Gastric Cancer Staging: Is It Time for Magnetic Resonance Imaging?
Abstract
1. Introduction
2. Tumor-Node-Metastasis Staging of Gastric Cancer
3. Magnetic Resonance Imaging in T- and N-Parameter Evaluation of Gastric Cancer
4. Magnetic Resonance Imaging of Metastases from Gastric Cancer
5. MRI in the Assessment of Peritoneal Metastases from Gastric Cancer
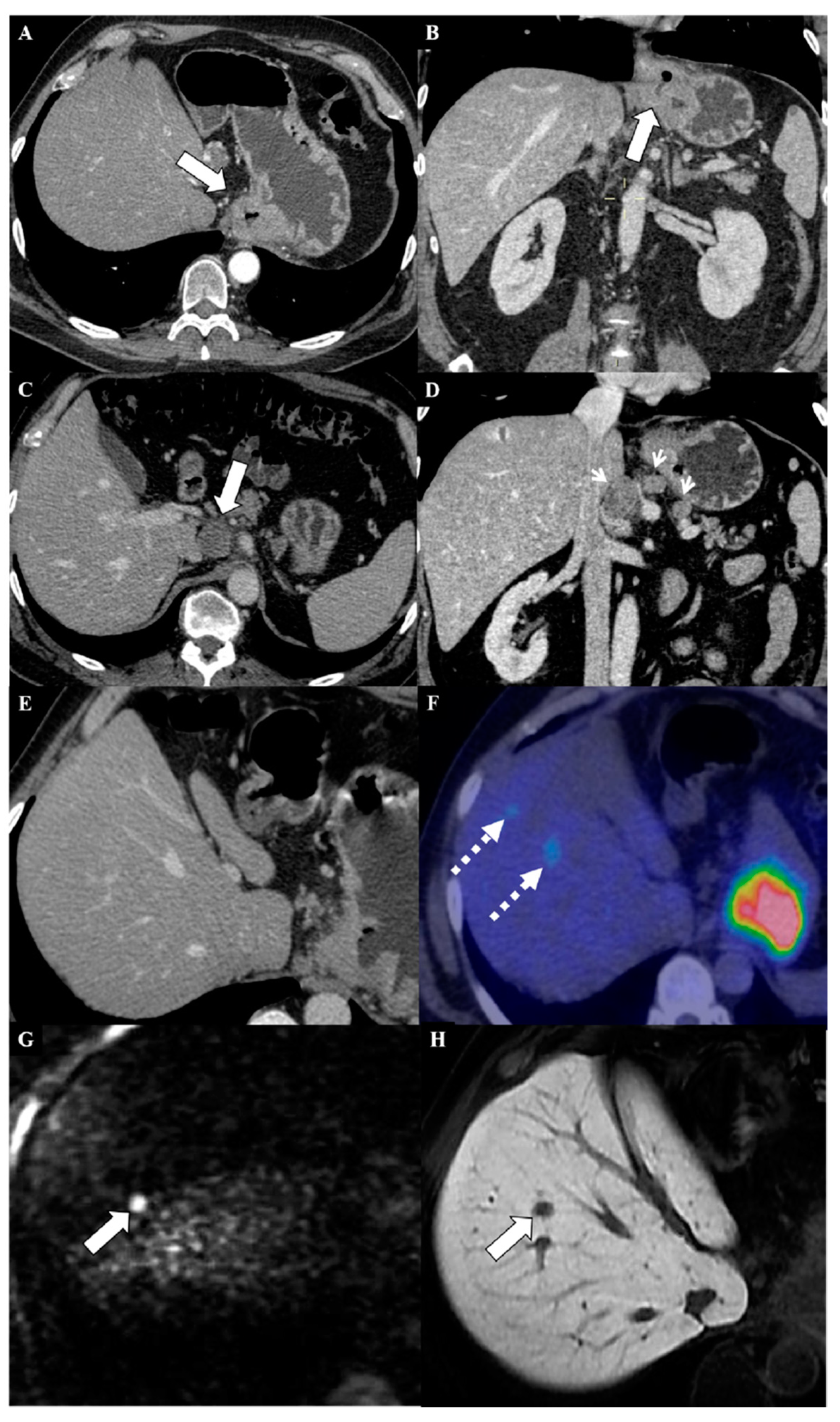
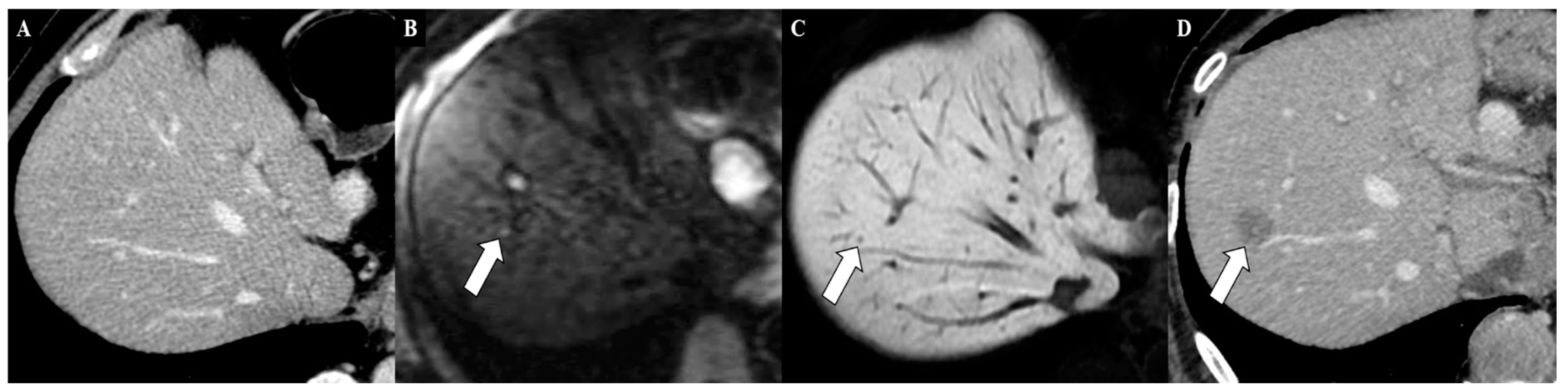
6. Magnetic Resonance Imaging in the Assessment of Liver Metastases from Gastric Cancer
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Borggreve, A.S.; Goense, L.; Brenkman, H.J.F.; Mook, S.; Meijer, G.J.; Wessels, F.J.; Verheij, M.; Jansen, E.P.M.; van Hillegersberg, R.; van Rossum, P.S.N.; et al. Imaging strategies in the management of gastric cancer: Current role and future potential of MRI. Br. J. Radiol. 2019, 92, 20181044. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Richman, D.M.; Tirumani, S.H.; Hornick, J.L.; Fuchs, C.S.; Howard, S.; Krajewski, K.; Ramaiya, N.; Rosenthal, M. Beyond gastric adenocarcinoma: Multimodality assessment of common and uncommon gastric neoplasms. Abdom. Radiol. (NY) 2017, 42, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Eusebi, L.H.; Telese, A.; Marasco, G.; Bazzoli, F.; Zagari, R.M. Gastric Cancer Prevention Strategies: A Global Perspective. J. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Crew, K.D.; Negut, A.I. Epidemiology of gastric cancer. World J. Gastroenterol. 