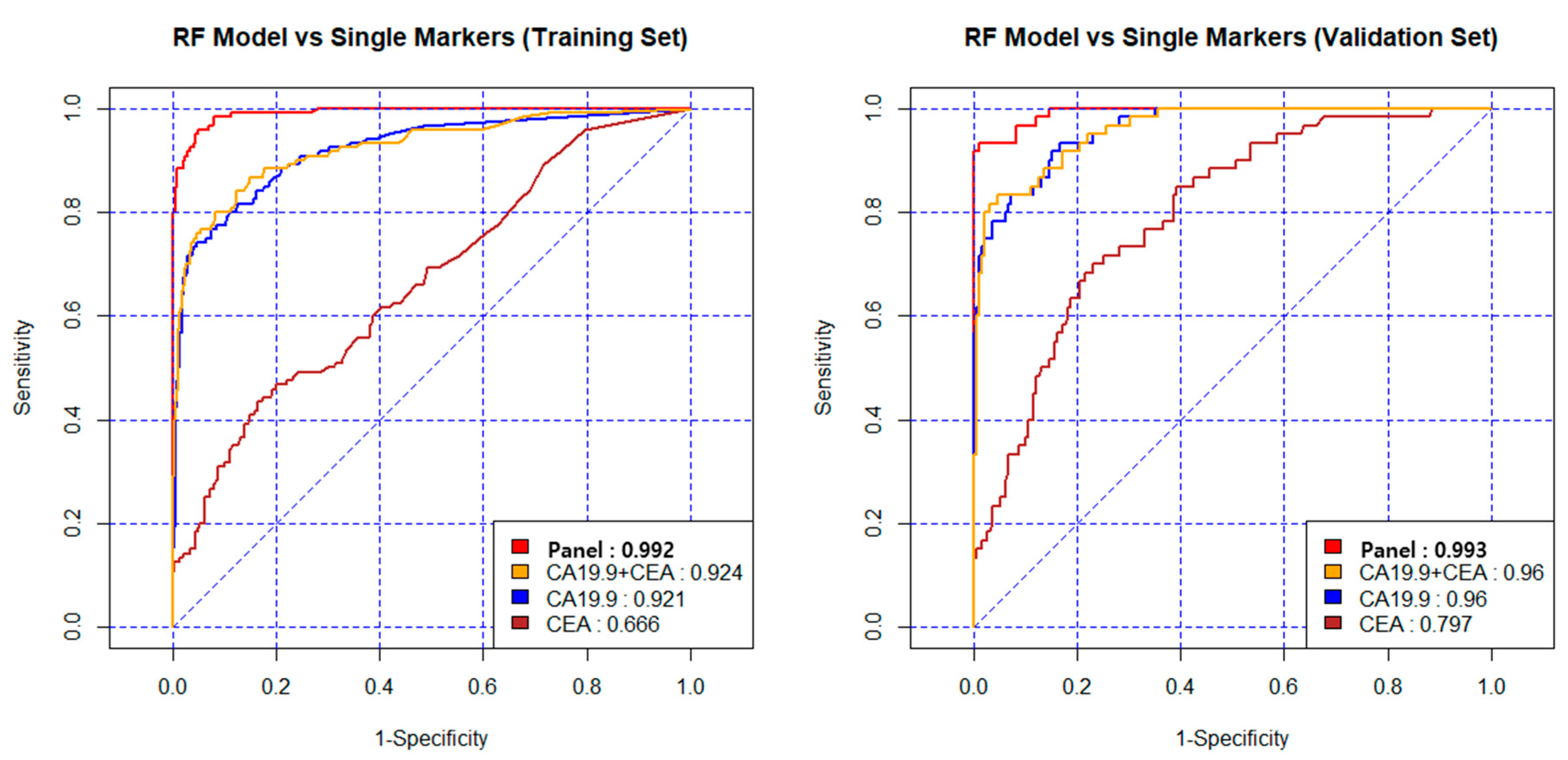
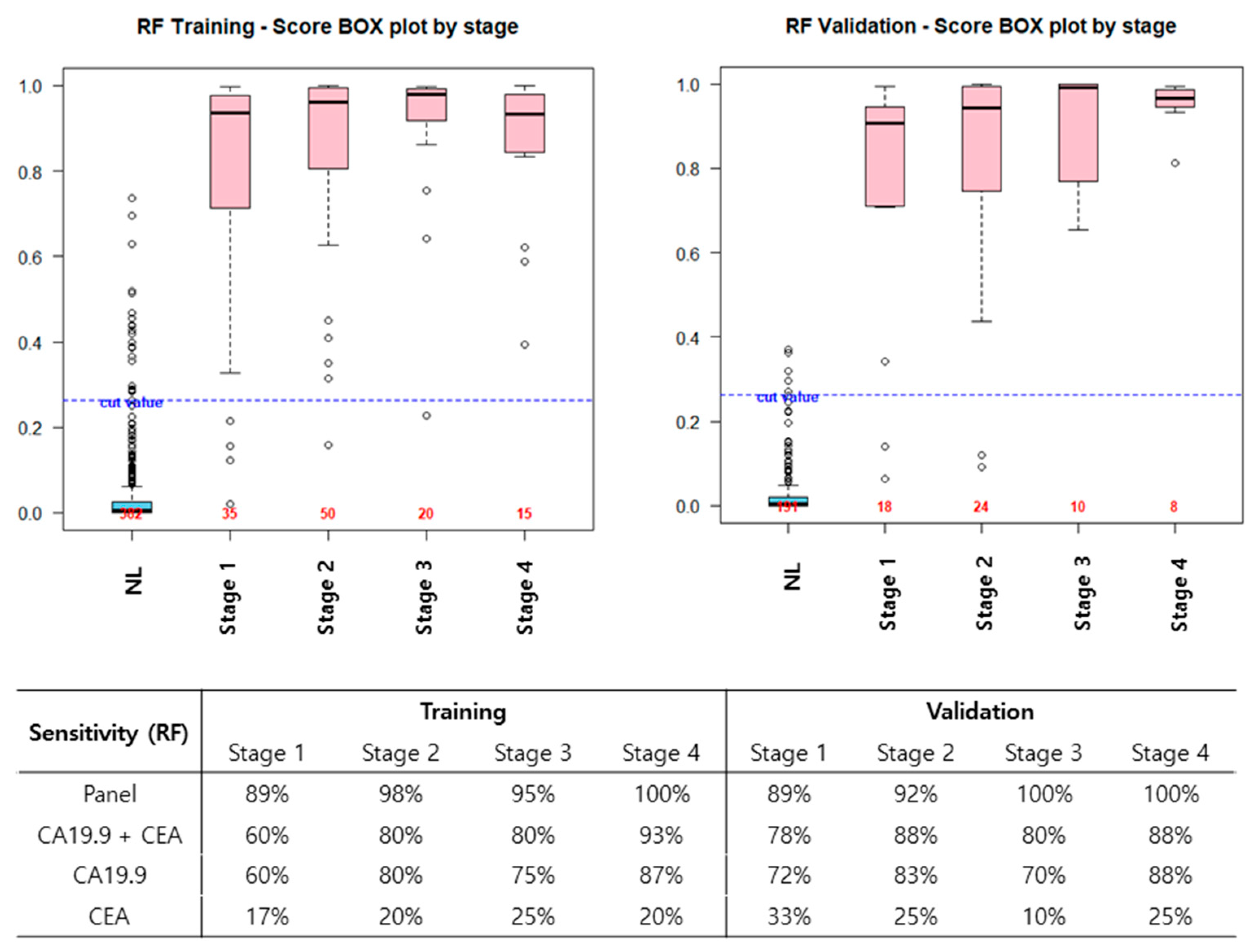
3.3. Diagnostic Performance of New Biomarker Combination Set
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.M.; Turk, T.; Al-Husseini, M.J.; Abdel-Rahman, O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer 2018, 18, 688. [Google Scholar] [CrossRef] [PubMed]
- Gillen, S.; Schuster, T.; Zum Büschenfelde, C.M.; Friess, H.; Kleeff, J. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010, 7, e1000267. [Google Scholar] [CrossRef] [PubMed]
- Issa, I.A.; Noureddine, M. Colorectal cancer screening: An updated review of the available options. World J. Gastroenterol. 2017, 23, 5086–5096. [Google Scholar] [CrossRef] [PubMed]
- Humphris, J.L.; Chang, D.K.; Johns, A.L.; Scarlett, C.J.; Pajic, M.; Jones, M.D.; Colvin, E.K.; Nagrial, A.; Chin, V.T.; Chantrill, L.A.; et al. The prognostic and predictive value of serum CA19-9 in pancreatic cancer. Ann. Oncol. 2012, 23, 1713–1722. [Google Scholar] [CrossRef]
- Goonetilleke, K.; Siriwardena, A. Systematic review of carbohydrate antigen (CA19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur. J. Surg. Oncol. (EJSO) 2007, 33, 266–270. [Google Scholar] [CrossRef]
- Rieser, C.J.; Zenati, M.; Hamad, A.; Al Abbas, A.I.; Bahary, N.; Zureikat, A.H.; Zeh, H.J.; Hogg, M.E. CA19-9 on postoperative surveillance in pancreatic ductal adenocarcinoma: Predicting recurrence and changing prognosis over time. Ann. Surg. Oncol. 2018, 25, 3483–3491. [Google Scholar] [CrossRef]
- Tsai, S.; George, B.; Wittmann, D.; Ritch, P.S.; Krepline, A.N.; Aldakkak, M.; Barnes, C.A.; Christians, K.K.; Dua, K.; Griffin, M.; et al. Importance of Normalization of CA19-9 Levels Following Neoadjuvant Therapy in Patients With Localized Pancreatic Cancer. Ann. Surg. 2020, 1, 740–747. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; Tarpey, P.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef]
