Surveillance for Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease: Universal or Selective?
Abstract
1. Introduction
2. The Global Burden of HCC in NAFLD
3. Incidence of HCC in NAFLD-Cirrhosis
4. Incidence of HCC in NAFLD Patients without Cirrhosis
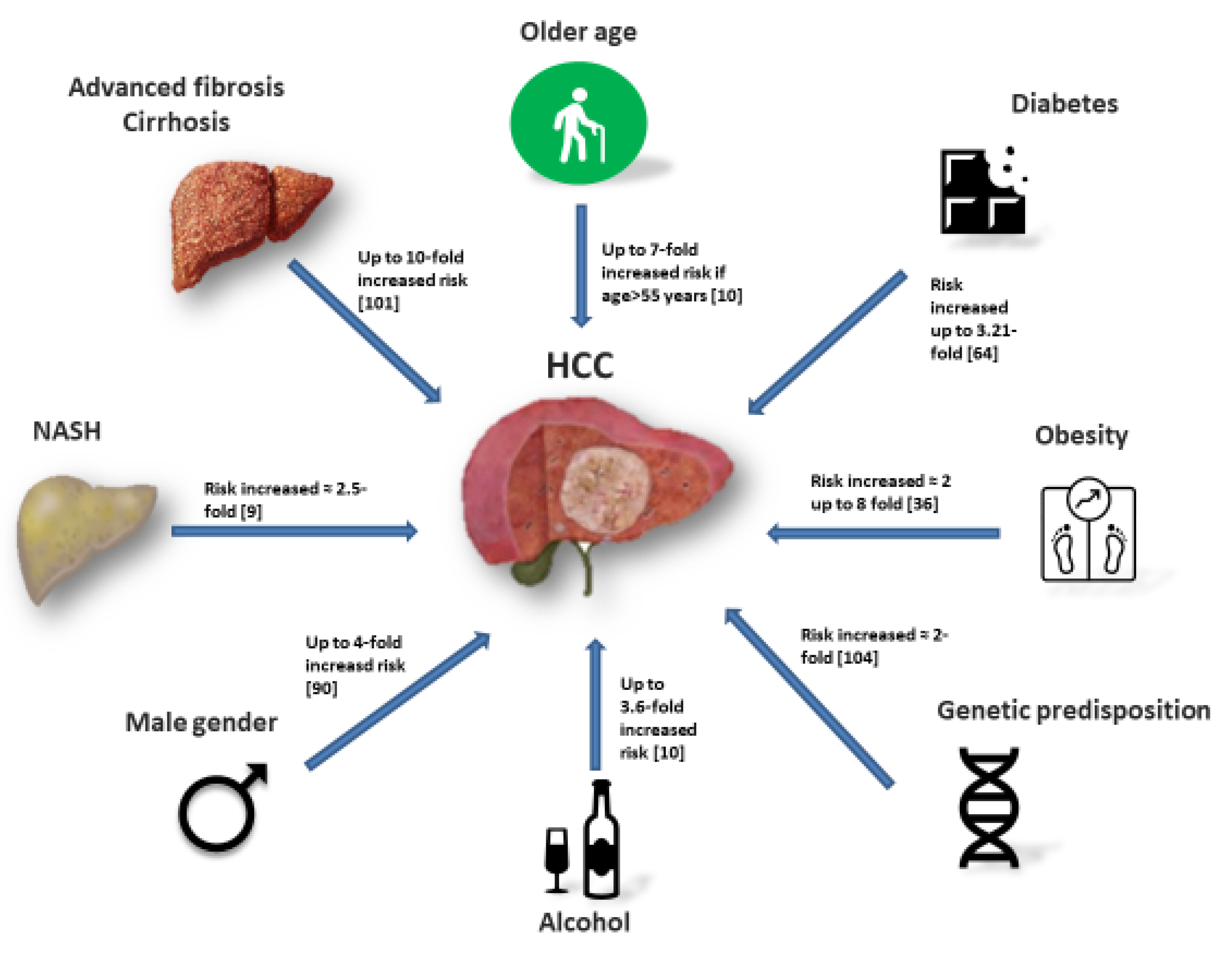
5. Additional Risk Factors for HCC in Non-Cirrhotic NAFLD
6. Diabetes
7. Obesity
8. Demographic Risk Factors
9. Genetic Predisposition
10. Lifestyle
11. The Issue of Surveillance
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Day, C.; Dufour, J.F.; Canbay, A.; Nobili, V.; Ratziu, V.; Tilg, H.; Roden, M.; Gastaldelli, A.; Ykijarvinen, H.; et al. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Li, J.; Zou, B.; Yeo, Y.H.; Feng, Y.; Xie, X.; Lee, D.H.; Fujii, H.; Wu, Y.; Kam, L.Y.; Ji, F.; et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2019, 4, 389–398. [Google Scholar] [CrossRef]
- Loomba, R.; Lim, J.K.; Patton, H.; El-Serag, H.B. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2020. [Google Scholar] [CrossRef]
- Mittal, S.; El-Serag, H.B.; Sada, Y.H.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans Is Associated With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 124–131. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1828–1837. [Google Scholar] [CrossRef]
- Stine, J.G.; Wentworth, B.J.; Zimmet, A.; Rinella, M.E.; Loomba, R.; Caldwell, S.H.; Argo, C.K. Systematic review with meta-analysis: Risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment. Pharmacol. Ther. 2018, 48, 696–703. [Google Scholar] [CrossRef]
- Lee, T.Y.; Wu, J.-C.; Yu, S.-H.; Lin, J.-T.; Wu, M.-S.; Wu, C.-Y. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically noncirrhotic nonalcoholic fatty liver disease. Int. J. Cancer 2017, 141, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Gambato, M.; Man, N.K.; Roberts, J.P.; Victor, D.; Orci, L.A.; Toso, C. Should Patients With NAFLD/NASH Be Surveyed for HCC? Transplantation 2019, 103, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Piscaglia, F.; Svegliati-Baroni, G.; Barchetti, A.; Pecorelli, A.; Marinelli, S.; Tiribelli, C.; Bellentani, S.; Group, H.-N.I.S. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology 2016, 63, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Naimark, D.; Naglie, G.; Detsky, A.S. The meaning of life expectancy: What is a clinically significant gain? J. Gen. Intern. Med. 1994, 9, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Laupacis, A.; Feeny, D.; Detsky, A.S.; Tugwell, P.X. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. Can. Med. Assoc. J. 1992, 146, 473–481. [Google Scholar]
- Sarasin, F.P.; Giostra, E.; Hadengue, A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am. J. Med. 1996, 101, 422–434. [Google Scholar] [CrossRef]
- Lin, O.S.; Keeffe, E.B.; Sanders, G.D.; Owens, D.K. Cost-effectiveness of screening for hepatocellular carcinoma in patients with cirrhosis due to chronic hepatitis C. Aliment. Pharmacol. Ther. 2004, 19, 1159–1172. [Google Scholar] [CrossRef]
- Mortality, G.B.D.; Causes of Death, C.; Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar]
- Akinyemiju, T.; Abera, S.F.; Ahmed, M.B.; Alam, N.; Alemayohu, M.A.; Allen, C.; Alraddadi, R.; Alvisguzman, N.; Amoako, Y.A.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar]
- Lampertico, P.; Agarwal, K.; Berg, T.; Buti, M.; Janssen, H.L.A.; Papatheodoridis, G.V.; Zoulim, F.; Tacke, F. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Stamm, L.M.; Brainard, D.M.; Mchutchison, J.G. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar]
- Thursz, M.; Gual, A.; Lackner, C.; Mathurin, P.; Moreno, C.; Spahr, L.; Sterneck, M.; Cortezpinto, H. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J. Hepatol. 2018, 69, 154–181. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Blissett, D.; Blissett, R.; Henry, L.; Stepanova, M.; Younossi, Y.; Racila, A.; Hunt, S.; Beckerman, R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016, 64, 1577–1586. [Google Scholar] [CrossRef]
