Multicenter Retrospective Analysis of Second-Line Therapy after Gemcitabine Plus Nab-Paclitaxel in Advanced Pancreatic Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Evaluation of Outcomes
2.3. Statistical Analysis
3. Results
3.1. Population
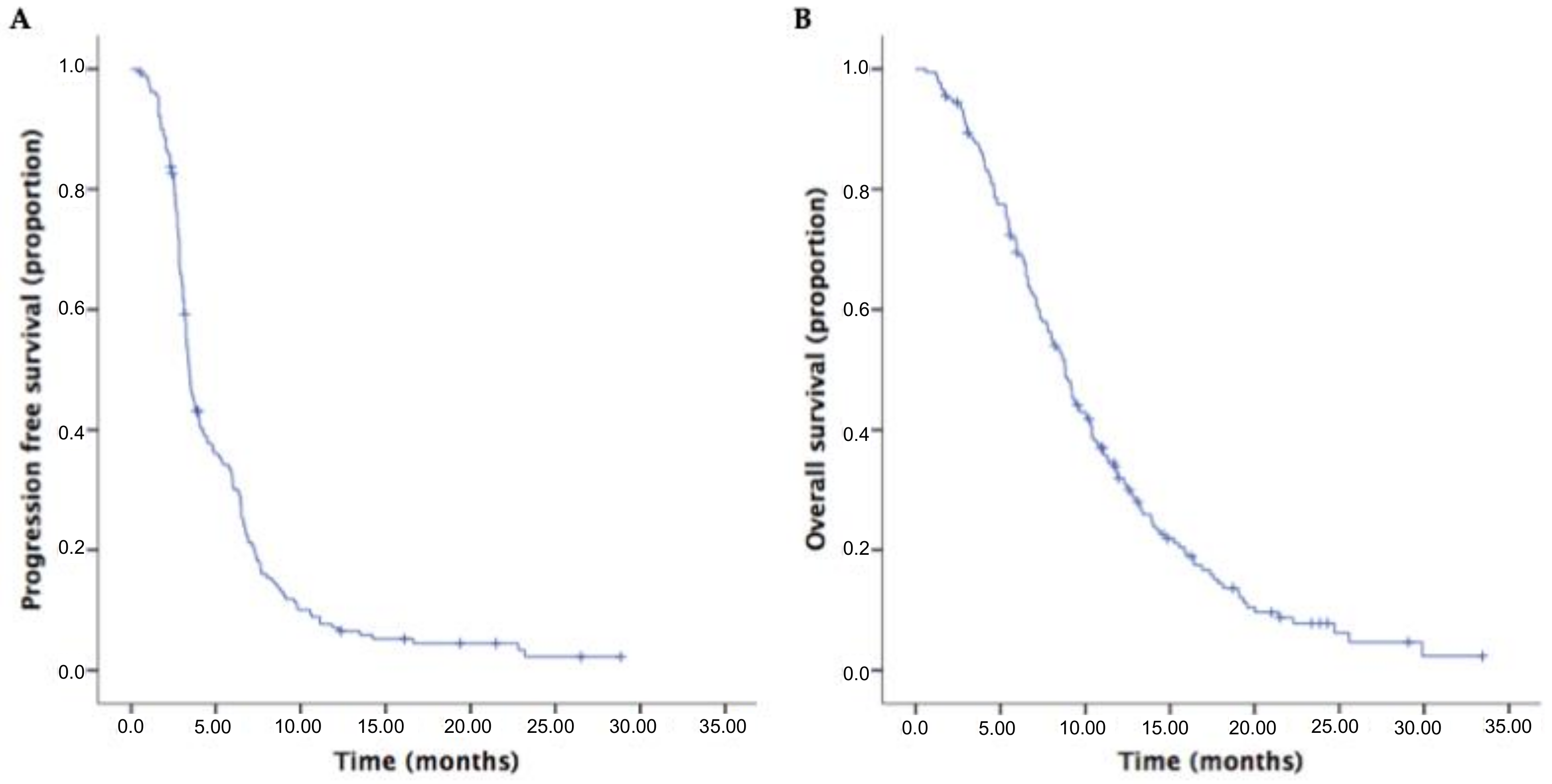
3.2. Survival Outcomes and Response Rates of Second-Line Therapies in the Overall Population
3.3. Second-Line Therapies in the Overall Population
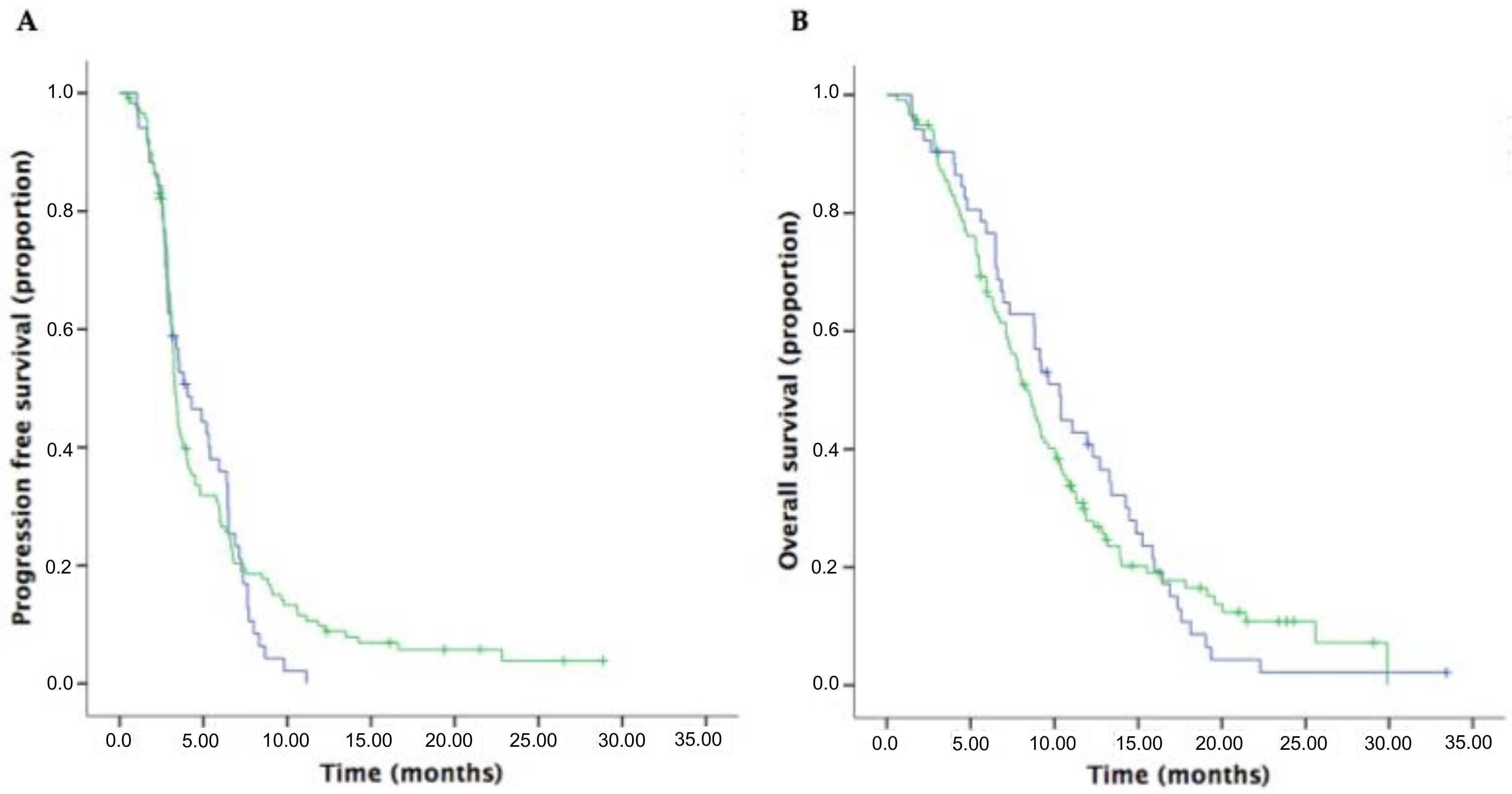
3.4. Survival Outcomes and Response Rates in Patients Receiving Oxaliplatin-Based and Irinotecan-Based Doublet Regimens in the Second-Line Setting
3.5. Survival Outcomes of First-Line Therapy in the Overall Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Melisi, D.; Calvetti, L.; Frizziero, M.; Tortora, G. Pancreatic cancer: Systemic combination therapies for a heterogeneous disease. Curr. Pharm. Des. 2014, 20, 6660–6669. [Google Scholar] [CrossRef]
- Melisi, D.; Budillon, A. Pancreatic cancer: Between bench and bedside. Curr. Drug Targets 2012, 13, 729–730. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Pourshams, A.; Sepanlou, S.G.; Ikuta, K.S.; Bisignano, C.; Safiri, S.; Roshandel, G.; Sharif, M.; Khatibian, M.; Fitzmaurice, C.; Nixon, M.R.; et al. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 934–947. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouche, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardiere, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Taieb, J.; Prager, G.W.; Melisi, D.; Westphalen, C.B.; D’Esquermes, N.; Ferreras, A.; Carrato, A.; Macarulla, T. First-line and second-line treatment of patients with metastatic pancreatic adenocarcinoma in routine clinical practice across Europe: A retrospective, observational chart review study. ESMO Open 2020, 5, e000587. [Google Scholar] [CrossRef]
