1. Introduction
BRCA1/BRCA2 pathogenic variants have a 17–44% ovarian cancer (OC) risk until age 80 years [
1]. Testing for OC susceptibility genes (CSGs)—
RAD51C (lifetime OC risk = 11%) [
2],
RAD51D (lifetime OC risk = 13%) [
2] and
BRIP1 (lifetime OC risk = 5.8%) [
3]—is now part of clinical practice. Genome-wide association studies (GWAS) have discovered ~30 validated single-nucleotide polymorphisms (SNPs) which modify OC risk [
4,
5]. Newer risk prediction models incorporating validated SNPs as a polygenic risk score with epidemiologic/family history(FH)/hormonal data and moderate–high-penetrance CSGs can be used to predict lifetime OC risk, improving the precision of risk estimation and allowing population division into risk strata, enabling targeted downstream risk-stratified prevention/screening for those at increased risk [
4,
6].
The current practice of identifying high-risk women uses clinical criteria/FH-based testing for CSGs, misses >50% CSG carriers who do not fulfil genetic testing criteria and requires people to get cancer before identifying unaffected family members who can benefit from prevention [
7,
8,
9,
10]. Given the effective cancer risk management/prevention options available, the adequacy of current practice, representing massive missed opportunities for risk-stratified prevention, is questionable. Unselected population genetic testing (PGT) overcomes these limitations and identifies many more individuals at increased OC risk. PGT can be cost effective and prevent thousands of more OC/BC cases than clinical criteria/FH-based genetic testing [
11].
Most PGT evidence comes from UK/Israeli/Canadian studies in Ashkenazi Jewish (AJ) populations [
9,
10,
12]. These show that AJ population-based
BRCA testing is acceptable, feasible, can be community based, doubles the
BRCA pathogenic variant individuals identified, does not harm psychological health/quality of life (QoL), reduces long-term anxiety, has high satisfaction rates (90–95%) [
9,
10,
13], and is extremely cost effective (potentially cost saving) for the UK/US health systems [
14]. However, prospective/unbiased PGT data and model-based OC risk stratification for a general (non-Jewish) low-risk population are lacking.
We describe results from a feasibility study in order to stratify a general population using predicted lifetime OC risk and offer risk management options of screening and prevention, within the Predicting Risk of Ovarian Malignancy Improved Screening and Early detection programme (PROMISE-FS, ISRCTN54246466). This article reports on (1) the acceptability, feasibility, and uptake of PGT/OC risk stratification; (2) perceived risks/limitations; (3) decision aid (DA)/telephone helpline use; (4) satisfaction; (5) cancer worry/risk perception; (6) impact on psychological health/QoL.
