Micrometastases in Sentinel Lymph Nodes Represent a Significant Negative Prognostic Factor in Early-Stage Cervical Cancer: A Single-Institutional Retrospective Cohort Study
Abstract
1. Introduction
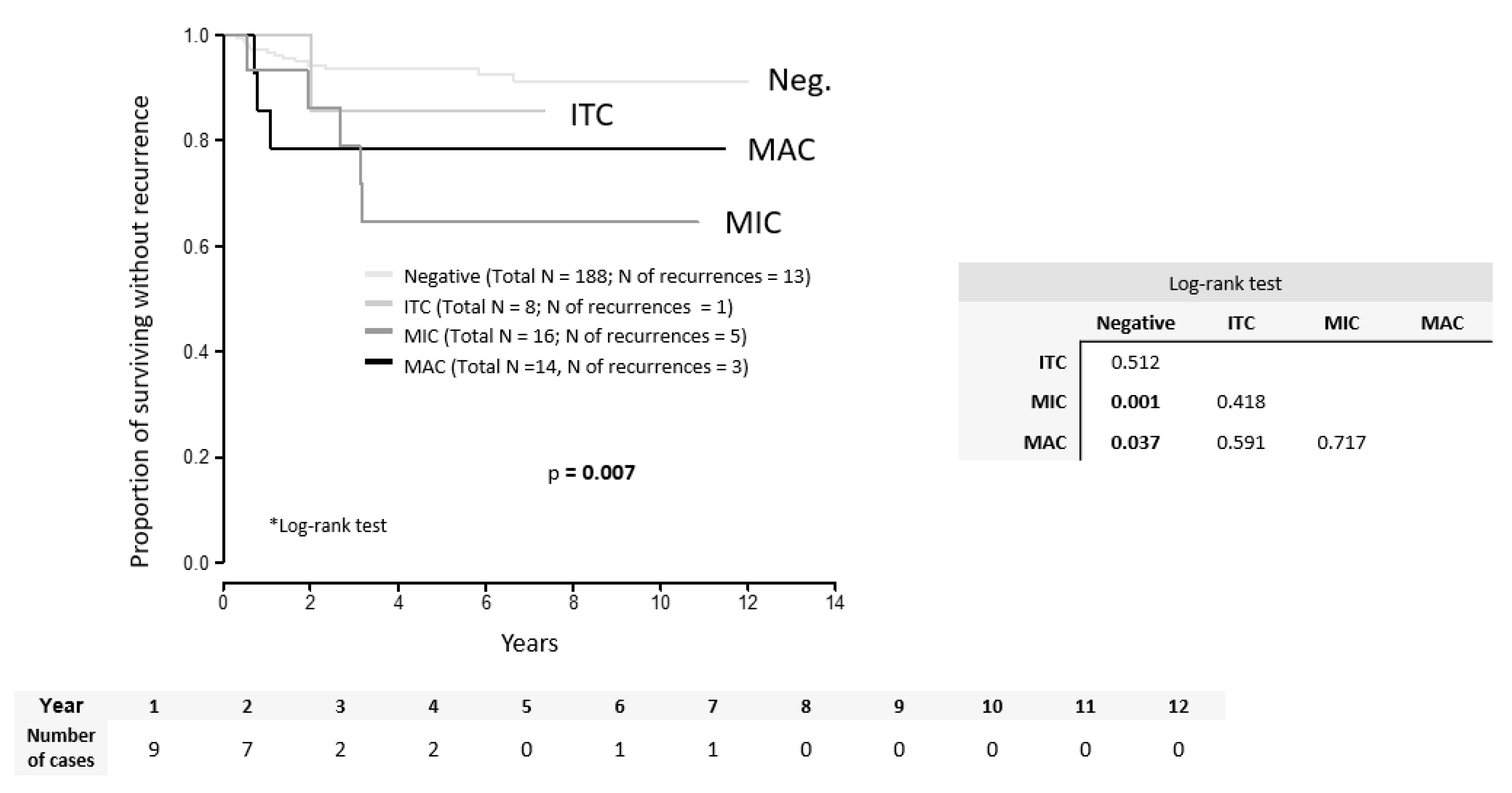
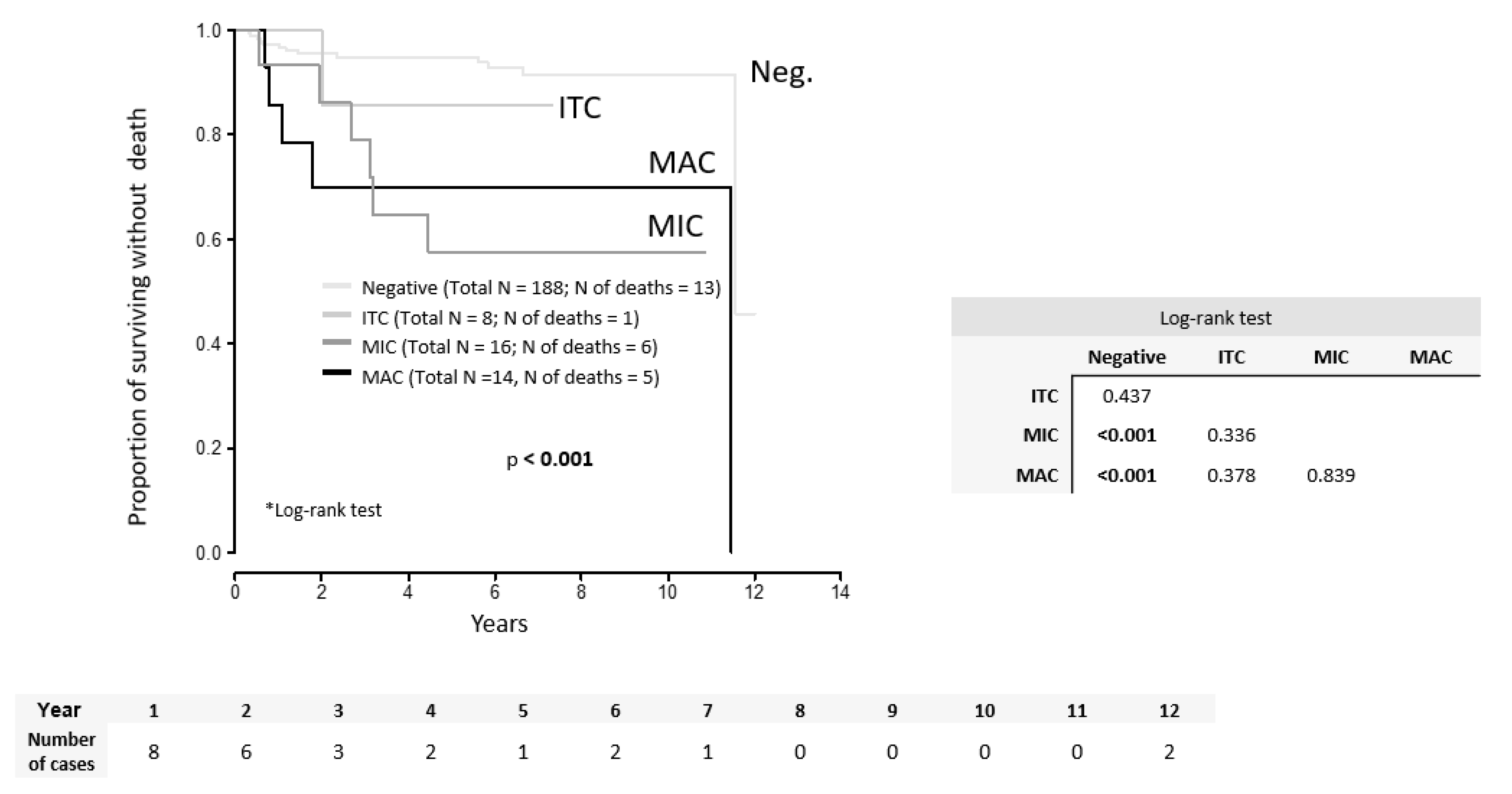
2. Results
3. Discussion
4. Materials and Methods
4.1. Methods
4.2. Pathology
4.3. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cibula, D.; Abu-Rustum, N.R.; Dusek, L.; Zikán, M.; Zaal, A.; Sevcik, L.; Kenter, G.G.; Querleu, D.; Jach, R.; Bats, A.S.; et al. Prognostic significance of low volume sentinel lymph node disease in early-stage cervical cancer. Gynecol. Oncol. 2012, 124, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Rob, L.; Charvat, M.; Robova, H.; Strnad, P.; Pluta, M.; Halaska, M.; Hrehorcak, M.; Schlegerova, D.; Taborska, K. Sentinel lymph node identification (SLNI) in the management of conservative surgery in early cervical cancer: Is it acceptable? Gynecol. Oncol. 2005, 99, S147–S148. [Google Scholar] [CrossRef] [PubMed]
- Wydra, D.; Sawicki, S.; Wojtylak, S.; Bandurski, T.; Emerich, J. Sentinel node identification in cervical cancer patients undergoing transperitoneal radical hysterectomy: A study of 100 cases. Int. J. Gynecol. Cancer 2006, 16, 649–654. [Google Scholar] [CrossRef]
- Darlin, L.; Persson, J.; Bossmar, T.; Lindahl, B.; Kannisto, P.; Måsbäck, A.; Borgfeldt, C. The sentinel node concept in early cervical cancer performs well in tumors smaller than 2 cm. Gynecol. Oncol. 2010, 117, 266–269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Altgassen, C.; Hertel, H.; Brandstädt, A.; Köhler, C.; Dürst, M.; Schneider, A. Multicenter Validation Study of the Sentinel Lymph Node Concept in Cervical Cancer: AGO Study Group. J. Clin. Oncol. 2008, 26, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- Marnitz, S.; Köhler, C.; Bongardt, S.; Braig, U.; Hertel, H.; Schneider, A. Topographic distribution of sentinel lymph nodes in patients with cervical cancer. Gynecol. Oncol. 2006, 103, 35–44. [Google Scholar] [CrossRef]
- Cibula, D.; Kuzel, D.; Slama, J.; Fischerova, D.; Dundr, P.; Freitag, P.; Zikán, M.; Pavlista, D.; Tomancova, V. Sentinel node (SLN) biopsy in the management of locally advanced cervical cancer. Gynecol. Oncol. 2009, 115, 46–50. [Google Scholar] [CrossRef]
- Du, X.L.; Sheng, X.G.; Jiang, T.; Li, Q.S.; Yu, H.; Pan, C.X.; Lu, C.H.; Wang, C.; Song, Q.Q. Sentinel lymph node biopsy as guidance for radical trachelectomy in young patients with early stage cervical cancer. BMC Cancer 2011, 11. [Google Scholar] [CrossRef]
