Anatomical Theories of the Pathophysiology of Cancer-Related Lymphoedema
Abstract
1. Introduction
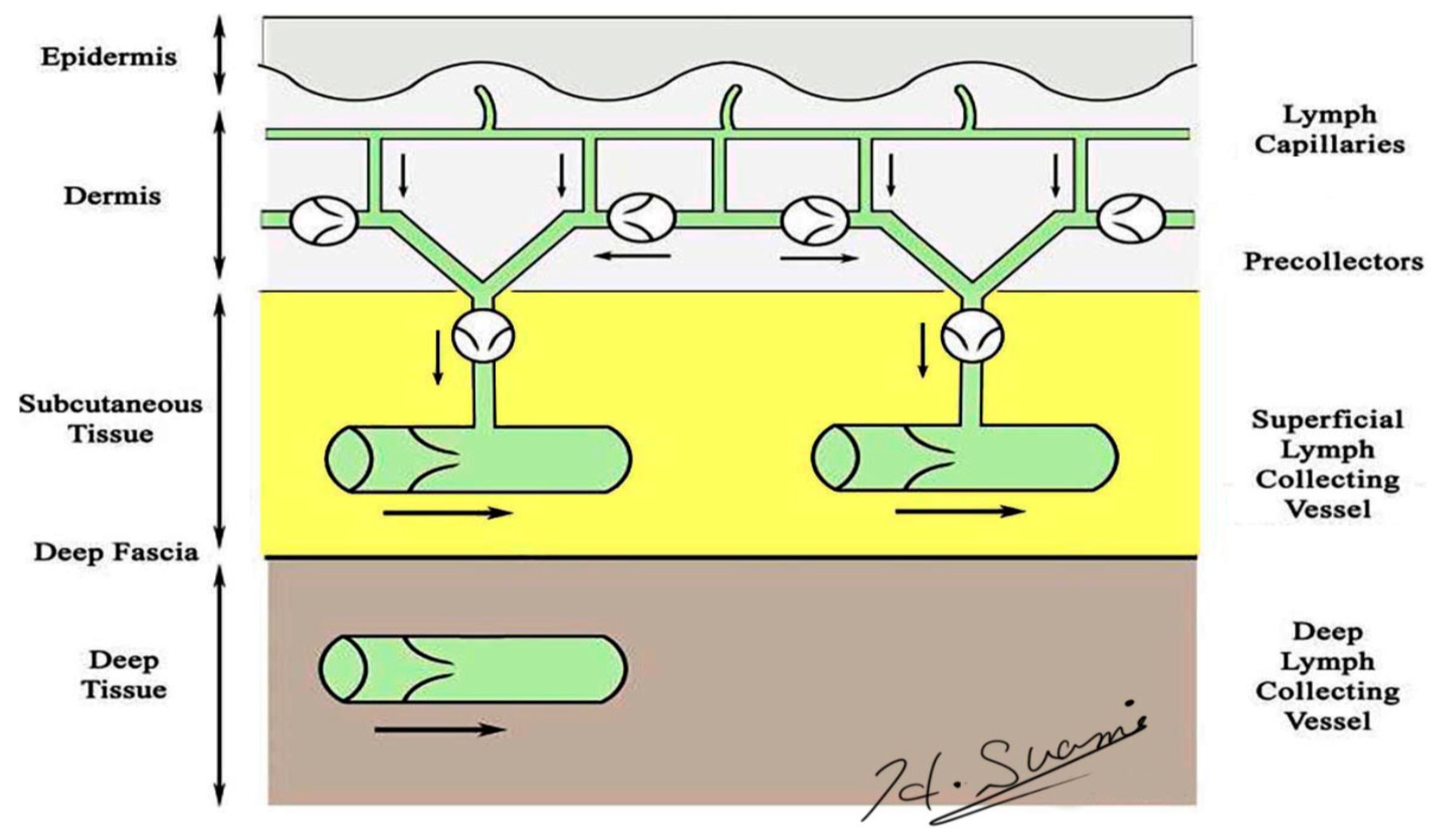
2. Normal Lymphatic Anatomy
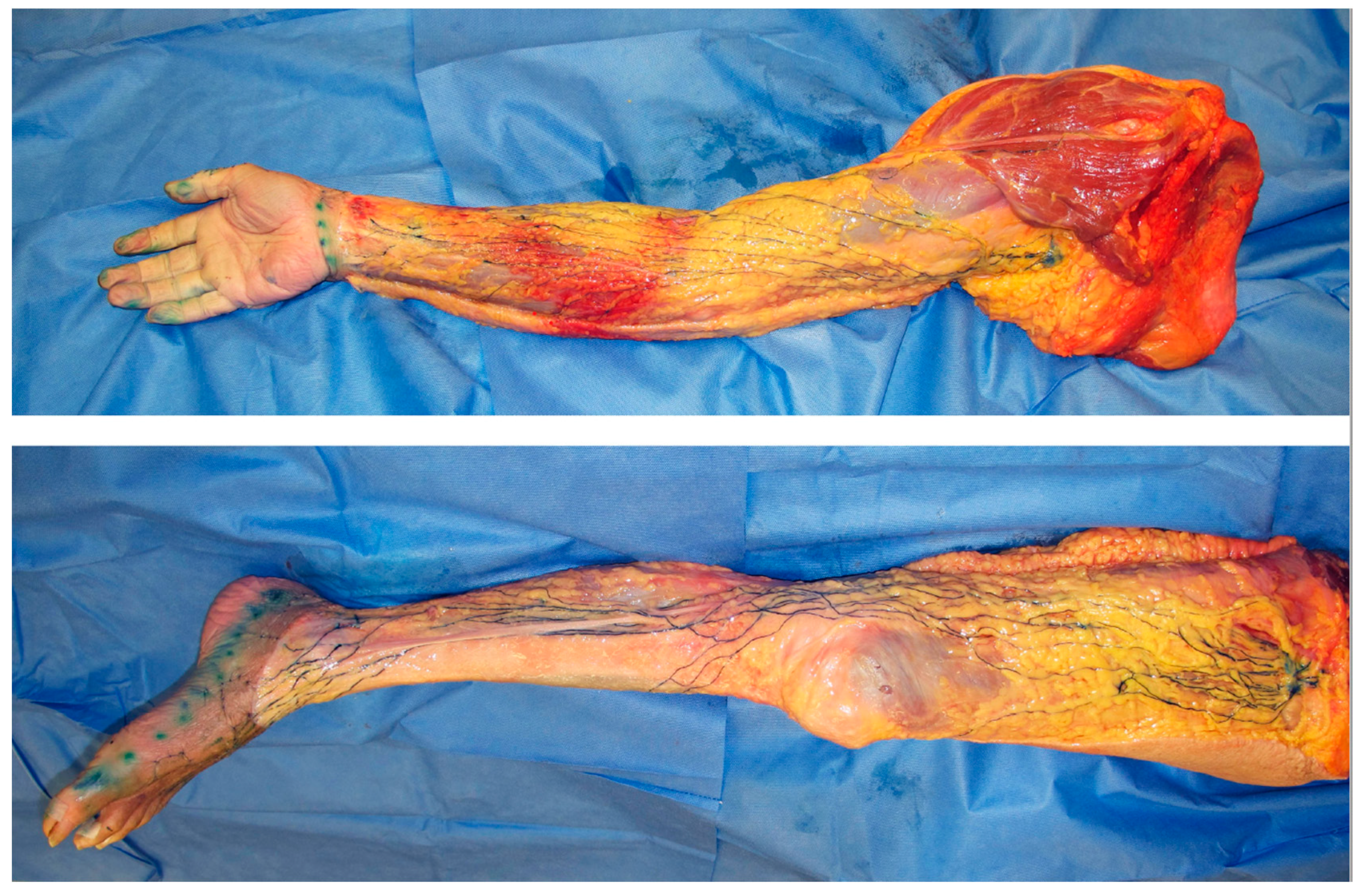
3. Anatomical Changes in Lymphoedema
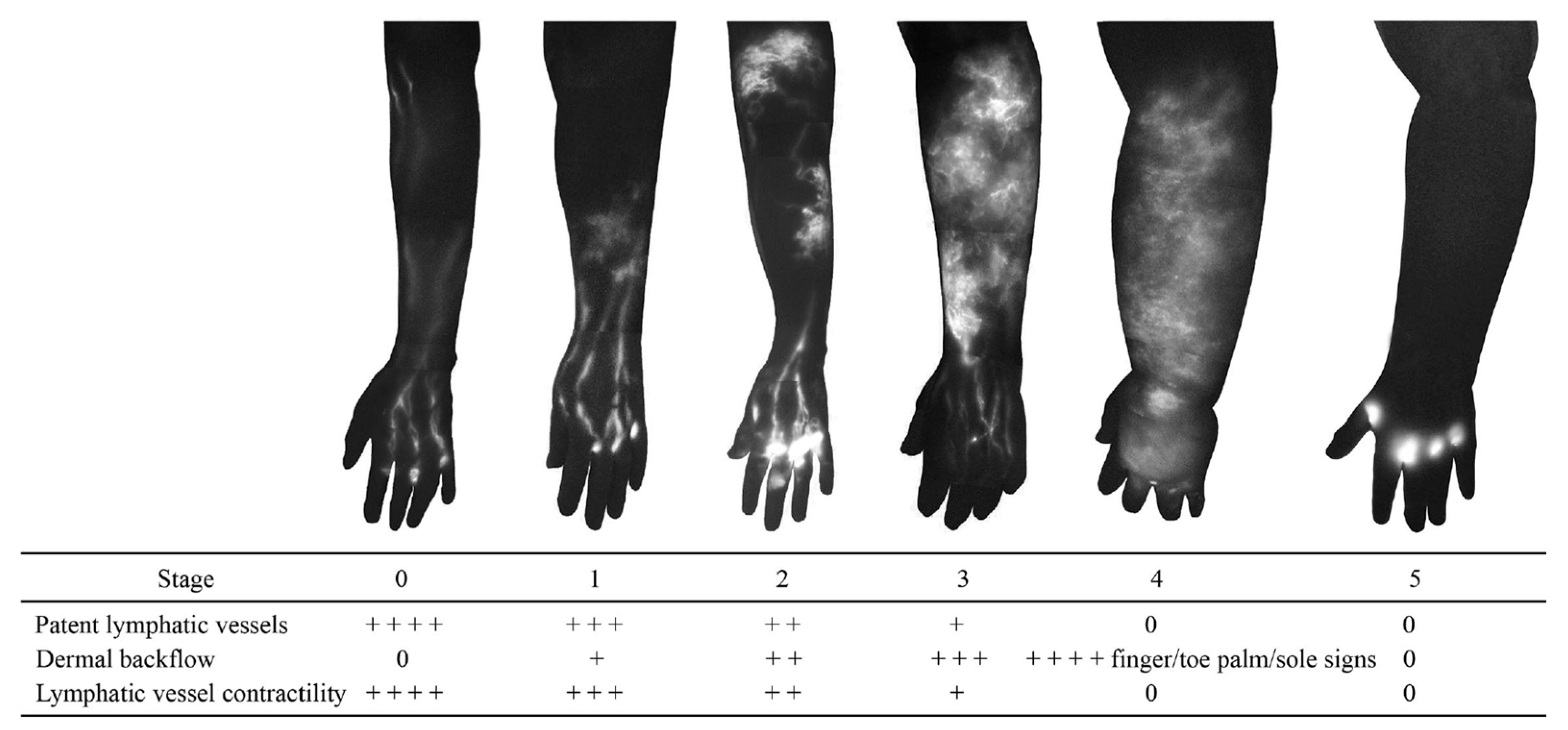
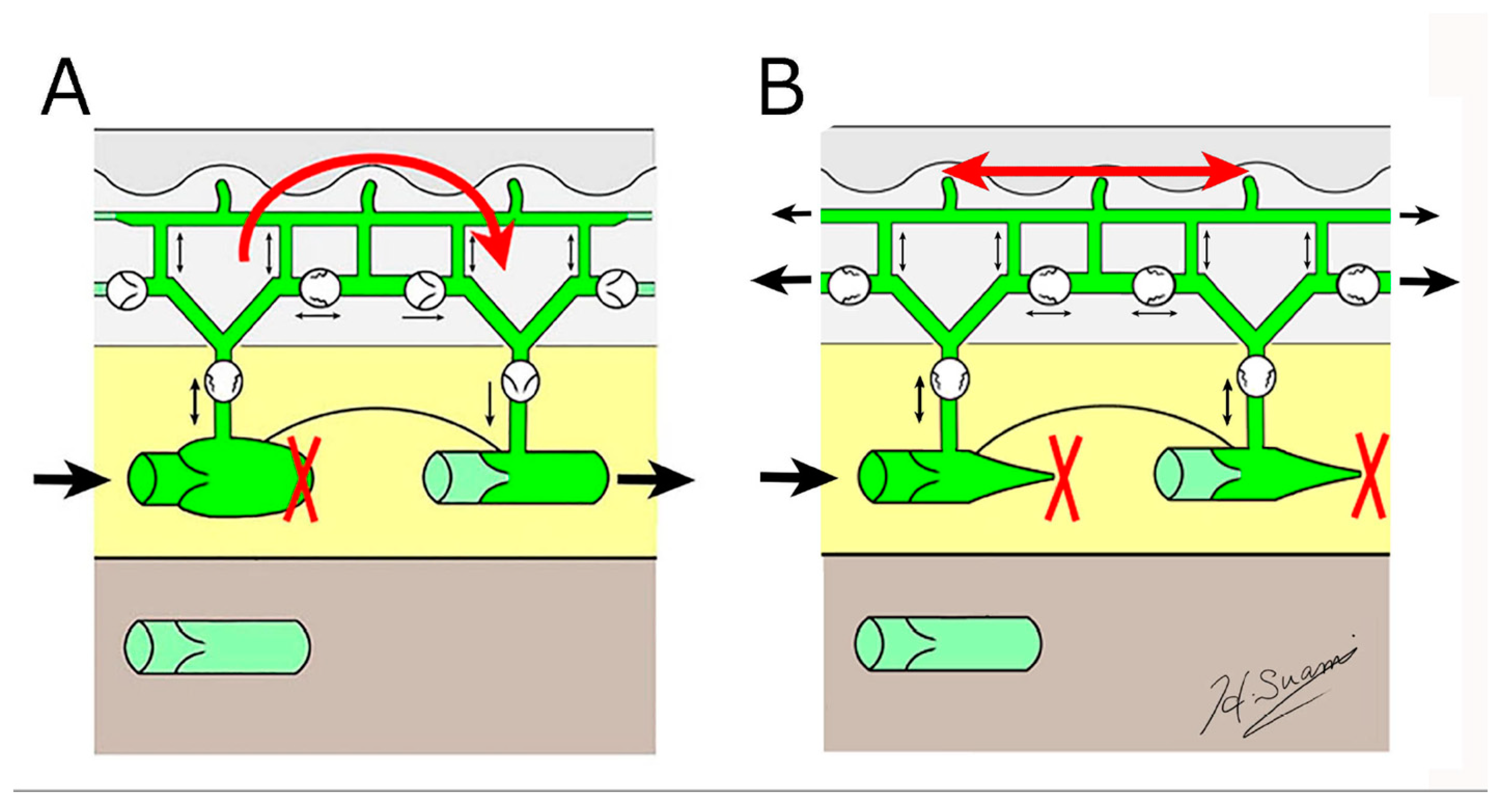
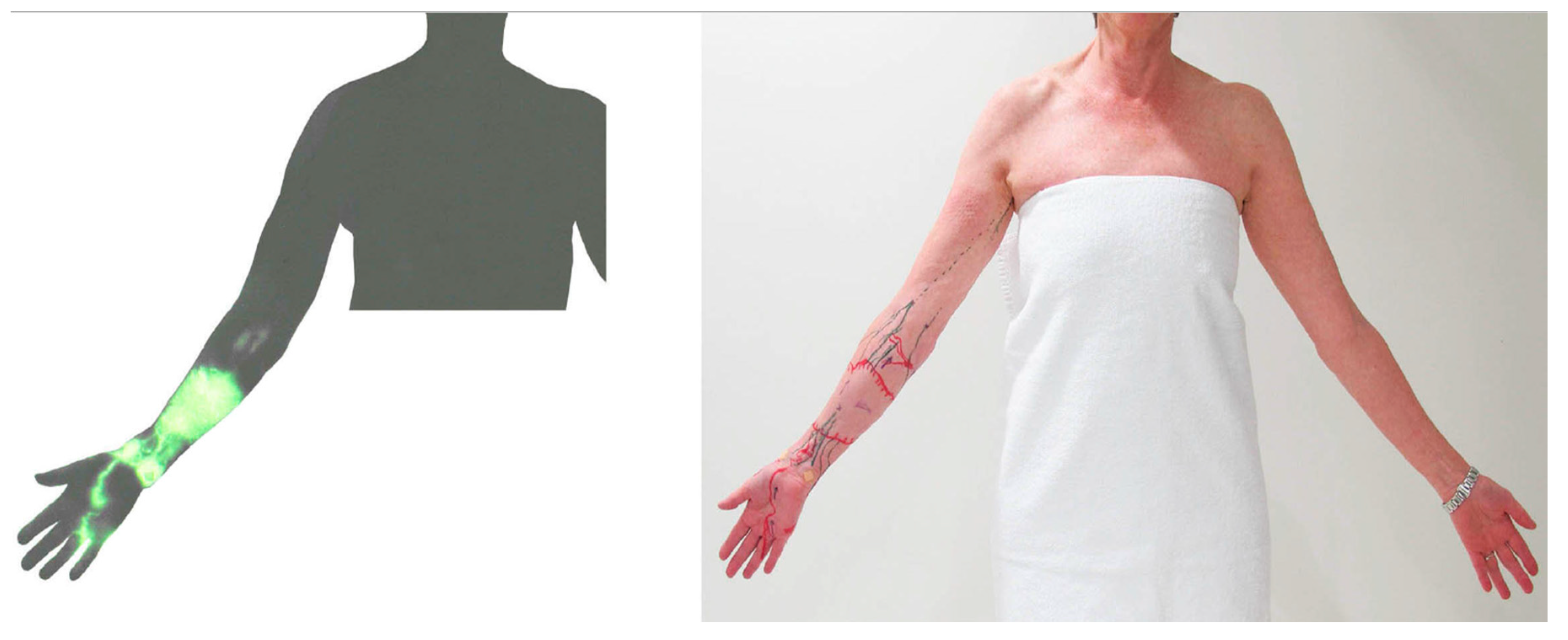
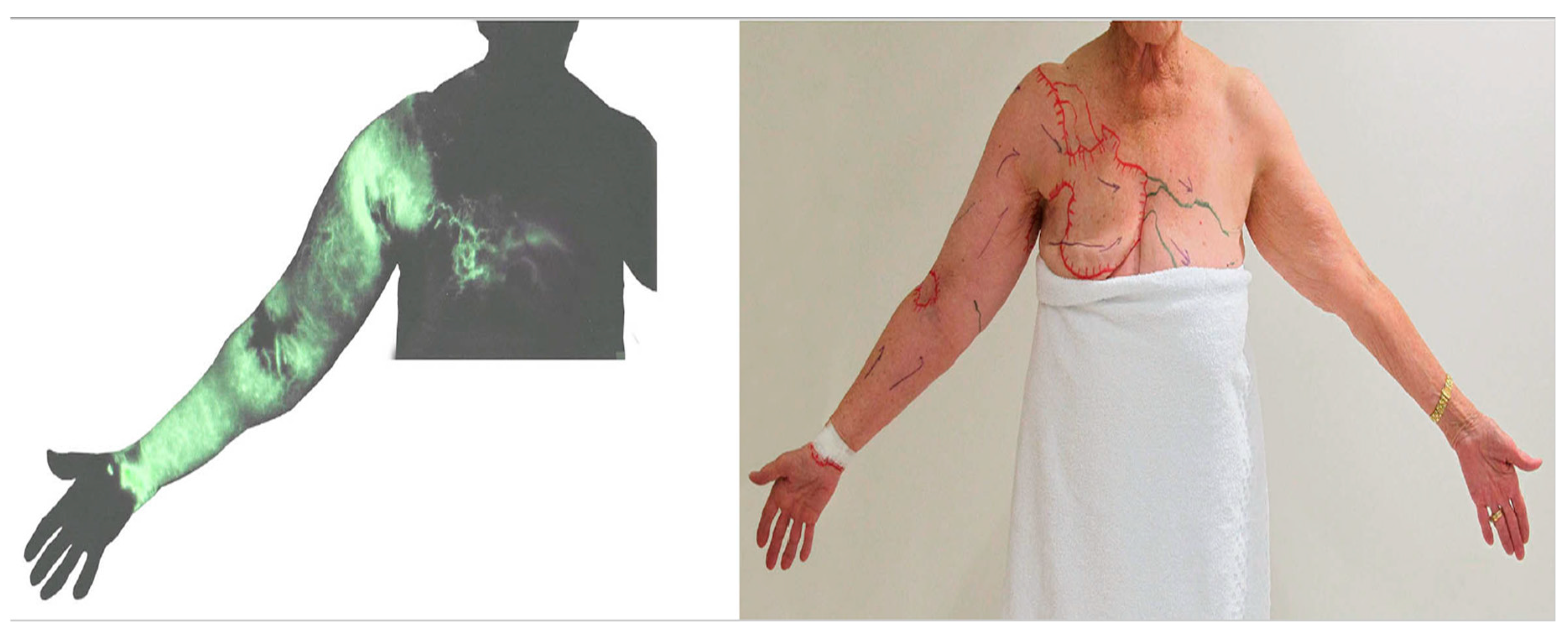
3.1. Dermal Backflow
3.2. Lymphangiogenesis
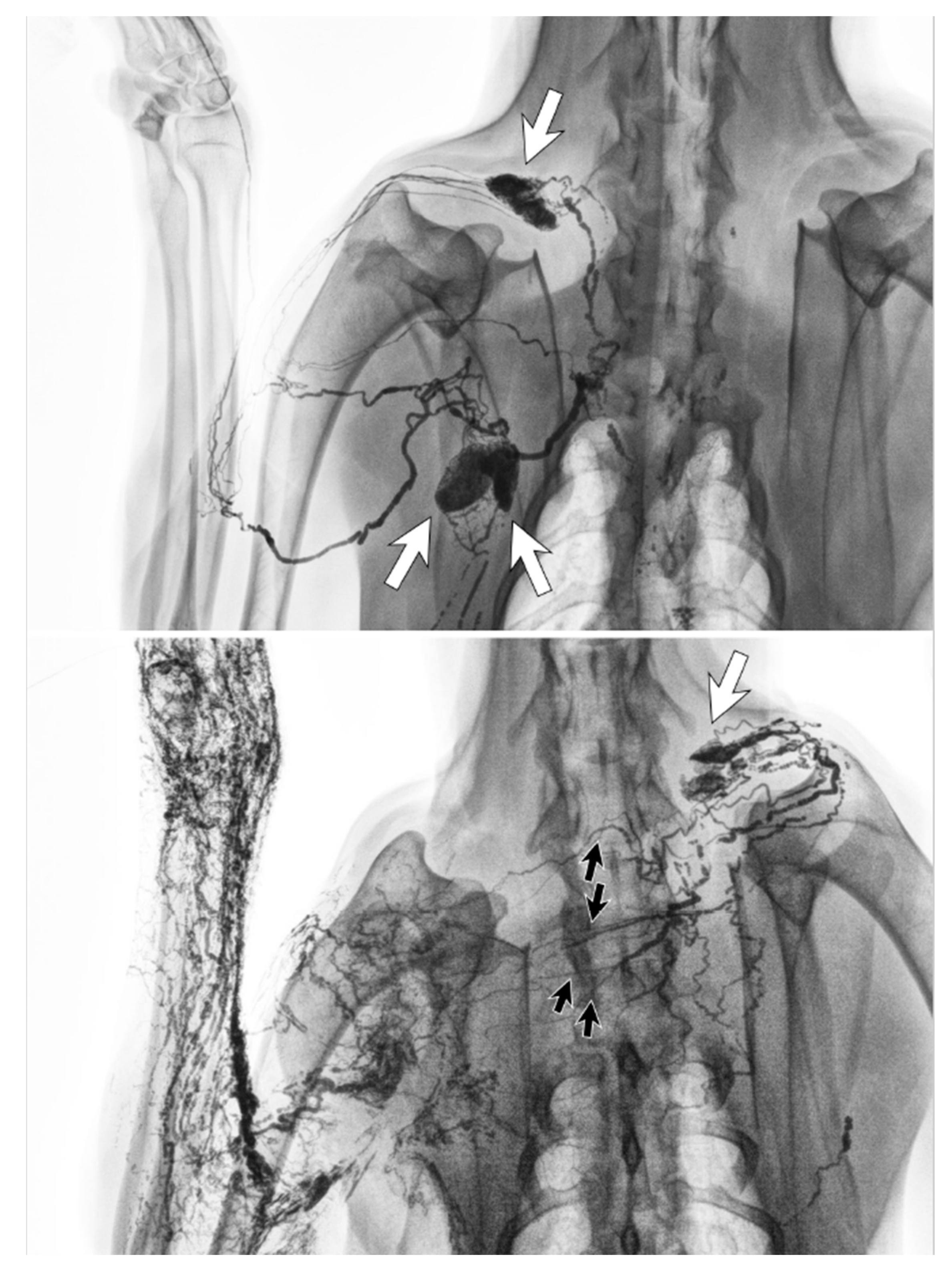
3.3. Detour via the Deep Lymphatic System
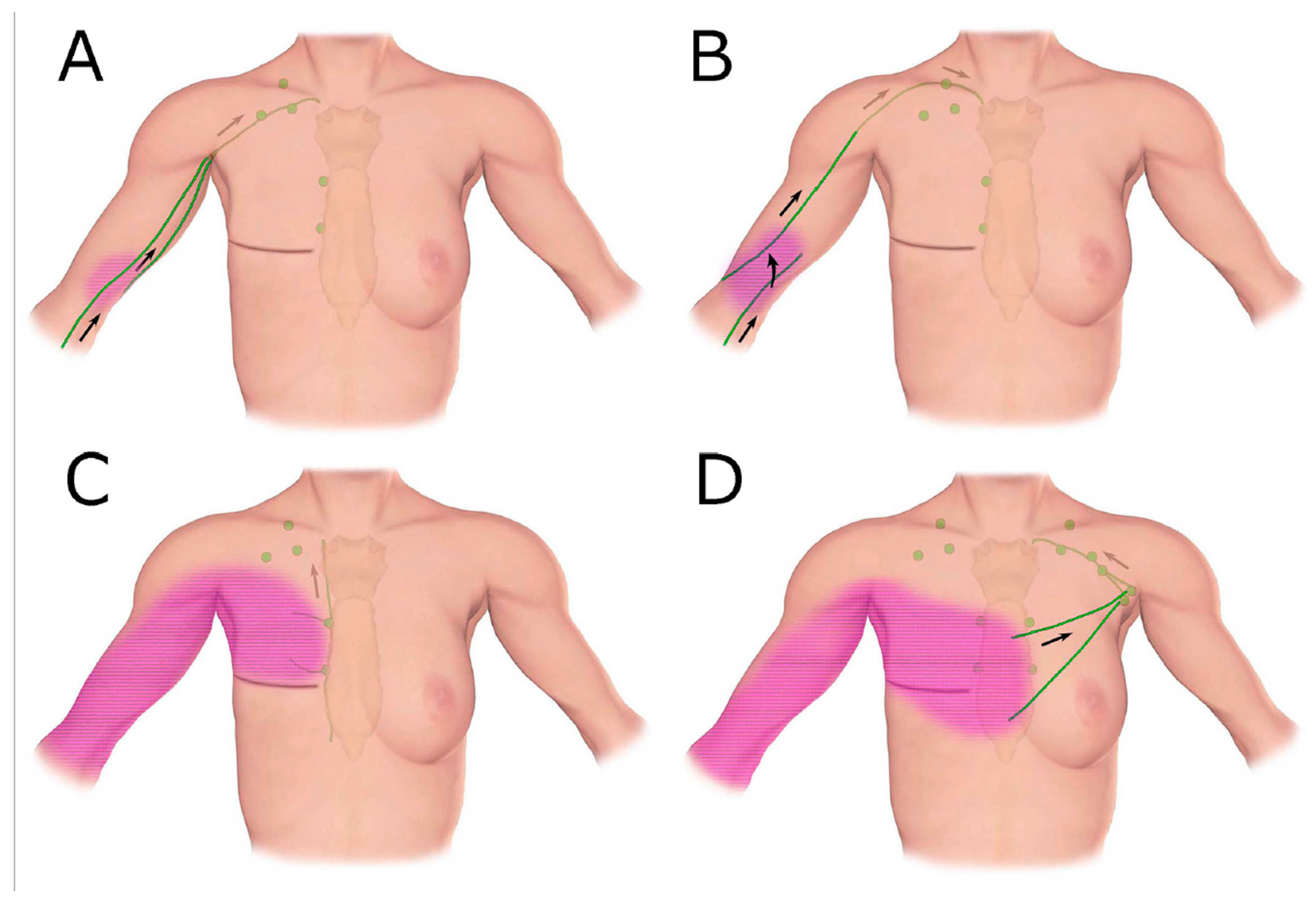
3.4. Detour via the Lymphatics in The Torso
4. Anatomical Theories of Cancer-Related Lymphoedema
4.1. Lymph Node Dissection
4.2. Lymphangiogenesis
4.3. Latent Phase
4.4. Development of Lymphoedema
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, B.B.; Andrade, M.; Antignani, P.L.; Boccardo, F.; Bunke, N.; Campisi, C.; Damstra, R.; Flour, M.; Forner-Cordero, I.; Gloviczki, P.; et al. Diagnosis and treatment of primary lymphedema. Consensus document of the International Union of Phlebology (IUP)-2013. Int. Angiol. 2013, 32, 541–574. [Google Scholar] [PubMed]
- Mehrara, B.; Zampell, J.C.; Suami, H.; Chang, D.W. Surgical Management of Lymphedema: Past, Present, and Future. Lymphat. Res. Biol. 2011, 9, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Suami, H.; Chang, D.W. Overview of Surgical Treatments for Breast Cancer–Related Lymphedema. Plast. Reconstr. Surg. 2010, 126, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Boyages, J.; Xu, Y.; Kalfa, S.; Koelmeyer, L.A.; Parkinson, B.; Mackie, H.; Viveros, H.; Gollan, P.; Taksa, L. Financial cost of lymphedema borne by women with breast cancer. Psycho-Oncology 2016, 26, 849–855. [Google Scholar] [CrossRef] [PubMed]