- Baffy, G. Hepatocellular Carcinoma in Non-alcoholic Fatty Liver Disease: Epidemiology, Pathogenesis, and Prevention. J. Clin. Transl. Hepatol. 2013, 1, 131–137. [Google Scholar]
- Sanyal, A.; Poklepovic, A.; Moyneur, E.; Barghout, V. Population-based risk factors and resource utilization for HCC: US perspective. Curr. Med. Res. Opin. 2010, 26, 2183–2191. [Google Scholar] [CrossRef]
- Dyson, J.; Jaques, B.; Chattopadyhay, D.; Lochan, R.; Graham, J.; Das, D.; Aslam, T.; Patanwala, I.; Gaggar, S.; Cole, M.; et al. Hepatocellular cancer: The impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 2014, 60, 110–117. [Google Scholar] [CrossRef]
- Paik, J.M.; Golabi, P.; Younossi, Y.; Mishra, A.; Younossi, Z.M. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of Nonalcoholic Fatty Liver Disease. Hepatology 2020. [Google Scholar] [CrossRef]
- Charlton, M.R.; Burns, J.M.; Pedersen, R.A.; Watt, K.D.; Heimbach, J.K.; Dierkhising, R.A. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011, 141, 1249–1253. [Google Scholar] [CrossRef]
- Wong, R.J.; Aguilar, M.; Cheung, R.; Perumpail, R.B.; Harrison, S.A.; Younossi, Z.M.; Ahmed, A. Nonalcoholic Steatohepatitis Is the Second Leading Etiology of Liver Disease Among Adults Awaiting Liver Transplantation in the United States. Gastroenterology 2015, 148, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Cheung, R.; Ahmed, A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014, 59, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Stepanova, M.; Ong, J.P.; Jacobson, I.M.; Bugianesi, E.; Duseja, A.; Eguchi, Y.; Wong, V.W.; Negro, F.; Yilmaz, Y.; et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin. Gastroenterol. Hepatol. 2019, 17, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Degasperi, E.; Colombo, M. Distinctive features of hepatocellular carcinoma in non-alcoholic fatty liver disease. Lancet Gastroenterol. Hepatol. 2016, 1, 156–164. [Google Scholar] [CrossRef]
- Welzel, T.M.; Graubard, B.I.; Quraishi, S.; Zeuzem, S.; Davila, J.A.; El-Serag, H.B.; McGlynn, K.A. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am. J. Gastroenterol. 2013, 108, 1314–1321. [Google Scholar] [CrossRef]
- Bhala, N.; Angulo, P.; van der Poorten, D.; Lee, E.; Hui, J.M.; Saracco, G.; Adams, L.A.; Charatcharoenwitthaya, P.; Topping, J.H.; Bugianesi, E.; et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: An international collaborative study. Hepatology 2011, 54, 1208–1216. [Google Scholar] [CrossRef]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef]
- Giannini, E.G.; Cucchetti, A.; Erroi, V.; Garuti, F.; Odaldi, F.; Trevisani, F. Surveillance for early diagnosis of hepatocellular carcinoma: How best to do it? World J. Gastroenterol. 2013, 19, 8808–8821. [Google Scholar] [CrossRef]
- Yatsuji, S.; Hashimoto, E.; Tobari, M.; Taniai, M.; Tokushige, K.; Shiratori, K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J. Gastroenterol. Hepatol. 2009, 24, 248–254. [Google Scholar] [CrossRef]
- White, D.L.; Kanwal, F.; El–Serag, H.B. Association Between Nonalcoholic Fatty Liver Disease and Risk for Hepatocellular Cancer, Based on Systematic Review. Clin. Gastroenterol. Hepatol. 2012, 10, 1342–1359. [Google Scholar] [CrossRef]
- Soderberg, C.; Stal, P.; Askling, J.; Glaumann, H.; Lindberg, G.; Marmur, J.; Hultcrantz, R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology 2010, 51, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Loomba, R.; Anstee, Q.M.; Rinella, M.E.; Bugianesi, E.; Marchesini, G.; Neuschwander-Tetri, B.A.; Serfaty, L.; Negro, F.; Caldwell, S.H.; et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology 2018, 68, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Loomba, R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Ahmed, F.; Mara, K.C.; Addissie, B.D.; Allen, A.M.; Gores, G.J.; Roberts, L.R. Diabetes Is Associated With Increased Risk of Hepatocellular Carcinoma in Patients With Cirrhosis From Nonalcoholic Fatty Liver Disease. Hepatology 2020, 71, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Delemos, A.; Dakhoul, L.; Roche, P.; Miller, E.; Sbeih, H.A.; Mao, E.; Scanga, A.; Gomez, E.V.; Gawrieh, S.; Chalasani, N.; et al. Nonalcoholic fatty liver disease is the most common cause of hepatocellular carcinoma (HCC) in individuals without cirrhosis: A United States multicenter study. J. Hepatol. 2018, 68, S419–S420. [Google Scholar] [CrossRef]
