Application of In Vivo Imaging Techniques for Monitoring Natural Killer Cell Migration and Tumor Infiltration
Abstract
1. Introduction
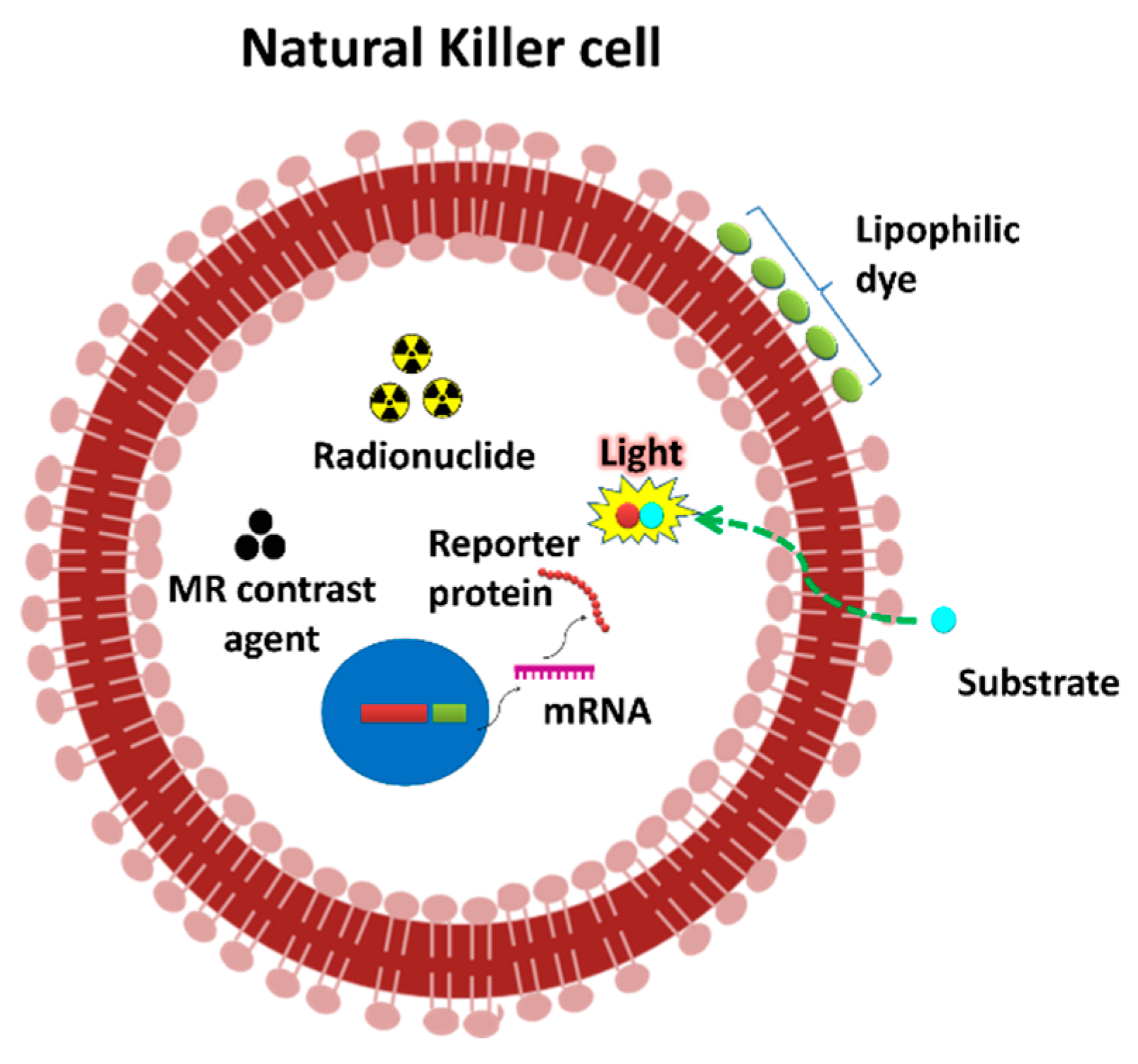
2. Non-Invasive In Vivo Imaging Modalities
3. In Vivo Monitoring of NK Cell Migration and Infiltration into a Tumor by Molecular Imaging
4. Challenges in the NK Cell Expansion and Labeling
5. Future Prospects of In Vivo NK Cell Imaging
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sutlu, T.; Alici, E. Natural killer cell-based immunotherapy in cancer: Current insights and future prospects. J. Intern. Med. 2009, 266, 154–181. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Colonna, M.; Yokoyama, W.M. Hidden talents of natural killers: NK cells in innate and adaptive immunity. EMBO Rep. 2009, 10, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Leung, W. Infusions of Allogeneic Natural Killer Cells as Cancer Therapy. Clin. Cancer Res. 2014, 20, 3390–3400. [Google Scholar] [CrossRef] [PubMed]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef]
- Albertsson, P.A.; Basse, P.H.; Hokland, M.; Goldfarb, R.H.; Nagelkerke, J.F.; Nannmark, U.; Kuppen, P.J.K. NK cells and the tumour microenvironment: Implications for NK-cell function and anti-tumour activity. Trends Immunol. 2003, 24, 603–609. [Google Scholar] [CrossRef]
- Vitale, M.; Cantoni, C.; Pietra, G.; Mingari, M.C.; Moretta, L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur. J. Immunol. 2014, 44, 1582–1592. [Google Scholar] [CrossRef]
- Vanherberghen, B.; Olofsson, P.E.; Forslund, E.; Sternberg-Simon, M.; Khorshidi, M.A.; Pacouret, S.; Guldevall, K.; Enqvist, M.; Malmberg, K.-J.; Mehr, R.; et al. Classification of human natural killer cells based on migration behavior and cytotoxic response. Blood 2013, 121, 1326–1334. [Google Scholar] [CrossRef]
- Gong, J.H.; Maki, G.; Klingemann, H.G. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 1994, 8, 652–658. [Google Scholar]
- Dahlberg, C.I.M.; Sarhan, D.; Chrobok, M.; Duru, A.D.; Alici, E. Natural Killer Cell-Based Therapies Targeting Cancer: Possible Strategies to Gain and Sustain Anti-Tumor Activity. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef]
- Rezvani, K.; Rouce, R.H. The Application of Natural Killer Cell Immunotherapy for the Treatment of Cancer. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef]
- Garrod, K.R.; Wei, S.H.; Parker, I.; Cahalan, M.D. Natural killer cells actively patrol peripheral lymph nodes forming stable conjugates to eliminate MHC-mismatched targets. Proc. Natl. Acad. Sci. USA 2007, 104, 12081–12086. [Google Scholar] [CrossRef]
- Fang, F.; Xiao, W.; Tian, Z. NK cell-based immunotherapy for cancer. Semin. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Mace, E.M. Acquisition of cell migration defines NK cell differentiation from hematopoietic stem cell precursors. bioRxiv 2017, 142380. [Google Scholar] [CrossRef]
