Efficacy and Safety of the Combination of Pravastatin and Sorafenib for the Treatment of Advanced Hepatocellular Carcinoma (ESTAHEP Clinical Trial)
Abstract
1. Introduction
2. Results
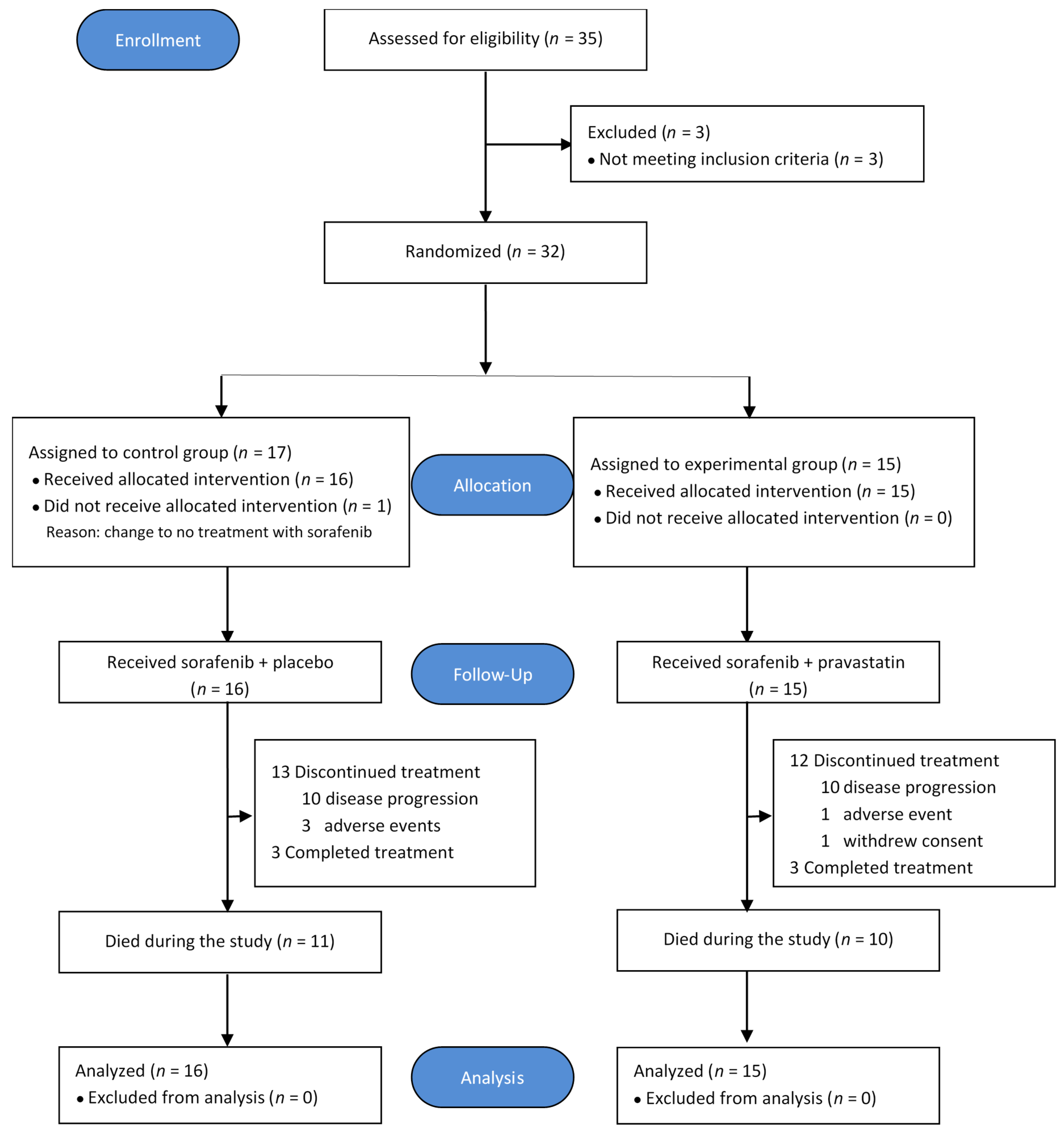
2.1. Patient Characteristics