- McDuff, S.G.; Mina, A.I.; Brunelle, C.L.; Salama, L.; Warren, L.E.; Abouegylah, M.; Swaroop, M.; Skolny, M.N.; Asdourian, M.; Gillespie, T.; et al. Timing of Lymphedema After Treatment for Breast Cancer: When Are Patients Most At Risk? Int. J. Radiat. Oncol. 2019, 103, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.; Chang, J.; Choi, S.; Kim, Y. Risk of Lymphedema Following Contemporary Treatment for Breast Cancer: An Analysis of 7,426 Consecutive Patients from a Multidisciplinary Perspective. Int. J. Radiat. Oncol. 2019, 105, E52. [Google Scholar] [CrossRef]
- Gillespie, T.C.; Sayegh, H.E.; Brunelle, C.L.; Daniell, K.M.; Taghian, A.G. Breast cancer-related lymphedema: Risk factors, precautionary measures, and treatments. Gland Surg. 2018, 7, 379–403. [Google Scholar] [CrossRef]
- Zou, L.; Liu, F.-H.; Shen, P.-P.; Hu, Y.; Liu, X.-Q.; Xu, Y.-Y.; Pen, Q.-L.; Wang, B.; Zhu, Y.-Q.; Tian, Y. The incidence and risk factors of related lymphedema for breast cancer survivors post-operation: A 2-year follow-up prospective cohort study. Breast Cancer 2018, 25, 309–314. [Google Scholar] [CrossRef]
- Frueh, F.S.; Gousopoulos, E.; Rezaeian, F.; Menger, M.D.; Lindenblatt, N.; Giovanoli, P.; Information, P.E.K.F.C. Animal models in surgical lymphedema research—A systematic review. J. Surg. Res. 2016, 200, 208–220. [Google Scholar] [CrossRef]
- Hadamitzky, C.; Pabst, R. Acquired Lymphedema: An Urgent Need for Adequate Animal Models. Cancer Res. 2008, 68, 343–345. [Google Scholar] [CrossRef]
