Targeted Osmotic Lysis of Highly Invasive Breast Carcinomas Using Pulsed Magnetic Field Stimulation of Voltage-Gated Sodium Channels and Pharmacological Blockade of Sodium Pumps
Abstract
1. Introduction
2. Results
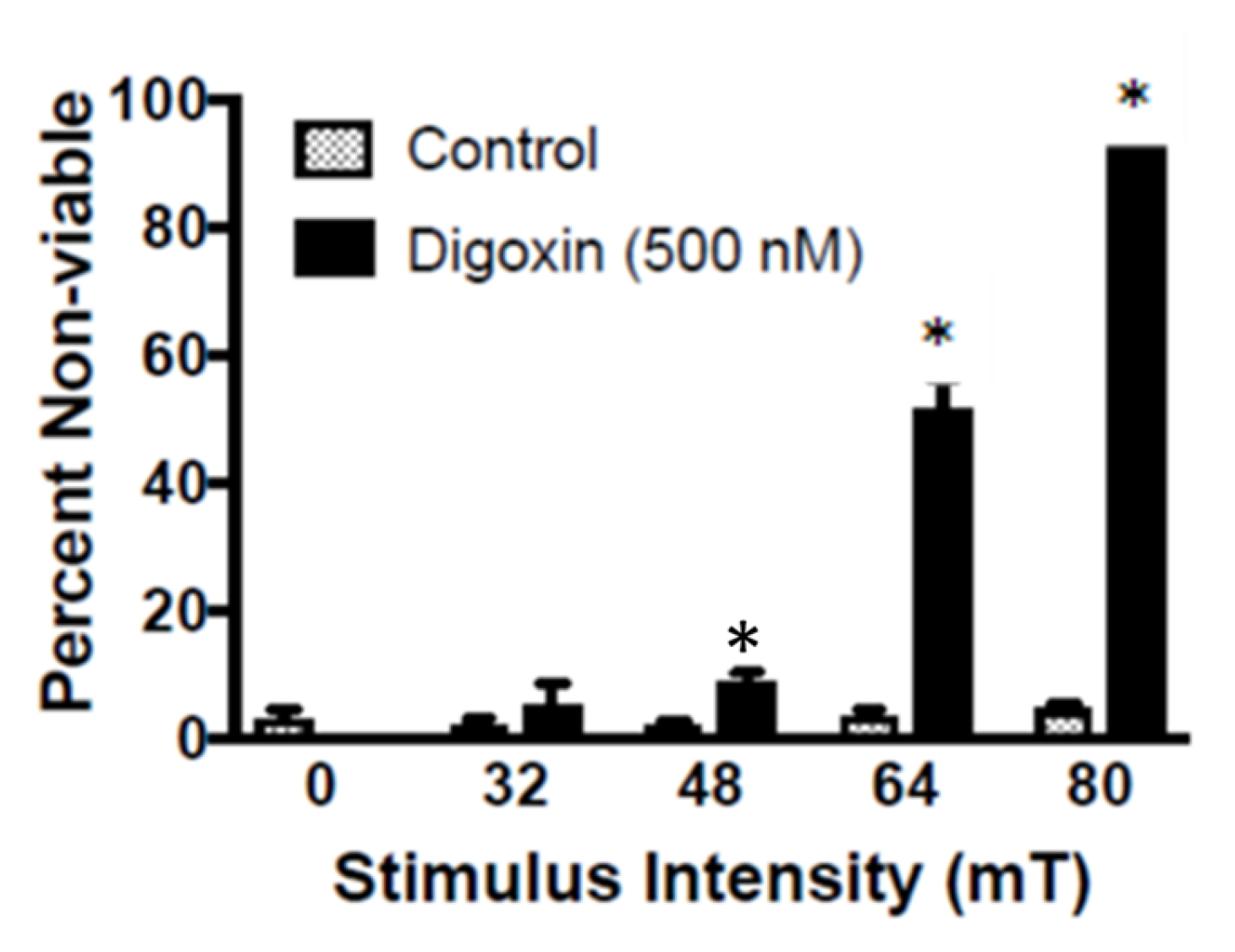
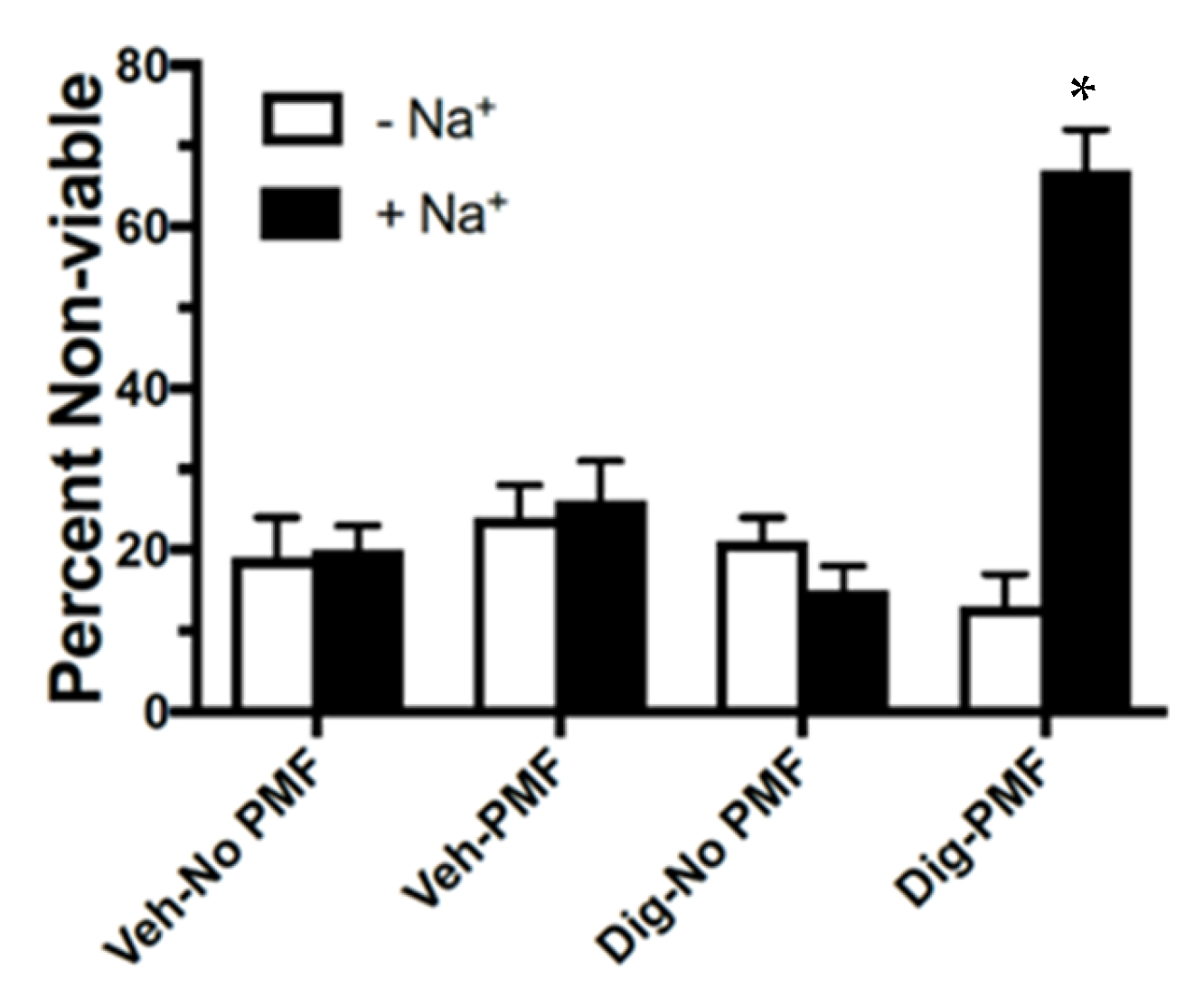
2.1. In Vitro TOL Treatment
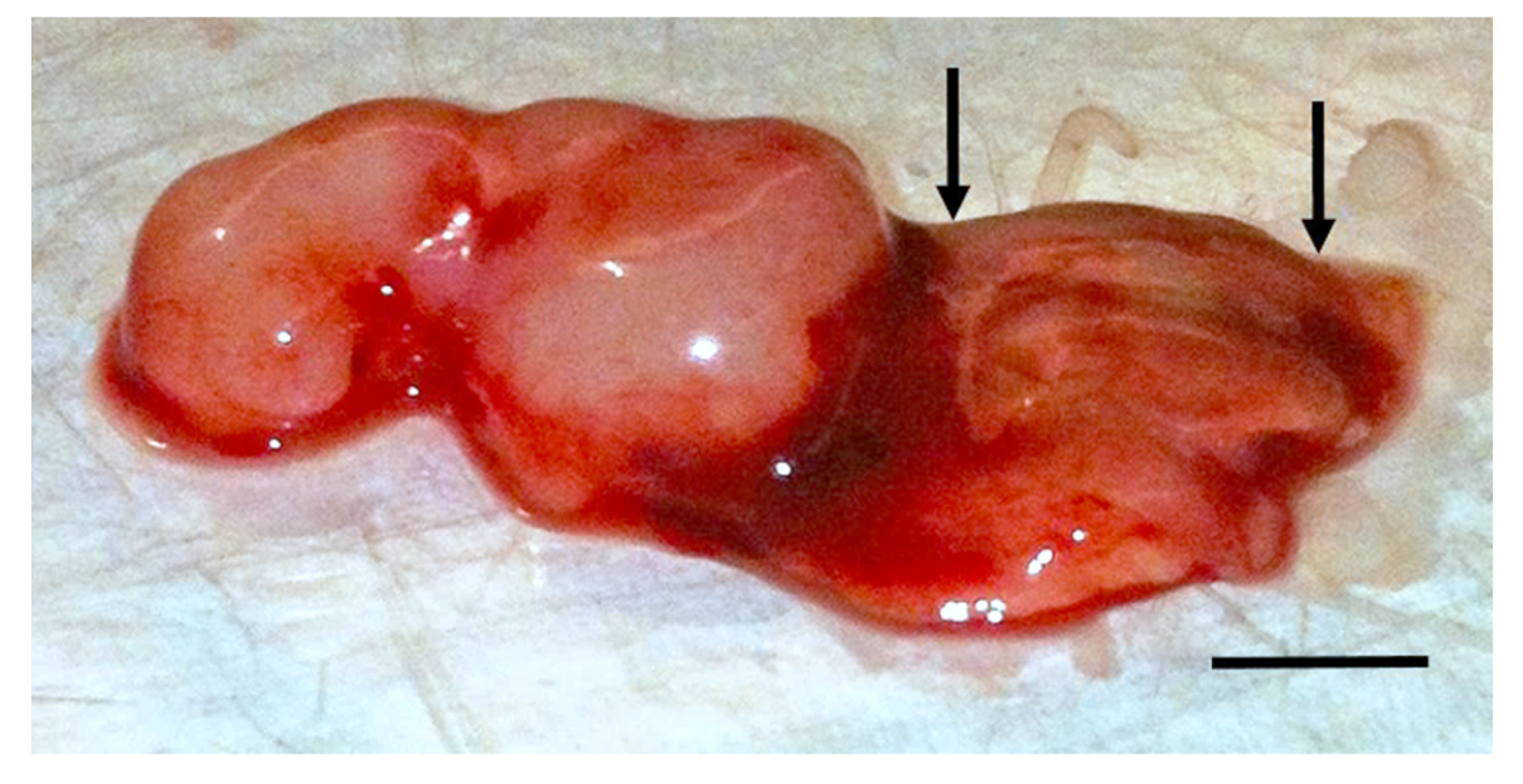
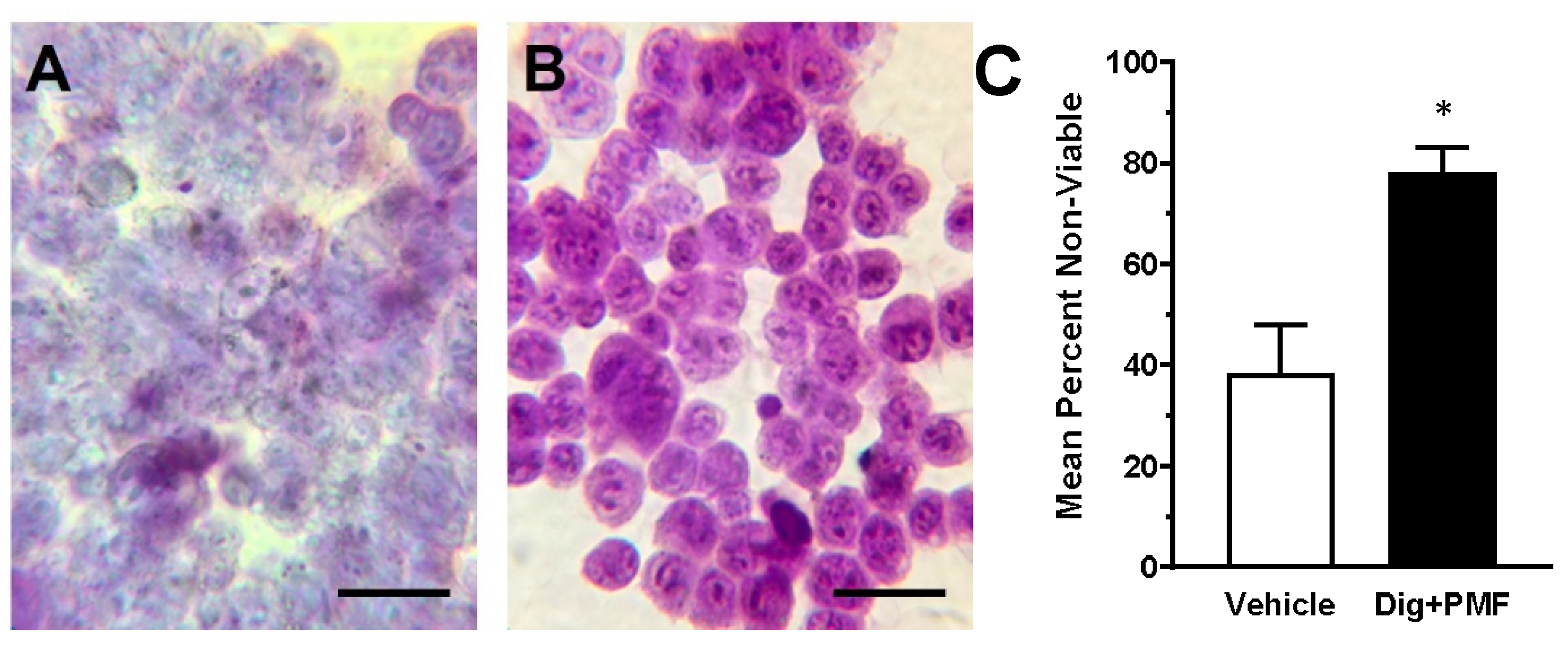
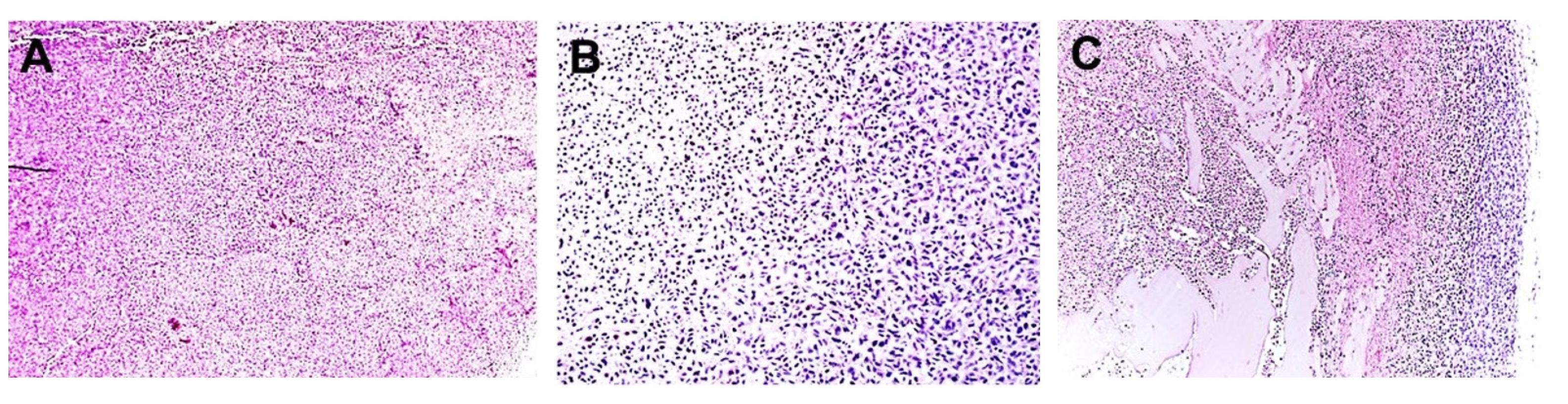
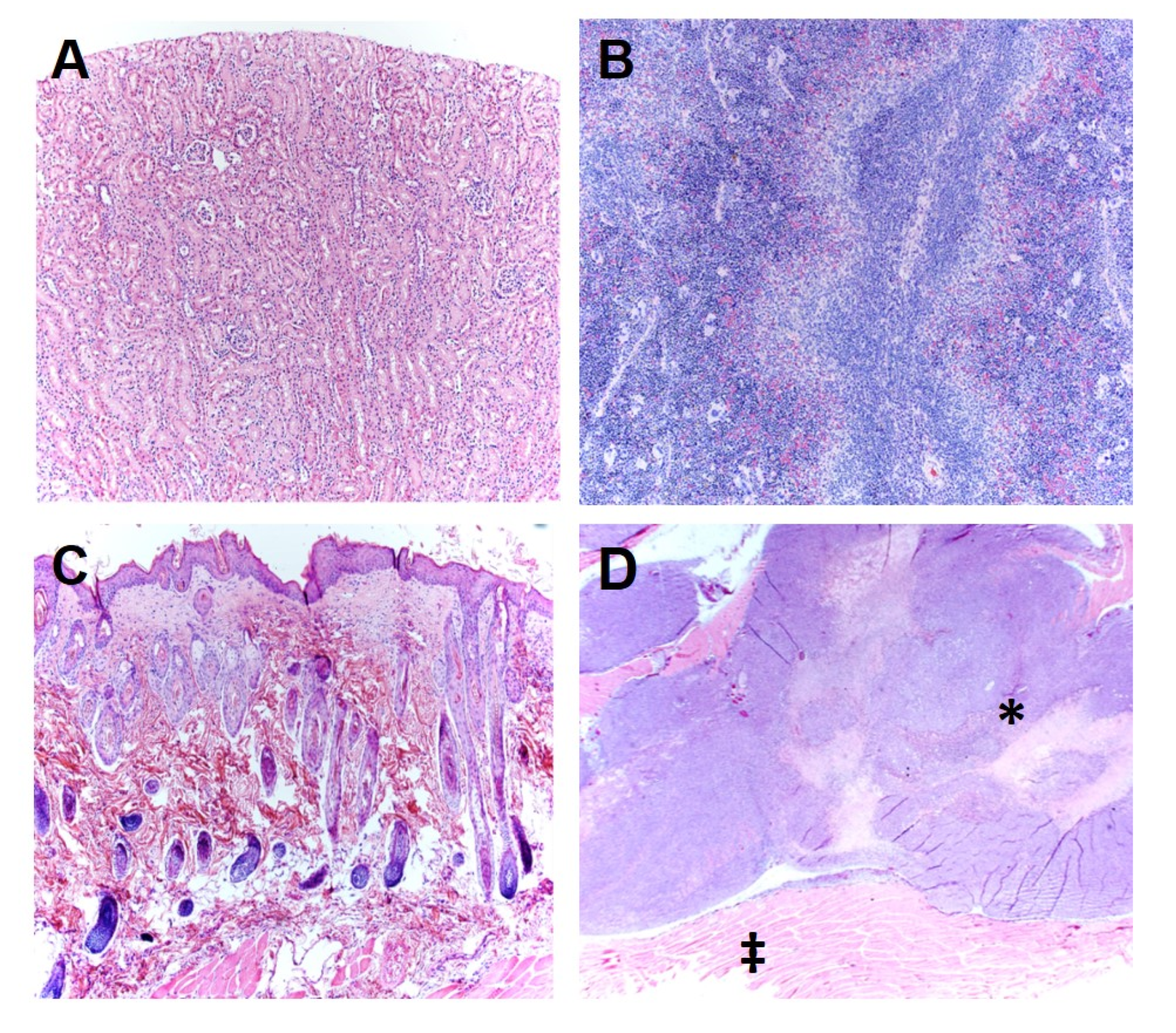
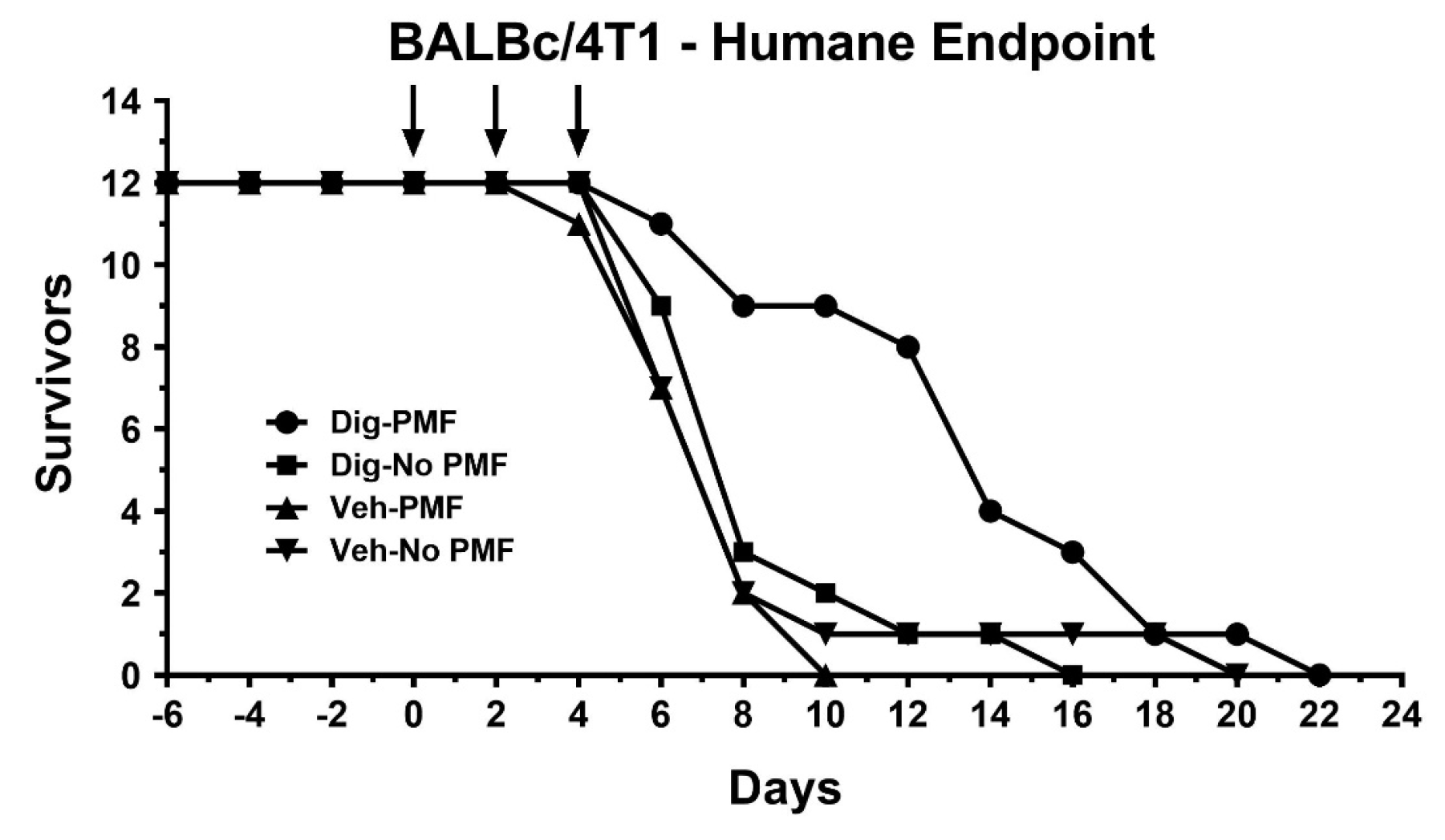
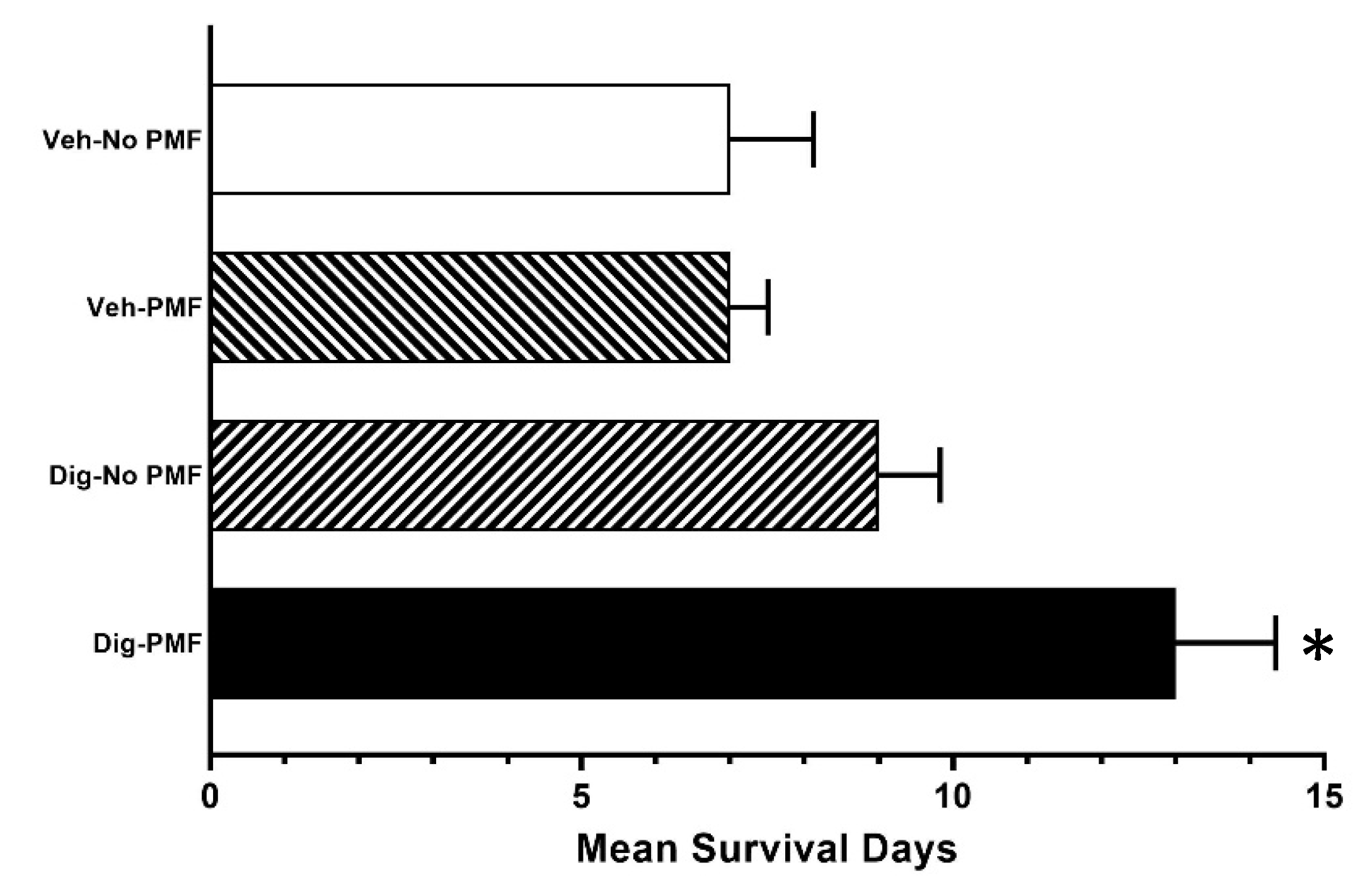
2.2. In Vivo TOL Treatment
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Cell Culture
4.3. Animals
4.4. Flow Cytometry
4.5. Image Analysis
4.6. In Vitro TOL Treatment
4.7. Sodium Dependency
4.8. In Vivo TOL Treatment
4.9. Survival Studies
4.10. Enhancement of Drug Penetration
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roger, S.; Rollin, J.; Barascu, A.; Besson, P.; Raynal, P.I.; Iochmann, S.; Lei, M.; Bougnoux, P.; Gruel, Y.; Le Guennec, J.Y. Voltage-gated sodium channels potentiate the invasive capacities of human non-small-cell lung cancer cell lines. Int. J. Biochem. Cell Biol. 2007, 39, 774–786. [Google Scholar] [CrossRef]
