Risk Assessment by Presurgical Tractography Using Navigated TMS Maps in Patients with Highly Motor- or Language-Eloquent Brain Tumors
Abstract
1. Introduction
2. Methods
2.1. Ethics
2.2. Study Design and Patient Inclusion
- -
- Written informed consent;
- -
- Age above 18 years;
- -
- Suspected right- or left-hemispheric motor-eloquent and/or left-hemispheric language-eloquent tumor location according to initial anatomical magnetic resonance imaging (MRI; suggesting infiltration or compression of anatomically suspected cortical motor-eloquent or language-eloquent areas and/or suspected close proximity to the CST or subcortical language-related pathways);
- -
- Availability of preoperative 3-Tesla MRI including diffusion tensor imaging (DTI) with 32 diffusion directions;
- -
- Clinical indication for preoperative nTMS language or motor mapping and nTMS-based DTI FT; surgery for tumor resection or biopsy;
- -
- Regular preoperative, postoperative, and follow-up (FU) examinations (at least until the three-month FU visit) including respective assessments of motor or language function.
- -
- Pregnancy;
- -
- Implanted devices (e.g., deep brain stimulation electrodes or cochlear implants);
- -
- Preoperative plegia and/or severe aphasia making motor or language mapping by nTMS impossible;
- -
- Infratentorial tumor location;
- -
- Relevant postoperative bleeding according to T2*- or susceptibility-weighted MRI with suspected affection of the motor cortex or course of the CST and/or language cortex or course of major subcortical language-related pathways.
2.3. Magnetic Resonance Imaging
2.4. Definition of Functional Deficits
- -
- Transient paresis/transient aphasia: any new or increased motor or language deficit owing to surgery that resolved within the regular FU interval;
- -
- Permanent paresis/permanent aphasia: any new or increased motor or language deficit owing to surgery that did not resolve to the preoperative status within the regular FU interval.
2.5. Mapping by Navigated Transcranial Magnetic Stimulation
2.5.1. Motor Mapping
2.5.2. Language Mapping
2.6. Tractography Based on Navigated Transcranial Magnetic Stimulation
2.6.1. Tracking of the Corticospinal Tract
2.6.2. Tracking of the Arcuate Fascicle and Other Language-Related Fiber Tracts
2.7. Statistics
3. Results
3.1. Patients
3.2. Mapping and Tractography
3.3. Lesion-To-Tract Distances
3.3.1. Corticospinal Tract
3.3.2. Arcuate Fascicle and Other Language-Related Fiber Tracts
3.4. Correlations between Lesion-To-Tract Distances and Deficits
4. Discussion
4.1. General Considerations
4.2. Lesion-To-Tract Distances for Motor Function
4.3. Lesion-To-Tract Distances for Language Function
4.4. Correlations to Functional Outcome
4.5. Limitations and Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| AF | Arcuate fascicle |
| BMRC | British Medical Research Council |
| CST | Corticospinal tract |
| DES | Direct electrical stimulation |
