Molecular Signatures of JMJD10/MINA53 in Gastric Cancer
Abstract
1. Introduction
2. Results
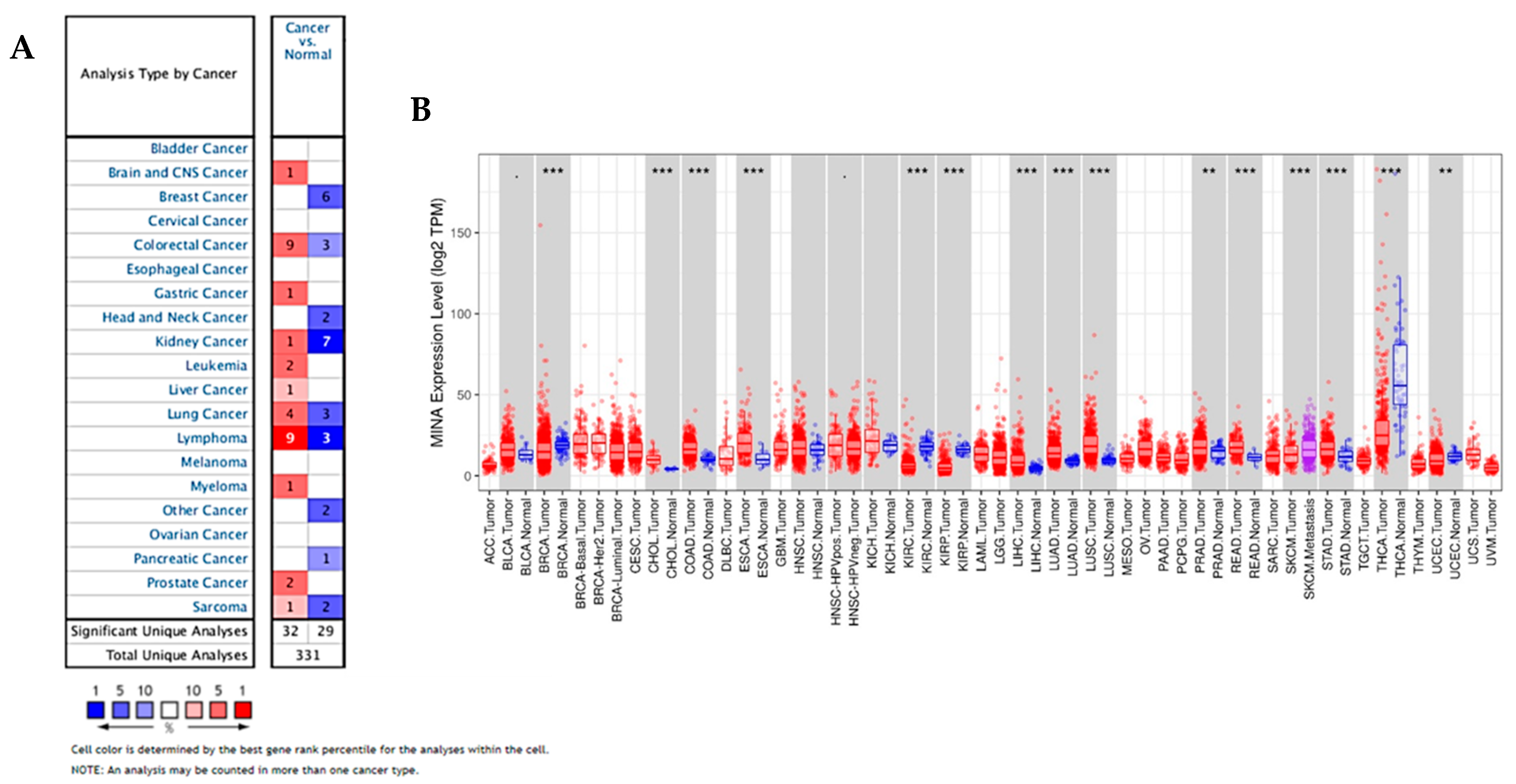
2.1. Oncomine and TCGA Data Indicate that JMJD10 is Overexpressed in Multiple Cancers Including Gastric Cancer
2.2. JMJD10 Expression in a Microarray of Gastric Cancer Patients from the ACRG Cohort
2.3. Genetic Alteration of JMJD10 in the Gastric Cancer TCGA Cohort
2.4. Analysis of MINA53 Protein Expression in Human Gastric Cancer Tissues
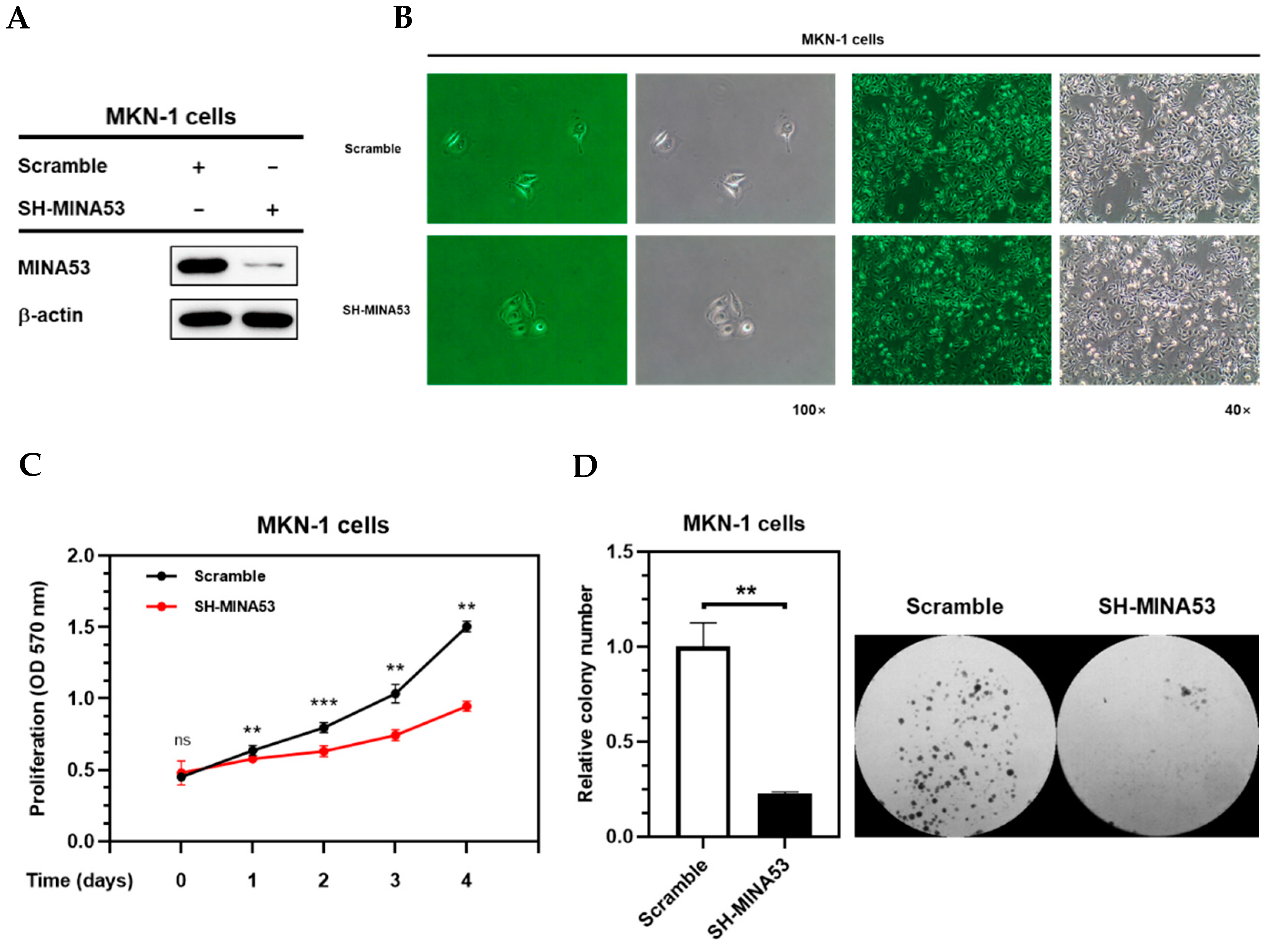
2.5. MINA53 Silencing Inhibited Proliferation Rate and Survival Ability of Gastric Cancer Cell Lines
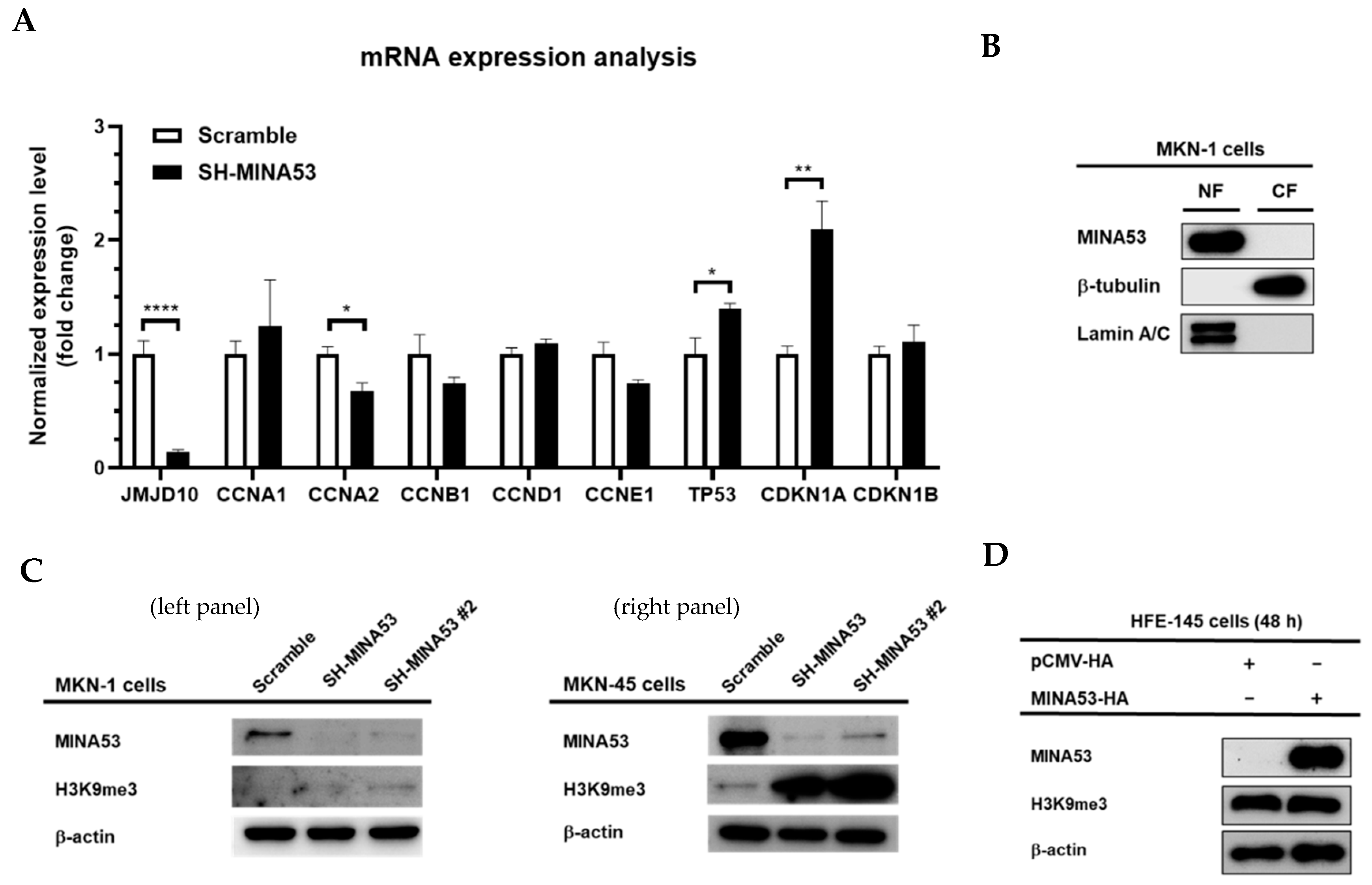
2.6. MINA53 is Involved in the Regulation of Cell Cycle-Related Gene Expression in Gastric Cancer Cell Lines
2.7. MINA53 Regulation of Histone 3 Lysine 9 Methylation in Gastric Cancer Cell Lines
2.8. Functional Enrichment Analysis of JMJD10 in Stomach Adenocarcinoma
2.9. The Regulatory Function of MINA53 in the AP-1 Signaling Pathway in Gastric Cancer Cell Lines
3. Discussion
4. Materials and Methods
4.1. JMJD10 mRNA Expression Analysis in Cancer Using Oncomine and TIMER
4.2. JMJD10 Gene Expression in the Asian Cancer Research Group (ACRG) GEO Array Dataset
4.3. Frequency of JMJD10 Gene Alteration Analysis
4.4. Preparation of Gastric Tumor Tissue Lysate and Immunoblotting
4.5. Cell Culture
4.6. shRNA-Mediated Silencing of JMJD10 in Gastric Cancer Cell Lines
4.7. Cell Proliferation Assay
4.8. Colony Formation Assay
4.9. Reverse-Transcriptase Real-Time PCR
4.10. Preparation of Nuclear and Cytosolic Fractions
4.11. Plasmid DNA Transfection
4.12. Functional and Pathway Enrichment Analysis of JMJD10 in Gastric Cancer