- Yang, M.; Brackenbury, W.J. Membrane potential and cancer progression. Front Physiol. 2013, 4, 185. [Google Scholar] [CrossRef] [PubMed]
- Leslie, T.K.; James, A.D.; Zaccagna, F.; Grist, J.T.; Deen, S.; Kennerley, A.; Riemer, F.; Kaggie, J.D.; Gallagher, F.A.; Gilbert, F.J.; et al. Sodium homeostasis in the tumour microenvironment. Biochim. Biophys. Acta. Rev. Cancer 2019, 1872, 188304. [Google Scholar] [CrossRef] [PubMed]
- Exadaktylos, A.K.; Buggy, D.J.; Moriarty, D.C.; Mascha, E.; Sessler, D.I. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiolog. 2006, 105, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Driffort, V.; Gillet, L.; Bon, E.; Marionneau-Lambot, S.; Oullier, T.; Joulin, V.; Collin, C.; Pagès, J.C.; Jourdan, M.L.; Chevalier, S.; et al. Ranolazine inhibits NaV1.5-mediated breast cancer cell invasiveness and lung colonization. Mol. Cancer 2014, 13, 264. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.; Yang, M.; Dowle, A.A.; Thomas, J.R.; Brackenbury, W.J. The sodium channel-blocking antiepileptic drug phenytoin inhibits breast tumour growth and metastasis. Mol. Cancer 2015, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Ufodiama, C.; Watt, I.; Bland, M.; Brackenbury, W.J. Therapeutic value of voltage-gated sodium channel inhibitors in breast, colorectal and prostate cancer: A systemic review. Front. Pharmacol. 2015, 6, 273. [Google Scholar] [CrossRef]
- Dutta, S.; Lopez Charcas, O.; Tanner, S.; Gradek, F.; Driffort, V.; Roger, S.; Selander, K.; Velu, S.E.; Brouillette, W. Discovery and evaluation of nNa1.5 sodium channel blockers with potent cell envasion inhibitory activity in breast cancer cells. Bioorg. Med. Chem. 2018, 26, 2428–2436. [Google Scholar] [CrossRef]
- Fraser, S.P.; Ozerlat-Gunduz, I.; Brackenbury, W.J.; Fitzgerald, E.M.; Campbell, T.M.; Coombes, R.C.; Djamgoz, M.B. Regulation of voltage-gated sodium channel expression in cancer: Hormones, growth factors and auto-regulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130105. [Google Scholar] [CrossRef]
