A Novel Serum Metabolomic Profile for the Differential Diagnosis of Distal Cholangiocarcinoma and Pancreatic Ductal Adenocarcinoma
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Population
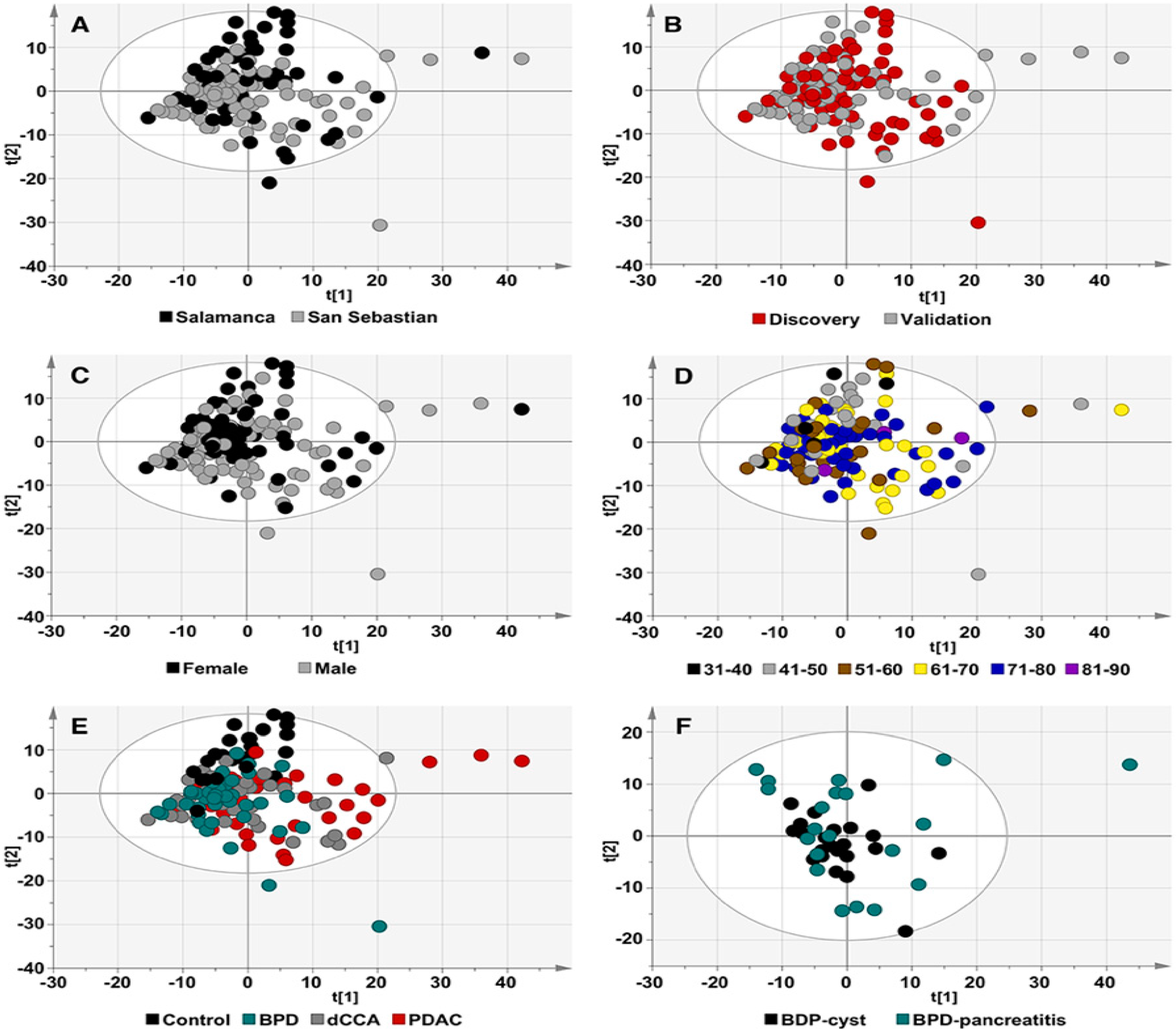
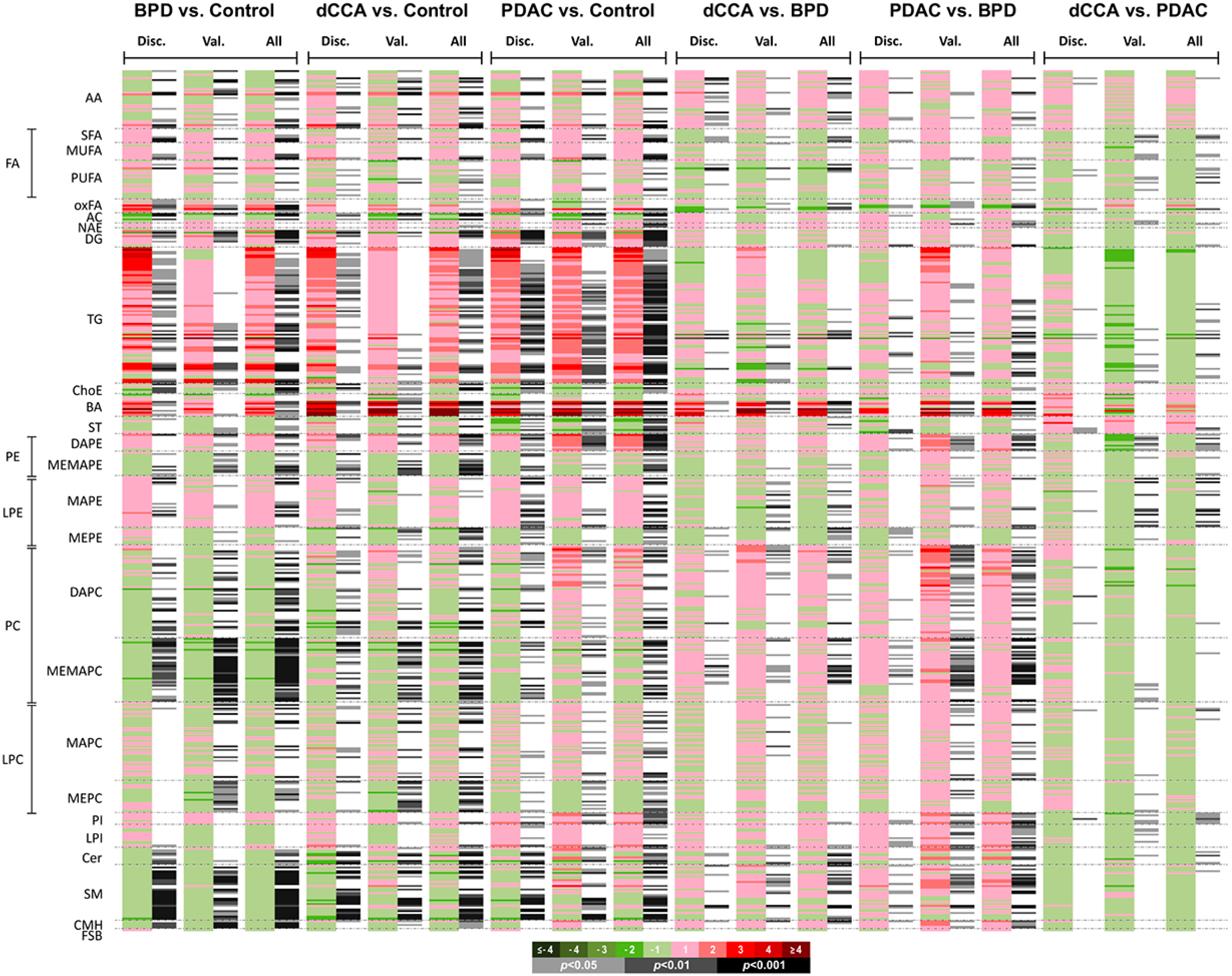
2.2. Serum Metabolomic Profiles of Patients with dCCA, PDAC and BPD and Healthy Individuals
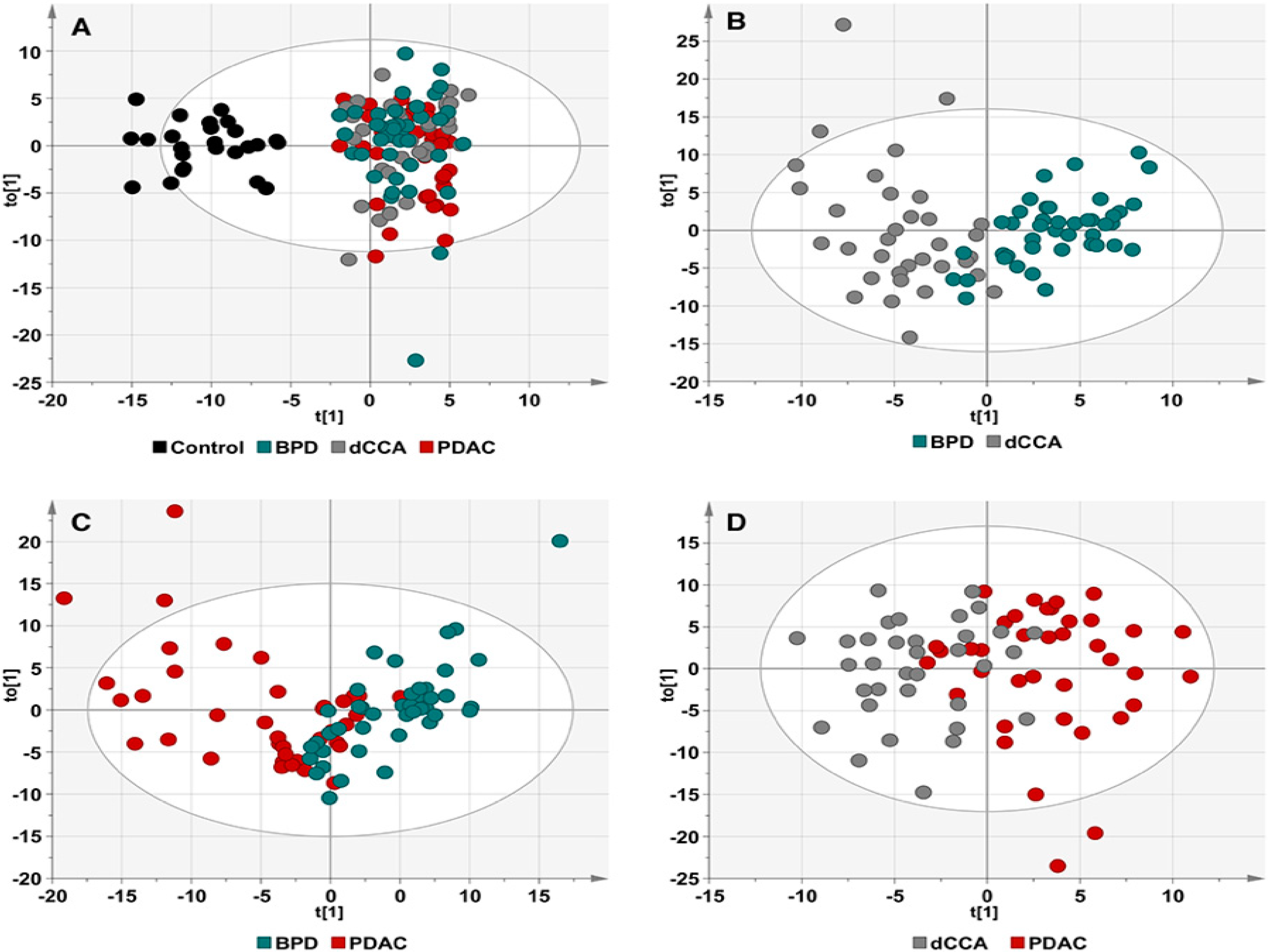
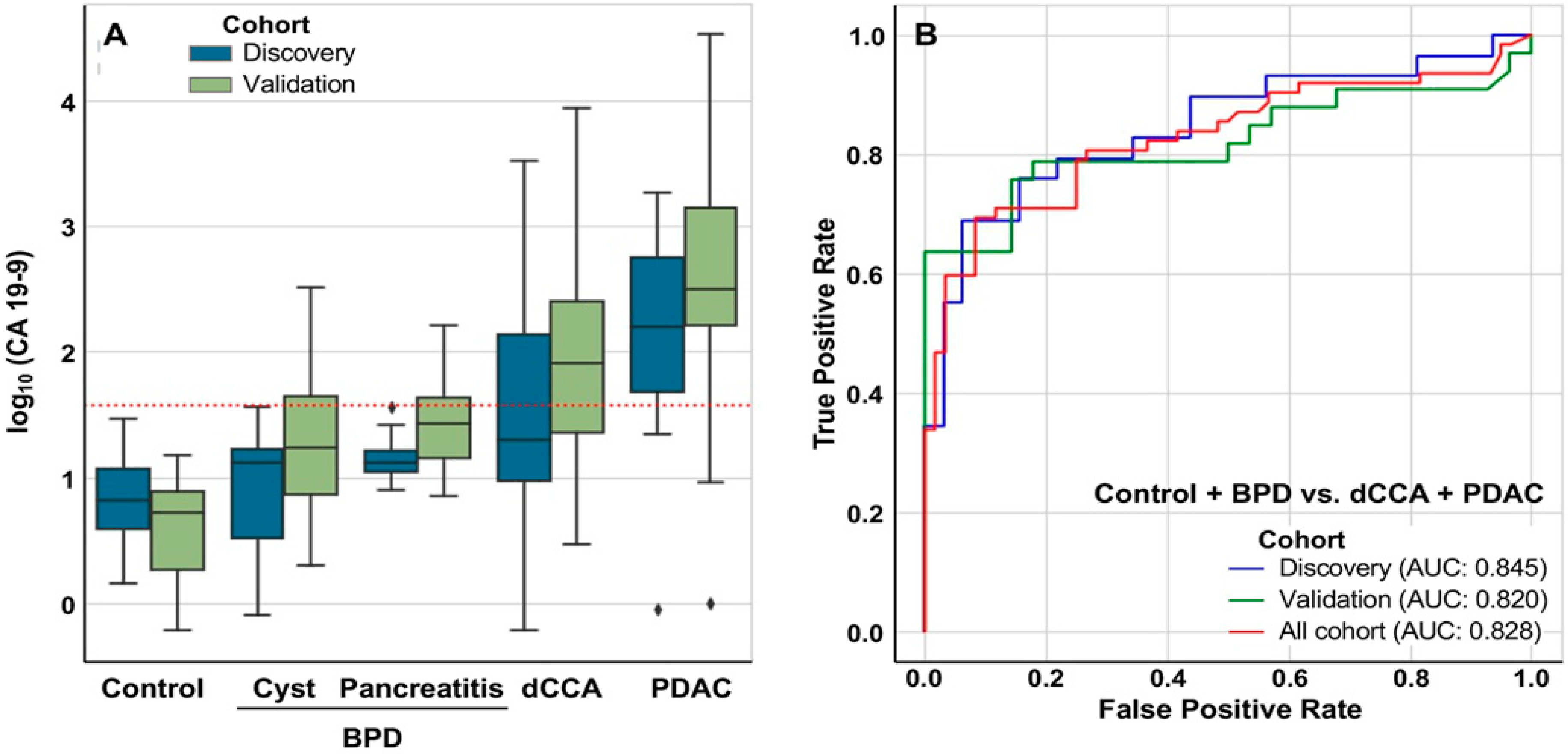
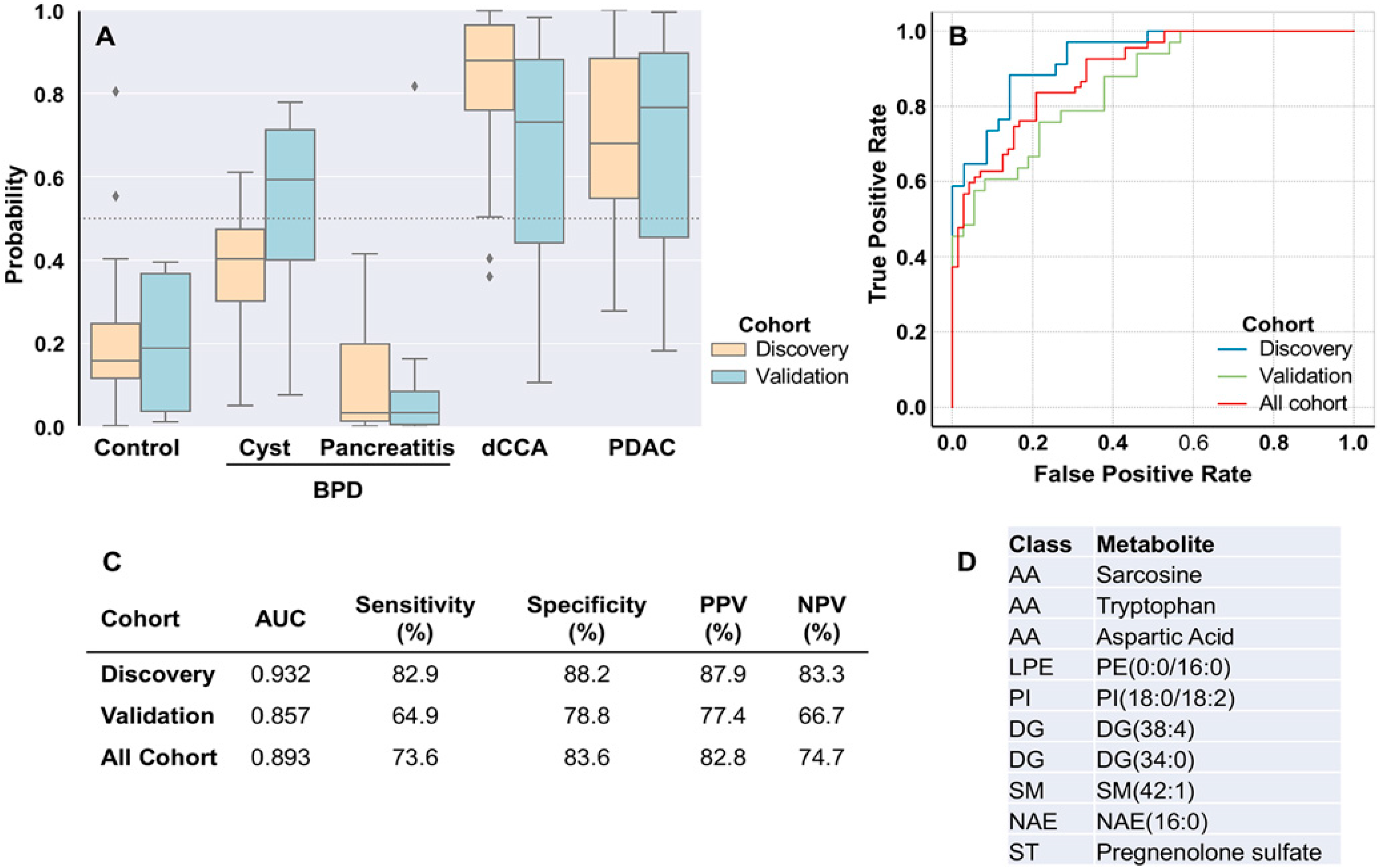
2.3. Discrimination between Patients with and without Tumors
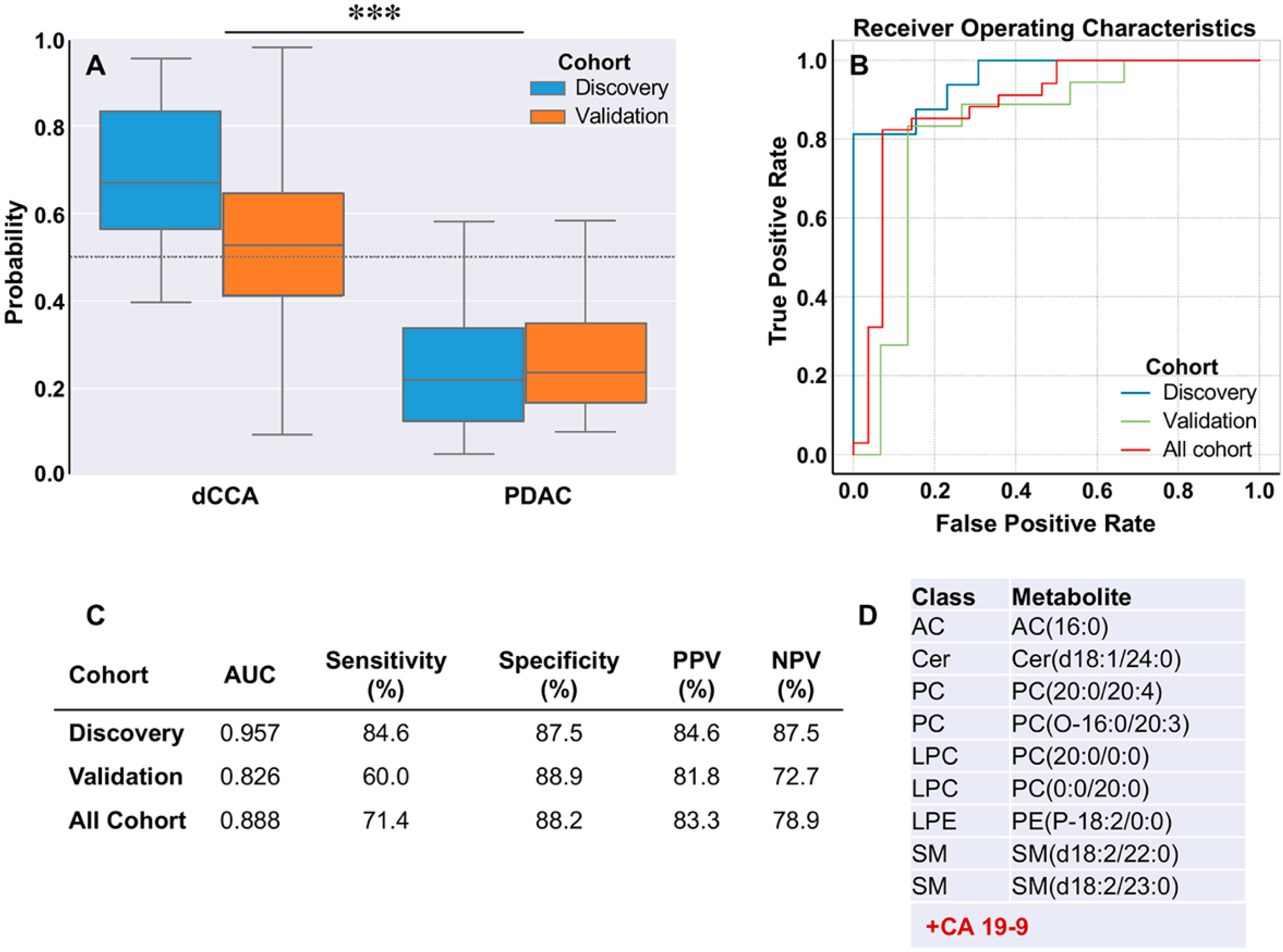
2.4. Discrimination between dCCA and PDAC
3. Discussion
4. Materials and Methods
4.1. Study Population and Eligibility
4.2. Metabolomic Analyses
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AUC | area under the receiver operating characteristic curve |
| BPD | benign pancreatic disease |
| CA 19-9 | carbohydrate antigen 19-9 |
| CCA | cholangiocarcinoma |
| dCCA | distal cholangiocarcinoma |
| OPLS | orthogonal partial least squares |
| PCA | principal component analysis |
| PDAC | pancreatic ductal adenocarcinoma |
| UHPLC-MS | ultra-high performance liquid chromatography coupled to mass spectrometry |
References
- Ethun, C.G.; Lopez-Aguiar, A.G.; Pawlik, T.M.; Poultsides, G.; Idrees, K.; Fields, R.C.; Weber, S.M.; Cho, C.; Martin, R.C.; Scoggins, C.R.; et al. Distal Cholangiocarcinoma and Pancreas Adenocarcinoma: Are They Really the Same Disease? A 13-Institution Study from the US Extrahepatic Biliary Malignancy Consortium and the Central Pancreas Consortium. J. Am. Coll. Surg. 2017, 224, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Marin, J.J.G.; Lozano, E.; Herraez, E.; Asensio, M.; Di Giacomo, S.; Romero, M.R.; Briz, O.; Serrano, M.A.; Efferth, T.; Macias, R.I.R. Chemoresistance and chemosensitization in cholangiocarcinoma. Biochim Biophys Acta Mol. Basis Dis. 2018, 1864, 1444–1453. [Google Scholar] [CrossRef]
- Forner, A.; Vidili, G.; Rengo, M.; Bujanda, L.; Ponz-Sarvise, M.; Lamarca, A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. 1), 98–107. [Google Scholar] [CrossRef]