- Bertot, L.C.; Adams, L.A. Trends in hepatocellular carcinoma due to non-alcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 179–187. [Google Scholar] [CrossRef]
- Ekstedt, M.; Hagstrom, H.; Nasr, P.; Fredrikson, M.; Stal, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef]
- Ekstedt, M.; Franzén, L.E.; Mathiesen, U.L.; Thorelius, L.; Holmqvist, M.; Bodemar, G.; Kechagias, S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006, 44, 865–873. [Google Scholar] [CrossRef]
- Perumpail, R.B.; Wong, R.J.; Ahmed, A.; Harrison, S.A. Hepatocellular Carcinoma in the Setting of Non-cirrhotic Nonalcoholic Fatty Liver Disease and the Metabolic Syndrome: US Experience. Dig. Dis. Sci. 2015, 60, 3142–3148. [Google Scholar] [CrossRef]
- Paradis, V.; Zalinski, S.; Chelbi, E.; Guedj, N.; Degos, F.; Vilgrain, V.; Bedossa, P.; Belghiti, J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: A pathological analysis. Hepatology 2009, 49, 851–859. [Google Scholar] [CrossRef]
- Yasui, K.; Hashimoto, E.; Komorizono, Y.; Koike, K.; Arii, S.; Imai, Y.; Shima, T.; Kanbara, Y.; Saibara, T.; Mori, T.; et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2011, 9, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Massoud, O.; Charlton, M. Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma. Clin. Liver Dis. 2018, 22, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, S.H.; Lee, V.D.; Kleiner, D.E.; Al-Osaimi, A.M.; Argo, C.K.; Northup, P.G.; Berg, C.L. NASH and cryptogenic cirrhosis: A histological analysis. Ann. Hepatol. 2009, 8, 346–352. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Green, P.; Kerr, K.F.; Berry, K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J. Hepatol. 2019, 71, 523–533. [Google Scholar] [CrossRef]
- Yang, T.; Hu, L.Y.; Li, Z.L.; Liu, K.; Wu, H.; Xing, H.; Lau, W.Y.; Pawlik, T.M.; Zeng, Y.Y.; Zhou, Y.H.; et al. Liver Resection for Hepatocellular Carcinoma in Non-alcoholic Fatty Liver Disease: A Multicenter Propensity Matching Analysis with HBV-HCC. J. Gastrointest. Surg. 2020, 24, 320–329. [Google Scholar] [CrossRef]
- Kawamura, Y.; Arase, Y.; Ikeda, K.; Seko, Y.; Imai, N.; Hosaka, T.; Kobayashi, M.; Saitoh, S.; Sezaki, H.; Akuta, N.; et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am. J. Gastroenterol. 2012, 107, 253–261. [Google Scholar] [CrossRef]
- Tokushige, K.; Hashimoto, E.; Kodama, K. Hepatocarcinogenesis in non-alcoholic fatty liver disease in Japan. J. Gastroenterol. Hepatol. 2013, 28, 88–92. [Google Scholar] [CrossRef]
- Liu, Y.L.; Patman, G.L.; Leathart, J.B.; Piguet, A.C.; Burt, A.D.; Dufour, J.F.; Day, C.P.; Daly, A.K.; Reeves, H.L.; Anstee, Q.M. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J. Hepatol. 2014, 61, 75–81. [Google Scholar] [CrossRef]
- Grimaudo, S.; Pipitone, R.M.; Pennisi, G.; Celsa, C.; Cammà, C.; Di Marco, V.; Barcellona, M.R.; Boemi, R.; Enea, M.; Giannetti, A.; et al. Association Between PNPLA3 rs738409 C>G Variant and Liver-Related Outcomes in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 935–944. [Google Scholar] [CrossRef]
- Tahergorabi, Z.; Khazaei, M.; Moodi, M.; Chamani, E. From obesity to cancer: A review on proposed mechanisms. Cell Biochem. Funct. 2016, 34, 533–545. [Google Scholar] [CrossRef]
- Noureddin, M.; Rinella, M.E. Nonalcoholic Fatty liver disease, diabetes, obesity, and hepatocellular carcinoma. Clin. Liver Dis. 2015, 19, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Reeves, H.L.; Zaki, M.Y.; Day, C.P. Hepatocellular Carcinoma in Obesity, Type 2 Diabetes, and NAFLD. Dig. Dis. Sci. 2016, 61, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Talamini, R.; Pelucchi, C.; Polesel, J.; Franceschi, S.; Crispo, A.; Izzo, F.; La Vecchia, C.; Boffetta, P.; Montella, M. Metabolic syndrome and hepatocellular carcinoma risk. Br. J. Cancer 2013, 108, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Welzel, T.M.; Graubard, B.I.; Zeuzem, S.; El-Serag, H.B.; Davila, J.A.; McGlynn, K.A. Metabolic syndrome increases the risk of primary liver cancer in the United States: A study in the SEER-Medicare database. Hepatology 2011, 54, 463–471. [Google Scholar] [CrossRef]