2. Results
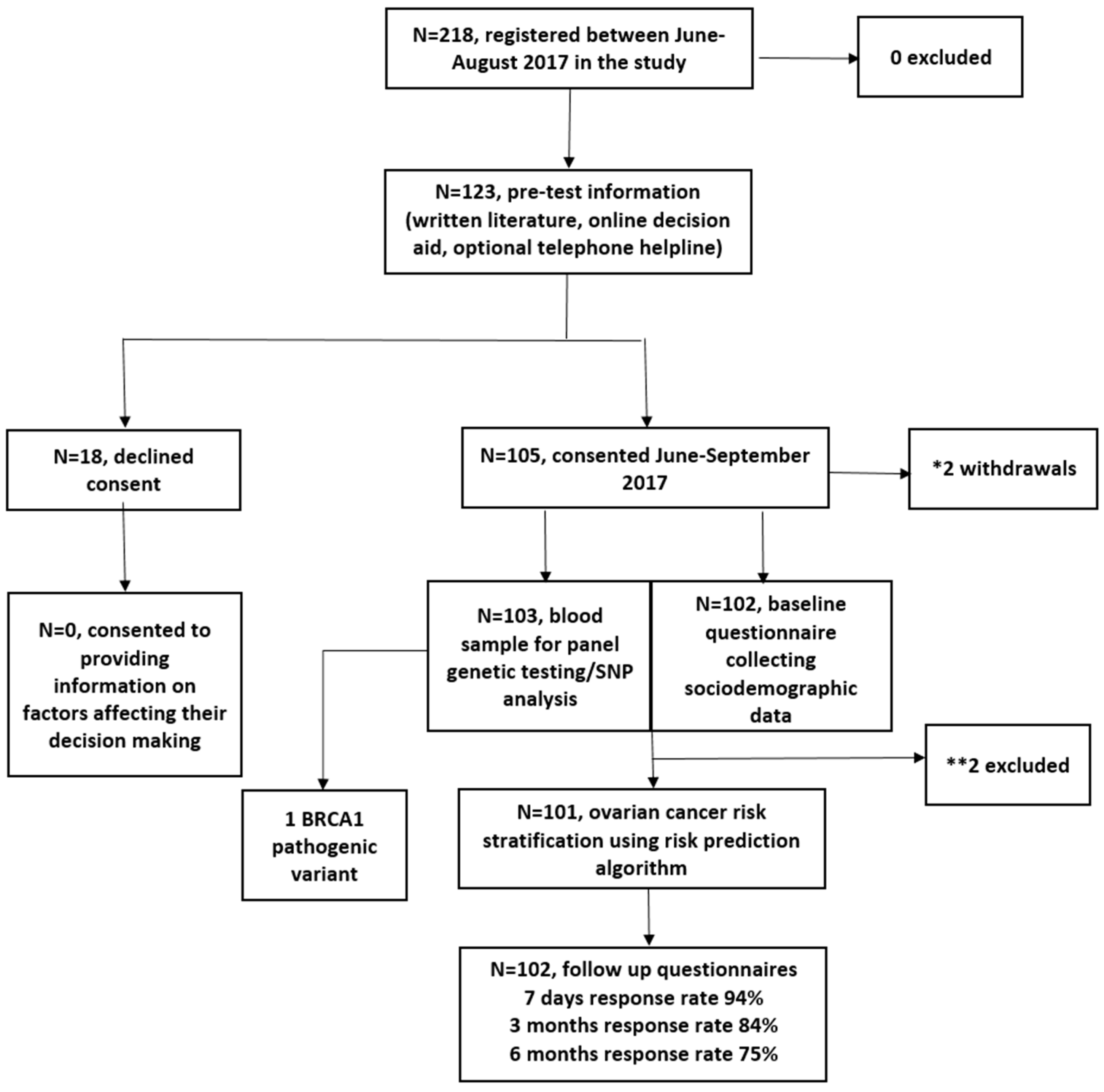
Between June 2017 and August 2017, 218 women registered and 123 viewed the online DA. In total, 105/123 (85%) DA users consented to genetic testing/risk assessment, and two withdrew. In total, 103 were eligible for analysis (
Figure 1). In total, 2/103 were excluded from RPA assessment (
Figure 1). Women who chose not to participate declined providing information on factors affecting decision making. The follow-up questionnaire response rate was 94%, 84%, and 75% at seven days, three months and six months post results, respectively.
Table 1 summarises cohort baseline characteristics. The mean age of participants was 48.5 (SD = 15.4; range = 18–85) years; 44.6% (
n = 45) had university level education; 55.7% (
n = 54) had a household income >£40,000; 74.5% (
n = 76) were Caucasian; 7% (
n = 7) were smokers; 64% (
n = 63) ate >5 portions of fruit/vegetables daily; 78% (
n = 80) were physically active over the last month. None had a clinically significant FH of cancer (fulfilling NHS genetic testing criteria). RPA revealed 1/103 at ≥10%, 0/103 at ≥5%–<10% and 100/103 at <5% lifetime OC risk. As expected using the algorithm, the epidemiological risk factors alone provide a greater level of OC risk stratification among the participants compared to the polygenic risk score (PRS) alone (
Figure S1). However, risk stratification is further improved when the full model incorporating both epidemiological risk factors and PRS is considered. One high-risk participant, aged 35 years, had a lifetime OC risk of 42%. She had a pathogenic duplication of exon-13 in BRCA1. History included one second-degree relative with OC—parity = 1, 10 years oral contraceptive pill (OCP) use, endometriosis, BMI = 30.4, and no tubal ligation/hormone-replacement therapy (HRT) use. Following results, the participant opted for Risk of Ovarian Cancer Algorithm (ROCA)-based screening (24) within a research study (ALDO,
https://www.uclh.nhs.uk/OurServices/ServiceA-Z/Cancer/NCV/Pages/TheALDOproject.aspx) and for risk-reducing early salpingectomy within a clinical trial (PROTECTOR, ISRCTN25173360,
http://www.protector.org.uk/). She underwent MRI screening for BC risk. Four Class-3 variants of uncertain significance (VUS) were detected (BRCA1:c.3328_3330delAAG, c.2998_3003del; BRCA2:c.1438T>G; RAD51D:c.482T>C).