- Marusyk, A.; Almendro, V.; Polyak, K. Intra-tumour heterogeneity: A looking glass for cancer? Nat. Rev. Cancer 2012, 12, 323–334. [Google Scholar] [CrossRef]
- Zhang, Z. An in vitro diagnostic multivariate index assay (IVDMIA) for ovarian cancer: Harvesting the power of multiple biomarkers. Rev. Obstet. Gynecol. 2012, 5, 35–41. [Google Scholar] [PubMed]
- Kim, B.K.; Lee, J.W.; Park, P.J.; Shin, Y.S.; Lee, W.Y.; Lee, K.A.; Ye, S.; Hyun, H.; Kang, K.N.; Yeo, D.; et al. The multiplex bead array approach to identifying serum biomarkers associated with breast cancer. Breast Cancer Res. 2009, 11, R22. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.S.; Shin, Y.S.; Park, P.J.; Kang, K.N.; Kim, Y.; Lee, H.J.; Yang, H.K.; Kim, C.W. Serum biomarker panels for the diagnosis of gastric adenocarcinoma. Br. J. Cancer 2012, 106, 733–739. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, Y.T.; Park, P.J.; Shin, Y.S.; Kang, K.N.; Kim, Y.; Kim, C.W. A novel detection method of non–small cell lung cancer using multiplexed bead-based serum biomarker profiling. J. Thorac. Cardiovasc. Surg. 2012, 143, 421–427. [Google Scholar] [CrossRef]
- Yoon, H.I.; Kwon, O.R.; Kang, K.N.; Shin, Y.S.; Shin, H.S.; Yeon, E.H.; Kwon, K.Y.; Hwang, I.; Jeon, Y.K.; Kim, Y.; et al. Diagnostic value of combining tumor and inflammatory markers in lung cancer. J. Cancer Prev. 2016, 21, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, Y.; Namkung, J.; Yi, S.G.; Kim, H.; Yu, J.; Kim, Y.; Kwon, M.S.; Kwon, W.; Oh, D.Y.; et al. Diagnostic performance enhancement of pancreatic cancer using proteomic multimarker panel. Oncotarget 2017, 8, 93117–93130. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Sato, S.; Kitajima, Y.; Tanaka, M.; Nakafusa, Y.; Miyazaki, K. False-positive findings for tumor markers after curative gastrectomy for gastric cancer. Dig. Dis. Sci. 2008, 53, 73–79. [Google Scholar] [CrossRef]