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Oh, S.; Shin, S.; Janknecht, R. The small members of the JMJD protein family: Enzymatic jewels or jinxes? Biochim. Biophys. Acta (BBA) Bioenerg. 2019, 1871, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Ciotta, A.; Altucci, L. The Jumonji family: Past, present and future of histone demethylases in cancer. Biomol. Concepts 2014, 5, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Johansson, C.; Tumber, A.; Che, K.; Cain, P.; Nowak, R.; Gileadi, C.; Oppermann, U. The roles of Jumonji-type oxygenases in human disease. Epigenomics 2014, 6, 89–120. [Google Scholar] [CrossRef] [PubMed]
- Cloos, P.A.; Christensen, J.; Agger, K.; Helin, K. Erasing the methyl mark: Histone demethylases at the center of cellular differentiation and disease. Genome Res. 2008, 22, 1115–1140. [Google Scholar] [CrossRef]
- Bundred, J.R.; Hendrix, E.; Coleman, M. The emerging roles of ribosomal histidyl hydroxylases in cell biology, physiology and disease. Cell. Mol. Life Sci. 2018, 75, 4093–4105. [Google Scholar] [CrossRef]
- Chowdhury, R.; Sekirnik, R.; Brissett, N.C.; Krojer, T.; Ho, C.-H.; Ng, S.S.; Clifton, I.J.; Ge, W.; Kershaw, N.J.; Fox, G.C.; et al. Ribosomal oxygenases are structurally conserved from prokaryotes to humans. Nature 2014, 510, 422–426. [Google Scholar] [CrossRef]
- Lu, Y.; Beezhold, K.; Chang, Q.; Zhang, Y.; Rojanasakul, Y.; Zhao, H.; Castranova, V.; Shi, X.; Chen, F. Lung cancer-associated JmjC domain protein mdig suppresses formation of tri-methyl lysine 9 of histone H3. Cell Cycle 2009, 8, 2101–2109. [Google Scholar] [CrossRef]
- Huang, M.-Y.; Xuan, F.; Liu, W.; Cui, H.-J. MINA controls proliferation and tumorigenesis of glioblastoma by epigenetically regulating cyclins and CDKs via H3K9me3 demethylation. Oncogene 2016, 36, 387–396. [Google Scholar] [CrossRef]
- Chen, B.; Yu, M.; Chang, Q.; Lu, Y.; Thakur, C.; Ma, D.; Yi, Z.; Chen, F. Mdig de-represses H19 large intergenic non-coding RNA (lincRNA) by down-regulating H3K9me3 and heterochromatin. Oncotarget 2013, 4, 1427–1437. [Google Scholar] [CrossRef]
- Huo, Q.; Ge, C.; Tian, H.; Sun, J.; Cui, M.; Li, H.; Zhao, F.; Chen, T.; Xie, H.; Cui, Y.; et al. Dysfunction of IKZF1/MYC/MDIG axis contributes to liver cancer progression through regulating H3K9me3/p21 activity. Cell Death Dis. 2017, 8, e2766. [Google Scholar] [CrossRef]