- Rosmorduc, O.; Fartoux, L. HCC and NASH: How strong is the clinical demonstration? Clin. Res. Hepatol. Gastroenterol. 2012, 36, 202–208. [Google Scholar] [CrossRef]
- Polesel, J.; Zucchetto, A.; Montella, M.; Dal Maso, L.; Crispo, A.; La Vecchia, C.; Serraino, D.; Franceschi, S.; Talamini, R. The impact of obesity and diabetes mellitus on the risk of hepatocellular carcinoma. Ann. Oncol. 2009, 20, 353–357. [Google Scholar] [CrossRef]
- Lawson, D.H.; Gray, J.M.; McKillop, C.; Clarke, J.; Lee, F.D.; Patrick, R.S. Diabetes mellitus and primary hepatocellular carcinoma. Q. J. Med. 1986, 61, 945–955. [Google Scholar]
- Adami, H.O.; Chow, W.H.; Nyren, O.; Berne, C.; Linet, M.S.; Ekbom, A.; Wolk, A.; McLaughlin, J.K.; Fraumeni, J.F., Jr. Excess risk of primary liver cancer in patients with diabetes mellitus. J. Natl. Cancer Inst. 1996, 88, 1472–1477. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Richardson, P.A.; Everhart, J.E. The role of diabetes in hepatocellular carcinoma: A case-control study among United States Veterans. Am. J. Gastroenterol. 2001, 96, 2462–2467. [Google Scholar] [CrossRef]
- Davila, J.A.; Morgan, R.O.; Shaib, Y.; McGlynn, K.A.; El-Serag, H.B. Diabetes increases the risk of hepatocellular carcinoma in the United States: A population based case control study. Gut 2005, 54, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Si, W.K.; Chung, J.W.; Cho, J.; Baeg, J.Y.; Jang, E.S.; Yoon, H.; Kim, J.; Shin, C.M.; Park, Y.S.; Hwang, J.H.; et al. Predictors of Increased Risk of Hepatocellular Carcinoma in Patients with Type 2 Diabetes. PLoS ONE 2016, 11, e0158066. [Google Scholar] [CrossRef]
- Pang, Y.; Kartsonaki, C.; Turnbull, I.; Guo, Y.; Clarke, R.; Chen, Y.; Bragg, F.; Yang, L.; Bian, Z.; Millwood, I.Y.; et al. Diabetes, Plasma Glucose, and Incidence of Fatty Liver, Cirrhosis, and Liver Cancer: A Prospective Study of 0.5 Million People. Hepatology 2018, 68, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Hampel, H.; Javadi, F. The association between diabetes and hepatocellular carcinoma: A systematic review of epidemiologic evidence. Clin. Gastroenterol. Hepatol. 2006, 4, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Gong, G.; Ben, Q.; Qiu, W.; Chen, Y.; Li, G.; Wang, L. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: A systematic review and meta-analysis of cohort studies. Int. J. Cancer 2012, 130, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Wang, P.; Wang, B.; Fu, Z.-J.; Zhao, W.-J.; Yan, S.-L. Diabetes mellitus and poorer prognosis in hepatocellular carcinoma: A systematic review and meta-analysis. PLoS ONE 2014, 9, e95485. [Google Scholar] [CrossRef]
- Oh, S.W.; Yoon, Y.S.; Shin, S.A. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J. Clin. Oncol. 2005, 23, 4742–4754. [Google Scholar] [CrossRef]
- Wolk, A.; Gridley, G.; Svensson, M.; Nyren, O.; McLaughlin, J.K.; Fraumeni, J.F.; Adam, H.O. A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control. 2001, 12, 13–21. [Google Scholar] [CrossRef]
- Moller, H.; Mellemgaard, A.; Lindvig, K.; Olsen, J.H. Obesity and cancer risk: A Danish record-linkage study. Eur. J. Cancer. 1994, 30, 344–350. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Overweight, obesity and risk of liver cancer: A meta-analysis of cohort studies. Br. J. Cancer 2007, 97, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, S.; Aleksandrova, K.; Pischon, T.; Fedirko, V.; Jenab, M.; Trepo, E.; Boffetta, P.; Dahm, C.C.; Overvad, K.; Tjonneland, A.; et al. Abdominal obesity, weight gain during adulthood and risk of liver and biliary tract cancer in a European cohort. Int. J. Cancer 2013, 132, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Berentzen, T.L.; Gamborg, M.; Holst, C.; Sorensen, T.I.; Baker, J.L. Body mass index in childhood and adult risk of primary liver cancer. J. Hepatol. 