- Onkal, R.; Djamgoz, M.B. Molecular pharmacology of voltage-gated sodium channel expression in metastatic disease: Clinical potential of neonatal NaV1.5 in breast cancer. Eur. J. Pharmacol. 2009, 625, 206–219. [Google Scholar] [CrossRef]
- Roger, S.; Potier, M.; Vandier, C.; Besson, P.; Le Guennec, J.Y. Voltage-gated sodium channels: New targets in cancer therapy? Curr. Pharm Des. 2006, 12, 3681–3695. [Google Scholar] [CrossRef] [PubMed]
- Brackenbury, W.J.; Isom, L.L. Voltage-gated Na+ channels: Potential for beta subunits as therapeutic targets. Expert Opin. Ther. Targets 2008, 12, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Lin, S.; Lin, J. The effects of anesthetics on tumor progression. Int. J. Physiol. Pathophysiol. Pharmacol. 2013, 5, 1–10. [Google Scholar] [PubMed]
- Djamgoz, M.B.; Onkal, R. Persistent current blockers of voltage-gated sodium channels: A clinical opportunity for controlling metastatic disease. Recent Pat. on Anticancer Drug Discov. 2013, 8, 66–84. [Google Scholar] [CrossRef]
- Lo, W.L.; Donermeyer, D.L.; Allen, P.M. A voltage-gated sodium channel is essential for the positive selection of CD4(+) T cells. Nat. Immunol. 2012, 13, 880–887. [Google Scholar] [CrossRef]