- Witte, C.L.; Witte, M.H.; Unger, E.C.; Williams, W.H.; Bernas, M.J.; McNeill, G.C.; Stazzone, A.M. Advances in Imaging of Lymph Flow Disorders. Radiographics 2000, 20, 1697–1719. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.F.; Lu, Q.; Jiang, Z.-H.; Wang, C.-G.; Zhou, J.-G. Anatomic and functional evaluation of the lymphatics and lymph nodes in diagnosis of lymphatic circulation disorders with contrast magnetic resonance lymphangiography. J. Vasc. Surg. 2009, 49, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Unno, N.; Inuzuka, K.; Suzuki, M.; Yamamoto, N.; Sagara, D.; Nishiyama, M.; Konno, H. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J. Vasc. Surg. 2007, 45, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Suami, H.; Chang, D.W.; Yamada, K.; Kimata, Y. Use of Indocyanine Green Fluorescent Lymphography for Evaluating Dynamic Lymphatic Status. Plast. Reconstr. Surg. 2011, 127, 74e–76e. [Google Scholar] [CrossRef]
- Aldrich, M.B.; Guilliod, R.; Fife, C.E.; Maus, E.A.; Smith, L.; Rasmussen, J.C.; Sevick-Muraca, E.M. Lymphatic abnormalities in the normal contralateral arms of subjects with breast cancer-related lymphedema as assessed by near-infrared fluorescent imaging. Biomed. Opt. Express 2012, 3, 1256–1265. [Google Scholar] [CrossRef]
- Yamamoto, T.; Narushima, M.; Doi, K.; Oshima, A.; Ogata, F.; Mihara, M.; Koshima, I.; Mundinger, G.S. Characteristic Indocyanine Green Lymphography Findings in Lower Extremity Lymphedema: The Generation of a Novel Lymphedema Severity Staging System Using Dermal Backflow Patterns. Plast. Reconstr. Surg. 2011, 127, 1979–1986. [Google Scholar] [CrossRef]
- Suami, H.; Heydon-White, A.; Mackie, H.; Czerniec, S.; Koelmeyer, L.A.; Boyages, J. A new indocyanine green fluorescence lymphography protocol for identification of the lymphatic drainage pathway for patients with breast cancer-related lymphoedema. BMC Cancer 2019, 19, 985–987. [Google Scholar] [CrossRef]