2014, 60, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Wang, J.; Yan, Z.; Luo, J. Excess body weight and the risk of primary liver cancer: An updated meta-analysis of prospective studies. Eur. J. Cancer 2012, 48, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tsuji, I.; Tamakoshi, A.; Matsuo, K.; Ito, H.; Wakai, K.; Nagata, C.; Mizoue, T.; Sasazuki, S.; Inoue, M.; et al. Obesity and liver cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2012, 42, 212–221. [Google Scholar] [CrossRef]
- Yao, K.F.; Ma, M.; Ding, G.Y.; Li, Z.M.; Chen, H.L.; Han, B.; Chen, Q.; Jiang, X.Q.; Wang, L.S. Meta-analysis reveals gender difference in the association of liver cancer incidence and excess BMI. Oncotarget 2017, 8, 72959–72971. [Google Scholar] [CrossRef] [PubMed]
- Ohki, T.; Tateishi, R.; Shiina, S.; Goto, E.; Sato, T.; Nakagawa, H.; Masuzaki, R.; Goto, T.; Hamamura, K.; Kanai, F.; et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut 2009, 58, 839. [Google Scholar] [CrossRef]
- Vucenik, I.; Stains, J.P. Obesity and cancer risk: Evidence, mechanisms, and recommendations. Ann. N. Y. Acad. Sci. 2012, 1271, 37–43. [Google Scholar] [CrossRef]
- Villa, E.; Baldini, G.M.; Pasquinelli, C.; Melegari, M.; Cariani, E.; Di Chirico, G.; Manenti, F. Risk factors for hepatocellular carcinoma in Italy. Male sex, hepatitis B virus, non-A non-B infection, and alcohol. Cancer 1988, 62, 611–615. [Google Scholar] [CrossRef]
- Lerose, R.; Molinari, R.; Rocchi, E.; Manenti, F.; Villa, E. Prognostic features and survival of hepatocellular carcinoma in Italy: Impact of stage of disease. Eur. J. Cancer 2001, 37, 239–245. [Google Scholar] [CrossRef]
- Park, J.W.; Chen, M.; Colombo, M.; Roberts, L.R.; Schwartz, M.; Chen, P.-J.; Kudo, M.; Johnson, P.; Wagner, S.; Orsini, L.S.; et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study. Liver Int. 2015, 35, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.W.; Ting, Y.W.; Chan, W.K. Epidemiology of non-alcoholic fatty liver disease-related hepatocellular carcinoma and its implications. JGH Open 2018, 2, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Wai-Sun Wong, V.; Castellanos, M.; Aller-de la Fuente, R.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Alvarez-Quiñones Sanz, M.; Conde-Martin, A.F.; et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018, 155, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Villa, E. Role of estrogen in liver cancer. Womens Health 2008, 4, 41–50. [Google Scholar] [CrossRef]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef]
- Mohamad, B.; Shah, V.; Onyshchenko, M.; Elshamy, M.; Aucejo, F.; Lopez, R.; Hanouneh, I.A.; Alhaddad, R.; Alkhouri, N. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol. Int. 2016, 10, 632–639. [Google Scholar] [CrossRef]
- Couto, C.A.; Gelape, C.L.; Calmet, F.; Martin, P.; Levy, C. Effect of ethnicity on liver transplant for hepatocellular carcinoma. Exp. Clin. Transpl. 2013, 11, 339–345. [Google Scholar] [CrossRef]
- Altekruse, S.F.; Henley, S.J.; Cucinelli, J.E.; McGlynn, K.A. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am. J. Gastroenterol. 2014, 109, 542–553. [Google Scholar] [CrossRef]
- Chang, E.T.; Yang, J.; Alfaro-Velcamp, T.; So, S.K.; Glaser, S.L.; Gomez, S.L. Disparities in liver cancer incidence by nativity, acculturation, and socioeconomic status in California Hispanics and Asians. Cancer Epidemiol. Biomark. Prev. 2010, 19, 3106–3118. [Google Scholar] [CrossRef]
- Kallwitz, E.R.; Daviglus, M.L.; Allison, M.A.; Emory, K.T.; Zhao, L.; Kuniholm, M.H.; Chen, J.; Gouskova, N.; Pirzada, A.; Talavera, G.A.; et al. Prevalence of suspected nonalcoholic fatty liver disease in Hispanic/Latino individuals differs by heritage. Clin. Gastroenterol. Hepatol. 2015, 13, 569–576. [Google Scholar] [CrossRef]