- Paul, D.; Soignier, R.D.; Minor, L.; Tau, H.; Songu-Mize, E.; Gould, H.J., 3rd. Regulation and pharmacological blockade of sodium-potassium ATPase: Inflammation may lead to neuropathy. J. Neurol. Sci. 2014, 340, 139–143. [Google Scholar] [CrossRef]
- Gould, H.J., 3rd; Norleans, J.; Ward, T.D.; Reid, C.; Paul, D. Selective lysis of breast carcinomas by simultaneous stimulation of sodium channels and blockade of sodium pumps. Oncotarget 2018, 9, 15606–15615. [Google Scholar] [CrossRef][Green Version]
- Merrill, D.R.; Bikson, M.; Jefferys, J.G.R. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J. Neurosci. Methods 2005, 141, 171–198. [Google Scholar] [CrossRef]
- Choi, I.K.; Strauss, R.; Richter, M.; Yun, C.O.; Lieber, A. Strategies to increase drug penetration in solid tumors. Front. Oncol. 2013, 3, 193. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Secomb, T.W. Transport of drugs from blood vessels to tumour tissue. Nat. Rev. Cancer 2017, 17, 738–750. [Google Scholar] [CrossRef]
- Diop-Frimpong, B.; Chauhan, V.P.; Krane, S.; Boucher, Y.; Jain, R.K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl. Acad. Sci USA 2011, 108, 2909–2914. [Google Scholar] [CrossRef] [PubMed]
- Greish, K.; Fateel, M.; Abdelghany, S.; Rachel, N.; Alimoradi, H.; Bakhiet, M.; Alsaie, A. Sildenafil citrate improves the delivery and anticancer activity of doxorubicin formulations in a mouse model of breast cancer. J. Drug Target. 2018, 26, 610–615. [Google Scholar] [CrossRef]
- Wieraszko, A.; Armani, J.; Maqsood, N.; Raja, H.; Philip, S. Modification of the synaptic glutamate turnover in the hippocampal tissue exposed to low-frequency, pulsed magnetic fields. Brain Res. 2005, 1052, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Marchionni, I.; Paffi, A.; Pellegrino, M.; Liberti, M.; Apollonio, F.; Abeti, R.; Fontana, F.; D’Inzeo, G.; Mazzanti, M. Comparison between low-level 50 Hz and 900 MHz electromagnetic stimulation on single channel ionic currents and on firing frequency in dorsal root ganglion isolated neurons. Biochim. Biophys. Acta 2006, 1758, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Wieraszko, A. Pulsed magnetic stimulation modifies amplitude