| DTI | Diffusion tensor imaging |
| DTI FT | Diffusion tensor imaging fiber tracking |
| FA | Fractional anisotropy |
| FAT | Fractional anisotropy threshold |
| FL | Fiber length |
| FLAIR | Fluid attenuated inversion recovery |
| FoF | Frontooccipital fascicle |
| FU | Follow-up |
| IDH | Isocitrate dehydrogenase |
| ILF | Inferior longitudinal fascicle |
| LAD | Lesion-to-activation distance |
| LTD | Lesion-to-tract distance |
| MEP | Motor evoked potential |
| MRI | Magnetic resonance imaging |
| nTMS | Navigated transcranial magnetic stimulation |
| rMT | Resting motor threshold |
| ROI | Region of interest |
| SD | Standard deviation |
| SLF | Superior longitudinal fascicle |
| TE | Echo time |
| TR | Repetition time |
| UC | Uncinate fascicle |
| WHO | World Health Organization |
References
- Duffau, H.; Moritz-Gasser, S.; Gatignol, P. Functional outcome after language mapping for insular World Health Organization Grade II gliomas in the dominant hemisphere: Experience with 24 patients. Neurosurg. Focus 2009, 27, E7. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.M.; Parney, I.F.; McDermott, M.; Barker, F.G., II; Schmidt, M.H.; Huang, W.; Laws, E.R., Jr.; Lillehei, K.O.; Bernstein, M.; Brem, H.; et al. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J. Neurosurg. 2003, 98, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Berger, M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008, 62, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.M.; Lam, D.; Babiak, M.C.; Perry, D.W.; Shih, T.; Hess, C.P.; Berger, M.S.; Chang, E.F. Transient aphasias after left hemisphere resective surgery. J. Neurosurg. 2015, 123, 581–593. [Google Scholar] [CrossRef]
- Duffau, H. Lessons from brain mapping in surgery for low-grade glioma: Insights into associations between tumour and brain plasticity. Lancet. Neurol. 2005, 4, 476–486. [Google Scholar] [CrossRef]
- Duffau, H.; Denvil, D.; Capelle, L. Long term reshaping of language, sensory, and motor maps after glioma resection: A new parameter to integrate in the surgical strategy. J. Neurol. Neurosurg. Psychiatry 2002, 72, 511–516. [Google Scholar]
- Briganti, C.; Sestieri, C.; Mattei, P.A.; Esposito, R.; Galzio, R.J.; Tartaro, A.; Romani, G.L.; Caulo, M. Reorganization of functional connectivity of the language network in patients with brain gliomas. AJNR Am. J. Neuroradiol. 2012, 33, 1983–1990. [Google Scholar] [CrossRef]
- Ius, T.; Angelini, E.; Thiebaut de Schotten, M.; Mandonnet, E.; Duffau, H. Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: Towards a “minimal common brain”. NeuroImage 2011, 56, 992–1000. [Google Scholar] [CrossRef]
- Silva, M.A.; See, A.P.; Essayed, W.I.; Golby, A.J.; Tie, Y. Challenges and techniques for presurgical brain mapping with functional MRI. NeuroImage. Clin. 2018, 17, 794–803. [Google Scholar] [CrossRef]