- Hester, D.; Golabi, P.; Paik, J.; Younossi, I.; Mishra, A.; Younossi, Z.M. Among Medicare Patients With Hepatocellular Carcinoma, Non-alcoholic Fatty Liver Disease is the Most Common Etiology and Cause of Mortality. J. Clin. Gastroenterol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Manjunath, H.; Yopp, A.C.; Beg, M.S.; Marrero, J.A.; Gopal, P.; Waljee, A.K. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: A meta-analysis. Am. J. Gastroenterol. 2014, 109, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Trepo, E.; Nahon, P.; Bontempi, G.; Valenti, L.; Falleti, E.; Nischalke, H.D.; Hamza, S.; Corradini, S.G.; Burza, M.A.; Guyot, E.; et al. Association between the PNPLA3 (rs738409 C>G) variant and hepatocellular carcinoma: Evidence from a meta-analysis of individual participant data. Hepatology 2014, 59, 2170–2177. [Google Scholar] [CrossRef] [PubMed]
- Mancina, R.M.; Dongiovanni, P.; Petta, S.; Pingitore, P.; Meroni, M.; Rametta, R.; Boren, J.; Montalcini, T.; Pujia, A.; Wiklund, O.; et al. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology 2016, 150, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Donati, B.; Dongiovanni, P.; Romeo, S.; Meroni, M.; McCain, M.; Miele, L.; Petta, S.; Maier, S.; Rosso, C.; De Luca, L.; et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci. Rep. 2017, 7, 4492. [Google Scholar]
- Lee, Y.C.A.; Cohet, C.; Yang, Y.-C.; Stayner, L.; Hashibe, M.; Straif, K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int. J. Epidemiol. 2009, 38, 1497–1511. [Google Scholar] [CrossRef]
- Petrick, J.L.; Campbell, P.T.; Koshiol, J.; Thistle, J.E.; Andreotti, G.; Beane-Freeman, L.E.; Buring, J.E.; Chan, A.T.; Chong, D.Q.; Doody, M.M.; et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. Br. J. Cancer 2018, 118, 1005–1012. [Google Scholar] [CrossRef]
- Kolly, P.; Knöpfli, M.; Dufour, J.F. Effect of smoking on survival of patients with hepatocellular carcinoma. Liver Int. 2017, 37, 1682–1687. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Del Poggio, P.; Olmi, S.; Ciccarese, F.; Di Marco, M.; Rapaccini, G.L.; Benvegnù, L.; Borzio, F.; Farinati, F.; Zoli, M.; Giannini, E.G.; et al. Factors That Affect Efficacy of Ultrasound Surveillance for Early Stage Hepatocellular Carcinoma in Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2014, 12, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Simmons, O.; Fetzer, D.T.; Yokoo, T.; Marrero, J.A.; Yopp, A.; Kono, Y.; Parikh, N.D.; Browning, T.; Singal, A.G. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment. Pharmacol. Ther. 2017, 45, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Samoylova, M.L.; Mehta, N.; Roberts, J.P.; Yao, F.Y. Predictors of Ultrasound Failure to Detect Hepatocellular Carcinoma. Liver Transpl. 2018, 24, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Libbrecht, L.; Bielen, D.; Verslype, C.; Vanbeckevoort, D.; Pirenne, J.; Nevens, F.; Desmet, V.; Roskams, T. Focal lesions in cirrhotic explant livers: Pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transpl. 2002, 8, 749–761. [Google Scholar] [CrossRef]
- Andersson, K.L.; Salomon, J.A.; Goldie, S.J.; Chung, R.T. Cost Effectiveness of Alternative Surveillance Strategies for Hepatocellular Carcinoma in Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2008, 6, 1418–1424. [Google Scholar] [CrossRef]
- Manghisi, G.; Elba, S.; Mossa, A.; Giorgio, A.; Aloisio, V.; Perrotta, A.; Tardio, B.; del Naja, C.; Caturelli, E.; Calandra, M.; et al. A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients. Hepatology 1998, 28, 751–755. [Google Scholar]
- Giannini, E.G.; Sammito, G.; Farinati, F.; Ciccarese, F.; Pecorelli, A.; Rapaccini, G.L.; Di Marco, M.; Caturelli, E.; Zoli, M.; Borzio, F.; et al. Determinants of alpha-fetoprotein levels in patients with hepatocellular carcinoma: Implications for its clinical use. Cancer 2014, 120, 2150–2157. [Google Scholar] [CrossRef]
- Beale, G.; Chattopadhyay, D.; Gray, J.; Stewart, S.; Hudson, M.; Day, C.; Trerotoli, P.; Giannelli, G.; Manas, D.; Reeves, H. AFP, PIVKAII, GP3, SCCA-1 and follisatin as surveillance biomarkers for hepatocellular cancer in non-alcoholic and alcoholic fatty liver disease. BMC Cancer 2008, 8, 200. [Google Scholar] [CrossRef]