- Somersalo, K.; Saksela, E. Fibronectin facilitates the migration of human natural killer cells. Eur. J. Immunol. 1991, 21, 35–42. [Google Scholar] [CrossRef]
- Taub, D.D.; Sayers, T.J.; Carter, C.R.; Ortaldo, J.R. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J. Immunol. 1995, 155, 3877–3888. [Google Scholar]
- Levi, I.; Amsalem, H.; Nissan, A.; Darash-Yahana, M.; Peretz, T.; Mandelboim, O.; Rachmilewitz, J. Characterization of tumor infiltrating natural killer cell subset. Oncotarget 2015, 6, 13835–13843. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, E.; Kremer, V.; Childs, R.; Lundqvist, A. CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol. Immunother. 2015, 64, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kalimuthu, S.; Ahn, B.-C. In vivo cell tracking with bioluminescence imaging. Nucl. Med. Mol. Imaging 2015, 49, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, S.; Jeong, J.H.; Oh, J.M.; Ahn, B.-C. Drug Discovery by Molecular Imaging and Monitoring Therapy Response in Lymphoma. Int. J. Mol. Sci. 2017, 18, 1639. [Google Scholar] [CrossRef]
- Lee, H.W.; Gangadaran, P.; Kalimuthu, S.; Ahn, B.-C. Advances in Molecular Imaging Strategies for In Vivo Tracking of Immune Cells. BioMed Res. Int. 2016, 2016, 1946585. [Google Scholar] [CrossRef]
- Zhu, L.; Li, X.J.; Kalimuthu, S.; Gangadaran, P.; Lee, H.W.; Oh, J.M.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; et al. Natural Killer Cell (NK-92MI)-Based Therapy for Pulmonary Metastasis of Anaplastic Thyroid Cancer in a Nude Mouse Model. Front. Immunol. 2017, 8, 816. [Google Scholar] [CrossRef]
- Li, X.J.; Gangadaran, P.; Kalimuthu, S.; Oh, J.M.; Zhu, L.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Role of pulmonary macrophages in initiation of lung metastasis in anaplastic thyroid cancer. Int. J. Cancer 2016, 139, 2583–2592. [Google Scholar] [CrossRef] [PubMed]
- Hillman, E.M.C.; Amoozegar, C.B.; Wang, T.; McCaslin, A.F.H.; Bouchard, M.B.; Mansfield, J.; Levenson, R.M. In vivo optical imaging and dynamic contrast methods for biomedical research. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 4620–4643. [Google Scholar] [CrossRef] [PubMed]
- Lassailly, F.; Griessinger, E.; Bonnet, D. Microenvironmental contaminations induced by fluorescent lipophilic dyes used for noninvasive in vitro and in vivo cell tracking. Blood 2010, 115, 5347–5354. [Google Scholar] [CrossRef] [PubMed]
- Zinn, K.R.; Chaudhuri, T.R.; Szafran, A.A.; O’Quinn, D.; Weaver, C.; Dugger, K.; Lamar, D.; Kesterson, R.A.; Wang, X.; Frank, S.J. Noninvasive bioluminescence imaging in small animals. ILAR J. 2008, 49, 103–115. [Google Scholar] [CrossRef]
- Ahn, B.-C. Requisites for successful theranostics with radionuclide-based reporter gene imaging. J. Drug Target. 2014, 22, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Matera, L.; Galetto, A.; Bello, M.; Baiocco, C.; Chiappino, I.; Castellano, G.; Stacchini, A.; Satolli, M.A.; Mele, M.; Sandrucci, S.; et al. In vivo migration of labeled autologous natural killer cells to liver metastases in patients with colon carcinoma. J. Transl. Med. 2006, 4, 49. [Google Scholar] [CrossRef]