- Ottenhausen, M.; Krieg, S.M.; Meyer, B.; Ringel, F. Functional preoperative and intraoperative mapping and monitoring: Increasing safety and efficacy in glioma surgery. Neurosurg. Focus 2015, 38, E3. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Picht, T. The value of preoperative functional cortical mapping using navigated TMS. Neurophysiol. Clin. Clin. Neurophysiol. 2016, 46, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Picht, T.; Mularski, S.; Kuehn, B.; Vajkoczy, P.; Kombos, T.; Suess, O. Navigated transcranial magnetic stimulation for preoperative functional diagnostics in brain tumor surgery. Neurosurgery 2009, 65, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Krings, T.; Chiappa, K.H.; Foltys, H.; Reinges, M.H.; Cosgrove, G.R.; Thron, A. Introducing navigated transcranial magnetic stimulation as a refined brain mapping methodology. Neurosurg. Rev. 2001, 24, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ruohonen, J.; Karhu, J. Navigated transcranial magnetic stimulation. Neurophysiol. Clin. Clin. Neurophysiol. 2010, 40, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Shiban, E.; Buchmann, N.; Gempt, J.; Foerschler, A.; Meyer, B.; Ringel, F. Utility of presurgical navigated transcranial magnetic brain stimulation for the resection of tumors in eloquent motor areas. J. Neurosurg. 2012, 116, 994–1001. [Google Scholar] [CrossRef]
- Krieg, S.M.; Shiban, E.; Buchmann, N.; Meyer, B.; Ringel, F. Presurgical navigated transcranial magnetic brain stimulation for recurrent gliomas in motor eloquent areas. Clin. Neurophysiol. 2013, 124, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Sollmann, N.; Obermueller, T.; Sabih, J.; Bulubas, L.; Negwer, C.; Moser, T.; Droese, D.; Boeckh-Behrens, T.; Ringel, F.; et al. Changing the clinical course of glioma patients by preoperative motor mapping with navigated transcranial magnetic brain stimulation. BMC Cancer 2015, 15, 231. [Google Scholar] [CrossRef]
- Krieg, S.M.; Sabih, J.; Bulubasova, L.; Obermueller, T.; Negwer, C.; Janssen, I.; Shiban, E.; Meyer, B.; Ringel, F. Preoperative motor mapping by navigated transcranial magnetic brain stimulation improves outcome for motor eloquent lesions. Neuro Oncol. 2014, 16, 1274–1282. [Google Scholar] [CrossRef]
- Sollmann, N.; Ille, S.; Hauck, T.; Maurer, S.; Negwer, C.; Zimmer, C.; Ringel, F.; Meyer, B.; Krieg, S.M. The impact of preoperative language mapping by repetitive navigated transcranial magnetic stimulation on the clinical course of brain tumor patients. BMC Cancer 2015, 15, 261. [Google Scholar] [CrossRef]
- Frey, D.; Schilt, S.; Strack, V.; Zdunczyk, A.; Rosler, J.; Niraula, B.; Vajkoczy, P.; Picht, T. Navigated transcranial magnetic stimulation improves the treatment outcome in patients with brain tumors in motor eloquent locations. Neuro Oncol. 2014, 16, 1365–1372. [Google Scholar] [CrossRef]