- Abu-Rustum, N.; Sonoda, Y. Fertility-Sparing Surgery in Early-Stage Cervical Cancer: Indications and Applications. J. Natl. Compr. Cancer Netw. 2010, 8, 1435–1438. [Google Scholar] [CrossRef]
- Cibula, D.; SlÁMa, J.; SvÁRovskÝ, J.; Fischerova, D.; Freitag, P.; ZikÁN, M.; PinkavovÁ, I.; Pavlista, D.; Dundr, P.; Hill, M. Abdominal Radical Trachelectomy in Fertility-Sparing Treatment of Early-Stage Cervical Cancer. Int. J. Gynecol. Cancer 2009, 19, 1407–1411. [Google Scholar] [CrossRef]
- Euscher, E.; Malpica, A.; Atkinson, E.; Levenback, C.; Frumovitz, M.; Deavers, M. Ultrastaging Improves Detection of Metastases in Sentinel Lymph Nodes of Uterine Cervix Squamous Cell Carcinoma. Am. J. Surg Pathol. 2008, 32, 1336–1343. [Google Scholar] [CrossRef]
- Lentz, S.; Muderspach, L.; Felix, J.; Ye, W.; Groshen, S.; Amezcua, C. Identification of Micrometastases in Histologically Negative Lymph Nodes of Early-Stage Cervical Cancer Patients. Obstet. Gynecol. 2004, 103, 1204–1210. [Google Scholar] [CrossRef]
- Daraï, E.; Rouzier, R.; Ballester, M.; Barranger, E.; Coutant, C. Sentinel lymph node biopsy in gynaecological cancers: The importance of micrometastases in cervical cancer. Surg. Oncol. 2008, 17, 227–235. [Google Scholar] [CrossRef]
- Horn, L.; Hentschel, B.; Fischer, U.; Peter, D.; Bilek, K. Detection of micrometastases in pelvic lymph nodes in patients with carcinoma of the cervix uteri using step sectioning: Frequency, topographic distribution and prognostic impact. Gynecol. Oncol. 2008, 111, 276–281. [Google Scholar] [CrossRef]
- Marchiolè, P.; Buénerd, A.; Benchaib, M.; Nezhat, K.; Dargent, D.; Mathevet, P. Clinical significance of lympho vascular space involvement and lymph node micrometastases in early-stage cervical cancer: A retrospective case-control surgico-pathological study. Gynecol. Oncol. 2005, 97, 727–732. [Google Scholar] [CrossRef]
- Juretzka, M.; Jensen, K.; Longacre, T.; Teng, N.; Husain, A. Detection of pelvic lymph node micrometastasis in stage IA2–IB2 cervical cancer by immunohistochemical analysis. Gynecol. Oncol. 2004, 93, 107–111. [Google Scholar] [CrossRef]
- Fregnani, J.; Latorre, M.; Novik, P.; Lopes, A.; Soares, F. Assessment of pelvic lymph node micrometastatic disease in stages IB and IIA of carcinoma of the uterine cervix. Int. J. Gynecol. Cancer 2006, 16, 1188–1194. [Google Scholar] [CrossRef]
- Stany, M.P.; Stone, P.J.; Felix, J.C.; Amezcua, C.A.; Groshen, S.; Ye, W.; Kyser, K.L.; Howard, R.S.; Zahn, C.M.; Muderspach, L.I.; et al. Lymph Node Micrometastases in Early-Stage Cervical Cancer are Not Predictive of Survival. Int. J. Gynecol. Pathol. 2015, 34, 379–384. [Google Scholar] [CrossRef]