2.2. Treatment
2.3. Efficacy
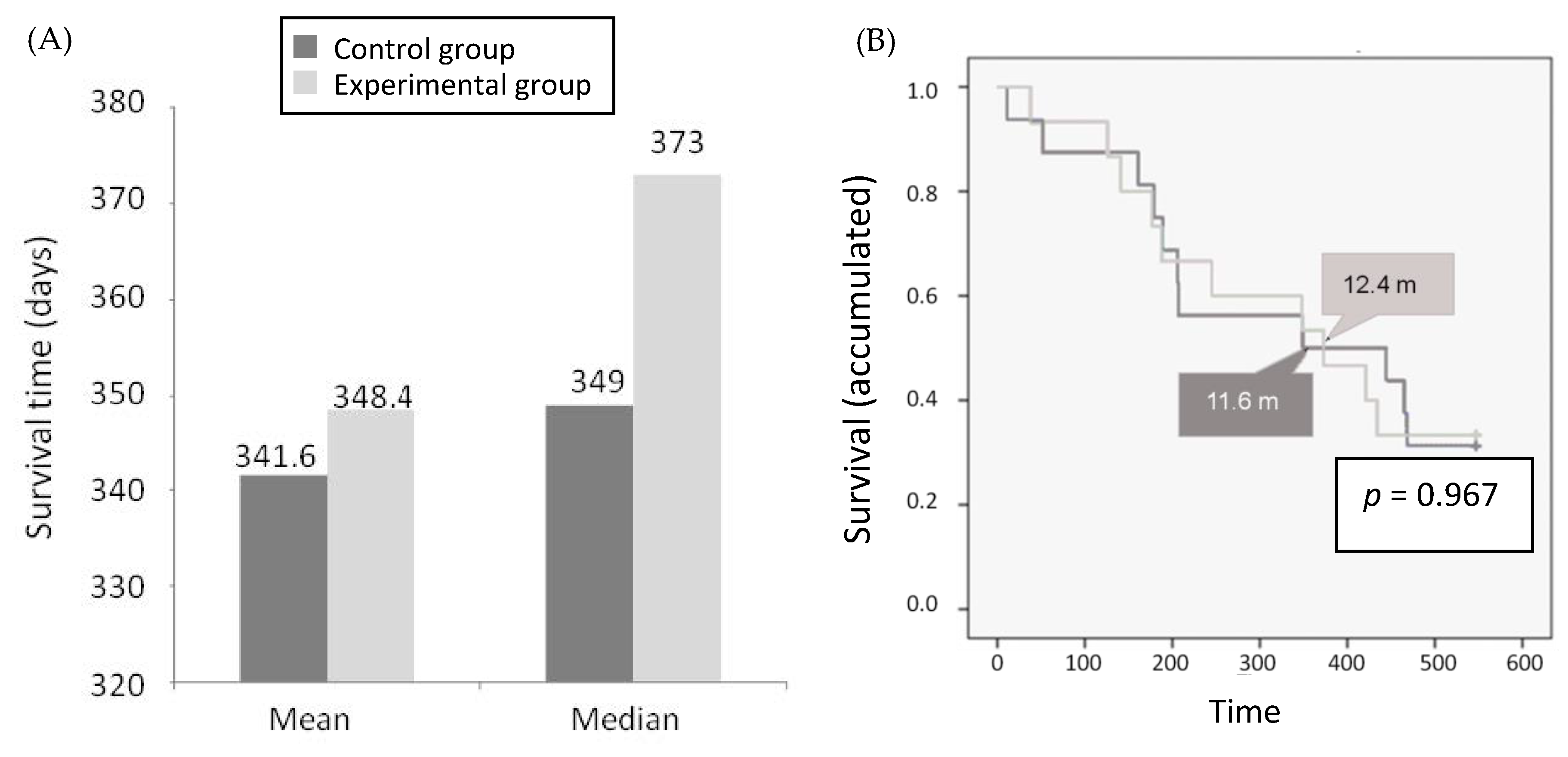
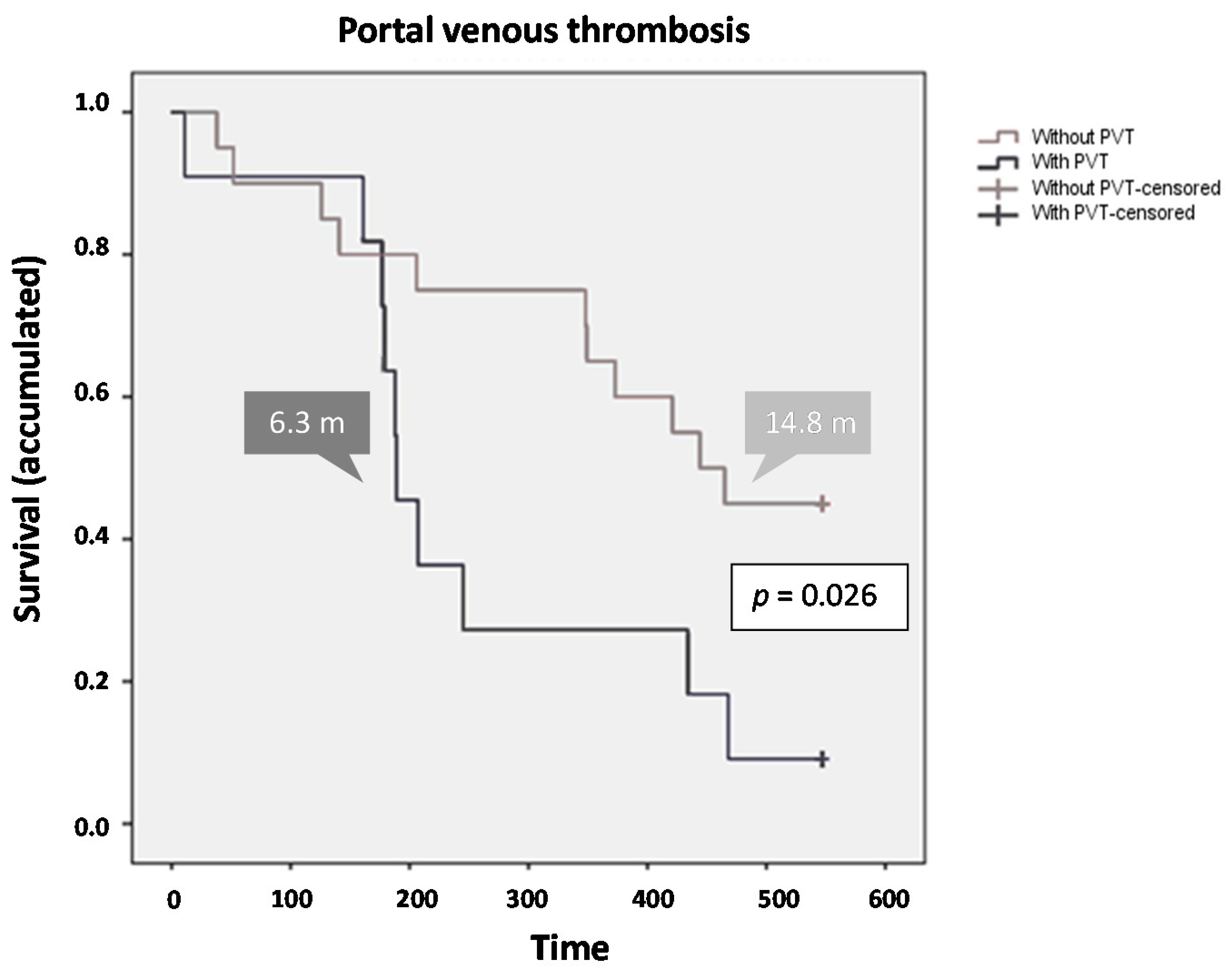
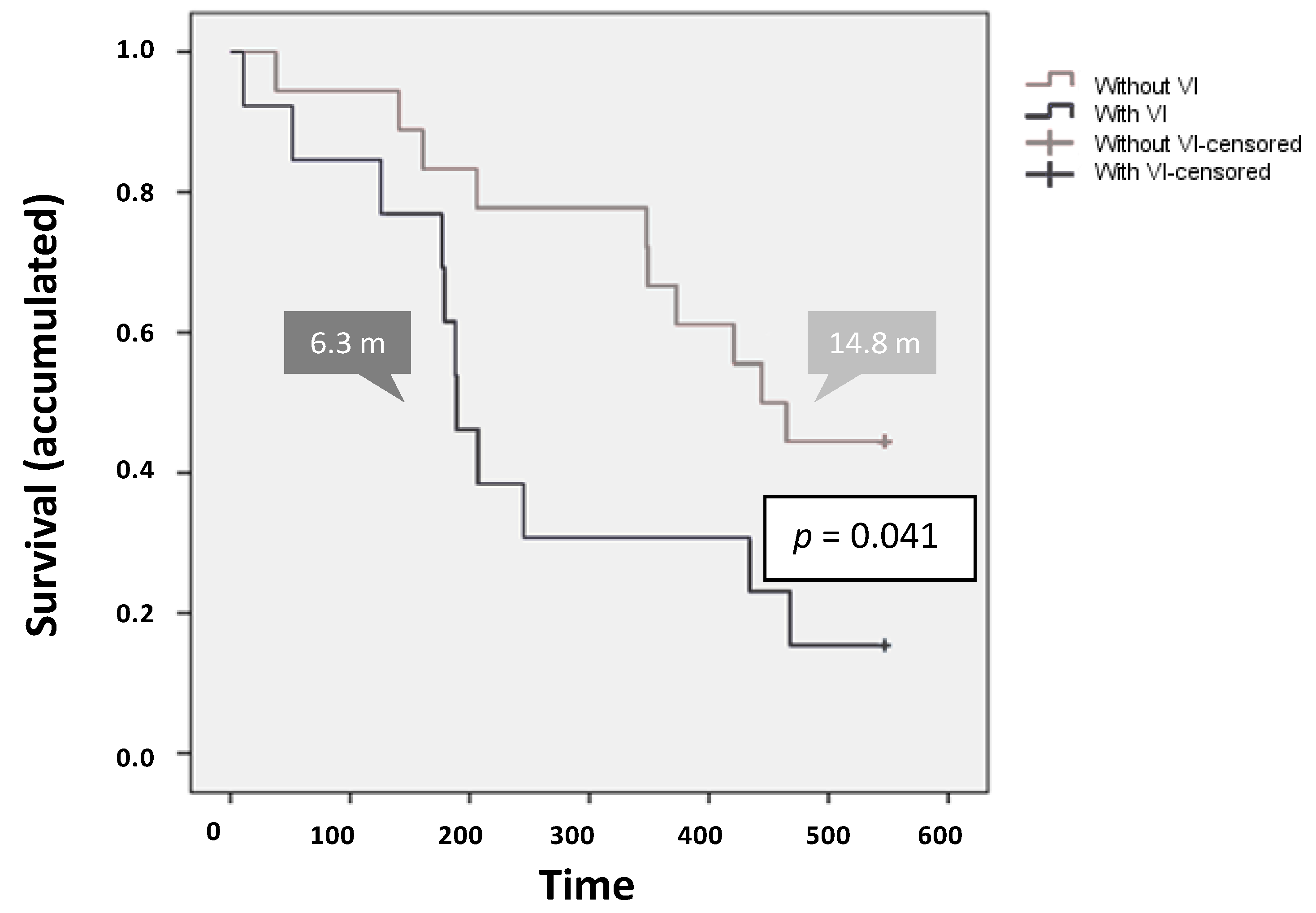
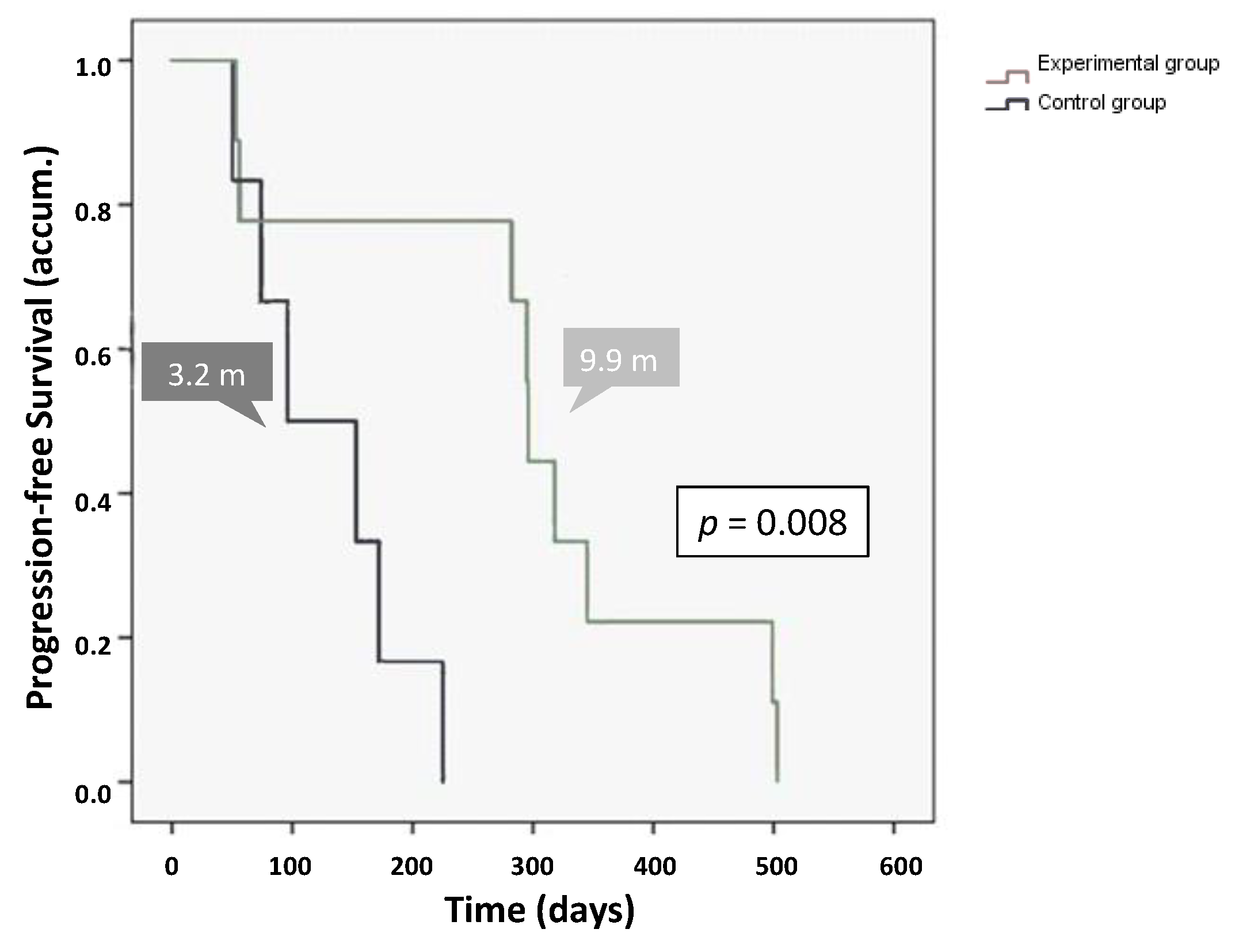
2.3.1. Overall Survival
2.3.2. Tumor Response