- Ogasawara, S.; Komuta, M.; Nakashima, O.; Akiba, J.; Tsuneoka, M.; Yano, H. Accelerated expression of a Myc target geneMina53in aggressive hepatocellular carcinoma. Hepatol. Res. 2010, 40, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Wang, K.; Liu, P.-W.; Miao, Q.; Chen, X.-Y. Mina53, a novel molecular marker for the diagnosis and prognosis of gastric adenocarcinoma. Oncol. Rep. 2013, 31, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-P.; Dong, W.-G.; Zhang, Q.; Yang, Z.-R.; Lei, X.-F.; Ai, M.-H. Potential effects of Mina53 on tumor growth in human pancreatic cancer. Cell Biophys. 2014, 69, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Li, L.; Thakur, C.; Lu, Y.; Zhang, X.; Yi, Z.; Chen, F. Proteomic Characterization of the World Trade Center dust-activated mdig and c-myc signaling circuit linked to multiple myeloma. Sci. Rep. 2016, 6, 36305. [Google Scholar] [CrossRef]
- Thakur, C.; Lu, Y.; Sun, J.; Yu, M.; Chen, B.; Chen, F. Increased expression of mdig predicts poorer survival of the breast cancer patients. Gene 2013, 535, 218–224. [Google Scholar] [CrossRef]
- Huang, H.; Kong, W.; Jean, M.; Fiches, G.; Zhou, D.; Hayashi, T.; Que, J.; Santoso, N.; Zhu, J. A CRISPR/Cas9 screen identifies the histone demethylase MINA53 as a novel HIV-1 latency-promoting gene (LPG). Nucleic Acids Res. 2019, 47, 7333–7347. [Google Scholar] [CrossRef]
- Zhang, Q.; Thakur, C.; Fu, Y.; Bi, Z.; Wadgaonkar, P.; Xu, L.; Liu, Z.; Liu, W.; Wang, J.; Kidder, B.L.; et al. Mdig promotes oncogenic gene expression through antagonizing repressive histone methylation markers. Theranostics 2020, 10, 602–614. [Google Scholar] [CrossRef]