- Meller, B.; Frohn, C.; Brand, J.-M.; Lauer, I.; Schelper, L.F.; von Hof, K.; Kirchner, H.; Richter, E.; Baehre, M. Monitoring of a new approach of immunotherapy with allogenic (111)In-labelled NK cells in patients with renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 403–407. [Google Scholar] [CrossRef]
- Rahmim, A.; Zaidi, H. PET versus SPECT: Strengths, limitations and challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef]
- Hengerer, A.; Grimm, J. Molecular magnetic resonance imaging. Biomed. Imaging Interv. J. 2006, 2, e8. [Google Scholar] [CrossRef]
- Schick, F. Whole-body MRI at high field: Technical limits and clinical potential. Eur. Radiol. 2005, 15, 946–959. [Google Scholar] [CrossRef] [PubMed]
- Sheu, A.Y.; Zhang, Z.; Omary, R.A.; Larson, A.C. MRI-monitored transcatheter intra-arterial delivery of SPIO-labeled natural killer cells to hepatocellular carcinoma: Preclinical studies in a rodent model. Investig. Radiol. 2013, 48, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Bouchlaka, M.N.; Ludwig, K.D.; Gordon, J.W.; Kutz, M.P.; Bednarz, B.P.; Fain, S.B.; Capitini, C.M. (19)F-MRI for monitoring human NK cells in vivo. Oncoimmunology 2016, 5, e1143996. [Google Scholar] [CrossRef] [PubMed]
- Mallett, C.L.; McFadden, C.; Chen, Y.; Foster, P.J. Migration of iron-labeled KHYG-1 natural killer cells to subcutaneous tumors in nude mice, as detected by magnetic resonance imaging. Cytotherapy 2012, 14, 743–751. [Google Scholar] [CrossRef]
- Galli, F.; Rapisarda, A.S.; Stabile, H.; Malviya, G.; Manni, I.; Bonanno, E.; Piaggio, G.; Gismondi, A.; Santoni, A.; Signore, A. In Vivo Imaging of Natural Killer Cell Trafficking in Tumors. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2015, 56, 1575–1580. [Google Scholar] [CrossRef]
- Lim, Y.T.; Cho, M.Y.; Noh, Y.-W.; Chung, J.W.; Chung, B.H. Near-infrared emitting fluorescent nanocrystals-labeled natural killer cells as a platform technology for the optical imaging of immunotherapeutic cells-based cancer therapy. Nanotechnology 2009, 20, 475102. [Google Scholar] [CrossRef]
- Daldrup-Link, H.E.; Meier, R.; Rudelius, M.; Piontek, G.; Piert, M.; Metz, S.; Settles, M.; Uherek, C.; Wels, W.; Schlegel, J.; et al. In vivo tracking of genetically engineered, anti-HER2/neu directed natural killer cells to HER2/neu positive mammary tumors with magnetic resonance imaging. Eur. Radiol. 2005, 15, 4–13. [Google Scholar] [CrossRef]
- Meier, R.; Golovko, D.; Tavri, S.; Henning, T.D.; Knopp, C.; Piontek, G.; Rudelius, M.; Heinrich, P.; Wels, W.S.; Daldrup-Link, H. Depicting adoptive immunotherapy for prostate cancer in an animal model with magnetic resonance imaging. Magn. Reson. Med. 2011, 65, 756–763. [Google Scholar] [CrossRef]