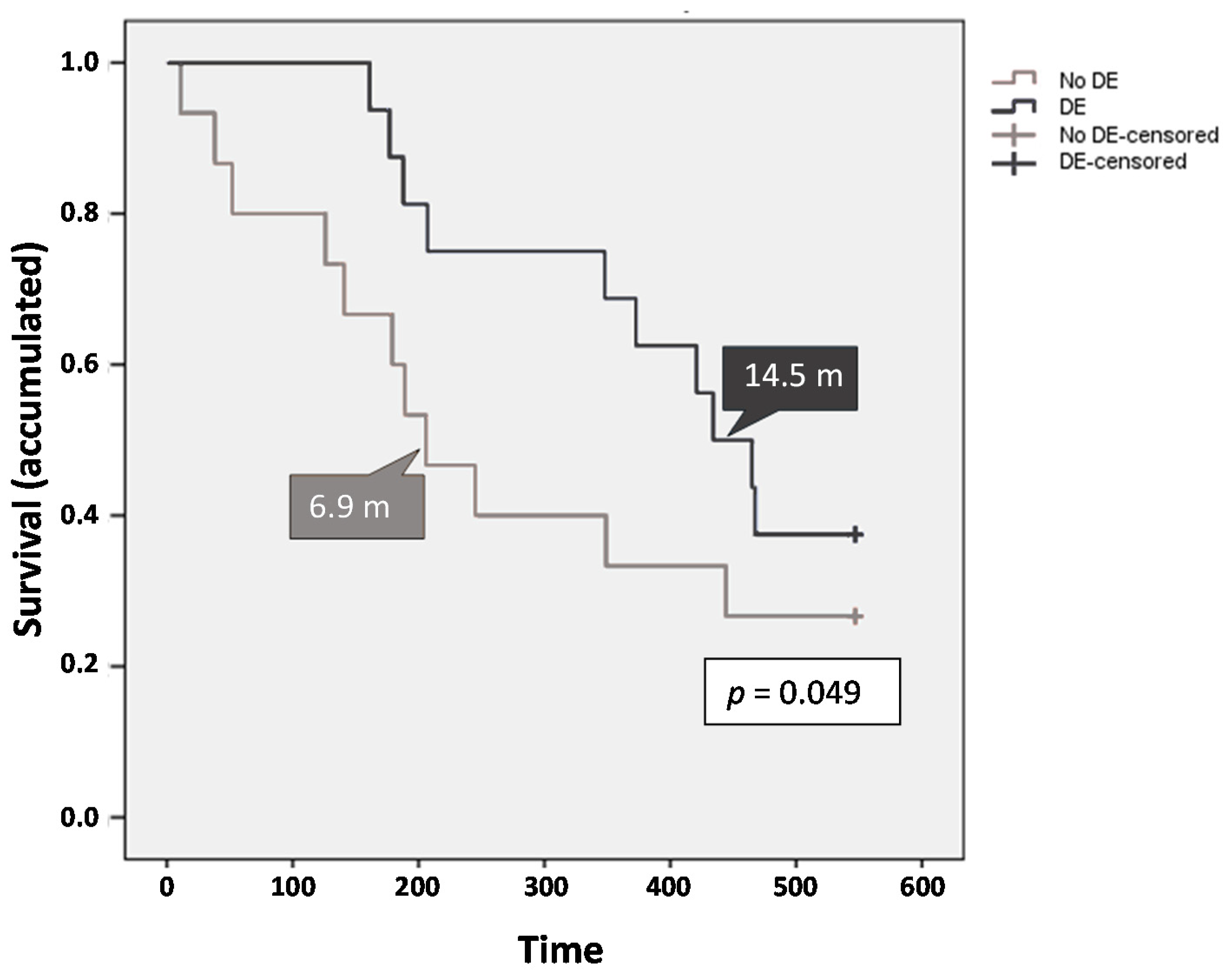
2.4. Safety
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Trial Design and Treatment Allocation
4.3. Procedures
4.4. Outcomes
4.5. Statistical Analysis
4.6. Ethical Consideration and Registration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baecker, A.; Liu, X.; La Vecchia, C.; Zhang, Z.F. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur. J. Cancer Prev. 2018, 27, 205–212. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 871–873. [Google Scholar] [CrossRef] [PubMed]
- WHO. Projections of Mortality and Causes of Death, 2016 to 2060. Available online: http://www.who.int/healthinfo/global_burden_disease/projections/en/ (accessed on 8 May 2020).