Key perceived benefits/risks of PGT/OC risk assessment are shown in
Table S1. Need for reassurance, reduction in uncertainty, enhancing cancer prevention, benefiting research, knowledge about enhanced screening/prevention and children’s risks were rated somewhat/very important by ~70–98% women. Important risks/limitations of PGT/OC risk assessment rated somewhat/very important included concern about effect on family (56.4%) and being unable to handle it emotionally (38.6%). A minority felt stigmatization (9%) or targeting of an ethnic group (11%) was a somewhat/very important risk. Insurance and confidentiality were highlighted as somewhat/very important by 28% and 24.7% respectively.
Participant responses to the ten DA items are shown in
Table S2. The mean number of times DA was viewed was non-significantly higher in consenters versus decliners (1.61 vs. 1.05;
p = 0.06). The mean DA score was not significantly different between consenters and decliners (8.1 vs. 7.4;
p = 0.14). Consenters were older than decliners (48.5 vs. 40,
p = 0.016). The mean age of volunteers who registered but did not view the DA was 45.5 years and not significantly different from consenters (
p = 0.16) or decliners (
p = 0.24). There was no statistically significant difference in 9/10 DA item responses between consenters and decliners. (
Table S2). In total, 88.3% of consenters versus 75% of decliners (
p = 0.036) would regret not participating if they developed OC in the future. In total, 23/123 viewed the DA on multiple occasions, and DA scores increased on repeat attempts (
Tables S3 and S4). For 122/123 participants, there was concordance between participant decision making and DA outcome category. One participant (85 years, Caucasian, no OC-FH) consented to PGT/OC risk stratification despite DA advice to the contrary (DA score = −1).
Table 2 summarises responses to the DA evaluation questionnaire. In total, 92.2% (94/102) were very satisfied/satisfied and 82.2% (83/101) would recommend the DA. The amount of information provided, length of time taken to view and level of detail available was deemed just right by 98% (100/102), 97.1% (99/102), and 97% (98/101), respectively. No part of the DA needed omitting.
In total, 13% (13/103) of consenters used the optional telephone helpline (
Table 3), and 8/13 filled in an evaluation questionnaire. No decliner used the telephone helpline. The mean number of calls to the telephone helpline was 1.38 (SD = 1.12; range = 1–5). In total, 12.5% (1/8) used the telephone helpline to aid decision making and 75% (6/8) had study specific queries—of which, DA technical assistance queries (4/8) were the most common. All helpline users were very satisfied/satisfied with their experience and 75% (6/8) would recommend the helpline. In total, 37.5% (3/8) felt that the helpline aided decision making. There was no difference in baseline characteristics between helpline users and non-users. When comparing how much the DA improved understanding of OC/gene testing/advantages and disadvantages of discovering personalised OC risk or DA satisfaction, there was no statistically significant difference between helpline users/non-users. Helpline users had a significantly greater degree of worry (2/13 vs. 0/89;
p = 0.02) and upset (1/13 vs. 0/89;
p = 0.003) when viewing the DA in comparison to non-users. Helpline users had a higher DA mean score than non-users (9.123 vs. 8.019;
p = 0.032)
Mean Hospital Anxiety and Depression Scale (HADS)/EuroQol-5D-5L (EQ-5D-5L)/Impact of Events Scale (IES)/Cancer Risk Perception questionnaire (CRP)/Cancer Worry Scale questionnaire (CWS)/Decision Regret Satisfaction questionnaire (DRS) questionnaire scores at baseline and at seven days/three months/six months follow up are shown in
Table 4.
Linear random-effects mixed-model outputs showing the association of covariates with different outcomes are shown in
Table 5. There was a transient increase in HADS anxiety at seven days (
p = 0.048), returning to baseline by three months (
p = 0.318). Compared to baseline, there was a small increase in HADS depression scores at individual time points of 3 months (
p = 0.027) and 6 months (
p ≤ 0.001), while QoL scores were marginally lower at three (
p = 0.025) and six months (
p = 0.036). However, the absolute level of change from baseline in all these scores was extremely small (HADS depression = 2.92 to 3.55; HADS anxiety = 6.11 to 7.02; EQ-5D-5L = 0.86 to 0.84) and not clinically meaningful. Additionally, contrast tests evaluating whether overall mean values at seven days, three months and six months were jointly different from the baseline suggested that anxiety, depression and QoL at these time points were not jointly different from the baseline value for the cohort (
Table 5). Distress scores decreased with time and were significantly lower at six months versus 7 days (
p = 0.042). Compared to baseline, OC worry was significantly lower at 7 days (
p ≤ 0.001), 3 months (
p ≤ 0.001) and 6 months (
p ≤ 0.001). Contrast tests evaluating the overall time effect showed a significant decrease in OC worry scores (
p = 0.02) but not distress scores (
p = 0.25) over time (
Table 5). General cancer risk perception showed a decrease at 7 days (
p = 0.012), returning to baseline by 6 months (
p = 0.45).