- Suami, H.; Kato, S. Anatomy of the Lymphatic System and Its Structural Disorders in Lymphoedema, 2nd ed.; Lee, B.B., Rockson, S.G., Bergan, J., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 57–78. [Google Scholar]
- Suami, H.; Taylor, G.I.; Pan, W.-R. The Lymphatic Territories of the Upper Limb: Anatomical Study and Clinical Implications. Plast. Reconstr. Surg. 2007, 119, 1813–1822. [Google Scholar] [CrossRef]
- Brorson, H.; Ohlin, K.; Olsson, G.; Nilsson, M. Adipose Tissue Dominates Chronic Arm Lymphedema Following Breast Cancer: An Analysis Using Volume Rendered CT Images. Lymphat. Res. Biol. 2006, 4, 199–210. [Google Scholar] [CrossRef]
- Brorson, H.; Ohlin, K.; Olsson, G.; Karlsson, M.K. Breast Cancer-Related Chronic Arm Lymphedema Is Associated with Excess Adipose and Muscle Tissue. Lymphat. Res. Biol. 2009, 7, 3–10. [Google Scholar] [CrossRef]
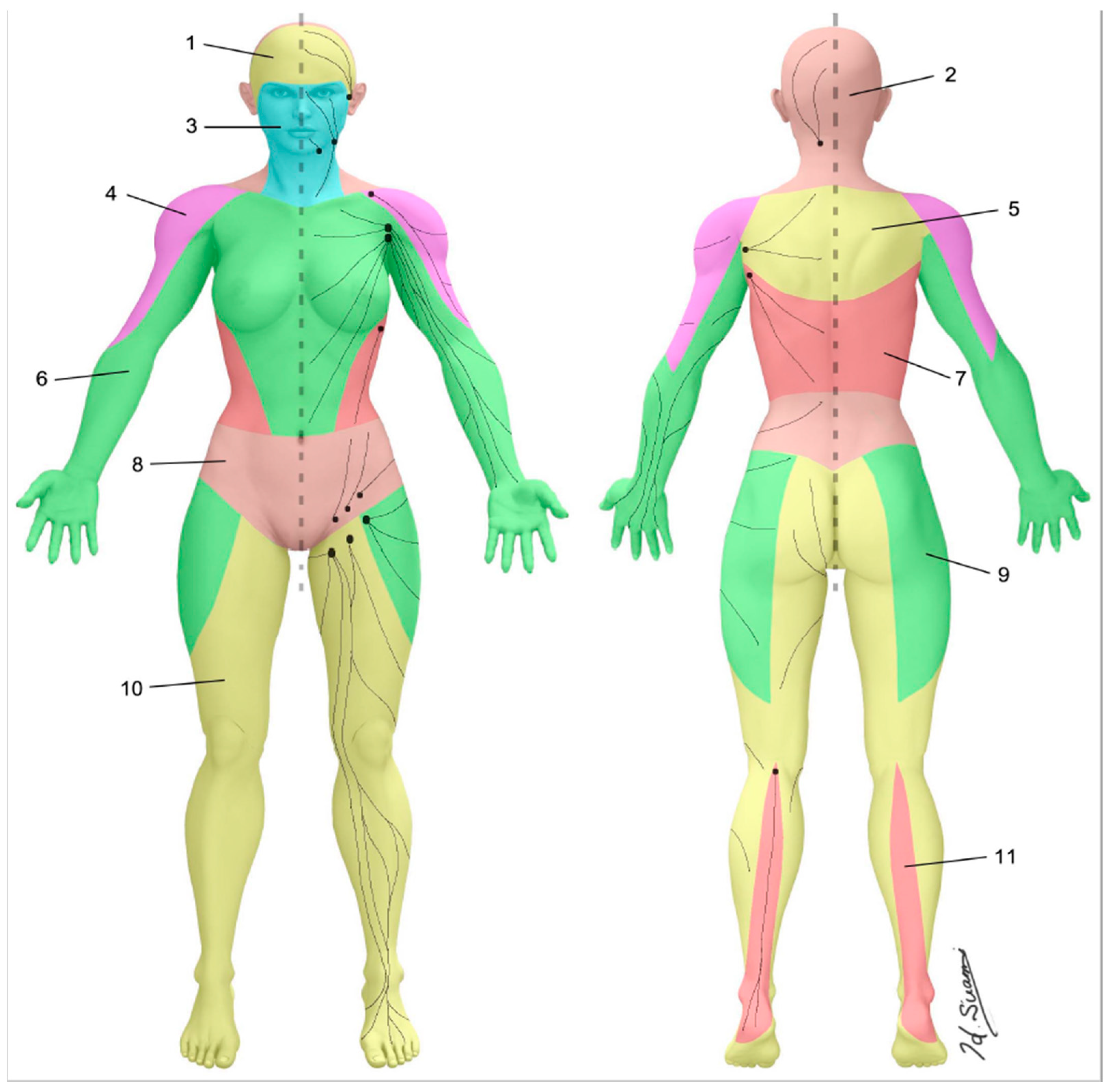
- Suami, H. Lymphosome concept: Anatomical study of the lymphatic system. J. Surg. Oncol. 2016, 115, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, M.F.; Suami, H. Anatomy of the Lymphatic System and the Lymphosome Concept with Reference to Lymphedema. Semin. Plast. Surg. 2018, 32, 005–011. [Google Scholar] [CrossRef] [PubMed]
- Kubik, S. The role of the lateral upper arm bundle and the lymphatic watersheds in the formation of collateral pathways in lymphedema. Acta Biol. Acad. Sci. Hung. 1980, 31, 191–200. [Google Scholar] [PubMed]
- Johnson, A.R.; Bravo, M.G.; James, T.A.; Suami, H.; Lee, B.T.; Singhal, D. The All but Forgotten Mascagni–Sappey Pathway: Learning from Immediate Lymphatic Reconstruction. J. Reconstr. Microsurg. 2019, 36, 028–031. [Google Scholar] [CrossRef]