- Teye, K.; Tsuneoka, M.; Arima, N.; Koda, Y.; Nakamura, Y.; Ueta, Y.; Shirouzu, K.; Kimura, H. Increased Expression of a Myc Target Gene Mina53 in Human Colon Cancer. Am. J. Pathol. 2004, 164, 205–216. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Yuan, B.-Z.; Castranova, V.; Shi, X.; Stauffer, J.L.; Demers, L.M.; Chen, F. The Human mineral dust-induced gene, mdig, is a cell growth regulating gene associated with lung cancer. Oncogene 2005, 24, 4873–4882. [Google Scholar] [CrossRef]
- Komiya, K.; Sueoka-Aragane, N.; Sato, A.; Hisatomi, T.; Sakuragi, T.; Mitsuoka, M.; Sato, T.; Hayashi, S.; Izumi, H.; Tsuneoka, M.; et al. Mina53, a novel c-Myc target gene, is frequently expressed in lung cancers and exerts oncogenic property in NIH/3T3 cells. J. Cancer Res. Clin. Oncol. 2009, 136, 465–473. [Google Scholar] [CrossRef]
- Tsuneoka, M.; Arima, N.; Teye, K.; Okamura, T.; Inutsuka, H.; Shirouzu, K.; Fujita, H.; Koda, Y.; Kimura, H. Mina53 as a Potential Prognostic Factor for Esophageal Squamous Cell Carcinoma. Clin. Cancer Res. 2004, 10, 7347–7356. [Google Scholar] [CrossRef] [PubMed]
- Teye, K.; Arima, N.; Nakamura, Y.; Sakamoto, K.; Sueoka, E.; Kimura, H.; Tsuneoka, M. Expression of Myc target gene mina53 in subtypes of human lymphoma. Oncol. Rep. 2007, 18, 841–848. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tan, X.-P.; Zhang, Q.; Dong, W.-G.; Lei, X.-W.; Yang, Z.-R. Upregulated expression of Mina53 in cholangiocarcinoma and its clinical significance. Oncol. Lett. 2012, 3, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Kuratomi, K.; Yano, H.; Tsuneoka, M.; Sakamoto, K.; Kusukawa, J.; Kojiro, M. Immunohistochemical expression of Mina53 and Ki67 proteins in human primary gingival squamous cell carcinoma. Kurume Med J. 2006, 53, 71–78. [Google Scholar] [CrossRef][Green Version]
- Fukahori, S.; Yano, H.; Tsuneoka, M.; Tanaka, Y.; Yagi, M.; Kuwano, M.; Tajiri, T.; Taguchi, T.; Tsuneyoshi, M.; Kojiro, M. Immunohistochemical expressions of Cap43 and Mina53 proteins in neuroblastoma. J. Pediatr. Surg. 2007, 42, 1831–1840. [Google Scholar] [CrossRef]