- Chiorean, E.G.; Von Hoff, D.D.; Tabernero, J.; El-Maraghi, R.; Ma, W.W.; Reni, M.; Manax, V. Second-line therapy after nab-paclitaxel plus gemcitabine or after gemcitabine for patients with metastatic pancreatic cancer. Br. J. Cancer 2016, 115, 188–194. [Google Scholar] [CrossRef]
- Aprile, G.; Negri, F.; Giuliani, F.; De Carlo, E.; Melisi, D.; Simionato, F.; Silvestris, N.; Brunetti, O.; Leone, F.; Marino, D.; et al. Second-line chemotherapy for advanced pancreatic cancer: Which is the best option? Crit. Rev. Oncol. 2017, 115, 1–12. [Google Scholar] [CrossRef]
- Pelzer, U.; Schwaner, I.; Stieler, J.M.; Adler, M.; Seraphin, J.; Dörken, B.; Riess, H.; Oettle, H. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: A phase III-study from the German CONKO-study group. Eur. J. Cancer 2011, 47, 1676–1681. [Google Scholar] [CrossRef]
- Oettle, H.; Riess, H.; Stieler, J.M.; Heil, G.; Schwaner, I.; Seraphin, J.; Görner, M.; Mölle, M.; Greten, T.F.; Lakner, V.; et al. Second-Line Oxaliplatin, Folinic Acid, and Fluorouracil Versus Folinic Acid and Fluorouracil Alone for Gemcitabine-Refractory Pancreatic Cancer: Outcomes From the CONKO-003 Trial. J. Clin. Oncol. 2014, 32, 2423–2429. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Ko, Y.-J.; Cripps, C.; Beaudoin, A.; Dhesy-Thind, S.; Zulfiqar, M.; Zalewski, P.; Do, T.; Cano, P.; Lam, W.Y.H.; et al. PANCREOX: A Randomized Phase III Study of Fluorouracil/Leucovorin With or Without Oxaliplatin for Second-Line Advanced Pancreatic Cancer in Patients Who Have Received Gemcitabine-Based Chemotherapy. J. Clin. Oncol. 2016, 34, 3914–3920. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Lonardi, S.; Bendell, J.C.; Sim, H.-W.; Macarulla, T.; Lopez, C.D.; Van Cutsem, E.; Martin, A.J.M.; Park, J.O.; Greil, R.; et al. Randomized Phase III Study of FOLFOX Alone and with Pegilodecakin as Second-line Therapy in Patients with Metastatic Pancreatic Cancer (SEQUOIA). J. Clin. Oncol. 2020, 38, 637. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Li, C.-P.; Bodoky, G.; Dean, A.; Shan, Y.-S.; Jameson, G.; Macarulla, T.; Lee, K.-H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Hubner, R.A.; Siveke, J.T.; Von Hoff, D.D.; Belanger, B.; de Jong, F.A.; Mirakhur, B.; Chen, L.T. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur. J. Cancer 2019, 108, 78–87. [Google Scholar] [CrossRef]