- European Association for the Study of the Liver. Electronic address, e.e.e.; European Association for the Study of the, L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Saffo, S.; Taddei, T.H. Systemic Management for Advanced Hepatocellular Carcinoma: A Review of the Molecular Pathways of Carcinogenesis, Current and Emerging Therapies, and Novel Treatment Strategies. Dig. Dis. Sci. 2019, 64, 1016–1029. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Rimassa, L.; Pressiani, T.; Merle, P. Systemic Treatment Options in Hepatocellular Carcinoma. Liver Cancer 2019, 8, 427–446. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Kim, G.; Kang, E.S. Prevention of Hepatocellular Carcinoma by Statins: Clinical Evidence and Plausible Mechanisms. Semin. Liver Dis. 2019, 39, 141–152. [Google Scholar] [CrossRef]
- Mullen, P.J.; Yu, R.; Longo, J.; Archer, M.C.; Penn, L.Z. The interplay between cell signalling and the mevalonate pathway in cancer. Nat. Rev. Cancer 2016, 16, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Kruzliak, P.; Rotrekl, V.; Jelinkova, S.; Mladosievicova, B. Statins in oncological research: From experimental studies to clinical practice. Crit. Rev. Oncol. Hematol. 2014, 92, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; Natarajan, Y.; Liu, Y.; El-Serag, H.B. Statin Use After Diagnosis of Hepatocellular Carcinoma Is Associated With Decreased Mortality. Clin. Gastroenterol. Hepatol. 2019, 17, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P.P.; Singh, A.G.; Murad, M.H.; Sanchez, W. Statins are associated with a reduced risk of hepatocellular cancer: A systematic review and meta-analysis. Gastroenterology 2013, 144, 323–332. [Google Scholar] [CrossRef]
- Tsan, Y.T.; Lee, C.H.; Ho, W.C.; Lin, M.H.; Wang, J.D.; Chen, P.C. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J. Clin. Oncol. 2013, 31, 1514–1521. [Google Scholar] [CrossRef]
- Hijona, E.; Banales, J.M.; Hijona, L.; Medina, J.F.; Arenas, J.; Herreros-Villanueva, M.; Aldazabal, P.; Bujanda, L. Pravastatin inhibits cell proliferation and increased MAT1A expression in hepatocarcinoma cells and in vivo models. Cancer Cell Int. 2012, 12, 5. [Google Scholar] [CrossRef]
- Sutter, A.P.; Maaser, K.; Höpfner, M.; Huether, A.; Schuppan, D.; Scherübl, H. Cell cycle arrest and apoptosis induction in hepatocellular carcinoma cells by HMG-CoA reductase inhibitors. Synergistic antiproliferative action with ligands of the peripheral benzodiazepine receptor. J. Hepatol. 2005, 43, 808–816. [Google Scholar] [CrossRef]
- Kawata, S.; Yamasaki, E.; Nagase, T.; Inui, Y.; Ito, N.; Matsuda, Y.; Inada, M.; Tamura, S.; Noda, S.; Imai, Y.; et al. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br. J. Cancer 2001, 84, 886–891. [Google Scholar] [CrossRef]
- Lersch, C.; Schmelz, R.; Erdmann, J.; Hollweck, R.; Schulte-Frohlinde, E.; Eckel, F.; Nader, M.; Schusdziarra, V. Treatment of HCC with pravastatin, octreotide, or gemcitabine--a critical evaluation. Hepatogastroenterology 2004, 51, 1099–1103. [Google Scholar] [PubMed]
- Graf, H.; Jüngst, C.; Straub, G.; Dogan, S.; Hoffmann, R.; Jakobs, T.; Reiser, M.; Waggershauser, T.; Helmberger, T.; Walter, A.; et al. Chemoembolization Combined with Pravastatin Improves Survival in Patients with Hepatocellular Carcinoma. Digestion 2008, 78, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Jouve, J.L.; Lecomte, T.; Bouche, O.; Barbier, E.; Khemissa Akouz, F.; Riachi, G.; Nguyen Khac, E.; Ollivier-Hourmand, I.; Debette-Gratien, M.; Faroux, R.; et al. Pravastatin combination with sorafenib does not improve survival in advanced hepatocellular carcinoma. J. Hepatol. 2019, 71, 516–522. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Bil, J.; Zapala, L.; Nowis, D.; Jakobisiak, M.; Golab, J. Statins potentiate cytostatic/cytotoxic activity of sorafenib but not sunitinib against tumor cell lines in vitro. Cancer Lett. 2010, 288, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Blanc, J.F.; Khemissa, F.; Bronowicki, J.P.; Monterymard, C.; Perarnau, J.M.; Bourgeois, V.; Obled, S.; Abdelghani, M.B.; Mabile-Archambeaud, I.; Faroux, R.; et al. Results of the Phase II randomized French trial PRODIGE 21 comparing sorafenib vs pravastatin vs sorafenib and pravastatin vs best supportive care for the palliative treatment of HCC in CHILD B cirrhotic patients. J. Hepatol. 2018, 68, S195. [Google Scholar] [CrossRef]
- Solms, A.; Reinecke, I.; Fiala-Buskies, S.; Keunecke, A.; Drenth, H.J.; Bruix, J.; Meinhardt, G.; Cleton, A.; Ploeger, B. Exposure-response relationship of regorafenib efficacy in patients with hepatocellular carcinoma. Eur. J. Pharm. Sci. 2017, 109S, S149–S153. [Google Scholar] [CrossRef]
- Bosch, J.; Gracia-Sancho, J.; Abraldes, J.G. Cirrhosis as new indication for statins. Guthrie 2020, 69, 953–962. [Google Scholar] [CrossRef]
- Kim, G.; Jang, S.Y.; Nam, C.M.; Kang, E.S. Statin use and the risk of hepatocellular carcinoma in patients at high risk: A nationwide nested case-control study. J. Hepatol. 2018, 68, 476–484. [Google Scholar] [CrossRef]
- Simon, T.G.; Bonilla, H.; Yan, P.; Chung, R.T.; Butt, A.A. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: Results from ERCHIVES. Hepatology 2016, 64, 47–57. [Google Scholar] [CrossRef]
- Weis, M.; Heeschen, C.; Glassford, A.J.; Cooke, J.P. Statins have biphasic effects on angiogenesis. Circulation 2002, 105, 739–745. [Google Scholar] [CrossRef]
- Ye, S.L.; Chen, X.; Yang, J.; Bie, P.; Zhang, S.; Liu, F.; Liu, L.; Zhou, J.; Dou, K.; Yip, C.S.; et al. Evaluation of sorafenib in Chinese unresectable hepatocellular carcinoma patients with prior surgery and portal vein tumor thrombosis: A subset analysis of GIDEON study data. Tumour Biol. 2017, 39, 1010428317695030. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, J.; Ai, D.L.; Fu, J.L.; Peng, X.M.; Zhang, L.Z.; Wang, J.Y.; Zhao, Y.; Yang, B.; Yu, Q.; et al. Enhanced therapeutic efficacy of combined use of sorafenib and transcatheter arterial chemoembolization for treatment of advanced hepatocellular carcinoma. Jpn. J. Clin. Oncol. 2014, 44, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Cerban, R.; Ester, C.; Iacob, S.; Grasu, M.; Paslaru, L.; Dumitru, R.; Lupescu, I.; Constantin, G.; Croitoru, A.; Gheorghe, L. Predictive Factors of Tumor Recurrence and Survival in Patients with Hepatocellular Carcinoma treated with Transarterial Chemoembolization. J. Gastrointest. Liver Dis. 2018, 27, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Branco, F.; Alencar, R.S.; Volt, F.; Sartori, G.; Dode, A.; Kikuchi, L.; Tani, C.M.; Chagas, A.L.; Pfiffer, T.; Hoff, P.; et al. The Impact of Early Dermatologic Events in the Survival of Patients with Hepatocellular Carcinoma Treated with Sorafenib. Ann. Hepatol. 2017, 16, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Torres, F.; Rodriguez-Lope, C.; Forner, A.; LLarch, N.; Rimola, J.; Darnell, A.; Rios, J.; Ayuso, C.; Bruix, J. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J. Hepatol. 2014, 61, 318–324. [Google Scholar] [CrossRef]
- Rimola, J.; Diaz-Gonzalez, A.; Darnell, A.; Varela, M.; Pons, F.; Hernandez-Guerra, M.; Delgado, M.; Castroagudin, J.; Matilla, A.; Sangro, B.; et al. Complete response under sorafenib in patients with hepatocellular carcinoma: Relationship with dermatologic adverse events. Hepatology 2018, 67, 612–622. [Google Scholar] [CrossRef]