- Fong, Z.V.; Winter, J.M. Biomarkers in pancreatic cancer: Diagnostic, prognostic, and predictive. Cancer J. 2012, 18, 530–538. [Google Scholar] [CrossRef]
- Macias, R.I.R.; Banales, J.M.; Sangro, B.; Muntane, J.; Avila, M.A.; Lozano, E.; Perugorria, M.J.; Padillo, F.J.; Bujanda, L.; Marin, J.J.G. The search for novel diagnostic and prognostic biomarkers in cholangiocarcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1468–1477. [Google Scholar] [CrossRef]
- Macias, R.I.R.; Kornek, M.; Rodrigues, P.M.; Paiva, N.A.; Castro, R.E.; Urban, S.; Pereira, S.P.; Cadamuro, M.; Rupp, C.; Loosen, S.H.; et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. 1), 108–122. [Google Scholar] [CrossRef]
- Boone, B.A.; Steve, J.; Zenati, M.S.; Hogg, M.E.; Singhi, A.D.; Bartlett, D.L.; Zureikat, A.H.; Bahary, N.; Zeh, H.J., 3rd. Serum CA 19-9 response to neoadjuvant therapy is associated with outcome in pancreatic adenocarcinoma. Ann. Surg. Oncol. 2014, 21, 4351–4358. [Google Scholar] [CrossRef]
- Tempero, M.A.; Uchida, E.; Takasaki, H.; Burnett, D.A.; Steplewski, Z.; Pour, P.M. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987, 47, 5501–5503. [Google Scholar] [PubMed]
- Frampton, A.E.; Giovannetti, E. Diagnosing pancreatic ductal adenocarcinoma using plasma extracellular vesicle RNA profiles. Gut 2020, 69, 404–405. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhong, L.; He, Q.; Wang, S.; Pan, Z.; Wang, T.; Zhao, Y. Diagnostic Accuracy of Serum CA19-9 in Patients with Cholangiocarcinoma: A Systematic Review and Meta-Analysis. Med. Sci. Monit. 2015, 21, 3555–3563. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Satriano, L.; Lewinska, M.; Rodrigues, P.M.; Banales, J.M.; Andersen, J.B. Metabolic rearrangements in primary liver cancers: Cause and consequences. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 748–766. [Google Scholar] [CrossRef]