- Colturato, L.; Signorini Filho, R.; Fernandes, R.; Gebrim, L.; Oliani, A. Lymph node micrometastases in initial stage cervical cancer and tumoral recurrence. Int. J. Gynecol. Obstet. 2015, 133, 69–75. [Google Scholar] [CrossRef]
- Guani, B.; Dorez, M.; Magaud, L.; Buenerd, A.; Lecuru, F.; Mathevet, P. Impact of micrometastasis or isolated tumor cells on recurrence and survival in patients with early cervical cancer: SENTICOL Trial. Int. J. Gynecol. Cancer 2019, 29, 447–452. [Google Scholar] [CrossRef]
- Bizzarri, N.; Anchora, L.P.; Zannoni, G.F.; Santoro, A.; Valente, M.; Inzani, F.; Gallotta, V.; Conte, C.; Chiantera, V.; Fanfani, F.; et al. Role of one-step nucleic acid amplification (OSNA) to detect sentinel lymph node low-volume metastasis in early-stage cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Dostálek, L.; Zikan, M.; Fischerova, D.; Kocian, R.; Germanova, A.; Frühauf, F.; Dusek, L.; Slama, J.; Dundr, P.; Nemejcova, K.; et al. SLN biopsy in cervical cancer patients with tumors larger than 2 cm and 4 cm. Gynecol. Oncol. 2018, 148, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.P.; Gemignani, M.L.; Pandit-Taskar, N.; Park, K.J.; Murray, M.P.; Chi, D.S.; Sonoda, Y.; Barakat, R.R.; Abu-Rustum, N.R. Sentinel lymph node biopsy in the management of early-stage cervical carcinoma. Gynecol. Oncol. 2011, 120, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Salvo, G.; Ramirez, P.T.; Levenback, C.F.; Munsell, M.F.; Euscher, E.D.; Soliman, P.T.; Frumovitz, M. Sensitivity and negative predictive value for sentinel lymph node biopsy in women with early-stage cervical cancer. Gynecol. Oncol. 2017, 145, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Van Trappen, P.O.; Gyselman, V.G.; Lowe, D.G.; Ryan, A.; Oram, D.H.; Bosze, P.; Weekes, A.R.; Shepherd, J.H.; Dorudi, S.; Bustin, S.A.; et al. Molecular quantification and mapping of lymph-node micrometastases in cervical cancer. Lancet 2001, 357, 15–20. [Google Scholar] [CrossRef]
- Bats, A.S.; Mathevet, P.; Buenerd, A.; Orliaguet, I.; Mery, E.; Zerdoud, S.; Le Frère-Belda, M.A.; Froissart, M.; Querleu, D.; Martinez, A.; et al. The Sentinel Node Technique Detects Unexpected Drainage Pathways and Allows Nodal Ultrastaging in Early Cervical Cancer: Insights from the Multicenter Prospective SENTICOL Study. Ann. Surg. Oncol. 2012, 20, 413–422. [Google Scholar] [CrossRef]
- Dundr, P.; Cibula, D.; Němejcová, K.; Tichá, I.; Bártů, M.; Jakša, R. Pathologic Protocols for Sentinel Lymph Nodes Ultrastaging in Cervical Cancer. Arch. Pathol. Lab. Med. 2019. [Google Scholar] [CrossRef]