- Bates, S.E.; Longo, D.L. Tumor markers: Value and limitations in the management of cancer patients. Cancer Treat. Rev. 1985, 12, 163–207. [Google Scholar] [CrossRef]
- Fung, E.T. A recipe for proteomics diagnostic test development: The OVA1 test, from biomarker discovery to FDA clearance. Clin. Chem. 2010, 56, 327–329. [Google Scholar] [CrossRef]
- Mellby, L.D.; Nyberg, A.P.; Johansen, J.S.; Wingren, C.; Nordestgaard, B.G.; Bojesen, S.E.; Mitchell, B.L.; Sheppard, B.C.; Sears, R.C.; Borrebaeck, C.A. Serum biomarker signature-based liquid biopsy for diagnosis of early-stage pancreatic cancer. J. Clin. Oncol. 2018, 36, 2887–2894. [Google Scholar] [CrossRef]
- Coppin, L.; Benomar, K.; Corfiotti, F.; Cattan, S.; Renaud, F.; Lapere, C.; Leteurtre, E.; Vantyghem, M.C.; Truant, S.; Pigny, P. CA-125, but not galectin-3, complements CA19-9 for discriminating ductal adenocarcinoma versus non-malignant pancreatic diseases. Pancreatology 2016, 16, 115–120. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.P.; Sandanayake, N.S.; Jenkinson, C.; Gentry-Maharaj, A.; Apostolidou, S.; Fourkala, E.O.; Camuzeaux, S.; Blyuss, O.; Gunu, R.; Dawnay, A.; et al. Serum CA19-9 is significantly upregulated up to 2 years before diagnosis with pancreatic cancer: Implications for early disease detection. Clin. Cancer Res. 2015, 21, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Li, S.; Wu, C.T.; Qi, Z.H.; Wang, W.Q.; Jin, W.; Gao, H.L.; Zhang, S.R.; Xu, J.Z.; Liu, C.; et al. Postoperative serum CA19-9, CEA and CA125 predicts the response to adjuvant chemoradiotherapy following radical resection in pancreatic