- OuYang, D.; Xu, J.; Huang, H.; Chen, Z. Metabolomic profiling of serum from human pancreatic cancer patients using 1H NMR spectroscopy and principal component analysis. Appl. Biochem. Biotechnol. 2011, 165, 148–154. [Google Scholar] [CrossRef]
- Akita, H.; Ritchie, S.A.; Takemasa, I.; Eguchi, H.; Pastural, E.; Jin, W.; Yamazaki, Y.; Goodenowe, D.B.; Nagano, H.; Monden, M.; et al. Serum Metabolite Profiling for the Detection of Pancreatic Cancer: Results of a Large Independent Validation Study. Pancreas 2016, 45, 1418–1423. [Google Scholar] [CrossRef]
- Di Gangi, I.M.; Mazza, T.; Fontana, A.; Copetti, M.; Fusilli, C.; Ippolito, A.; Mattivi, F.; Latiano, A.; Andriulli, A.; Vrhovsek, U.; et al. Metabolomic profile in pancreatic cancer patients: A consensus-based approach to identify highly discriminating metabolites. Oncotarget 2016, 7, 5815–5829. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, H.; Zhang, T.; Jiang, Y.; Xing, H.; Zhang, H. Serum metabolomics uncovering specific metabolite signatures of intra- and extrahepatic cholangiocarcinoma. Mol. Biosyst. 2016, 12, 334–340. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kobayashi, T.; Chayahara, N.; Imamura, Y.; Toyoda, M.; Kiyota, N.; Mukohara, T.; Nishiumi, S.; Azuma, T.; Yoshida, M.; et al. Metabolomics evaluation of serum markers for cachexia and their intra-day variation in patients with advanced pancreatic cancer. PLoS ONE 2014, 9, e113259. [Google Scholar] [CrossRef]
- Itoi, T.; Sugimoto, M.; Umeda, J.; Sofuni, A.; Tsuchiya, T.; Tsuji, S.; Tanaka, R.; Tonozuka, R.; Honjo, M.; Moriyasu, F.; et al. Serum Metabolomic Profiles for Human Pancreatic Cancer Discrimination. Int. J. Mol. Sci. 2017, 18, 767. [Google Scholar] [CrossRef] [PubMed]
- Mayerle, J.; Kalthoff, H.; Reszka, R.; Kamlage, B.; Peter, E.; Schniewind, B.; Gonzalez Maldonado, S.; Pilarsky, C.; Heidecke, C.D.; Schatz, P.; et al. Metabolic biomarker signature to differentiate pancreatic ductal adenocarcinoma from chronic pancreatitis. Gut 2018, 67, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.J.; Macias, R.I.; Briz, O.; Banales, J.M.; Monte, M.J. Bile Acids in Physiology, Pathology and Pharmacology. Curr. Drug Metab. 2015, 17, 4–29. [Google Scholar] [CrossRef] [PubMed]