| Characteristic | Control Group (Sorafenib + Placebo) (n = 16) | Experimental Group (Sorafenib + Pravastatin) (n = 15) | Total (n = 31) |
|---|---|---|---|
| Age (years) | 59.94 ± 12.57 | 63.00 ± 10.39 | 61.42 ± 11.48 |
| Sex (M/F) | 14 (87.5%)/2 (12.5%) | 15 (100%)/0 (0%) | 29 (93.5%)/2 (6.5%) |
| Albumin (g/L) | 37.32 ± 8.38 | 40.33 ± 4.59 | 38.83 ± 6.82 |
| Prothrombin activity (INR) | 1.24 ± 0.39 | 1.07 ± 0.10 | 1.16 ± 0.30 |
| Platelets (103/μL) | 180.62 ± 81.38 | 127.53 ± 64.90 | 154.93 ± 77.49 |
| Total bilirubin ≤1.2 mg/dL >1.2 mg/dL Missing | 1.07 ± 0.59 11 (68.8%) 4 (25.0%) 1 (6.2%) | 1.24 ± 0.70 8 (53.3%) 7 (46.7%) 0 (0.0%) | 1.16 ± 0.64 19 (61.3%) 11 (35.5%) 1 (3.2%) |
| Serum AFP ≤100 IU/mL >100 IU/mL Missing | 2710.18 ± 4093.59 6 (37.5%) 8 (50.0%) 2 (12.5%) | 1315.08 ± 3584.00 10 (66.7%) 4 (26.7%) 1 (6.6%) | 2012.63 ± 3841.57 16 (51.6%) 12 (38.7%) 3 (9.7%) |
| Child-Pugh classification: Stage A Stage B | 13 (81.2%) 3 (18.8%) | 15 (100.0%) 0 (0.0%) | 28 (90.3%) 3 (9.7%) |
| ECOG-PS: Grade 0 Grade 1 | 12 (75.0%) 4 (25.0%) | 12 (80.0%) 3 (20.0%) | 24 (77.4%) 7 (22.6%) |
| Portal thrombosis (Yes/No) | 6 (37.5%)/10 (62.5%) | 5 (33.3%)/10 (66.7%) | 11 (35.48%)/20 (64.5%) |
| Vascular invasion (Yes/No) | 8 (50.0%)/8 (50.0%) | 5 (33.3%)/10 (66.7%) | 13 (41.9%)/18 (58.1%) |
| Extrahepatic metastases (Yes/No) | 6 (37.5%)/10 (62.5%) | 6 (40.0%)/9 (60.0%) | 12 (38.7%)/19 (61.3%) |
| Adverse Event | Total, n (%) | Control Group n (%) | Experimental Group n (%) |
|---|---|---|---|
| Total AE incidence | 182 | 84 (46.2%) | 98 (53.8%) |
| Total SAE incidence | 19 (10.4%) | 12 (63.2%) | 7 (36.8%) |
| Gastrointestinal disorders | 66 (36.3%) | 39 (59.1%) | 27 (40.9%) |
| Diarrhea | 20 | 11 (55.0%) | 9 (45.0%) |
| Abdominal pain | 14 | 6 (42.9%) | 8 (57.1%) |
| Anorexia/Hyporexia | 9 | 3 (33.3%) | 6 (66.7%) |
| Ascitis | 8 | 6 (75.0%) | 2 (25.0%) |
| Gastrointestinal hemorrhage | 7 | 7 (100.0%) | 0 (0.0%) |
| General disorders | 33 (18.1%) | 15 (45.4%) | 18 (54.6%) |
| Asthenia | 20 | 11 (55.0%) | 9 (45.0%) |
| Weight loss | 8 | 2 (25.0%) | 6 (75.0%) |
| Skin and subcutaneous tissue disorders | 30 (16.5%) | 11 (36.7%) | 19 (63.3%) |
| Hand-foot syndrome | 9 | 5 (55.6%) | 4 (44.4%) |
| Rash | 8 | 1 (12.5%) | 7 (87.5%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaño, I.; Martín, L.; Varela, M.; Serrano, T.; Núñez, O.; Mínguez, B.; Rodrigues, P.M.; Perugorria, M.J.; Banales, J.M.; Arenas, J.I. Efficacy and Safety of the Combination of Pravastatin and Sorafenib for the Treatment of Advanced Hepatocellular Carcinoma (ESTAHEP Clinical Trial). Cancers 2020, 12, 1900. https://doi.org/10.3390/cancers12071900
Riaño I, Martín L, Varela M, Serrano T, Núñez O, Mínguez B, Rodrigues PM, Perugorria MJ, Banales JM, Arenas JI. Efficacy and Safety of the Combination of Pravastatin and Sorafenib for the Treatment of Advanced Hepatocellular Carcinoma (ESTAHEP Clinical Trial). Cancers. 2020; 12(7):1900. https://doi.org/10.3390/cancers12071900
Chicago/Turabian StyleRiaño, Ioana, Leticia Martín, Maria Varela, Trinidad Serrano, Oscar Núñez, Beatriz Mínguez, Pedro M. Rodrigues, Maria J. Perugorria, Jesus M. Banales, and Juan I. Arenas. 2020. "Efficacy and Safety of the Combination of Pravastatin and Sorafenib for the Treatment of Advanced Hepatocellular Carcinoma (ESTAHEP Clinical Trial)" Cancers 12, no. 7: 1900. https://doi.org/10.3390/cancers12071900
APA StyleRiaño, I., Martín, L., Varela, M., Serrano, T., Núñez, O., Mínguez, B., Rodrigues, P. M., Perugorria, M. J., Banales, J. M., & Arenas, J. I. (2020). Efficacy and Safety of the Combination of Pravastatin and Sorafenib for the Treatment of Advanced Hepatocellular Carcinoma (ESTAHEP Clinical Trial). Cancers, 12(7), 1900. https://doi.org/10.3390/cancers12071900