In total, 85.5% strongly agreed and 13.2% agreed that their decision to undergo PGT/OC risk stratification was the right decision and that they were satisfied with it. In total, 95% would make the same choice again. Only 1.3% regretted their decision.
Table 6 summarises responses to the DRS questionnaire.
A FH of BC (p = 0.034) but not OC (p = 0.20) was negatively associated with QOL. Having a FH of OC was not associated with an increase in OC worry or general cancer risk perception. However, women with a FH of BC perceived themselves to be at higher cancer risk (p = 0.002) but did not have increased OC worry.
Results from contrast tests assessing the joint effect of between-group and within-group differences in various outcomes over six months compared to baseline are shown in
Table 7. There was no statistically significant between-group difference for groups ‘with’ and ‘without’ a FH of OC for HADS total/HADS depression/HADS anxiety/QoL/distress/OC worry/general cancer risk perception over time. There was no statistically significant within-group difference for groups ‘with’ and ‘without’ a FH of OC for HADS total/HADS anxiety/QoL/general cancer risk perception over six months. However, there was a statistically significant within-group difference for individuals ‘without’ a FH of OC but not ‘with’ a FH of OC for HADS depression (
p = 0.003,
p = 0.866, respectively), distress (
p = 0.043,
p = 0.524 respectively) and OC worry (
p ≤ 0.001,
p = 0.582, respectively) over six months. Viewing the contrast tests together in combination with the linear random-effects mixed-model outputs would suggest a small increase in HADS depression scores not of clinical significance and a decrease in distress and OC worry over six months for the ‘without’ a FH of OC group.
3. Discussion
This is the first unselected population-based, prospective cohort study recruiting participants without cancer history in self/family, evaluating the feasibility of personalised lifetime OC risk stratification followed by offering risk management options. Data suggest that OC risk stratification using genetic/non-genetic (epidemiological/hormonal) factors in general population women is feasible and acceptable.
The 85% uptake of PGT and OC risk stratification suggests high acceptability, similar to previously published data indicating putative 85% uptake of PGT (
n = 734/829 in a survey study assessing attitudes of a general population of women to unselected PGT and risk-stratified OC screening [
15,
16]. Findings are also similar to data showing the high acceptability of unselected
BRCA testing in AJ populations (up to 88% uptake) [
17]. The 85%–98% overall satisfaction we found with PGT is similar to rates reported with population-based
BRCA testing in AJ populations [
9,
12].
Data from unselected
BRCA testing in the AJ population [
9,
10,
14,
18,
19] show acceptability/feasibility/effectiveness/cost effectiveness/lack of detrimental impact on psychological health/QoL, and support the concept of population-based
BRCA testing in Jewish populations. However, these inferences cannot be directly generalized to a non-Jewish general population. Our findings of overall time effect contrast tests showing levels of anxiety/depression/QoL/distress not being jointly different from baseline values but a significant reduction in OC-specific worry/general cancer risk perception following OC risk stratification are reassuring. Small changes in scores observed in some outcomes at individual time points were not clinically meaningful. While larger studies are warranted, these initial findings concur with short-/long-term outcome data following unselected
BRCA testing in AJ populations [
9,
13] and are similar to findings amongst high-risk individuals undergoing clinical criteria-based genetic testing [
20,
21,
22]. In total, 25.5% of our cohort was non-Caucasian (13.7% Asian). We found no difference in psychological health/QoL outcomes amongst non-Caucasians versus Caucasians. More research is required for understanding the role of various risk factors in non-Europeans.
Our online DA was successfully completed by women from a wide range of ages (18–85), education levels, and ethnicities, with high levels (92.2%) of satisfaction. Women who used the optional telephone helpline reported higher levels of worry/upset when viewing the DA. In total, 75% of women using the telephone helpline did so for technical DA assistance. All went on to successfully view the online DA. The telephone helpline appears to have been used as a source of emotional/technical support, emphasising the importance/need for a telephone helpline as an adjunct to online web applications to facilitate access/decision making for PGT/OC risk stratification. That one volunteer consented despite her DA score (−1) indicating she was “leaning against taking part”, highlights that whilst decision aids are adjuncts aiding decision making, individuals retain ultimate autonomy. While we showed the feasibility of using an online DA and helpline approach for PGT, this has not been compared in randomised trials to more standard/established methods (face-to-face/telephone-based/DVD-assisted counselling).