- Tonack, S.; Jenkinson, C.; Cox, T.; Elliott, V.; Jenkins, R.E.; Kitteringham, N.R.; Greenhalf, W.; Shaw, V.; Michalski, C.W.; Friess, H.; et al. iTRAQ reveals candidate pancreatic cancer serum biomarkers: Influence of obstructive jaundice on their performance. Br. J. Cancer 2013, 108, 1846–1853. [Google Scholar] [CrossRef]
- Lowenfels, A.B.; Maisonneuve, P.; Cavallini, G.; Ammann, R.W.; Lankisch, P.G.; Andersen, J.R.; Dimagno, E.P.; Andren-Sandberg, A.; Domellof, L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N. Engl. J. Med. 1993, 328, 1433–1437. [Google Scholar] [CrossRef]
- Gao, R.; Cheng, J.; Fan, C.; Shi, X.; Cao, Y.; Sun, B.; Ding, H.; Hu, C.; Dong, F.; Yan, X. Serum Metabolomics to Identify the Liver Disease-Specific Biomarkers for the Progression of Hepatitis to Hepatocellular Carcinoma. Sci. Rep. 2015, 5, 18175. [Google Scholar] [CrossRef]
- Banales, J.M.; Inarrairaegui, M.; Arbelaiz, A.; Milkiewicz, P.; Muntane, J.; Munoz-Bellvis, L.; La Casta, A.; Gonzalez, L.M.; Arretxe, E.; Alonso, C.; et al. Serum Metabolites as Diagnostic Biomarkers for Cholangiocarcinoma, Hepatocellular Carcinoma, and Primary Sclerosing Cholangitis. Hepatology 2019, 70, 547–562. [Google Scholar] [CrossRef]
- Eniu, D.T.; Romanciuc, F.; Moraru, C.; Goidescu, I.; Eniu, D.; Staicu, A.; Rachieriu, C.; Buiga, R.; Socaciu, C. The decrease of some serum free amino acids can predict breast cancer diagnosis and progression. Scand. J. Clin. Lab. Investig. 2019, 79, 17–24. [Google Scholar] [CrossRef]
- Kozar, N.; Kruusmaa, K.; Bitenc, M.; Argamasilla, R.; Adsuar, A.; Goswami, N.; Arko, D.; Takac, I. Metabolomic profiling suggests long chain ceramides and sphingomyelins as a possible diagnostic biomarker of epithelial ovarian cancer. Clin. Chim. Acta 2018, 481, 108–114. [Google Scholar] [CrossRef]
- Morad, S.A.; Cabot, M.C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer 2013, 13, 51–65. [Google Scholar] [CrossRef]