- Hubner, R.A.; Cubillo, A.; Blanc, J.-F.; Melisi, D.; Von Hoff, D.D.; Wang-Gillam, A.; Chen, L.-T.; Becker, C.; Mamlouk, K.; Belanger, B.; et al. Quality of life in metastatic pancreatic cancer patients receiving liposomal irinotecan plus 5-fluorouracil and leucovorin. Eur. J. Cancer 2019, 106, 24–33. [Google Scholar] [CrossRef]
- Pelzer, U.; Blanc, J.-F.; Melisi, D.; Cubillo, A.; Von Hoff, D.D.; Wang-Gillam, A.; Chen, L.-T.; Siveke, J.T.; Wan, Y.; Solem, C.T.; et al. Quality-adjusted survival with combination nal-IRI+5-FU/LV vs 5-FU/LV alone in metastatic pancreatic cancer patients previously treated with gemcitabine-based therapy: A Q-TWiST analysis. Br. J. Cancer 2017, 116, 1247–1253. [Google Scholar] [CrossRef]
- Zaniboni, A.; Aitini, E.; Barni, S.; Ferrari, D.; Cascinu, S.; Catalano, V.; Valmadre, G.; Ferrara, D.; Veltri, E.; Codignola, C.; et al. FOLFIRI as second-line chemotherapy for advanced pancreatic cancer: A GISCAD multicenter phase II study. Cancer Chemother. Pharmacol. 2012, 69, 1641–1645. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 1491–1492. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Lee, J.W.; Zalupski, M.; Capanu, M.; Park, J.; Golan, T.; Tahover, E.; Lowery, M.A.; Chou, J.F.; Sahai, V.; et al. Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin With or Without Veliparib in Patients With Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation. J. Clin. Oncol. 2020, JCO1902931. [Google Scholar] [CrossRef]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmaña, J.; Rosengarten, O. Olaparib Monotherapy in Patients with Advanced Cancer and a Germline BRCA1/2 Mutation. J. Clin. Oncol. 