- Sollmann, N.; Kelm, A.; Ille, S.; Schroder, A.; Zimmer, C.; Ringel, F.; Meyer, B.; Krieg, S.M. Setup presentation and clinical outcome analysis of treating highly language-eloquent gliomas via preoperative navigated transcranial magnetic stimulation and tractography. Neurosurg. Focus 2018, 44, E2. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Buchmann, N.H.; Gempt, J.; Shiban, E.; Meyer, B.; Ringel, F. Diffusion tensor imaging fiber tracking using navigated brain stimulation—A feasibility study. Acta Neurochir. 2012, 154, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Negwer, C.; Ille, S.; Maurer, S.; Hauck, T.; Kirschke, J.S.; Ringel, F.; Meyer, B.; Krieg, S.M. Feasibility of nTMS-based DTI fiber tracking of language pathways in neurosurgical patients using a fractional anisotropy threshold. J. Neurosci. Methods 2016, 267, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Frey, D.; Strack, V.; Wiener, E.; Jussen, D.; Vajkoczy, P.; Picht, T. A new approach for corticospinal tract reconstruction based on navigated transcranial stimulation and standardized fractional anisotropy values. NeuroImage 2012, 62, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, T.; Grittner, U.; Acker, G.; Schwarzer, V.; Kulchytska, N.; Vajkoczy, P.; Picht, T. Risk stratification in motor area-related glioma surgery based on navigated transcranial magnetic stimulation data. J. Neurosurg. 2017, 126, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Wildschuetz, N.; Kelm, A.; Conway, N.; Moser, T.; Bulubas, L.; Kirschke, J.S.; Meyer, B.; Krieg, S.M. Associations between clinical outcome and navigated transcranial magnetic stimulation characteristics in patients with motor-eloquent brain lesions: A combined navigated transcranial magnetic stimulation-diffusion tensor imaging fiber tracking approach. J. Neurosurg. 2018, 128, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Fratini, A.; Zhang, H.; Zimmer, C.; Meyer, B.; Krieg, S.M. Associations between clinical outcome and tractography based on navigated transcranial magnetic stimulation in patients with language-eloquent brain lesions. J. Neurosurg. 2019, 1–10. [Google Scholar] [CrossRef]
- Sollmann, N.; Negwer, C.; Tussis, L.; Hauck, T.; Ille, S.; Maurer, S.; Giglhuber, K.; Bauer, J.S.; Ringel, F.; Meyer, B.; et al. Interhemispheric connectivity revealed by diffusion tensor imaging fiber tracking derived from navigated transcranial magnetic stimulation maps as a sign of language function at risk in patients with brain tumors. J. Neurosurg. 2017, 126, 222–233. [Google Scholar] [CrossRef]
- Krieg, S.M.; Lioumis, P.; Makela, J.P.; Wilenius, J.; Karhu, J.; Hannula, H.; Savolainen, P.; Lucas, C.W.; Seidel, K.; Laakso, A.; et al. Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochir. 2017, 159, 1187–1195. [Google Scholar] [CrossRef]
- Sollmann, N.; Meyer, B.; Krieg, S.M. Implementing Functional Preoperative Mapping in the Clinical Routine of a Neurosurgical Department: Technical Note. World Neurosurg. 2017, 103, 94–105. [Google Scholar] [CrossRef]