2006, 12, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Rothenbacher, D.; Arndt, V. Epidemiology of stomach cancer. Methods Mol. Biol. 2009, 472, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N. WHO Classification of Tumours of the Digestive System, 4th ed.; IARC Press: Lyon, France, 2010. [Google Scholar]
- Roder, D.M. The epidemiology of gastric cancer. Gastric Cancer 2002, 5 (Suppl. 1), 5–11. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.M.; Kim, K.W.; Park, H.S.; Choi, J.Y.; Kim, S.H.; Kim, M.A.; Lee, J.Y.; Han, J.K.; Choi, B.I. Ectopic pancreas: CT findings with emphasis on differentiation from small gastrointestinal stromal tumor and leiomyoma. Radiology 2009, 252, 92–100. [Google Scholar] [CrossRef]
- Fishman, E.K.; Urban, B.A.; Hruban, R.H. CT of the stomach: Spectrum of disease. RadioGraphics 1996, 16, 1035–1054. [Google Scholar] [CrossRef]
- McGhan, L.J.; Pockaj, B.A.; Gray, R.J.; Bagaria, S.P.; Wasif, N. Validation of the updated 7th edition AJCC TNM staging criteria for gastric adenocarcinoma. J. Gastrointest. Surg. 2012, 16, 53–61, discussion 61. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. Stomach, 8th ed.; AJCC Cancer Staging Manual; Springer: New York, NY, USA, 2017. [Google Scholar]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Gastric Cancer v. 4, Volume 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 19 March 2020).