- Jang, E.-S.; Shin, J.-H.; Ren, G.; Park, M.-J.; Cheng, K.; Chen, X.; Wu, J.C.; Sunwoo, J.B.; Cheng, Z. The manipulation of natural killer cells to target tumor sites using magnetic nanoparticles. Biomaterials 2012, 33, 5584–5592. [Google Scholar] [CrossRef]
- Tavri, S.; Jha, P.; Meier, R.; Henning, T.D.; Müller, T.; Hostetter, D.; Knopp, C.; Johansson, M.; Reinhart, V.; Boddington, S.; et al. Optical imaging of cellular immunotherapy against prostate cancer. Mol. Imaging 2009, 8, 15–26. [Google Scholar] [CrossRef]
- Uong, T.N.T.; Lee, K.-H.; Ahn, S.-J.; Kim, K.W.; Min, J.-J.; Hyun, H.; Yoon, M.S. Real-Time Tracking of Ex Vivo-Expanded Natural Killer Cells Toward Human Triple-Negative Breast Cancers. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, T.H.; Yoo, W.; Choi, H.; Jo, S.; Kong, K.; Lee, S.-R.; Kim, S.-U.; Kim, J.-S.; Cho, D.; et al. An Antibody Designed to Improve Adoptive NK-Cell Therapy Inhibits Pancreatic Cancer Progression in a Murine Model. Cancer Immunol. Res. 2019, 7, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cabello, J.; Barnett, B.P.; Bottomley, P.A.; Bulte, J.W.M. Fluorine (19F) MRS and MRI in biomedicine. NMR Biomed. 2011, 24, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Wang, X.; Zheng, L.; Lyu, T.; Figini, M.; Wang, B.; Procissi, D.; Shangguan, J.; Sun, C.; Pan, L.; et al. MRI-guided interventional natural killer cell delivery for liver tumor treatment. Cancer Med. 2018, 7, 1860–1869. [Google Scholar] [CrossRef]
- Melder, R.J.; Brownell, A.L.; Shoup, T.M.; Brownell, G.L.; Jain, R.K. Imaging of activated natural killer cells in mice by positron emission tomography: Preferential uptake in tumors. Cancer Res. 1993, 53, 5867–5871. [Google Scholar]
- Parashurama, N.; Ahn, B.-C.; Ziv, K.; Ito, K.; Paulmurugan, R.; Willmann, J.K.; Chung, J.; Ikeno, F.; Swanson, J.C.; Merk, D.R.; et al. Multimodality Molecular Imaging of Cardiac Cell Transplantation: Part II. In Vivo Imaging of Bone Marrow Stromal Cells in Swine with PET/CT and MR Imaging. Radiology 2016, 280, 826–836. [Google Scholar] [CrossRef]
- Townsend, D.W. Physical principles and technology of clinical PET imaging. Ann. Acad. Med. Singap. 2004, 33, 133–145. [Google Scholar]
- Meier, R.; Piert, M.; Piontek, G.; Rudelius, M.; Oostendorp, R.A.; Senekowitsch-Schmidtke, R.; Henning, T.D.; Wels, W.S.; Uherek, C.; Rummeny, E.J.; et al. Tracking of [18F]FDG-labeled natural killer cells to HER2/neu-positive tumors. Nucl. Med. Biol. 2008, 35, 579–588. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, H.; Diao, Y. Natural Killer Cells and Current Applications of Chimeric Antigen Receptor-Modified NK-92 Cells in Tumor Immunotherapy. Int. J. Mol. Sci. 2019, 20, 317. [Google Scholar] [CrossRef]
- Klingemann, H.; Boissel, L.; Toneguzzo, F. Natural Killer Cells for Immunotherapy—Advantages of the NK-92 Cell Line over Blood NK Cells. Front. Immunol. 2016, 7, 91. [Google Scholar] [CrossRef]