- Lioumis, P.; Zhdanov, A.; Makela, N.; Lehtinen, H.; Wilenius, J.; Neuvonen, T.; Hannula, H.; Deletis, V.; Picht, T.; Makela, J.P. A novel approach for documenting naming errors induced by navigated transcranial magnetic stimulation. J. Neurosci. Methods 2012, 204, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Diehl, C.D.; Schwendner, M.J.; Sollmann, N.; Oechsner, M.; Meyer, B.; Combs, S.E.; Krieg, S.M. Application of presurgical navigated transcranial magnetic stimulation motor mapping for adjuvant radiotherapy planning in patients with high-grade gliomas. Radiother. Oncol. 2019, 138, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Pontoriero, A.; Ricciardi, G.K.; Granata, F.; Vinci, S.; Angileri, F.F.; Pergolizzi, S.; Alafaci, C.; Rizzo, V.; Quartarone, A.; et al. Integration of functional neuroimaging in CyberKnife radiosurgery: Feasibility and dosimetric results. Neurosurg. Focus 2013, 34, E5. [Google Scholar] [CrossRef] [PubMed]
- Schwendner, M.J.; Sollmann, N.; Diehl, C.D.; Oechsner, M.; Meyer, B.; Krieg, S.M.; Combs, S.E. The Role of Navigated Transcranial Magnetic Stimulation Motor Mapping in Adjuvant Radiotherapy Planning in Patients With Supratentorial Brain Metastases. Front. Oncol. 2018, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Raabe, A.; Hattingen, E.; Szelenyi, A.; Yahya, H.; Hermann, E.; Zimmermann, M.; Seifert, V. Functional magnetic resonance imaging-integrated neuronavigation: Correlation between lesion-to-motor cortex distance and outcome. Neurosurgery 2004, 55, 904–914. [Google Scholar] [CrossRef]
- Haberg, A.; Kvistad, K.A.; Unsgard, G.; Haraldseth, O. Preoperative blood oxygen level-dependent functional magnetic resonance imaging in patients with primary brain tumors: Clinical application and outcome. Neurosurgery 2004, 54, 902–914. [Google Scholar] [CrossRef]
- Wood, J.M.; Kundu, B.; Utter, A.; Gallagher, T.A.; Voss, J.; Nair, V.A.; Kuo, J.S.; Field, A.S.; Moritz, C.H.; Meyerand, M.E.; et al. Impact of brain tumor location on morbidity and mortality: A retrospective functional MR imaging study. AJNR Am. J. Neuroradiol. 2011, 32, 1420–1425. [Google Scholar] [CrossRef]
- Bailey, P.D.; Zaca, D.; Basha, M.M.; Agarwal, S.; Gujar, S.K.; Sair, H.I.; Eng, J.; Pillai, J.J. Presurgical fMRI and DTI for the Prediction of Perioperative Motor and Language Deficits in Primary or Metastatic Brain Lesions. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2015, 25, 776–784. [Google Scholar] [CrossRef]
- Ulmer, J.L.; Salvan, C.V.; Mueller, W.M.; Krouwer, H.G.; Stroe, G.O.; Aralasmak, A.; Prost, R.W. The role of diffusion tensor imaging in establishing the proximity of tumor borders to functional brain systems: Implications for preoperative risk assessments and postoperative outcomes. Technol. Cancer Res. Treat. 2004, 3, 567–576. [Google Scholar] [CrossRef]