- Gray, J.; Chattopadhyay, D.; Beale, G.S.; Patman, G.L.; Miele, L.; King, B.P.; Stewart, S.; Hudson, M.; Day, C.P.; Manas, D.M.; et al. A proteomic strategy to identify novel serum biomarkers for liver cirrhosis and hepatocellular cancer in individuals with fatty liver disease. BMC Cancer 2009, 9, 271. [Google Scholar] [CrossRef]
- Fujiwara, N.; Nakagawa, H.; Enooku, K.; Kudo, Y.; Hayata, Y.; Nakatsuka, T.; Tanaka, Y.; Tateishi, R.; Hikiba, Y.; Misumi, K.; et al. CPT2 downregulation adapts HCC to lipid-rich environment and promotes carcinogenesis via acylcarnitine accumulation in obesity. Gut 2018, 67, 1493–1504. [Google Scholar] [CrossRef]
- Enooku, K.; Nakagawa, H.; Fujiwara, N.; Kondo, M.; Minami, T.; Hoshida, Y.; Shibahara, J.; Tateishi, R.; Koike, K. Altered serum acylcarnitine profile is associated with the status of nonalcoholic fatty liver disease (NAFLD) and NAFLD-related hepatocellular carcinoma. Sci. Rep. 2019, 9, 10663. [Google Scholar] [CrossRef] [PubMed]
- Elemeery, M.N.; Mohamed, M.A.; Madkour, M.A.; Shamseya, M.M.; Issa, N.M.; Badr, A.N.; Ghareeb, D.A.; Pan, C.H. MicroRNA signature in patients with hepatocellular carcinoma associated with type 2 diabetes. World J. Gastroenterol. 2019, 25, 6322–6341. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Celsa, C.; Giammanco, A.; Spatola, F.; Petta, S. The Burden of Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease: Screening Issue and Future Perspectives. Int. J. Mol. Sci. 2019, 20, 5613. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, F.; Salvi, A.; Gramantieri, L.; Sangiovanni, A.; Guerriero, P.; De Petro, G.; Bassi, C.; Lupini, L.; Sattari, A.; Cheung, D.; et al. Circulating miR-106b-3p, miR-101-3p and miR-1246 as diagnostic biomarkers of hepatocellular carcinoma. Oncotarget 2018, 9, 15350–15364. [Google Scholar] [CrossRef]

| First Author (Country, Year) | Type of Study | Number and Type of Patients | Diagnostic Method for NAFLD/NASH | NAFLD Patients with Cirrhosis | Mean Follow Up | HCC Incidence | HCC-Independent Risk Factors (HR, 95% CI) among NAFLD (Multivariate Analysis) |
|---|---|---|---|---|---|---|---|
| Ascha (US, 2010) [37] | Retrospective cohort study | 195 NASH-cirrhosis 315 HCV-cirrhosis | Histology or cryptogenic cirrhosis and MetS | 100% | 3.2 years | NASH: 2.6% | Any alcohol consumption (HR 3.6 (1.6–8.9)) Older age (HR 1.08 (1.02–1.1)) |
| Yatsuji (Japan, 2009) [39] | Prospective cohort study, observational | 68 NASH-cirrhosis 69 HCV-cirrhosis | Histology | 100% | NR | NASH: 5-year occurrence rate = 11.3% | NA |
| Kanwal (US, 2018) [9] | Retrospective cohort study | 296,707 NAFLD 296,707 matched controls | Elevated ALT and exclusion of other etiologies of liver disease | 0.4% at baseline | 9 years | NAFLD: 0.08 per 1000 person-years (PY) Subgroup analyses: -NAFLD + diabetes = 0.45 per 1000 PY -NAFLD + age > 65 = 0.41 per 1000 PY -NAFLD + age > 65 + Hispanic ethnicity = 0.93 per 1000 PY NASH-cirrhosis: 10.6 per 1000 PY (range 1.6–23, highest in older (>65 years) Hispanics). If cirrhosis + high FIB-4 = 13.55 per 1000 PY (11.93–15.33) | Cirrhosis Age ≥ 65 years Hispanic ethnicity Diabetes Male sex Among cirrhosis, risk highest if: -Male sex -Hispanic ethnicity and age ≥ 65 years -Diabetes-FIB-4 score > 2.67 |
| Ioannou (US, 2019) [54] | Retrospective cohort study | 7068 NAFLD-cirrhosis 16,175 ALD-cirrhosis | If comorbid with diabetes or BMI > 30 | 100% | 3.7 years | Annual incidence = 1.56% If FIB-4 > 3.65, annual incidence = 2.68% | Older age (aHR ≈ 2.09 if age > 60) Male sex (Ahr = 1 versus 0.25 for female) Platelet count < 150 × 103 µL (aHR ≈ 2 to ≈3) Albumin < 3.7 g/dL (aHR ≈ 2 to ≈ 3) AST/ALT ratio > 8.8 (aHR ≈ 2 to ≈ 5) |
| Yang (US, 2020) [55] | Retrospective cohort study | 354 NASH-cirrhosis | Histology or history of steatosis or fatty liver at radiology | 100% | 47 months | 5-year cumulative incidence rate = 7.8% | Older age (per decade, HR = 1.8 (1.2–2.6)) Low albumin (HR 2.1 (1.5–2.9)) Diabetes (HR 4.2 (1.2–14.2)) |