- Lécuru, F.; Mathevet, P.; Querleu, D.; Leblanc, E.; Morice, P.; Daraï, E.; Marret, H.; Magaud, L.; Gillaizeau, F.; Chatellier, G.; et al. Bilateral Negative Sentinel Nodes Accurately Predict Absence of Lymph Node Metastasis in Early Cervical Cancer: Results of the SENTICOL Study. J. Clin. Oncol. 2011, 29, 1686–1691. [Google Scholar] [CrossRef]
- Rob, L.; Robova, H.; Halaska, M.; Hruda, M.; Skapa, P. Current status of sentinel lymph node mapping in the management of cervical cancer. Expert Rev. Anticancer Ther. 2013, 13, 861–870. [Google Scholar] [CrossRef]
- Bats, A.S.; Buénerd, A.; Querleu, D.; Leblanc, E.; Daraï, E.; Morice, P.; Marret, H.; Gillaizeau, F.; Mathevet, P.; Lécuru, F. Diagnostic value of intraoperative examination of sentinel lymph node in early cervical cancer: A prospective, multicenter study. Gynecol. Oncol. 2011, 123, 230–235. [Google Scholar] [CrossRef]
- Martínez, A.; Mery, E.; Filleron, T.; Boileau, L.; Ferron, G.; Querleu, D. Accuracy of intraoperative pathological examination of SLN in cervical cancer. Gynecol. Oncol. 2013, 130, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Delgado, G.; Bundy, B.; Zaino, R.; Sevin, B.; Creasman, W.; Major, F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: A Gynecologic Oncology Group study. Gynecol. Oncol. 1990, 38, 352–357. [Google Scholar] [CrossRef]
- Hellebrekers, B.W.; Zwinderman, A.H.; Kenter, G.G.; Peters, A.A.; Snijders-Keilholz, A.; Graziosi, G.C.; Fleuren, G.J.; Trimbos, J.B. Surgically-treated early cervical cancer: Prognostic factors and the significance of depth of tumor invasion. Int. J. Gynecol. Cancer 1999, 9, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.; Frumovitz, M.; Pareja, R. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.W.; Chang, S.J.; Piao, X.; Paek, J.; Lee, Y.; Lee, E.J.; Chun, M.; Ryu, H.S. Patterns of recurrence and survival after abdominal versus laparoscopic/robotic radical hysterectomy in patients with early cervical cancer. J. Obstet. Gynaecol. Res. 2015, 42, 77–86. [Google Scholar] [CrossRef]
- Cibula, D.; McCluggage, W. Sentinel lymph node (SLN) concept in cervical cancer: Current limitations and unanswered questions. Gynecol. Oncol. 2019, 152, 202–207. [Google Scholar] [CrossRef]
- Brar, H.; Hogen, L.; Covens, A. Cost-effectiveness of sentinel node biopsy and pathological ultrastaging in patients with early-stage cervical cancer. Cancer 2017, 123, 1751–1759. [Google Scholar] [CrossRef]
- Cibula, D.; Abu-Rustum, N. Pelvic lymphadenectomy in cervical cancer—Surgical anatomy and proposal for a new classification system. Gynecol. Oncol. 2010, 116, 33–37. [Google Scholar] [CrossRef]