- Williams, B.A.; Law, A.D.; Routy, B.; denHollander, N.; Gupta, V.; Wang, X.-H.; Chaboureau, A.; Viswanathan, S.; Keating, A. A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget 2017, 8, 89256–89268. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Meagher, R.; Swearingen, M.; Myint, H.; Rich, E.; Martinson, J.; Klingemann, H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: A phase I trial. Cytotherapy 2008, 10, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.C.; Zhang, C.; Harter, P.N.; Romanski, A.; Strassheimer, F.; Senft, C.; Tonn, T.; Steinbach, J.P.; Wels, W.S. CAR-Engineered NK Cells for the Treatment of Glioblastoma: Turning Innate Effectors Into Precision Tools for Cancer Immunotherapy. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiang, J.; Wu, C. CAR-NK for tumor immunotherapy: Clinical transformation and future prospects. Cancer Lett. 2020, 472, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Golovko, D.; Bains, S.; Hostetter, D.; Meier, R.; Wendland, M.F.; Daldrup-Link, H.E. Monitoring of natural killer cell immunotherapy using noninvasive imaging modalities. Cancer Res. 2010, 70, 6109–6113. [Google Scholar] [CrossRef]
- Gangadaran, P.; Ahn, B.-C. Molecular Imaging: A Useful Tool for the Development of Natural Killer Cell-Based Immunotherapies. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Scarfe, L.; Taylor, A.; Sharkey, J.; Harwood, R.; Barrow, M.; Comenge, J.; Beeken, L.; Astley, C.; Santeramo, I.; Hutchinson, C.; et al. Non-invasive imaging reveals conditions that impact distribution and persistence of cells after in vivo administration. Stem Cell Res. Ther. 2018, 9, 332. [Google Scholar] [CrossRef]
- Shapovalova, M.; Pyper, S.R.; Moriarity, B.S.; LeBeau, A.M. The Molecular Imaging of Natural Killer Cells. Mol. Imaging 2018, 17. [Google Scholar] [CrossRef]

| Imaging | Imaging Modality | Labeling Method/Agent | Cell Type | Naïve/Modified Cell | Subject | Route of Injection | Duration | Migration/Infiltration to Tumor | Clinical Translation | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Optical Imaging | FLI | NIR dye | NK92MI | Naïve | Mice | Intratumor | 24 h | Infiltrated to melanoma | Limited | [36] |
| Cy5.5 | NK92MI | Naïve | Mice | Intravenous | Immediate | Migrated and Infiltrated to B cell lymphoma | Limited | [39] | ||
| DiR | Primary NK | Naïve | Mice | Intravenous | 5 days | Migrated to CXCL10 expressing melanoma | Limited | [17] | ||
| DiD | NK-92 | NK-92-scFv(MOC31)-zeta | Mice | Intravenous | 1.5, 8 and 25 h | Migrated to EpCAM expressing prostate cancer | Limited | [40] | ||
| DiR | Primary NK | NRP-body | Mice | Intravenous | 5 days | Infiltrated to pancreatic cancer | Limited | [42] | ||
| ESNF13 | Primary NK | Naïve | Mice | Intravenous | 0.5, 1, 2 and 4 h | Migrated to lung metastatic and xenograft breast cancer | Limited | [41] | ||