adenocarcinoma. Pancreatology 2018, 18, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, W.; Wang, W.; Shen, H.; Liu, L.; Lou, W.; Wang, X.; Yang, P. A new panel of pancreatic cancer biomarkers discovered using a mass spectrometry-based pipeline. Br. J. Cancer 2018, 118, e15. [Google Scholar] [CrossRef]
- Honda, K.; Katzke, V.A.; Hüsing, A.; Okaya, S.; Shoji, H.; Onidani, K.; Olsen, A.; Tjønneland, A.; Overvad, K.; Weiderpass, E.; et al. CA19-9 and apolipoprotein-A2 isoforms as detection markers for pancreatic cancer: A prospective evaluation. Int. J. Cancer 2019, 144, 1877–1887. [Google Scholar] [CrossRef]
- Tang, P.; Tao, L.; Yuan, C.; Zhang, L.; Xiu, D. Serum derived exosomes from pancreatic cancer patients promoted metastasis: An iTRAQ-based proteomic analysis. Onco Targets Ther. 2019, 12, 9329–9339. [Google Scholar] [CrossRef]

| Pancreatic Ductal Adenocarcinoma | |||||
|---|---|---|---|---|---|
| Total | Training set | Validation set | p-value | ||
| n = 180 | n = 120 | n = 60 | |||
| Age | 64.4 (9.8) | 63.6 (9.9) | 66.0 (9.5) | 0.109 | |
| Sex | M | 117 (65.0) | 75 (62.5) | 42 (70) | 0.320 |
| F | 63 (35.0) | 45 (37.5) | 18 (30) | ||
| Operation | PPPD | 62 (34.4) | 44 (36.7) | 18 (30) | 0.996 |
| PD | 38 (21.1) | 25 (20.8) | 13 (21.7) | ||
| DP | 50 (27.8) | 32 (26.7) | 18 (30) | ||
| TP | 15 (8.3) | 11 (9.2) | 4 (6.7) | ||
| Others * | 15 (8.3) | 8 (6.7) | 7 (11.7) | ||
| Stage | 1 | 53 (29.4) | 35 (29.2) | 18 (30) | 0.996 |
| 2 | 74 (41.1) | 50 (41.7) | 24 (40) | ||
| 3 | 30 (16.7) | 20 (16.7) | 10 (16.7) | ||
| 4 | 23 (12.8) | 15 (12.5) | 8 (13.3) | ||
| T stage | 1 | 22 (12.2) | 13 (10.8) | 9 (15.0) | 0.711 |
| 2 | 94 (52.2) | 66 (55.0) | 28 (46.7) | ||
| 3 | 38 (21.1) | 26 (21.7) | 12 (20.0) | ||
| 4 | 10 (5.6) | 6 (5.0) | 4 (6.7) | ||
| NA | 16 (8.9) | 9 (7.5) | 7 (11.7) | 0.779 | |