- Vila-Navarro, E.; Duran-Sanchon, S.; Vila-Casadesus, M.; Moreira, L.; Gines, A.; Cuatrecasas, M.; Lozano, J.J.; Bujanda, L.; Castells, A.; Gironella, M. Novel Circulating miRNA Signatures for Early Detection of Pancreatic Neoplasia. Clin. Transl. Gastroenterol. 2019, 10, e00029. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.A.; Dehlendorff, C.; Jensen, B.V.; Bjerregaard, J.K.; Nielsen, K.R.; Bojesen, S.E.; Calatayud, D.; Nielsen, S.E.; Yilmaz, M.; Hollander, N.H.; et al. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA 2014, 311, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Meijer, L.L.; Puik, J.R.; Le Large, T.Y.S.; Heger, M.; Dijk, F.; Funel, N.; Wurdinger, T.; Garajova, I.; van Grieken, N.C.T.; van de Wiel, M.A.; et al. Unravelling the Diagnostic Dilemma: A MicroRNA Panel of Circulating MiR-16 and MiR-877 as A Diagnostic Classifier for Distal Bile Duct Tumors. Cancers 2019, 11, 1181. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jang, C.Y.; Kim, S.A.; Park, S.B.; Jung, D.E.; Kim, B.O.; Kim, H.Y.; Chung, M.J.; Park, J.Y.; Bang, S.; et al. Combined use of CEMIP and CA 19-9 enhances diagnostic accuracy for pancreatic cancer. Sci. Rep. 2018, 8, 3383. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.; Caballeria, J.; Martinez-Arranz, I.; Dominguez-Diez, A.; Alonso, C.; Muntane, J.; Perez-Cormenzana, M.; Garcia-Monzon, C.; Mayo, R.; Martin-Duce, A.; et al. Obesity-dependent metabolic signatures associated with nonalcoholic fatty liver disease progression. J. Proteome Res. 2012, 11, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Arranz, I.; Mayo, R.; Perez-Cormenzana, M.; Minchole, I.; Salazar, L.; Alonso, C.; Mato, J.M. Enhancing metabolomics research through data mining. J. Proteom. 2015, 127, 275–288. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal Component Analysis; Springer: New York, NY, USA, 2002. [Google Scholar]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]

| Variable | Control | BPD | dCCA | PDAC | ||||
|---|---|---|---|---|---|---|---|---|