| Yasui (Japan, 2011) [51] | Cross-sectional multicenter study | 87 NASH-related HCC cases | Histology | 51% | NR | NR | Advanced fibrosis (21%) and cirrhosis (51%), male sex (62%) and diabetes (59%), obesity (62%) and hypertension (55%) were highly prevalent in the population. Risk analysis was not performed. |
| Piscaglia (Italy, 2016) [13] | Multicenter observational prospective study | 145 NAFLD- related HCC cases 611 HCV-related HCC cases | Histology or radiology | 53.8% | NR | NR | Causality not assessed but in comparison with the HCV cohort, NAFLD patients showed significantly higher prevalence of male gender, diabetes, hypertension and dyslipidemia |
| Kawamura (Japan, 2011) [56] | Retrospective cohort study | 6508 NAFLD patients | Ultrasound scan Only 16 patients had NAFLD-related HCC | NR | 5.6 years | Overall incidence = 0.25% Annual incidence = 0.043% Cumulative HCC incidence: -4-year = 0.02% -8-year = 0.19% -12-year = 0.51% | AST ≥ 40 IU/L (HR 8.20 (2.56–26.26)) Age > 60 (HR 4.27 (1.30–14.01)) Platelet count < 150 × 103/µL (HR 7.19 (2.26–23.26)) Diabetes (HR 3.21 (1.09–9.50)) APRI > 1.5 (i.e., significant fibrosis) (HR 25.03 (9.02–69.52)) |
| Lee (Taiwan, 2017) [11] | Population-based retrospective cohort study | 18,080 NAFLD patients | Not reported | NR | 6.3 years | Cumulative incidence at 1-year = 0.18%, increasing until up to 2.73% at 10 years | Age > 55 years (HR 7.78 (3.12–19.44)) ALT elevation (HR 6.80 (3.00–15.42)) 10-year cumulative incidence 4-fold higher in patients aged over 55 with ALT elevation |
| Tokushige (Japan, 2013) [57] | Prospective cohort study | 14,530 HCC cases: 84.1% viral aetiology; 7.2% alcoholic, 2% NAFLD; 5.1% cryptogenic | Histology | 62% | NR | 5-years incidence = 11.3% | Older age (HR 1.103 (1.050–1.159) + Male gender (HR 4.680 (1.803–12.146)) Advanced liver fibrosis (HR 2.718 (1.745–4.233)) Higher GGT (HR 1.005 (1.001–1.009)) |
| Liu (UK, Switzerland, 2014) [58] | Prospective cohort study | 100 NAFLD-related HCC cases 275 NAFLD cases w/o HCC | Histology or radiology | Among NAFLD-HCCs, 67% Among NAFLD w/o HCC, 26% | NR | NR | Carriage of the PNPLA3 rs-738409 G > C polymorphism (2.26 (1.23–4.14)) Male gender (HR 11.11 (4.17–33.33)) Age (HR 1.24 (1.17–1.32)) Cirrhosis (HR 9.37 (3.82–23.00)) |
| Grimaudo (Italy, 2020) [59] | Prospective cohort study | 471 NAFLD cases | Histology or radiology | 11.5% | 64.6 months | Incidence rate in the non-cirrhotic vs. cirrhotic: - 1-year: 0.2% vs. 1.3% - 5 -years: 3.0% vs. 9.3% - 10-years: 4.2% vs. 13.5% | Advanced fibrosis and cirrhosis (HR not reported) PNPLA3 G variant (HR 2.68 (1.01–7.26)). Among the subgroup of patients with F3–F4 fibrosis it was the only independent risk factor: HR 2.66 (1.02–7.13) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaz Torres, M.C.; Bodini, G.; Furnari, M.; Marabotto, E.; Zentilin, P.; Strazzabosco, M.; Giannini, E.G. Surveillance for Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease: Universal or Selective? Cancers 2020, 12, 1422. https://doi.org/10.3390/cancers12061422
Plaz Torres MC, Bodini G, Furnari M, Marabotto E, Zentilin P, Strazzabosco M, Giannini EG. Surveillance for Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease: Universal or Selective? Cancers. 2020; 12(6):1422. https://doi.org/10.3390/cancers12061422
Chicago/Turabian StylePlaz Torres, Maria Corina, Giorgia Bodini, Manuele Furnari, Elisa Marabotto, Patrizia Zentilin, Mario Strazzabosco, and Edoardo G. Giannini. 2020. "Surveillance for Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease: Universal or Selective?" Cancers 12, no. 6: 1422. https://doi.org/10.3390/cancers12061422
APA StylePlaz Torres, M. C., Bodini, G., Furnari, M., Marabotto, E., Zentilin, P., Strazzabosco, M., & Giannini, E. G. (2020). Surveillance for Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease: Universal or Selective? Cancers, 12(6), 1422. https://doi.org/10.3390/cancers12061422