| BLI | Fluc | NK92MI | Naïve | Mice | Intravenous | 1, 3, 24 and 48 h | Migrated to lung metastatic thyroid cancer | Limited | [21] | |
| Fluc | NK92MI | Naïve | Mice | Intravenous | 1, 3, 24 and 48 h | Migrated to xenograft thyroid cancer | Limited | [21] | ||
| Magnetic Resonance Imaging | MRI | ferumoxides | NK-92 | NK-92-scFv (MOC31)-zeta | Rat | Intraperitoneal | 1 and 24 h | Migrated to EpCAM expressing prostate cancer | Yes | [38] |
| ferucarbotran | NK-92 | NK-92-scFv (FRP5)-zeta | Mice | Intravenous | 12 and 24 h | Migrated to HER2/neu positive NIH3T3 mammary tumors | Yes | [37] | ||
| USPIO | KHYG-1 | Naïve | Mice | Subcutaneous | Migrated to prostate cancer | Yes | [34] | |||
| 19F | Primary NK | Naïve | Mice | Intratumor | 0–2 days | Infiltrated to neuroblastoma | Yes | [33] | ||
| 19F | Primary NK | Naïve | Mice | Intratumor | 0–8 days | Infiltrated to Mantle cell lymphoma | Yes | [33] | ||
| 19F | Primary NK | Naïve | Mice | Subcutaneous | 0–15 days | Infiltrated to melanoma | Yes | [33] | ||
| SPIO | NK92MI | Naïve | Rat | Intraarterial | Immediate | Infiltrated to hepatocellular carcinoma | Yes | [32] | ||
| ferumoxytol | LNK | Naive | Rat | Intravenous and transcatheter | 1, 2 and 8 days | Migrated to hepatocellular carcinoma | Yes | [44] | ||
| Nuclear Imaging | PET | 11C | Murine NK | Naïve | Mice | Intravenous | 30, 60 min | Migrated to fibrosarcoma | Yes | [45] |
| SPECT | 111In-oxine | Primary NK | Naïve | human | Intravenous | 1.5–144 h | Migrated to renal cell carcinoma | Yes | [28] | |
| 111In-oxine | Primary NK | Naïve | human | Intraarterial | 6, 24, 72, 96 h | Liver metastases with colon carcinoma | Yes | [27] | ||
| AR | [18F] FDG | NK-92 | Naïve | mice | Intravenous | 30 min | Migrated to HER2/neu positive mouse sarcoma cell line | Yes | [48] | |
| GC | 99mTc-oxine | Primary NK | Naïve | mice | Intravenous | 1–24 h | Migrated to thyroid cancer | Yes | [35] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangadaran, P.; Rajendran, R.L.; Ahn, B.-C. Application of In Vivo Imaging Techniques for Monitoring Natural Killer Cell Migration and Tumor Infiltration. Cancers 2020, 12, 1318. https://doi.org/10.3390/cancers12051318
Gangadaran P, Rajendran RL, Ahn B-C. Application of In Vivo Imaging Techniques for Monitoring Natural Killer Cell Migration and Tumor Infiltration. Cancers. 2020; 12(5):1318. https://doi.org/10.3390/cancers12051318
Chicago/Turabian StyleGangadaran, Prakash, Ramya Lakshmi Rajendran, and Byeong-Cheol Ahn. 2020. "Application of In Vivo Imaging Techniques for Monitoring Natural Killer Cell Migration and Tumor Infiltration" Cancers 12, no. 5: 1318. https://doi.org/10.3390/cancers12051318
APA StyleGangadaran, P., Rajendran, R. L., & Ahn, B.-C. (2020). Application of In Vivo Imaging Techniques for Monitoring Natural Killer Cell Migration and Tumor Infiltration. Cancers, 12(5), 1318. https://doi.org/10.3390/cancers12051318