| Discovery (n = 12) | Validation (n = 13) | Discovery (n = 22) | Validation (n = 20) | Discovery (n = 16) | Validation (n = 18) | Discovery (n = 19) | Validation (n = 19) | |
| Age, mean ± SD | 53.7 ± 9.8 | 52.2 ± 8.6 | 60.9 ± 12.3 | 59.9 ± 11.6 | 70.9 ± 7.0 | 68.6 ± 10.9 | 68.5 ± 9.3 | 63.1 ± 9.4 |
| Males, n (%) | 5 (41.7) | 6 (46.1) | 12 (54.5) | 10 (50) | 12 (75) | 10 (55.6) | 12 (63.1) | 12 (63.1) |
| Tumor stage *, n (%) | ||||||||
| I | - | - | - | - | 0 (0) | 1 (5.6) | 1 (5.3) | 2 (10.5) |
| II | - | - | - | - | 13 (81.2) | 16 (88.8) | 7 (36.8) | 7 (36.8) |
| III | - | - | - | - | 1 (6.3) | 0 (0) | 2 (10.5) | 5 (26.3) |
| IV | - | - | - | - | 2 (12.5) | 1 (5.6) | 9 (47.4) | 5 (26.3) |
| Biochemistry, mean ± SD | ||||||||
| ALT (IU/L) | 21.3 ± 8.0 | 17.1 ± 6.7 | 38.9 ± 64.3 | 32.0 ± 27.0 | 107 ± 118 a,b | 38.5 ± 94.5 | 207 ± 203 a,b | 208 ± 318 a,b |
| GGT (IU/L) | 24.8 ± 20.4 | 19.0 ± 9.8 | 125 ± 206 a | 100 ± 142 a | 589 ± 592 a,b | 411 ± 816 a,b | 775 ± 1037 a,b | 784 ± 978 a,b |
| Alkaline phosphatase (IU/L) | 56.1.8 ± 15.4 | 58.5 ± 22.9 | 102 ± 115 | 94 ± 68 | 301 ± 220 a,b | 207 ± 153 a | 442 ± 427 a,b | 380 ± 359 a,b |
| Total bilirubin (mg/dL) | 0.5 ± 0.3 | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.8 ± 1.7 | 6.9 ± 7.5 a,b | 2.8 ± 4.9 a | 7.0 ± 7.6 a,b | 7.3 ± 6.2, a,b |
| CA 19-9 (IU/mL) | 5.4 ± 4.5 | 8.6 ±7.4 | 46.6 ± 74.9 a | 15.1 ± 10.5 | 893 ± 2405 a,b | 328 ± 855 a,b | 2983 ± 8024 a,b | 431 ± 561 a,b |
| BPD vs. Control | Metabolite | AUC | Sensitivity | Specificity | log2FC |
| Glutamic acid | 0.926 | 90 | 84 | 1.112 | |
| Tryptophan | 0.910 | 92 | 83 | −0.441 | |
| DG(34:0) | 0.910 | 84 | 86 | −1.731 | |
| PE(16:0/18:1) | 0.909 | 88 | 88 | 1.475 | |
| SM(32:1) | 0.909 | 96 | 74 | −0.722 | |
| AC(8:0) | 0.906 | 92 | 86 | −1.379 | |
| PC(O-16:0/18:2) | 0.903 | 92 | 71 | −1.087 | |
| Arachidic acid | 0.898 | 69 | 96 | 0.606 | |
| SM(d18:2/22:0) | 0.896 | 84 | 83 | −0.749 | |
| SM(38:1) | 0.889 | 96 | 76 | −0.612 | |
| dCCA vs. Control | Metabolite | AUC | Sensitivity | Specificity | log2FC |
| SM(d18:2/22:0) | 0.967 | 92 | 94 | −0.992 | |
| SM(d18:2/23:0) | 0.959 | 88 | 97 | −1.204 | |