of action potentials in vitro via ionic channels-dependent mechanism. Bioelectromagnetics 2015, 36, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cai, D.; Wang, J.H.; Li, G.; Lin, L. Effect of pulse magnetic field on distribution of neuronal action potential. Sheng Li Xue Bao 2014, 66, 438–448. [Google Scholar]
- Edwards, M.J.; Talelli, P.; Rothwell, J.C. Clinical applications of transcranial magnetic stimulation in patients with movement disorders. Lancet Neurol. 2008, 7, 827–840. [Google Scholar] [CrossRef]
- Özdemir, A.; Simay, Y.D.; Ibisog˘lu, B.; Yaren, B.; Bülbül, D.; Ark, M. Cardiac glycoside-induced cell death and Rho/Rho kinase pathway: Implication of different regulation in cancer cell lines. Steroids 2016, 109, 29–43. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, D.; Maggi, P.; Piero, F.D.; Scahill, S.D.; Sherman, K.J.; Edenfield, S.; Gould, H.J., III. Targeted Osmotic Lysis of Highly Invasive Breast Carcinomas Using Pulsed Magnetic Field Stimulation of Voltage-Gated Sodium Channels and Pharmacological Blockade of Sodium Pumps. Cancers 2020, 12, 1420. https://doi.org/10.3390/cancers12061420