- Shinaoka, A.; Koshimune, S.; Yamada, K.; Kumagishi, K.; Suami, H.; Kimata, Y.; Ohtsuka, A. Correlations between Tracer Injection Sites and Lymphatic Pathways in the Leg. Plast. Reconstr. Surg. 2019, 144, 634–642. [Google Scholar] [CrossRef]
- Shinaoka, A.; Koshimune, S.; Suami, H.; Yamada, K.; Kumagishi, K.; Boyages, J.; Kimata, Y.; Ohtsuka, A. Lower-Limb Lymphatic Drainage Pathways and Lymph Nodes: A CT Lymphangiography Cadaver Study. Radiology 2019, 294, 223–229. [Google Scholar] [CrossRef]
- Suami, H.; Pan, W.-R.; Taylor, G.I. Changes in the Lymph Structure of the Upper Limb after Axillary Dissection: Radiographic and Anatomical Study in a Human Cadaver. Plast. Reconstr. Surg. 2007, 120, 982–991. [Google Scholar] [CrossRef]
- Sappey, M.P.C. Amatomie, Physiologie, Pathologie des Vaisseaux lymphatiques Consideres Chez l’Homme et les Vertebres; A Delahaye & E Lecroisnier: Paris, France, 1874. [Google Scholar]
- Kinmonth, J.B. The Lymphatics: Diseases, Lymphography and Surgery; Edward Arnold: London, UK, 1972. [Google Scholar]
- Weissleder, H.; Weissleder, R. Lymphedema: Evaluation of qualitative and quantitative lymphoscintigraphy in 238 patients. Radiology 1988, 167, 729–735. [Google Scholar] [CrossRef]
- Suami, H.; Pan, W.-R.; I Taylor, G. The lymphatics of the skin filled by a dermal backflow: An observation in a scarred cadaver leg. Lymphology 2007, 40, 122–126. [Google Scholar]
- Chang, D.W.; Suami, H. Discussion: Comparison of indocyanine green lymphographic findings with the conditions of collecting lymphatic vessels of limbs in patients with lymphedema. Plast. Reconstr. Surg. 2013, 132, 1619–1621. [Google Scholar] [CrossRef]