| SM(39:1) | 0.958 | 96 | 91 | −1.052 | |
| Aspartic acid | 0.955 | 79 | 100 | 1.671 | |
| Glycocholic acid | 0.954 | 94 | 88 | 4.779 | |
| SM(38:1) | 0.951 | 96 | 94 | −0.750 | |
| SM(d18:1/23:0) | 0.929 | 80 | 94 | −0.900 | |
| SM(d18:1/22:0) | 0.928 | 92 | 88 | −0.810 | |
| SM(d18:2/20:0) | 0.921 | 84 | 88 | −0.581 | |
| Taurocholic acid | 0.919 | 76 | 100 | 8.035 | |
| PDAC vs. Control | Metabolite | AUC | Sensitivity | Specificity | log2FC |
| Glutamic acid | 0.937 | 92 | 88 | 1.570 | |
| Aspartic acid | 0.937 | 79 | 96 | 1.473 | |
| PE(16:0/18:1) | 0.919 | 82 | 88 | 2.295 | |
| SM(d18:2/22:0) | 0.919 | 92 | 89 | −0.810 | |
| SM(39:1) | 0.915 | 88 | 82 | −0.907 | |
| SM(d18:2/23:0) | 0.911 | 92 | 87 | −1.059 | |
| ChoE(18:3) | 0.907 | 76 | 92 | −1.178 | |
| AC(8:0) | 0.903 | 80 | 92 | −1.312 | |
| SM(38:1) | 0.899 | 96 | 76 | −0.603 | |
| PE(16:0/0:0) | 0.897 | 79 | 92 | 0.601 |
| dCCA vs. BPD | Metabolite | AUC | Sensitivity | Specificity | log2FC |
| SM(d18:1/23:1) | 0.858 | 79 | 81 | 0.839 | |
| Glycocholic acid | 0.834 | 94 | 62 | 2.579 | |
| Taurocholic acid | 0.823 | 73 | 83 | 4.096 | |
| PC(16:0/16:0) | 0.811 | 76 | 79 | 0.753 | |
| PC(31:0) | 0.805 | 71 | 81 | 1.233 | |
| TG(54:7) | 0.800 | 60 | 91 | −3.358 | |
| 18:3n-3 | 0.790 | 69 | 82 | −1.368 | |
| CMH(d18:1/16:0) | 0.788 | 82 | 71 | 0.720 | |
| Phenylalanine | 0.785 | 56 | 90 | 0.482 | |
| TG(54:6) | 0.783 | 51 | 100 | −2.154 | |
| PDAC vs. BPD | Metabolite | AUC | Sensitivity | Specificity | log2FC |
| PC(O-34:1) | 0.814 | 66 | 90 | 0.951 | |
| SM(d18:1/23:1) | 0.813 | 79 | 79 | 0.898 | |
| PC(P-16:0/16:0) | 0.795 | 74 | 74 | 0.730 | |
| PC(16:0/16:0) | 0.794 | 66 | 86 | 0.950 | |
| PC(31:0) | 0.794 | 68 | 81 | 1.376 | |
| PC(O-16:0/16:0) | 0.782 | 68 | 81 | 0.941 | |
| PC(O-38:5) | 0.782 | 58 | 93 | 0.446 | |
| PC(O-18:1/18:1) | 0.776 | 63 | 81 | 0.760 | |
| PC(O-22:1/20:4) | 0.768 | 74 | 71 | 0.677 | |
| SM(d18:0/15:0) | 0.766 | 55 | 93 | 1.058 | |
| dCCA vs. PDAC | Metabolite | AUC | Sensitivity | Specificity | log2FC |