- Querleu, D.; Cibula, D.; Abu-Rustum, N. 2017 Update on the Querleu–Morrow Classification of Radical Hysterectomy. Ann. Surg. Oncol. 2017, 24, 3406–3412. [Google Scholar] [CrossRef]
- Querleu, D.; Morrow, C. Classification of radical hysterectomy. Lancet Oncol. 2008, 9, 297–303. [Google Scholar] [CrossRef]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Meder, C.H.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients with Cervical Cancer. Virchows Archiv. 2018, 472, 919–936. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Whole Cohort 1 | |
|---|---|---|
| Age (years) | 42.2 (26.2; 67.9) | |
| BMI | 24.3 (18.4; 36.2) | |
| Stage pT | 1a1 | 8 (3.5%) |
| 1a2 | 7 (3.1%) | |
| 1b1 | 157 (69.4%) | |
| 1b2 | 42 (18.6%) | |
| 2a | 3 (1.3%) | |
| 2b | 9 (4.0%) | |
| Tumor type | Adenocarcinoma | 49 (21.7%) |
| Adenosquamous | 6 (2.7%) | |
| Squamous | 171 (75.7%) | |
| Grade | 1 | 21 (9.3%) |
| 2 | 95 (42.0%) | |
| 3 | 91 (40.3%) | |
| missing | 19 (8.4%) | |
| LVSI | 98 (43.4%) | |
| Fertility sparing treatment | Conisation ST RT | 11 (4.9%) 4 (1.8%) 12 (5.3%) |
| Surgical approach | Open Laparoscopic | 196 (86.7%) 30 (13.3%) |
| Type of parametrectomy | A | 5 (2.2%) |
| B | 13 (5.8%) | |
| C | 2 (0.9%) | |
| C1 | 106 (46.9%) | |
| C2 | 82 (36.3%) | |
| missing | 18 (8.0%) | |
| SLNB | Bilateral Unilateral | 196 (86.7%) 30 (13.3%) |
| Pelvic lymphadenectomy | 212 (93.8%) | |
| Number of LN per patient | 36.0 (4.0; 59.0) | |
| Type of LN positivity | MAC | 14 (6.2%) |
| MIC | 16 (7.1%) | |
| ITC | 8 (3.5%) | |
| Negative | 188 (83.2%) | |
| Largest tumor size (US) 2 | 25.5 (3.4; 52.0) | |
| Largest tumor size (P) 3 | 26.0 (6.0; 65.0) | |
| Depth of stromal invasion (P) 3 | 15.0 (5.0; 25.0) | |
| Tumor volume (P) 3 | 4336.3 (113.1; 43,987.6) | |
| Adjuvant treatment | 37 (16.4%) | |
| Combined RT | 13 (5.8%) | |
| Chemoradiation | 24 (10.6%) | |
| Follow-up length (months) | 64.5 (7.0; 123.0) | |
| Time to recurrence (months) | 61.5 (6.4; 123.0) | |
| Recurrences | 22 (9.7%) | |
| Deaths | DOD DOC | 25 (9.7%) 18 (7.9%) 7 (3.1%) |
| SLN | Non SLN | Final LN Status | n (%) |
|---|---|---|---|
| Negative | Negative | Negative | 188 (83.2%) |
| Negative | Positive (MAC) | Positive | 2 (0.9%) |
| Positive MIC 1 | Positive (MAC) | Positive | 1 (0.4%) |
| Positive ITC 1 MIC 2 MAC 1 MAC 2 | Positive ITC 1 MIC 2 MIC 1 MAC 2 | Positive | 6 (2.7%) |
| Positive ITC 7 MIC 14 MAC 8 | Negative | Positive | 29 (12.8%) |