| N stage | 0 | 69 (38.3) | 47 (39.2) | 22 (36.7) | |
| 1 | 73 (40.6) | 47 (39.2) | 26 (43.3) | ||
| 2 | 26 (14.4) | 19 (15.8) | 7 (11.7) | ||
| NA | 12 (6.7) | 7 (5.8) | 5 (8.3) | ||
| Differentiation | WD | 14 (7.8) | 9 (7.5) | 5 (8.3) | 0.862 |
| MD | 118 (65.6) | 80 (66.7) | 38 (63.3) | ||
| PD | 26 (14.4) | 18 (15.0) | 8 (13.3) | ||
| NA | 22 (12.2) | 13 (10.8) | 9 (15.0) | ||
| Lymphatic | No | 78 (43.3) | 52 (43.3) | 26 (43.3) | 0.948 |
| invasion | Yes | 77 (42.8) | 52 (43.3) | 25 (41.7) | |
| NA | 25 (13.9) | 16 (13.3) | 9 (15.0) | ||
| Venous | No | 61 (33.9) | 41 (34.2) | 20 (33.3) | 0.575 |
| invasion | Yes | 85 (47.2) | 54 (45.0) | 31 (51.7) | |
| NA | 34 (18.9) | 25 (20.8) | 9 (15.0) | ||
| Perineural | No | 19 (10.6) | 14 (11.7) | 5 (8.3) | 0.547 |
| invasion | Yes | 145 (80.6) | 97 (80.8) | 48 (80.0) | 0.547 |
| NA | 16 (8.9) | 9 (7.5) | 7 (11.7) | ||
| Healthy control | |||||
| Total | Training set | Validation set | p-value | ||
| n = 573 | n = 382 | n = 191 | |||
| Age | 56.9 (8.8) | 56.6 (8.9) | 57.5 (8.6) | 0.250 | |
| Sex | M | 334 (58.3) | 218 (57.1) | 116 (60.7) | 0.420 |
| F | 239 (41.7) | 164 (42.9) | 75 (39.3) | ||
| Marker | Training and Test Set | Validation Set | ||||||
|---|---|---|---|---|---|---|---|---|
| AUC | Accuracy (%) | Specificity (%) | Sensitivity (%) | AUC | Accuracy (%) | Specificity (%) | Sensitivity (%) | |
| RF | ||||||||
| Panel | 0.992 | 95 | 95 | 96 | 0.993 | 95 | 96 | 93 |
| CA19-9 + CEA | 0.924 | 90 | 95 | 76 | 0.960 | 92 | 94 | 83 |
| CA19-9 | 0.921 | 90 | 95 | 74 | 0.960 | 90 | 94 | 78 |
| CEA | 0.666 | 77 | 95 | 20 | 0.797 | 78 | 95 | 25 |
| GLM | ||||||||
| Panel | 0.983 | 94 | 95 | 92 | 0.983 | 94 | 95 | 92 |
| CA19-9 + CEA | 0.852 | 87 | 95 | 62 | 0.928 | 91 | 94 | 80 |
| CA19-9 | 0.848 | 88 | 95 | 66 | 0.923 | 92 | 94 | 83 |