2015, 33, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Hendifar, A.; McWilliams, R.R.; Geva, R.; Epelbaum, R.; Rolfe, L.; Goble, S.; Lin, K.K.; Biankin, A.V.; Giordano, H.; et al. Rucaparib Monotherapy in Patients With Pancreatic Cancer and a Known Deleterious BRCA Mutation. JCO Precis. Oncol. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v56–v68. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A. NCCN Guidelines Updates: Pancreatic Cancer. J. Natl. Compr. Canc. Netw. 2019, 17, 603–605. [Google Scholar] [CrossRef]

| Baseline Characteristics | n (%) |
|---|---|
| Sex | |
| female | 82 (45.4) |
| male | 99 (54.6) |
| Median age (years) | 64 (34-81) |
| ECOG PS | |
| 0 | 114 (62.9) |
| 1 | 49 (27.1) |
| 2 | 11 (6.0) |
| unknown | 7 (3.9) |
| Tumor location | |
| head | 92 (50.9) |
| body-tail | 87 (48.1) |
| unknown | 2 (1.2) |
| CA19.9 | |
| <40 U/mL | 24 (13.2) |
| ≥40 U/mL | 142 (78.5) |
| unknown | 15 (8.3) |
| BMI | |
| ≥25 | 42 (23.2) |
| 18.5–24.9 | 113 (62.5) |
| <18.5 | 13 (7.2) |
| unknown | 13 (7.2) |
| Metastatic sites | |
| liver | 130 (71.8) |
| peritoneum | 43 (23.8) |
| lung | 59 (32.6) |
| other | 16 (8.8) |
| Number of metastatic sites | |
| 0 | 3 (1.6) |
| 1 | 83 (45.9) |
| 2 | 59 (32.6) |
| ≥3 | 36 (19.9) |
| Previous procedures | |
| radiotherapy | 19 (10.4) |
| surgery | 42 (23.2) |
| Prior neoadjuvant therapy | 14 (7.7) |
| Prior adjuvant therapy | 16 (8.8) |
| Response | Overall Population n (%) | Oxaliplatin-Based Doublet n (%) | Irinotecan-Based Doublet n (%) |
|---|---|---|---|
| ORR | 18 (10.5) | 7 (13.7) | 11 (9.6) |
| PR | 16 (9.3) | 7 (13.7) | 9 (7.8) |
| CR | 2 (1.2) | 0 | 2 (1.7) |
| DCR | 56 (32.3) | 18 (35.2) | 35 (30.7) |
| SD | 38 (22.0) | 11 (21.5) | 24 (21.1) |
| PR | 16 (9.3) | 7 (13.7) | 9 (7.8) |
| CR | 2 (1.2) | 0 | 2 (1.7) |
| Variables | PFS | OS | ||
|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |
| p | p | p | p | |
| Sex | ||||
| Male vs. female | 0.935 | 0.274 | ||
| PS | ||||
| 0 vs. 1–2 | 0.062 | 0.077 | ||
| Pancreatic tumor site | ||||