| No | Age | FST | Tumor Type | Stage pT | LN Status | LVSI | Largest Tumour Size | DSI | Type of Parametrectomy | Adjuvant Treatment | Disease Free Interval | Site of Recurrence | Current Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 33 | SCC | 1b1 | 0 | Yes | 20 | 1/3 | C1 | 0 | 71 | Comb | DOD | |
| 2 | 30 | ART | A | 1b1 | 0 | 0 | 23 | 1/3 | C1 | 0 | 7 | Pelvic | NED |
| 3 | 53 | A | 1b1 | ITC | 0 | 34 | 2/3 | C2 | 0 | 25 | Comb | DOD | |
| 4 | 65 | SCC | 1b1 | 0 | Yes | 22 | 1/3 | C1 | 0 | 28 | Pelvic | DOD | |
| 5 | 37 | ART | A | 1b1 | 0 | 0 | 20 | 1/3 | C1 | 0 | 8 | Pelvic | NED |
| 6 | 29 | ART | A | 1b1 | 0 | 0 | 10 | 1/3 | C1 | 0 | 22 | Pelvic | NED |
| 7 | 62 | SCC | 1b1 | 0 | Yes | 22 | 3/3 | C1 | CombRT | 69 | Distant | DOD | |
| 8 | 46 | AS | 1b1 | 0 | Yes | 36 | 2/3 | C2 | 0 | 4 | Comb | DOD | |
| 9 | 25 | SCC | 1b2 | MIC | Yes | 55 | 3/3 | C2 | CHRT | 16 | Pelvic | DOD | |
| 10 | 41 | AS | 1b2 | MIC | Yes | 45 | 3/3 | C2 | CHRT | 25 | Distant | DOD | |
| 11 | 35 | SCC | 1b1 | MAC | Yes | 45 | 3/3 | C2 | CHRT | 10 | Comb | DOD | |
| 12 | 20 | Cone | SCC | 1b1 | 0 | Yes | 22 | 1/3 | NA | 0 | 7 | Pelvic | DOD |
| 13 | 65 | SCC | 1b2 | MIC | Yes | 45 | 3/3 | C2 | CombRT | 21 | Distant | DOD | |
| 14 | 30 | SCC | 1b1 | 0 | 0 | 26 | 2/3 | C1 | 0 | 17 | Pelvic | NED | |
| 15 | 32 | Cone | A | 1b1 | 0 | 0 | 13 | 1/3 | NA | 0 | 11 | Comb | DOD |
| 16 | 43 | SCC | 1b1 | 0 | Yes | 23 | 1/3 | C2 | 0 | 14 | Comb | DOD | |
| 17 | 29 | Cone | SCC | 1b1 | 0 | 0 | 25 | 3/3 | NA | 0 | 6 | Pelvic | DOD |
| 18 | 42 | SCC | 1b1 | 0 | Yes | 30 | 2/3 | C2 | 0 | 7 | Comb | DOD | |
| 19 | 34 | SCC | 1b2 | MIC | Yes | 68 | 3/3 | C2 | CHRT | 3 | Comb | DOD | |
| 20 | 44 | A | 1b1 | MAC | Yes | 25 | 2/3 | C2 | CHRT | 6 | Distant | DOD | |
| 21 | 61 | SCC | 2b | MAC | Yes | 25 | 2/3 | C2 | CombRT | 2 | Comb | DOD | |
| 22 | 50 | SCC | 2b | MIC | Yes | 32 | 3/3 | C2 | CHRT | 26 | Comb | DOD |
| Predictor | Total n (n Recurrence) | HR (95% CI) | p-Value 1 | |
|---|---|---|---|---|
| Tumor type | Squamous | 171 (15) | ref. | |
| Adenocarcinoma | 49 (5) | 1.19 (0.43; 3.29) | 0.731 | |
| Adenosquamous | 6 (2) | 5.08 (1.15; 22.35) | 0.032 | |
| LVSI | No | 128 (7) | ref. | |
| Yes | 98 (15) | 2.95 (1.20; 7.23) | 0.018 | |
| Number of positive LN | 226 (22) | 1.50 (1.08; 2.09) | 0.015 | |
| LN positivity, variant A | No | 188 (13) | ref. | |
| Yes (Any type) | 38 (9) | 3.71 (1.59; 8.69) | 0.003 | |
| LN positivity, variant B | ITC, negative | 196 (14) | ref. | |
| MAC, MIC | 30 (8) | 4.03 (1.69; 9.62) | 0.002 | |
| LN positivity, variant C | Negative | 188 (13) | ref. | |
| ITC | 8 (1) | 1.96 (0.26; 14.97) | 0.518 | |
| MAC | 14 (3) | 3.61 (1.03; 12.69) | 0.046 | |