- Renzulli, M.; Clemente, A.; Ierardi, A.M.; Pettinari, I.; Tovoli, F.; Brocchi, S.; Peta, G.; Cappabianca, S.; Carrafiello, G.; Golfieri, R. Imaging of Colorectal Liver Metastases: New Developments and Pending Issues. Cancers 2020, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.J.; Semelka, R.C.; Martin, D.R.; Marcos, H.B. Colon Diseases: MR Evaluation Using Combined T2-Weighted Single-Shot Echo Train Spinecho and Gadolinium-Enhanced Spoiled Gradient-Echo Sequences. J. Magn. Reson. Imaging 2000, 12, 297–305. [Google Scholar] [CrossRef]
- Coburn, N.; Seevaratnam, R.; Paszat, L.; Helyer, L.; Law, C.; Swallow, C.; Cardosa, R.; Mahar, A.; Lourenco, L.G.; Dixon, M.; et al. Optimal management of gastric cancer: Results from an international RAND/UCLA expert panel. Ann. Surg. 2014, 259, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011, 14, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Kinoshita, T.; Saiura, A.; Esaki, M.; Sakamoto, H.; Yamanaka, T. Multicentre analysis of long-term outcome after surgical resection for gastric cancer liver metastases. Br. J. Surg. 2015, 102, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Okano, K.; Maeba, T.; Ishimura, K.; Karasawa, Y.; Goda, F.; Wakabayashi, H.; Usuki, H.; Maeta, H. Hepatic resection for metastatic tumors from gastric cancer. Ann. Surg. 2002, 235, 86–91. [Google Scholar] [CrossRef]
- Mocellin, S.; Marchet, A.; Nitti, D. EUS for the staging of gastric cancer: A meta-analysis. Gastrointest. Endosc. 2011, 73, 1122–1134. [Google Scholar] [CrossRef]
- Seevaratnam, R.; Cardoso, R.; McGregor, C.; Lourenco, L.; Mahar, A.; Sutradhar, R.; Law, C.; Paszat, L.; Coburn, N. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging of gastric cancer? A meta-analysis. Gastric Cancer 2012, 15, S3–S18. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, D.H.; Guo, L.; Hu, C.H.; Fang, X.; Meng, Q.; Ping, X.X.; Lu, Z.W. The utility of MRI for pre-operative T and N staging of gastric carcinoma: A systematic review and meta-analysis. Br. J. Radiol. 2015, 88, 20140552. [Google Scholar] [CrossRef]
- Cai, J.S.; Chen, H.Y.; Chen, J.Y.; Lu, Y.F.; Sun, J.Z.; Zhou, Y.; Yu, R.S. Reduced field-of-view diffusion-weighted imaging (DWI) in patients with gastric cancer: Comparison with conventional DWI techniques at 3.0T: A preliminary study. Medicine 2020, 99, 1. [Google Scholar] [CrossRef]
- Tang, L.; Sun, Y.; Li, Z.; Cao, K.; Zhang, X.; Li, X.; Ji, J. Diffusion-weighted magnetic resonance imaging in the depiction of gastric cancer: Initial experience. Abdom. Radiol. 2016, 41, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Soydan, L.; Demir, A.A.; Torun, M.; Cikrikcioglu, M.A. Use of Diffusion-Weighted Magnetic Resonance Imaging and Apparent Diffusion Coefficient in Gastric Cancer Staging. Curr. Imaging 2020. [Google Scholar] [CrossRef] [PubMed]