- Chang, D.W.; Suami, H.; Skoracki, R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast. Reconstr. Surg. 2013, 132, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Suami, H.; Hanasono, M.M.; Womack, V.A.; Wong, F.C.; Chang, E.I. Long-term outcomes of the minimally invasive free vascularized omental lymphatic flap for the treatment of lymphedema. J. Surg. Oncol. 2016, 115, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Suami, H.; Scaglioni, M.F.; Dixon, K.A.; Tailor, R.C. Interaction between vascularized lymph node transfer and recipient lymphatics after lymph node dissection-a pilot study in a canine model. J. Surg. Res. 2016, 204, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Suami, H.; Yamashita, S.; Soto-Miranda, M.A.; Chang, D.W. Lymphatic Territories (Lymphosomes) in a Canine: An Animal Model for Investigation of Postoperative Lymphatic Alterations. PLoS ONE 2013, 8, e69222. [Google Scholar] [CrossRef] [PubMed]
- Tsangaris, N.T.; Yutzy, C.V. A lymphangiographic study of postmastectomy lymphedema. Surg. Gynecol. Obstet. 1966, 123, 1228–1232. [Google Scholar]
- Abe, R. A Study on the Pathogenesis of Postmastectomy Lymphedema. Tohoku J. Exp. Med. 1976, 118, 163–171. [Google Scholar] [CrossRef]
- Bourgeois, P.; Frühling, J.; Henry, J. Jacques Postoperative axillary lymphoscintigraphy in the management of breast cancer. Int. J. Radiat. Oncol. 1983, 9, 29–32. [Google Scholar] [CrossRef]
- Szuba, A.; Chachaj, Z.; Koba-Wszedybylb, M.; Hawro, R.; Jasinski, R.; Tarkowski, R.; Szewczyk, K.; Bebenek, M.; Forgacz, J.; Jodkowska, A.; et al. Axillary lymph nodes and arm lymphatic drainage pathways are spared during routine complete axillary clearance in majority of women undergoing breast cancer surgery. Lymphology 2011, 44, 103–112. [Google Scholar]