| MIC | 16 (5) | 4.62 (1.65; 12.95) | 0.004 | |
| Minimal TFD | 196 (18) | 0.87 (0.74; 1.03) | 0.116 | |
| TFD binarized 2 | > 3.45 | 65 (1) | ref. | |
| ≤ 3.45 | 131 (17) | 9.00 (1.20; 67.63) | 0.033 | |
| Tumor size binarized 2 | ≤ 33.5 | 151 (10) | ref. | |
| > 33.5 | 75 (12) | 2.56 (1.10; 5.94) | 0.029 | |
| Adjuvant treatment | No | 194 (14) | ref. | |
| Yes | 32 (8) | 3.46 (1.45; 8.25) | 0.005 | |
| Stage pT | 1a | 15 (0) | - | - |
| 1b1 | 157 (15) | ref. | ||
| ≥1b2 | 54 (7) | 1.37 (0.56; 3.36) | 0.491 | |
| Predictor | OR (95% IS) | p-Value | HR (95% IS) | p-Value 1 | |
|---|---|---|---|---|---|
| Area = 0.799; p < 0.001 | |||||
| Tumor type | Adenocarcinoma (ref. Squamous) | 1.36 (0.33; 5.69) | 0.670 | 1.24 (0.33; 4.61) | 0.749 |
| Adenosquamous (ref. Squamous) | 7.29 (0.86; 62.07) | 0.069 | 4.86 (1.00; 23.61) | 0.050 | |
| LVSI | Yes (ref. No) | 2.13 (0.59; 7.74) | 0.250 | 1.85 (0.56; 6.14) | 0.318 |
| Number of positive LN | 0.82 (0.37; 1.82) | 0.624 | 0.80 (0.39; 1.65) | 0.546 | |
| LN positivity | MAC, MIC (ref. ITC, negative) | 3.62 (0.46; 28.51) | 0.222 | 3.56 (0.54; 23.63) | 0.188 |
| TFD binarized 2 | ≤3.45 (ref. >3.45) | 5.27 (0.63; 43.92) | 0.125 | 5.25 (0.65; 42.34) | 0.119 |
| Tumor size binarized 2 | > 32.5 (ref. ≤ 32.5) | 0.64 (0.18; 2.25) | 0.486 | 0.72 (0.24; 2.17) | 0.554 |
| Adjuvant treatment | Yes (ref. No) | 2.45 (0.48; 12.39) | 0.279 | 1.78 (0.37; 8.57) | 0.472 |
| Author (year) | No. | Stage | Tumor Type | SLNB | Adj. Tx (%) | mF/U (m) | LN Positivity | Recurrence Rate | Reported Impact of MIC on the Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % N1 | %ITC | %MIC | %MAC | All | N0 | N1 * | ITC | MIC | MAC | ||||||||
| Juretzka (2004) | 49 | IA2-IB2 | SCC, A, AS, UD | no | 22 | 39 | 8% (4/49) | n/a | 8% (4/49) | n/a | 10% (5/49) | 6.7% (3/45) | 4% (2/49) | n/a | 50% (2/4) | n/a | ↑RecR |
| Marchiole (2005) | 52 (292) | IB1-IIB | SCC, A | no | 15 | 122 | n/a | 11.5% (6/52) | 23% (12/52) | n/a | 8,9% (26/292) | n/a | n/a | n/a | 21% (11/52) | n/a | ↑RecR (RR = 2.44 (95% CI 1.58-3.78)) |
| Fregnani (2006) | 289 | IB-IIA | SCC, A | no | 13 | 102 | 17% (48/289) | 2% (5/289) | 2% (6/289) | 13% (37/289) | 14.9% (43/289) | n/a | n/a | n/a | n/a | n/a | ↑RecR (RR N1mic = 3.2 (95% CI 1.1–9.6)), ↓5y DFS (88.7% N0, 50% N1mic) |
| Horn (2008) | 894 | IB-IIB | SCC, A | no | 31 | 82 | 31.4% (281/894) | n/a | 6,5% (59/894) | 23% (207/894) | 17.8% (135/894) | n/a | n/a | n/a | n/a | n/a | ↓5y DFS (91,4% for pN0, 69% pN1mic), ↓5y OS (86,6% pN0, 63,8% pN1mic) |