- Joo, I.; Lee, J.M.; Kim, J.H.; Shin, C.I.; Han, J.K.; Choi, B.I. Prospective Comparison of 3T MRI with Diffusion-Weighted Imaging and MDCT for the Preoperative TNM Staging of Gastric Cancer. J. Magn. Reson. Imaging 2015, 41, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, Y.; Deng, J.; Mccarthy, R.J.; Wang, G.; Wang, H.; Ye, Y. Discrimination of Metastatic Lymph Nodes in Patients with Gastric Carcinoma Using Diffusion Weighted Imaging. J. Magn. Reson. Imaging 2013, 37, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Canadian Cancer Statistics 2012; Canadian Cancer Society: Toronto, ON, Canada, 2012.
- Cancer Stat Facts: Stomach Cancer. National Cancer Institute Bethesda. 2011. Available online: http://seer.cancer.gov/statfacts/html/stomach.html (accessed on 2 March 2020).
- Coburn, N.G.; Lourenco, L.G.; Rossi, S.E.; Gunraj, N.; Mahar, A.L.; Helyer, L.K.; Law, C.; Rabeneck, L.; Paszat, L.J. Management of gastric cancer in Ontario. Surg. Oncol. 2010, 102, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kwee, R.M.; Kwee, T.C. Modern imaging techniques for preoperative detection of distant metastases in gastric cancer. World J. Gastroenterol. 2015, 21, 10502–10509. [Google Scholar] [CrossRef]
- Dixon, M.; Mahar, A.L.; Helyer, L.K.; Vasilevska-Ristovska, J.; Law, C.; Coburn, N.G. Prognostic factors in metastatic gastric cancer: Results of a population-based, retrospective cohort study in Ontario. Gastric Cancer 2016, 19, 150–159. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, K.W.; Baek, S.K.; Chang, H.J.; Kim, Y.J.; Park, D.J.; Kim, J.H.; Kim, H.H.; Lee, J.S. Survival benefit of gastrectomy ± metastasectomy in patients with metastatic gastric cancer receiving chemotherapy. Gastric Cancer 2011, 14, 130–138. [Google Scholar] [CrossRef][Green Version]
- Sarela, A.I.; Yelluri, S.; Leeds Upper Gastrointestinal Cancer Multidisciplinary Team. Gastric adenocarcinoma with distant metastasis: Is gastrectomy necessary? Arch. Surg. 2007, 142, 143–149, discussion 149. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.Q. Imaging in assessing hepatic and peritoneal metastases of gastric cancer: A systematic review. BMC Gastroenterol. 2011, 11, 19. [Google Scholar] [CrossRef]
- Roth, Y.; Tichler, T.; Kostenich, G.; Ruiz-Cabello, J.; Maier, S.E.; Cohen, J.S.; Orenstein, A.; Mardor, Y. High-b-Value Diffusion-Weighted MR Imaging for Pretreatment Prediction and Early Monitoring of Tumor Response to Therapy in Mice. Radiology 2004, 232, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Laghi, A.; Bellini, D.; Rengo, M.; Accarpio, F.; Caruso, D.; Biacchi, D.; Di Giorgio, A.; Sammartino, P. Diagnostic performance of computed tomography and magnetic resonance imaging for detecting peritoneal metastases: Systematic review and meta-analysis. Radiol. Med. 2017, 122, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-L.; He, L.; Zhu, Y.C.; Wu, K.; Yuan, F. Comparison between multi-slice spiral CT and magnetic resonance imaging in the diagnosis of peritoneal metastasis in primary ovarian carcinoma. Onco Targets Ther. 2018, 11, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Leake, P.A.; Cardoso, R.; Seevaratnam, R.; Lourenco, L.; Helyer, L.; Mahar, A.; Law, C.; Coburn, N.G. A systematic review of the accuracy and indications for diagnostic laparoscopy prior to curative-intent resection of gastric cancer. Gastric Cancer 2012, 15, S38–S47. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, D.; Chen, X.; Yu, P.; Wu, J.; Song, B.; Hu, J.; Wu, B. Retrospective imaging studies of gastric cancer: Study protocol clinical trial (SPIRIT Compliant). Medicine 2020, 99, e19157. [Google Scholar] [CrossRef]
- Vreugdenburg, T.D.; Ma, N.; Duncan, J.K.; Riitano, D.; Cameron, A.L.; Maddern, G.J. Comparative diagnostic accuracy of hepatocyte-specific gadoxetic acid (Gd-EOB-DTPA) enhanced MR imaging and contrast enhanced CT for the detection of liver metastases: A systematic review and meta-analysis. Int. J. Colorectal Dis. 2016, 31, 1739–1749. [Google Scholar] [CrossRef]
- Vilgrain, V.; Esvan, M.; Ronot, M.; Caumont-Prim, A.; Aubé, C.; Chatellier, G. A meta-analysis of diffusion-weighted and gadoxetic acid-enhanced MR imaging for the detection of liver metastases. Eur. Radiol. 2016, 26, 4595–4615. [Google Scholar] [CrossRef]
- Asato, N.; Tsurusaki, M.; Sofue, K.; Hieda, Y.; Katsube, T.; Kitajima, K.; Murakami, T. Comparison of gadoxetic acid-enhanced dynamic MR imaging and contrast-enhanced computed tomography for preoperative evaluation of colorectal liver metastases. Jpn. J. Radiol. 2017, 35, 197–205. [Google Scholar] [CrossRef]
- Karhunen, P.J.J. Benign hepatic tumours and tumour like conditions in men. Clin. Pathol. 1986, 39, 183–188. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Sharma, M.; Gibson, R.N.; Schreiber-Dietrich, D.; Jenssen, C. Fortuitously discovered liver lesions. World J. Gastroenterol. 2013, 19, 3173–3188. [Google Scholar] [CrossRef]
- Hammerstingl, R.; Huppertz, A.; Breuer, J.; Balzer, T.; Blakeborough, A.; Carter, R.; Fusté, L.C.; Heinz-Peer, G.; Judmaier, W.; Laniado, M.; et al. Diagnostic Efficacy of Gadoxetic Acid (Primovist)-Enhanced MRI and Spiral CT for a Therapeutic Strategy: Comparison with Intraoperative and Histopathologic Findings in Focal Liver Lesions. Eur. Radiol. 2008, 18, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Golfieri, R.; Garzillo, G.; Ascanio, S.; Renzulli, M. Focal Lesions in the Cirrhotic Liver: Their Pivotal Role in Gadoxetic Acid-Enhanced MRI and Recognition by the Western Guidelines. Dig. Dis. 2014, 32, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, M.; Buonfiglioli, F.; Conti, F.; Brocchi, S.; Serio, I.; Foschi, F.G.; Caraceni, P.; Mazzella, G.; Verucchi, G.; Golfieri, R.; et al. Imaging Features of Microvascular Invasion in Hepatocellular Carcinoma Developed After Direct-Acting Antiviral Therapy in HCV-Related Cirrhosis. Eur. Radiol. 2018, 28, 506–513. [Google Scholar] [CrossRef] [PubMed]
| 8th AJCC Gastric Cancer TNM Staging | Definition | Imaging |
|---|---|---|
| T | ||
| TX | Primary tumor cannot be assessed | EUS is the modality of choice in assessing the invasion depth of gastric cancer. |
| T0 | No evidence of primary tumor | |