| CEA | 0.732 | 78 | 95 | 24 | 0.814 | 80 | 96 | 28 |
| GLM + RF | ||||||||
| Panel | 0.984 | 94 | 95 | 92 | 0.986 | 95 | 96 | 92 |
| CA19-9 + CEA | 0.934 | 91 | 95 | 78 | 0.962 | 91 | 93 | 87 |
| CA19-9 | 0.933 | 90 | 95 | 75 | 0.964 | 90 | 94 | 80 |
| CEA | 0.732 | 78 | 95 | 24 | 0.814 | 80 | 96 | 28 |
| RIDGE | ||||||||
| Panel | 0.987 | 95 | 95 | 93 | 0.985 | 95 | 96 | 92 |
| CA19-9 + CEA | 0.852 | 87 | 95 | 62 | 0.928 | 91 | 94 | 80 |
| CA19-9 | 0.848 | 88 | 95 | 67 | 0.924 | 92 | 94 | 83 |
| CEA | 0.732 | 78 | 95 | 24 | 0.816 | 80 | 96 | 28 |
| SVM | ||||||||
| Panel | 0.990 | 95 | 95 | 95 | 0.991 | 97 | 98 | 92 |
| CA19-9 + CEA | 0.900 | 89 | 95 | 71 | 0.964 | 92 | 97 | 77 |
| CA19-9 | 0.912 | 88 | 95 | 68 | 0.967 | 92 | 96 | 77 |
| CEA | 0.627 | 78 | 95 | 25 | 0.692 | 78 | 95 | 27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kang, K.N.; Shin, Y.S.; Byun, Y.; Han, Y.; Kwon, W.; Kim, C.W.; Jang, J.-Y. Biomarker Panel for the Diagnosis of Pancreatic Ductal Adenocarcinoma. Cancers 2020, 12, 1443. https://doi.org/10.3390/cancers12061443
Kim H, Kang KN, Shin YS, Byun Y, Han Y, Kwon W, Kim CW, Jang J-Y. Biomarker Panel for the Diagnosis of Pancreatic Ductal Adenocarcinoma. Cancers. 2020; 12(6):1443. https://doi.org/10.3390/cancers12061443
Chicago/Turabian StyleKim, Hongbeom, Kyung Nam Kang, Yong Sung Shin, Yoonhyeong Byun, Youngmin Han, Wooil Kwon, Chul Woo Kim, and Jin-Young Jang. 2020. "Biomarker Panel for the Diagnosis of Pancreatic Ductal Adenocarcinoma" Cancers 12, no. 6: 1443. https://doi.org/10.3390/cancers12061443
APA StyleKim, H., Kang, K. N., Shin, Y. S., Byun, Y., Han, Y., Kwon, W., Kim, C. W., & Jang, J.-Y. (2020). Biomarker Panel for the Diagnosis of Pancreatic Ductal Adenocarcinoma. Cancers, 12(6), 1443. https://doi.org/10.3390/cancers12061443