- Xuan, F.; Huang, M.; Zhao, E.; Cui, H. MINA53 deficiency leads to glioblastoma cell apoptosis via inducing DNA replication stress and diminishing DNA damage response. Cell Death Dis. 2018, 9, 1062. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Morera, L.; Lübbert, M.; Jung, M. Targeting histone methyltransferases and demethylases in clinical trials for cancer therapy. Clin. Epigenetics 2016, 8, 57. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef]
- Van Dam, S.; Võsa, U.; Van Der Graaf, A.; Franke, L.; De Magalhães, J.P. Gene co-expression analysis for functional classification and gene-disease predictions. Briefings Bioinform. 2018, 19, 575–592. [Google Scholar] [CrossRef]
- Supek, F.; Bosnjak, M.; Skunca, N.; Šmuc, T. REVIGO Summarizes and Visualizes Long Lists of Gene Ontology Terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef]
- Wagner, E.F.; Nebreda, A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.R.; Yu, J.; Shanker, K.; Deshpande, N.; Varambally, R.; Ghosh, D.; Barrette, T.; Pander, A.; Chinnaiyan, A.M. ONCOMINE: A Cancer Microarray Database and Integrated Data-Mining Platform. Neoplasia 2004, 6, 1–6. [Google Scholar] [CrossRef]
- Rhodes, D.R.; Kalyana-Sundaram, S.; Mahavisno, V.; Varambally, R.; Yu, J.; Briggs, B.B.; Barrette, T.R.; Anstet, M.J.; Kincead-Beal, C.; Kulkarni, P.; et al. Oncomine 3.0: Genes, Pathways, and Networks in a Collection of 18,000 Cancer Gene Expression Profiles. Neoplasia 2007, 9, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—update. Nucleic Acids Res. 2012, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.C.; Sohn, B.H.; Cheong, J.-H.; Kim, S.-B.; Lee, J.E.; Park, K.C.; Lee, S.H.; Park, J.-L.; Park, Y.-Y.; Lee, H.-S.; et al. Clinical and genomic landscape of gastric cancer with a mesenchymal phenotype. Nat. Commun. 2018, 9, 1777. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Skanderup, A.J.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Skanderup, A.J.