- Suami, H.; Koelmeyer, L.A.; Mackie, H.; Boyages, J. Patterns of lymphatic drainage after axillary node dissection impact arm lymphoedema severity: A review of animal and clinical imaging studies. Surg. Oncol. 2018, 27, 743–750. [Google Scholar] [CrossRef]
- Suami, H.; O’neill, J.K.; Pan, W.-R.; Taylor, G.I. Superficial Lymphatic System of the Upper Torso: Preliminary Radiographic Results in Human Cadavers. Plast. Reconstr. Surg. 2008, 121, 1231–1239. [Google Scholar] [CrossRef]
- Lagendijk, M.; Van Maaren, M.C.; Saadatmand, S.; Strobbe, L.J.; Poortmans, P.M.; Koppert, L.B.; Tilanus-Linthorst, M.M.; Siesling, S. Breast conserving therapy and mastectomy revisited: Breast cancer-specific survival and the influence of prognostic factors in 129,692 patients. Int. J. Cancer 2017, 142, 165–175. [Google Scholar] [CrossRef]
- Berry, M.; Fitoussi, A.; Curnier, A.; Couturaud, B.; Salmon, R. Oncoplastic breast surgery: A review and systematic approach. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, N.; Belsack, D.; Buyl, R.; Ruggiero, L.; Breucq, C.; De Mey, J.; Lievens, P.; Lamote, J. Ultrasound elastography as an objective diagnostic measurement tool for lymphoedema of the treated breast in breast cancer patients following breast conserving surgery and radiotherapy. Radiol. Oncol. 2012, 46, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, W. Pathophysiological and clinical observations of obstructive lymphedema of the limbs. In Lymphedema; Clodius, L., Ed.; Georg Thieme Verlag: Stuttgar, Germany, 1977; pp. 79–102. [Google Scholar]