Paul D, Maggi P, Piero FD, Scahill SD, Sherman KJ, Edenfield S, Gould HJ III. Targeted Osmotic Lysis of Highly Invasive Breast Carcinomas Using Pulsed Magnetic Field Stimulation of Voltage-Gated Sodium Channels and Pharmacological Blockade of Sodium Pumps. Cancers. 2020; 12(6):1420. https://doi.org/10.3390/cancers12061420
Chicago/Turabian StylePaul, Dennis, Paul Maggi, Fabio Del Piero, Steven D. Scahill, Kelly Jean Sherman, Samantha Edenfield, and Harry J. Gould, III. 2020. "Targeted Osmotic Lysis of Highly Invasive Breast Carcinomas Using Pulsed Magnetic Field Stimulation of Voltage-Gated Sodium Channels and Pharmacological Blockade of Sodium Pumps" Cancers 12, no. 6: 1420. https://doi.org/10.3390/cancers12061420
APA StylePaul, D., Maggi, P., Piero, F. D., Scahill, S. D., Sherman, K. J., Edenfield, S., & Gould, H. J., III. (2020). Targeted Osmotic Lysis of Highly Invasive Breast Carcinomas Using Pulsed Magnetic Field Stimulation of Voltage-Gated Sodium Channels and Pharmacological Blockade of Sodium Pumps. Cancers, 12(6), 1420. https://doi.org/10.3390/cancers12061420