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Moffat, J.; Grueneberg, R.A.; Yang, X.; Kim, S.Y.; Kloepfer, A.M.; Hinkle, G.; Piqani, B.; Eisenhaure, T.; Luo, B.; Grenier, J.K.; et al. A Lentiviral RNAi Library for Human and Mouse Genes Applied to an Arrayed Viral High-Content Screen. Cell 2006, 124, 1283–1298. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Yi, Y.-S.; Son, Y.-J.; Han, S.Y.; Kim, N.H.; Nam, G.; Hossain, M.A.; Kim, J.-H.; Park, J.; Cho, J. BIOGF1K, a compound K-rich fraction of ginseng, plays an antiinflammatory role by targeting an activator protein-1 signaling pathway in RAW264.7 macrophage-like cells. J. Ginseng Res. 2018, 42, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Cho, J.; Cho, J. Thymoquinone Suppresses IRF-3-Mediated Expression of Type I Interferons via Suppression of TBK1. Int. J. Mol. Sci. 2018, 19, 1355. [Google Scholar] [CrossRef]
- Byeon, S.E.; Lee, Y.G.; Kim, B.H.; Shen, T.; Lee, S.Y.; Park, H.J.; Park, S.-C.; Rhee, M.H.; Cho, J.Y. Surfactin blocks NO production in lipopolysaccharide-activated macrophages by inhibiting NF-kappaB activation. J. Microbiol. Biotechnol. 2008, 18, 1984–1989. [Google Scholar]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]




| Characteristic | n (%) |
|---|---|
| Age | |
| Median | 60.5 |
| Range | 22–86 |
| Sex | |
| Male | 75 |
| Female | 25 |
| Stage | |
| Ib | 13.46 |
| II | |
| III | 34.62 |
| IV | 51.92 |
| Location | |
| Upper third | 19.23 |
| Mid third | 46.15 |
| Lower third | 48.08 |
| Lauren Classification | |
| Intestinal | 42.31 |
| Diffuse | 44.23 |
| Mixed | 9.62 |
| Indeterminate | 3.85 |
| Differentiation | |
| Well-differentiated carcinoma | 1.92 |
| Moderately differentiated carcinoma | 30.77 |
| Poorly differentiated carcinoma | 26.92 |
| Mucinous carcinoma | 15.38 |
| Signet ring cell carcinoma | 40.38 |
| Others | 7.69 |
| Lymphovascular invasion | |
| Absent | 26.92 |
| Present | 73.08 |
| Neural/perineural invasion | |
| Absent | 36.54 |
| Present | 63.46 |