- Jiao, Y.; Lin, F.; Wu, J.; Li, H.; Wang, L.; Jin, Z.; Wang, S.; Cao, Y. A supplementary grading scale combining lesion-to-eloquence distance for predicting surgical outcomes of patients with brain arteriovenous malformations. J. Neurosurg. 2018, 128, 530–540. [Google Scholar] [CrossRef]
- Meyer, E.J.; Gaggl, W.; Gilloon, B.; Swan, B.; Greenstein, M.; Voss, J.; Hussain, N.; Holdsworth, R.L.; Nair, V.A.; Meyerand, M.E.; et al. The Impact of Intracranial Tumor Proximity to White Matter Tracts on Morbidity and Mortality: A Retrospective Diffusion Tensor Imaging Study. Neurosurgery 2017, 80, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.M.; Sollmann, N.; Hauck, T.; Ille, S.; Foerschler, A.; Meyer, B.; Ringel, F. Functional language shift to the right hemisphere in patients with language-eloquent brain tumors. PLoS ONE 2013, 8, e75403. [Google Scholar] [CrossRef] [PubMed]
- Rosler, J.; Niraula, B.; Strack, V.; Zdunczyk, A.; Schilt, S.; Savolainen, P.; Lioumis, P.; Makela, J.; Vajkoczy, P.; Frey, D.; et al. Language mapping in healthy volunteers and brain tumor patients with a novel navigated TMS system: Evidence of tumor-induced plasticity. Clin. Neurophysiol. 2014, 125, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Negwer, C.; Sollmann, N.; Ille, S.; Hauck, T.; Maurer, S.; Kirschke, J.S.; Ringel, F.; Meyer, B.; Krieg, S.M. Language pathway tracking: Comparing nTMS-based DTI fiber tracking with a cubic ROIs-based protocol. J. Neurosurg. 2017, 126, 1006–1014. [Google Scholar] [CrossRef]
- Duffau, H. Diffusion tensor imaging is a research and educational tool, but not yet a clinical tool. World Neurosurg. 2014, 82, e43–e45. [Google Scholar] [CrossRef]
- Conti Nibali, M.; Rossi, M.; Sciortino, T.; Riva, M.; Gay, L.G.; Pessina, F.; Bello, L. Preoperative surgical planning of glioma: Limitations and reliability of fMRI and DTI tractography. J. Neurosurg. Sci. 2019, 63, 127–134. [Google Scholar] [CrossRef]
- Wende, T.; Hoffmann, K.T.; Meixensberger, J. Tractography in Neurosurgery: A Systematic Review of Current Applications. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2020. [Google Scholar] [CrossRef]
- Nimsky, C.; Bauer, M.; Carl, B. Merits and Limits of Tractography Techniques for the Uninitiated. Adv. Tech. Stand. Neurosurg. 2016, 37–60. [Google Scholar] [CrossRef]
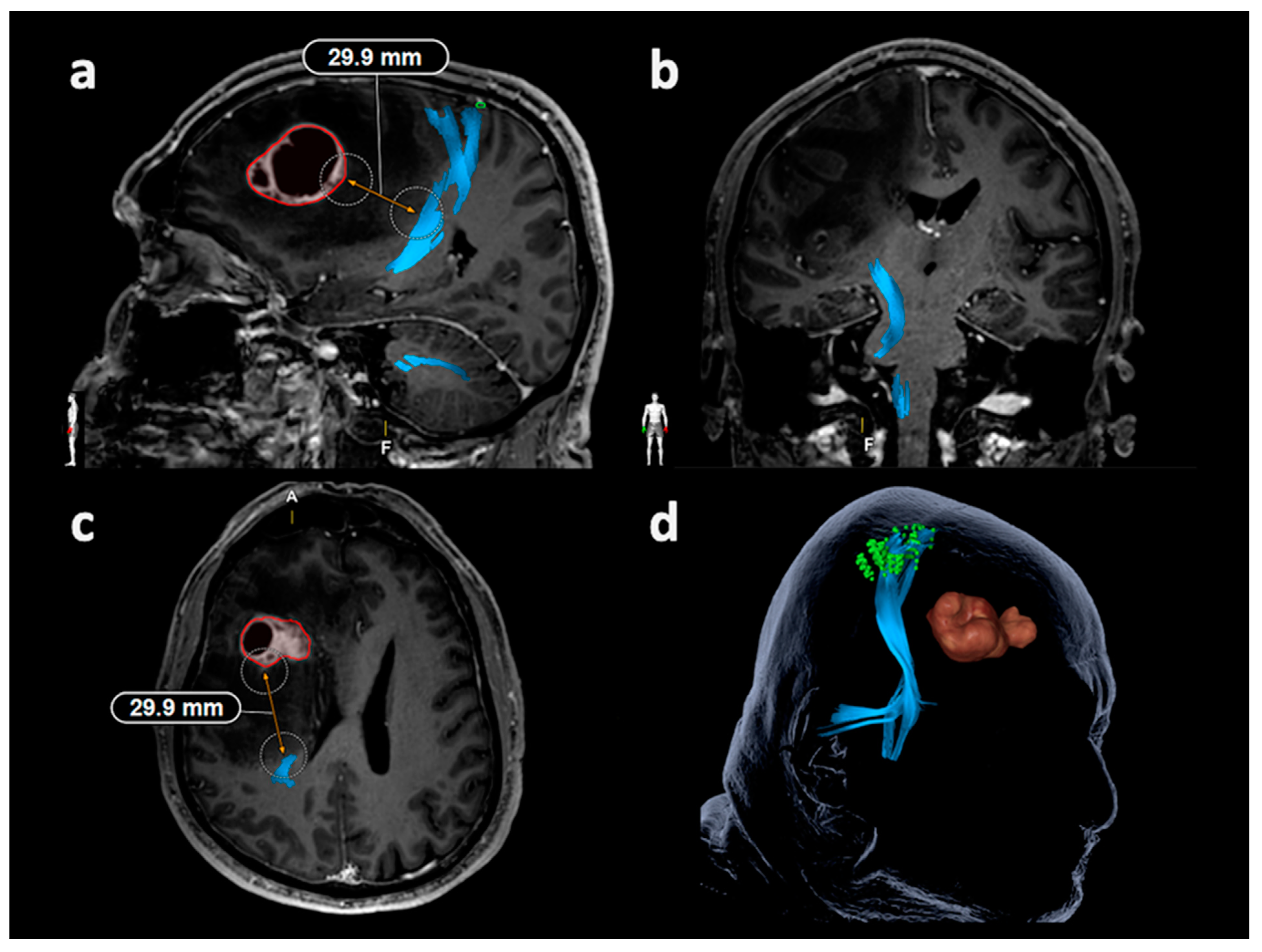
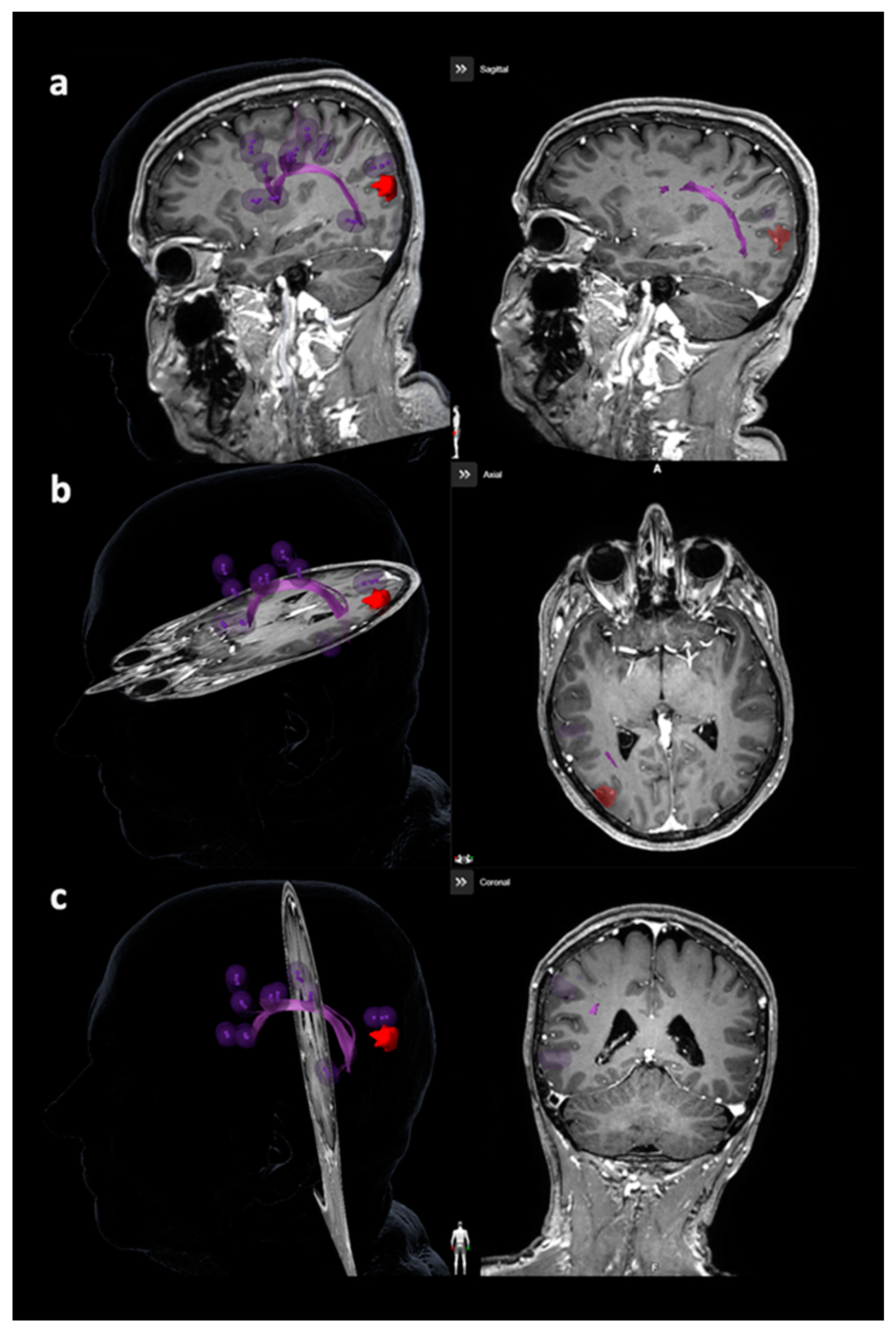
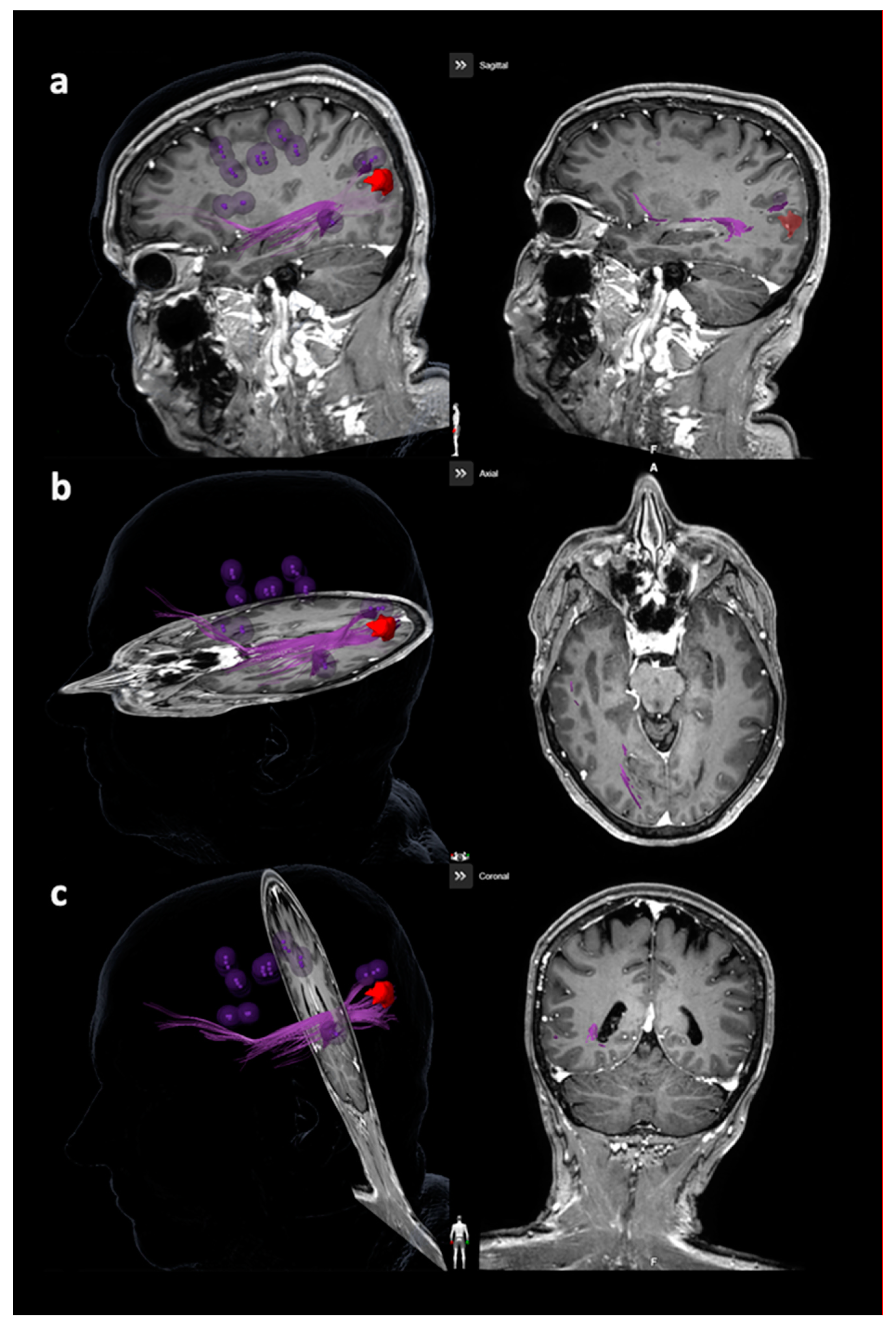
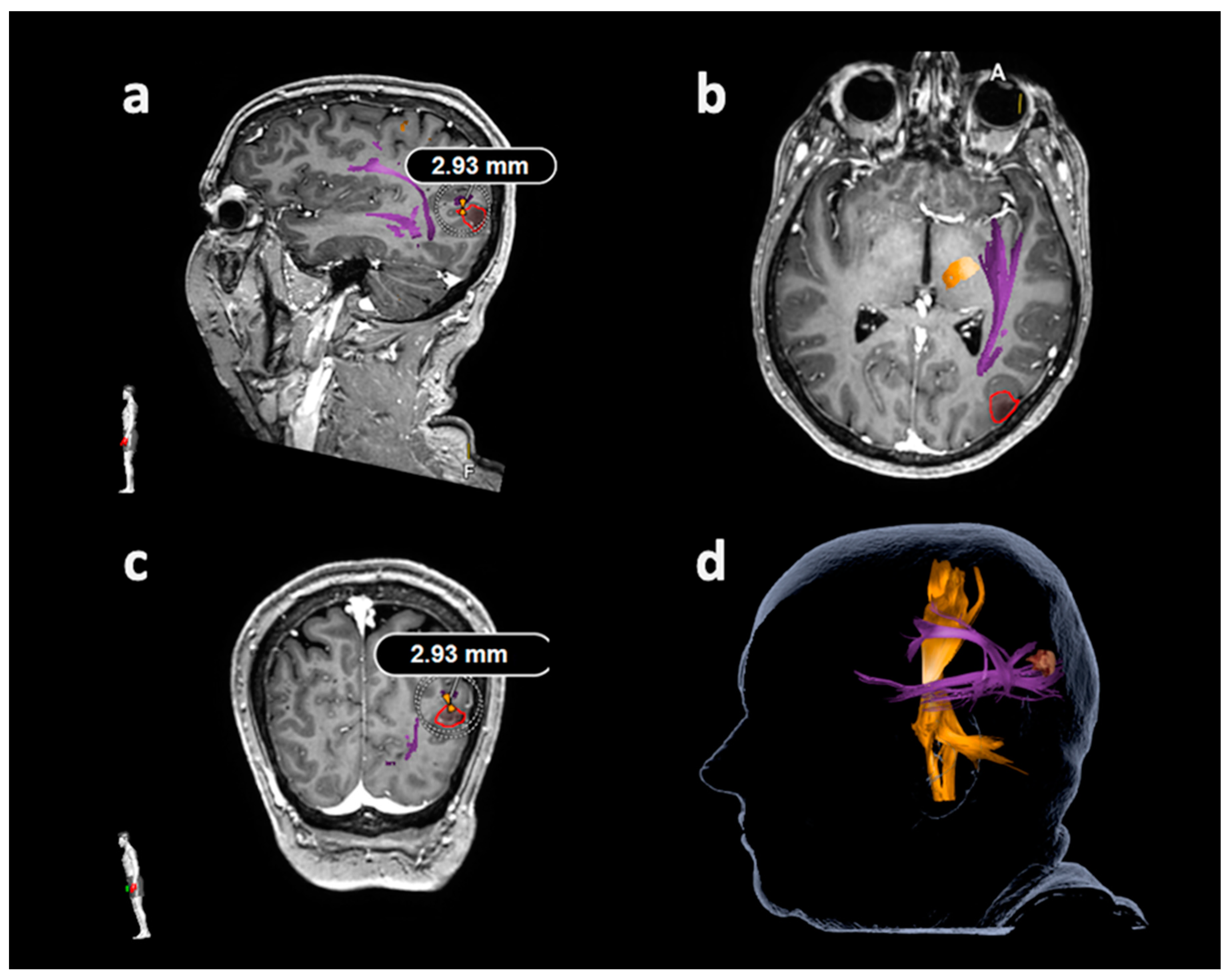
| Item | Motor Mappings | Language Mappings | ||
|---|---|---|---|---|
| Number of Mappings (N) | 150 | 100 | ||
| Number of Patients (N) | 118 | 98 | ||
| Age | 58.2 ± 15.2 [21.1–89.7] | 55.6 ± 15.6 [18.9–82.7] | ||
| (in years, mean ± SD [range]) | ||||
| Gender | 64.4/35.6 | 52.0/48.0 | ||
| (in %, male/female) | ||||
| Maximum Follow-Up | 10.8 ± 6.5 [3.0–28.4] | 8.8 ± 5.6 [3.0–33.7] | ||
| (in months, mean ± SD [range]) | ||||
| Affected Hemisphere | 39.0/61.0 | 100.0/0.0 | ||
| (in %, left/right) | ||||
| Awake Surgery (in %) | 0.0 | 21.0 | ||