- Akita, S.; Nakamura, R.; Yamamoto, N.; Tokumoto, H.; Ishigaki, T.; Yamaji, Y.; Sasahara, Y.; Kubota, Y.; Mitsukawa, N.; Satoh, K. Early Detection of Lymphatic Disorder and Treatment for Lymphedema following Breast Cancer. Plast. Reconstr. Surg. 2016, 138, 192e–202e. [Google Scholar] [CrossRef] [PubMed]
- Tervala, T.V.; Hartiala, P.; Tammela, T.; Visuri, M.T.; Ylä-Herttuala, S.; Alitalo, K.; Saarikko, A.M. Growth factor therapy and lymph node graft for lymphedema. J. Surg. Res. 2015, 196, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Boccardo, F.; Casabona, F.; Decian, F.; Friedman, D.; Murelli, F.; Puglisi, M.; Campisi, C.; Molinari, L.; Spinaci, S.; Dessalvi, S.; et al. Lymphatic Microsurgical Preventing Healing Approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: Over 4 years follow-up. Microsurgery 2014, 34, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Rochlin, D.H.; Inchauste, S.; Zelones, J.; Nguyen, D. The role of adjunct nanofibrillar collagen scaffold implantation in the surgical management of secondary lymphedema: Review of the literature and summary of initial pilot studies. J. Surg. Oncol. 2019, 121, 121–128. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suami, H. Anatomical Theories of the Pathophysiology of Cancer-Related Lymphoedema. Cancers 2020, 12, 1338. https://doi.org/10.3390/cancers12051338
Suami H. Anatomical Theories of the Pathophysiology of Cancer-Related Lymphoedema. Cancers. 2020; 12(5):1338. https://doi.org/10.3390/cancers12051338
Chicago/Turabian StyleSuami, Hiroo. 2020. "Anatomical Theories of the Pathophysiology of Cancer-Related Lymphoedema" Cancers 12, no. 5: 1338. https://doi.org/10.3390/cancers12051338
APA StyleSuami, H. (2020). Anatomical Theories of the Pathophysiology of Cancer-Related Lymphoedema. Cancers, 12(5), 1338. https://doi.org/10.3390/cancers12051338