Our study strengths include population-based recruitment in a non-Jewish, ethnically diverse general population. We engaged and worked with primary care networks prior to study commencement. They helped increase awareness of this study, identify eligible women and facilitate recruitment. Engagement with primary care would be vital for the implementation of any national population-based model for PGT/OC risk stratification. Other advantages include a good questionnaire response rate, ranging from 99% (baseline) to 75% (six months follow up).
Limitations include the small sample size, lack of long-term follow up on QoL/psychological health/health behaviours. Additionally, this study was non-randomised and a control arm (without genetic testing) to compare any change in outcomes was lacking. However, the high-risk individual identified did opt for appropriate screening and preventive interventions to reduce OC/BC risk. Lack of intermediate-risk women identified probably reflects the small sample size.
In our cohort, 45% vs. 40% [
23] of the UK general population had a university level education; 7% vs. 15% [
23] were current smokers; 64% vs. 32% [
23] ate the recommended ≥5 portions of fruit/vegetables daily; 78% vs. 64% [
23] were physically active over the last month; median total household income was >£50,000 vs. £29,000 in the UK general population [
23]. Higher income, education levels and healthy lifestyle behaviour in our study participants compared to the UK’s general population may indicate a London bias. The income/education levels/lifestyle choices are similar to those of the UK Jewish population [
9,
17]. Significant associations of some study outcome variables seen with demographic variables of income/age are consistent with observations from population-based data reported in other population cohorts.
Precision prevention is a prevention strategy incorporating individual variation in genetic, epi-genetic and non-genetic (e.g., environment, hormonal, lifestyle, behavioural) risk factors. This comprises primary prevention to prevent occurrence of disease and, secondary prevention for screening/early detection of pre-symptomatic disease. Next-generation sequencing technologies, falling costs and advances in computational bioinformatics makes personalised risk-stratified prevention feasible. Improvements in the precision of risk estimation, genetic understanding of disease and increasing awareness offers an opportunity to apply this knowledge and technology at a broad population scale to make an important shift in health care towards disease prevention. Over 50% of OCs occur in 9% of the population, which is at >5% OC risk [
4]. This provides a huge opportunity for population stratification for precision prevention. Identification of unaffected women at increased risk offers opportunities for risk-stratified prevention to reduce cancer burden. Women at increased OC risk can opt for risk-reducing salpingo-oophorectomy (RRSO) to prevent tubal cancer/OC [
24], now advocated at >4–5% lifetime OC risk [
25,
26,
27].
Access to and uptake of testing for CSGs remains restricted. Only a small proportion of at-risk
BRCA carriers have been identified [
7,
8]. Our approach offers opportunities to maximise pathogenic variant identification and population stratification for OC prevention. While recent data suggest that population-based genetic testing for OC/BC gene pathogenic variants could be cost effective in general population women [
11], additional research including general population implementation studies are needed to address knowledge gaps before considering this. Additional looked for findings have recently been offered and returned following post hoc sequencing and/or analysis of some large genomic study cohorts. These studies would enable evaluation of CSG pathogenic variant carriage rates. However, this would not address in a prospective unbiased fashion key questions around the (i) logistics of population testing; (ii) information giving, a priori informed consent, and uptake of testing; (iii) uptake of preventive options. This ‘bolt-on’ paradigm of returning additional ‘secondary findings’ cannot be equated to prospective uptake of testing CSGs in an unselected unaffected population.
A prospective, Canadian cohort study offering BRCA1/BRCA2 testing to unselected men/women (The Screen Project) is ongoing. The study is evaluating the feasibility of a direct-to-consumer approach, satisfaction, OC worry, prevalence of BRCA1/BRCA2 pathogenic variants and the number of OCs/BCs prevented. Results from our feasibility study would inform the development of a larger UK-wide study that implements PGT/OC risk-stratified prevention. An important challenge is identifying optimum implementation pathways. It is likely that different context-specific models are needed for various health care systems internationally. Risk assessment pathways could be established through a community/primary care-based approach outside the traditional hospital-based genetics clinic model. A key issue that needs resolving is a system for monitoring/managing VUS. Commissioning/funding of a system where laboratory reports can be reviewed and re-issued in light of new evidence is needed. A framework/structure for data management and legal and regulatory protections will also need to be established.