| Type of Surgery | 96.0/4.0 | 96.0/4.0 | ||
| (in %, resection/biopsy) | ||||
| Tumor Entity (in %) | Glioma WHO grade I | 0.0 | 5.1 | |
| Glioma WHO grade II | 0.0 | 4.1 | ||
| Glioma WHO grade III | 21.2 | 14.3 | ||
| Glioma WHO grade IV | 78.8 | 49.0 | ||
| Arteriovenous malformation | 0.0 | 4.1 | ||
| Cavernoma | 0.0 | 5.1 | ||
| Metastasis | 0.0 | 18.4 | ||
| IDH Status (in % for patients with glioma, IDH-wildtype/IDH-mutant) | 71.2/28.8 | 71.8/28.2 | ||
| Functional Deficits (in %) | Preoperative | No | 69.3 | 58.0 |
| Yes | 30.7 | 42.0 | ||
| Postoperative | No | 56.0 | 38.0 | |
| Yes | 44.0 | 62.0 | ||
| Follow-up | No | 58.7 | 57.0 | |
| Yes | 41.3 | 43.0 | ||
| Surgery-related | None | 76.0 | 62.0 | |
| Transient | 2.0 | 17.0 | ||
| Permanent | 22.0 | 21.0 | ||
| Perioperative ischemia (in patients with surgery-related transient or permanent deficits) | 44.4 | 57.9 | ||
| Motor Mappings and nTMS-Based Tractography | |||
|---|---|---|---|
| Resting Motor Threshold | Unaffected hemisphere | 33.5 ± 8.2 [21.0–56.0] | p = 0.1719 |
| (in % of stimulator output, mean ± SD [range]) | Tumor-affected hemisphere | 35.3 ± 10.1 [19.0–99.0] | |
| 100% Fractional Anisotropy Threshold (mean ± SD [range]) | 0.34 ± 0.07 [0.21–0.57] | ||
| Language Mappings and nTMS-Based Tractography | |||
| Resting Motor Threshold | Unaffected hemisphere | 35.6 ± 6.9 [22.0–55.0] | p = 0.0012 |
| (in % of stimulator output, mean ± SD [range]) | Tumor-affected hemisphere | 36.4 ± 9.1 [21.0–75.0] | |
| 100% Fractional Anisotropy Threshold (mean ± SD [range]) | 0.28 ± 0.06 [0.15–0.47] | ||
| AF is Closest Tract to Tumor (in %) | 27.0 | ||
| Other Closest Language-Related Tract (in %) | SLF | 15.0 | |
| ILF | 32.0 | ||
| FoF | 52.0 | ||
| UC | 1.0 | ||
| LTD—CST (in mm) | 25% FAT | 50% FAT | 75% FAT | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD [Range] | p (vs. None/Transient Deficits) | Mean ± SD [Range] | p (vs. None/Transient Deficits) | Mean ± SD [Range] | p (vs. None/Transient Deficits) | ||
| Surgery-Related Motor Deficits | None/transient | 10.0 ± 9.9 [0.0–42.4] | - | 12.7 ± 10.8 [0.0–48.2] | - | 14.9 ± 10.8 [0.0–48.2] | - |
| Permanent (all patients) | 2.0 ± 3.3 [0.0–12.2] | <0.0001 | 3.1 ± 3.6 [0.0–12.4] | <0.0001 | 4.3 ± 4.1 [0.0–13.4] | <0.0001 | |
| Permanent (excluding patients with perioperative ischemia) | 0.6 ± 1.1 [0.0–3.0] | <0.0001 | 1.8 ± 2.2 [0.0–5.5] | <0.0001 | 3.1 ± 3.4 [0.0–11.8] | <0.0001 | |
| LTD—AF (in mm) | 25% FAT | 50% FAT | 75% FAT | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD [Range] | p (vs. None/Transient Deficits) | Mean ± SD [Range] | p (vs. None/Transient Deficits) | Mean ± SD [Range] | p (vs. None/Transient Deficits) | ||
| Surgery-Related Language Deficits | None/transient | 12.2 ± 11.5 [0.0–41.1] | - | 16.0 ± 13.2 [0.0–57.7] | - | 18.4 ± 13.7 [0.0–58.3] | - |
| Permanent (all patients) | 2.8 ± 6.8 [0.0–24.6] | 0.0005 | 4.4 ± 8.7 [0.0–25.8] | 0.0012 | 8.3 ± 10.0 [0.0–27.1] | 0.0491 | |