| PCR Type | Genes Name | Sequence (5′-3′) | |
|---|---|---|---|
| qPCR | GAPDH | Forward | CAATGAATACGGCTACAGCA |
| Reverse | AGGGAGATGCTCAGTGTTGG | ||
| JMJD10 | Forward | TACCGAGGCTGGACAGTGTA | |
| Reverse | TTCATCCTCTCCTCGGCTCA | ||
| CCNA1 | Forward | AGAAAGATAACGACGGGAAGAG | |
| Reverse | CTGGAAGACGAAATCTGGGAG | ||
| CCNA2 | Forward | TCTGTGTTCTGTGAATAAAGCA | |
| Reverse | TTCTTGGATGCCAGTCTTAC | ||
| CCNB1 | Forward | CAACTTGAGGAAGAGCAAGC | |
| Reverse | TCTCCTGCAACAACCTGAAT | ||
| CCND1 | Forward | GAAGTTGCAAAGTCCTGGAGC | |
| Reverse | ATGGTTTCCACTTCGCAGCA | ||
| CCNE1 | Forward | GATGAAGAAATGGCCAAAATCG | |
| Reverse | GCACGTTGATGAGTTTGGGTAAA | ||
| TP53 | Forward | AAGCAGTCACAGCACATGACGGAG | |
| Reverse | GAGTCTTCCAGTGAGATGATGGT | ||
| CDKN1A | Forward | TGCCGAAGTCAGTTCCTTGTG | |
| Reverse | GTTCTGACATGGCGCCTCCT | ||
| CDKN1B | Forward | TCACAAGGCAGTGATGAAGCA | |
| Reverse | GGTGTTCACAGAGCCGAACT | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, N.; Hong, Y.H.; Jo, M.K.; Kim, J.K.; Kim, K.-H.; Ashktorab, H.; Smoot, D.T.; Hur, H.; Yoo, B.C.; Cho, J.Y. Molecular Signatures of JMJD10/MINA53 in Gastric Cancer. Cancers 2020, 12, 1141. https://doi.org/10.3390/cancers12051141
Aziz N, Hong YH, Jo MK, Kim JK, Kim K-H, Ashktorab H, Smoot DT, Hur H, Yoo BC, Cho JY. Molecular Signatures of JMJD10/MINA53 in Gastric Cancer. Cancers. 2020; 12(5):1141. https://doi.org/10.3390/cancers12051141
Chicago/Turabian StyleAziz, Nur, Yo Han Hong, Min Kyeong Jo, Jin Kyeong Kim, Kyung-Hee Kim, Hassan Ashktorab, Duane T. Smoot, Hoon Hur, Byong Chul Yoo, and Jae Youl Cho. 2020. "Molecular Signatures of JMJD10/MINA53 in Gastric Cancer" Cancers 12, no. 5: 1141. https://doi.org/10.3390/cancers12051141
APA StyleAziz, N., Hong, Y. H., Jo, M. K., Kim, J. K., Kim, K.-H., Ashktorab, H., Smoot, D. T., Hur, H., Yoo, B. C., & Cho, J. Y. (2020). Molecular Signatures of JMJD10/MINA53 in Gastric Cancer. Cancers, 12(5), 1141. https://doi.org/10.3390/cancers12051141