| PE(18:0/0:0) | 0.769 | 82 | 71 | −0.367 | |
| PE(0:0/18:0) | 0.763 | 84 | 68 | −0.358 | |
| PE(16:0/0:0) | 0.742 | 74 | 68 | −0.356 | |
| PE(20:4/0:0) | 0.739 | 74 | 71 | −0.317 | |
| PE(0:0/20:4) | 0.735 | 76 | 71 | −0.302 | |
| PE(0:0/16:0) | 0.732 | 89 | 47 | −0.365 | |
| PE(38:5) | 0.719 | 95 | 44 | −0.844 | |
| TG(52:5) | 0.705 | 71 | 68 | −0.620 | |
| PE 20:4 | 0.704 | 95 | 38 | −0.520 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macias, R.I.R.; Muñoz-Bellvís, L.; Sánchez-Martín, A.; Arretxe, E.; Martínez-Arranz, I.; Lapitz, A.; Gutiérrez, M.L.; La Casta, A.; Alonso, C.; González, L.M.; et al. A Novel Serum Metabolomic Profile for the Differential Diagnosis of Distal Cholangiocarcinoma and Pancreatic Ductal Adenocarcinoma. Cancers 2020, 12, 1433. https://doi.org/10.3390/cancers12061433
Macias RIR, Muñoz-Bellvís L, Sánchez-Martín A, Arretxe E, Martínez-Arranz I, Lapitz A, Gutiérrez ML, La Casta A, Alonso C, González LM, et al. A Novel Serum Metabolomic Profile for the Differential Diagnosis of Distal Cholangiocarcinoma and Pancreatic Ductal Adenocarcinoma. Cancers. 2020; 12(6):1433. https://doi.org/10.3390/cancers12061433
Chicago/Turabian StyleMacias, Rocio I. R., Luis Muñoz-Bellvís, Anabel Sánchez-Martín, Enara Arretxe, Ibon Martínez-Arranz, Ainhoa Lapitz, M. Laura Gutiérrez, Adelaida La Casta, Cristina Alonso, Luis M. González, and et al. 2020. "A Novel Serum Metabolomic Profile for the Differential Diagnosis of Distal Cholangiocarcinoma and Pancreatic Ductal Adenocarcinoma" Cancers 12, no. 6: 1433. https://doi.org/10.3390/cancers12061433
APA StyleMacias, R. I. R., Muñoz-Bellvís, L., Sánchez-Martín, A., Arretxe, E., Martínez-Arranz, I., Lapitz, A., Gutiérrez, M. L., La Casta, A., Alonso, C., González, L. M., Avila, M. A., Martinez-Chantar, M. L., Castro, R. E., Bujanda, L., Banales, J. M., & Marin, J. J. G. (2020). A Novel Serum Metabolomic Profile for the Differential Diagnosis of Distal Cholangiocarcinoma and Pancreatic Ductal Adenocarcinoma. Cancers, 12(6), 1433. https://doi.org/10.3390/cancers12061433