| Tis | Carcinoma in situ: intraepithelial tumor without invasion of the lamina propria | |
| T1 | Tumor invades lamina propria, muscularis mucosae, or submucosa | |
| T1a | Tumor invades lamina propria or muscularis mucosae | |
| T1b | Tumor invades submucosa | |
| T2 | Tumor invades muscularis propria | |
| T3 | Tumor penetrates subserosal connective tissue without invasion of visceral peritoneum or adjacent structures T3 tumors also include those extending into the gastrocolic or gastrohepatic ligaments or into the greater or lesser omentum, without perforation of the visceral peritoneum covering these structures | |
| T4 | Tumor invades serosa (visceral peritoneum) or adjacent structures | CT is more useful in advanced T stages to evaluate the tumor nodularity outside |
| T4a | Tumor invades serosa (visceral peritoneum) | the stomach or invasion of the adjacent organs. |
| T4b | Tumor invades adjacent structures, such as the spleen, transverse colon, liver, diaphragm, pancreas, abdominal wall, adrenal gland, kidney, small intestine, and retroperitoneum | |
| N | ||
| NX | Regional lymph node(s) cannot be assessed | CT, PET/CT, and EUS can be used for nodal staging. Radiological criterion for nodal involvement is lymph node enlargement. |
| N0 | No regional lymph node metastasis | |
| N1 | Metastasis in 1 to 2 regional lymph nodes | |
| N2 | Metastasis in 3 to 6 regional lymph nodes | |
| N3 | Metastasis in 7 or more regional lymph nodes | |
| N3a | Metastasis in 7–15 regional lymph nodes | |
| N3b | Metastasis in 16 or more regional lymph nodes | |
| M | ||
| M0 | No distant metastasis | CT or PET/CT are recommended to assess metastatic disease. |
| M1 | Distant metastasis |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renzulli, M.; Clemente, A.; Spinelli, D.; Ierardi, A.M.; Marasco, G.; Farina, D.; Brocchi, S.; Ravaioli, M.; Pettinari, I.; Cescon, M.; et al. Gastric Cancer Staging: Is It Time for Magnetic Resonance Imaging? Cancers 2020, 12, 1402. https://doi.org/10.3390/cancers12061402
Renzulli M, Clemente A, Spinelli D, Ierardi AM, Marasco G, Farina D, Brocchi S, Ravaioli M, Pettinari I, Cescon M, et al. Gastric Cancer Staging: Is It Time for Magnetic Resonance Imaging? Cancers. 2020; 12(6):1402. https://doi.org/10.3390/cancers12061402
Chicago/Turabian StyleRenzulli, Matteo, Alfredo Clemente, Daniele Spinelli, Anna Maria Ierardi, Giovanni Marasco, Davide Farina, Stefano Brocchi, Matteo Ravaioli, Irene Pettinari, Matteo Cescon, and et al. 2020. "Gastric Cancer Staging: Is It Time for Magnetic Resonance Imaging?" Cancers 12, no. 6: 1402. https://doi.org/10.3390/cancers12061402
APA StyleRenzulli, M., Clemente, A., Spinelli, D., Ierardi, A. M., Marasco, G., Farina, D., Brocchi, S., Ravaioli, M., Pettinari, I., Cescon, M., Reginelli, A., Cappabianca, S., Carrafiello, G., & Golfieri, R. (2020). Gastric Cancer Staging: Is It Time for Magnetic Resonance Imaging? Cancers, 12(6), 1402. https://doi.org/10.3390/cancers12061402