| Cibula (2012) | 645 | IA-IIB | SCC, A, AS | yes | 33 | 40 | 29.3% (189/645) | 4.5% (29/645) | 10.1% (65/645) | 14.7% (95/645) | n/a | n/a | n/a | n/a | n/a | n/a | ↓OS (HR = 6.86 (95% CI 2.09-22.61)), →DFS |
| Colturato (2015) | 83 | IB1-IIA | SCC, A, AS | no | 0 | 60 | n/a | 7% (6/83) # | n/a | 18% (15/83) | 18% (15/83) | n/a | 27% (4/15) # | n/a | ↑RecR (OR = 11.73 (95% CI 1.57-87.8)) | ||
| Stany (2015) | 129 | IA2-IB2 | SCC, A, AS | no | 23 | 70 | n/a | 20% (26/129) # | n/a | 8,5% (11/129) | n/a | n/a | 18% (2/11) # | n/a | →3y DFS | ||
| Guani (2019) | 139 | IA-IB1 | SCC, A, AS | yes | 19 | 36 | 15% (21/139) | 4.3% (6/139) | 5.7% (8/139) | 5.7% (8/139) | 9% (13/139) | 9% (11/118) | 10% (2/21) | 0% (0/6) | 14% (1/7) | 12.5% (1/8) | →3y DFS |
| This study (2020) | 226 | IA-IIB | SCC, A, AS | yes | 16 | 65 | 17% (38/226) | 3.5% (8/226) | 7% (16/226) | 6% (14/226) | 10% (22/226) | 7% (13/188) | 24% (9/38) | 12.5% (1/8) | 31% (5/16) | 21% (3/14) | ↓DFS (HR = 4.62 (95% CI 1.65-12.95)), ↓OS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocian, R.; Slama, J.; Fischerova, D.; Germanova, A.; Burgetova, A.; Dusek, L.; Dundr, P.; Nemejcova, K.; Jarkovsky, J.; Sebestova, S.; et al. Micrometastases in Sentinel Lymph Nodes Represent a Significant Negative Prognostic Factor in Early-Stage Cervical Cancer: A Single-Institutional Retrospective Cohort Study. Cancers 2020, 12, 1438. https://doi.org/10.3390/cancers12061438
Kocian R, Slama J, Fischerova D, Germanova A, Burgetova A, Dusek L, Dundr P, Nemejcova K, Jarkovsky J, Sebestova S, et al. Micrometastases in Sentinel Lymph Nodes Represent a Significant Negative Prognostic Factor in Early-Stage Cervical Cancer: A Single-Institutional Retrospective Cohort Study. Cancers. 2020; 12(6):1438. https://doi.org/10.3390/cancers12061438
Chicago/Turabian StyleKocian, Roman, Jiri Slama, Daniela Fischerova, Anna Germanova, Andrea Burgetova, Ladislav Dusek, Pavel Dundr, Kristyna Nemejcova, Jiri Jarkovsky, Silvie Sebestova, and et al. 2020. "Micrometastases in Sentinel Lymph Nodes Represent a Significant Negative Prognostic Factor in Early-Stage Cervical Cancer: A Single-Institutional Retrospective Cohort Study" Cancers 12, no. 6: 1438. https://doi.org/10.3390/cancers12061438
APA StyleKocian, R., Slama, J., Fischerova, D., Germanova, A., Burgetova, A., Dusek, L., Dundr, P., Nemejcova, K., Jarkovsky, J., Sebestova, S., Fruhauf, F., Dostalek, L., Ballaschova, T., & Cibula, D. (2020). Micrometastases in Sentinel Lymph Nodes Represent a Significant Negative Prognostic Factor in Early-Stage Cervical Cancer: A Single-Institutional Retrospective Cohort Study. Cancers, 12(6), 1438. https://doi.org/10.3390/cancers12061438