| Permanent (excluding patients with perioperative ischemia) | 1.0 ± 2.2 [0.0–4.9] | 0.0119 | 4.7 ± 6.3 [0.0–13.3] | 0.0579 | 7.8 ± 5.8 [1.3–15.4] | 0.1095 | |
| LTD—Other Closest Language-Related Tract (in mm) | 25% FAT | 50% FAT | 75% FAT | ||||
| Mean ± SD [Range] | p (vs. None/Transient Deficits) | Mean ± SD [Range] | p (vs. None/Transient Deficits) | Mean ± SD [Range] | p (vs. None/Transient Deficits) | ||
| Surgery-Related Language Deficits | None/transient | 10.3 ± 13.0 [0.0–53.0] | - | 13.0 ± 13.3 [0.0–54.2] | - | 16.1 ± 14.5 [0.0–54.7] | - |
| Permanent (all patients) | 3.4 ± 5.9 [0.0–23.1] | 0.0113 | 5.2 ± 6.7 [0.0–23.9] | 0.0147 | 7.6 ± 7.4 [0.0–24.1] | 0.0435 | |
| Permanent (excluding patients with perioperative ischemia) | 6.2 ± 8.1 [0.0–23.1] | 0.4179 | 7.6 ± 8.2 [0.0–23.9] | 0.2829 | 9.4 ± 8.5 [0.0–24.1] | 0.2299 | |
| Correlations | Surgery-Related Deficits (All Patients) | Surgery-Related Deficits (Excluding Patients with Perioperative Ischemia) | |||||
|---|---|---|---|---|---|---|---|
| 25% FAT | 50% FAT | 75% FAT | 25% FAT | 50% FAT | 75% FAT | ||
| LTD—CST | r | −0.3933 | −0.4136 | −0.4473 | −0.3910 | −0.3923 | −0.3994 |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| LTD—AF | r | −0.3590 | −0.3775 | −0.2888 | −0.2918 | −0.2662 | −0.2592 |
| p | 0.0006 | 0.0014 | 0.0490 | 0.0135 | 0.0473 | 0.1111 | |
| LTD—Other Closest Language-Related Tract | r | −0.2525 | −0.2551 | −0.2622 | −0.0853 | −0.1168 | −0.1683 |
| p | 0.0112 | 0.0147 | 0.0430 | 0.4575 | 0.3215 | 0.2478 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sollmann, N.; Zhang, H.; Fratini, A.; Wildschuetz, N.; Ille, S.; Schröder, A.; Zimmer, C.; Meyer, B.; Krieg, S.M. Risk Assessment by Presurgical Tractography Using Navigated TMS Maps in Patients with Highly Motor- or Language-Eloquent Brain Tumors. Cancers 2020, 12, 1264. https://doi.org/10.3390/cancers12051264
Sollmann N, Zhang H, Fratini A, Wildschuetz N, Ille S, Schröder A, Zimmer C, Meyer B, Krieg SM. Risk Assessment by Presurgical Tractography Using Navigated TMS Maps in Patients with Highly Motor- or Language-Eloquent Brain Tumors. Cancers. 2020; 12(5):1264. https://doi.org/10.3390/cancers12051264
Chicago/Turabian StyleSollmann, Nico, Haosu Zhang, Alessia Fratini, Noémie Wildschuetz, Sebastian Ille, Axel Schröder, Claus Zimmer, Bernhard Meyer, and Sandro M. Krieg. 2020. "Risk Assessment by Presurgical Tractography Using Navigated TMS Maps in Patients with Highly Motor- or Language-Eloquent Brain Tumors" Cancers 12, no. 5: 1264. https://doi.org/10.3390/cancers12051264
APA StyleSollmann, N., Zhang, H., Fratini, A., Wildschuetz, N., Ille, S., Schröder, A., Zimmer, C., Meyer, B., & Krieg, S. M. (2020). Risk Assessment by Presurgical Tractography Using Navigated TMS Maps in Patients with Highly Motor- or Language-Eloquent Brain Tumors. Cancers, 12(5), 1264. https://doi.org/10.3390/cancers12051264