| Head vs. body-tail | 0.639 | 0.318 | ||
| Previous surgery | ||||
| No vs. yes | 0.304 | 0.126 | ||
| Previous radiotherapy | ||||
| No vs. yes | 0.213 | 0.901 | ||
| Site of metastasis | ||||
| Liver (no vs. yes) | 0.865 | 0.051 | ||
| Lung (no vs. yes) | 0.048 | 0.060 | 0.624 | |
| Peritoneum (no vs. yes) | 0.123 | 0.237 | ||
| Bone (no vs. yes) | 0.002 | 0.004 | 0.070 | |
| BMI | ||||
| ≤25 vs. >25 kg/m2 | 0.099 | 0.058 | ||
| CA19.9 | ||||
| <40 vs. ≥40 U/mL | 0.069 | 0.172 | ||
| N/L ratio | ||||
| ≤5 vs. >5 | 0.770 | 0.809 | ||
| PFS | OS | |||||
|---|---|---|---|---|---|---|
| Characteristic | Oxaliplatin-Based Doublet Months (95%CI) | Irinotecan-Based Doublet Months (95%CI) | p | Oxaliplatin-Based Doublet Months (95%CI) | Irinotecan-Based Doublet Months (95%CI) | p |
| Sex | ||||||
| Male | 4.0 (2.2–6.0) | 3.2 (3.1–3.5) | 0.446 | 8.8 (8.0–9.6) | 8.0 (5.3–10.7) | 0.972 |
| Female | 3.5 (0.6–6.5) | 3.3 (3.0–3.7) | 0.901 | 11.9 (7.8–16) | 8.5 (6.9–10.1) | 0.550 |
| PS | ||||||
| 0 | 4.0 (1.4–6.7) | 3.6 (2.8–4.5) | 0.323 | 11.0 (8.1–14.0) | 9.1 (7.3–10.9) | 0.843 |
| 1–2 | 2.8 (1.7–4.1) | 2.8 (2.5–3.2) | 0.871 | 6.8 (5.2–8.5) | 5.9 (3.6–8.3) | 0.905 |
| Pancreatic tumor site | ||||||
| Head | 5.3 (3.9–6.9) | 3.3 (2.9–3.6) | 0.532 | 10.3 (6.3–14.3) | 8.0 (6.3–9.7) | 0.211 |
| Body-tail | 3.1 (2.2–4.1) | 3.5 (3.0–4.0) | 0.085 | 9.1 (7.2–11.1) | 8.9 (1.2–6.7) | 0.355 |
| Previous surgery | ||||||
| Yes | 5.1 (1.1–9.2) | 3.7 (2.6–4.9) | 0.450 | 10.3 (7.7–13.1) | 10.0 (6.9–13.2) | 0.621 |
| No | 3.8 (2.6–4.9) | 3.2 (3.0–3.5) | 0.855 | 9.2 (7.4–11.1) | 7.7 (6.2–9.3) | 0.417 |
| Previous radiotherapy | ||||||
| Yes | 7.1 (3.2–11.1) | 4.5 (1.1–7.8) | 0.813 | 12.7 (4.2–21.2) | 9.1 (7.9–10.6) | 0.679 |
| No | 3.5 (2.6–4.4) | 3.2 (3.1–3.4) | 0.428 | 9.2 (6.4–11.9) | 8.0 (6.6–9.4) | 0.789 |
| Site of metastasis | ||||||
| Liver | 3.5 (2.5–4.4) | 3.3 (2.9–3.9) | 0.361 | 9.2 (7.5–10.9) | 7.7 (6.6–8.9) | 0.831 |
| Lung | 3.0 (2.5–3.6) | 3.0 (2.6–3.5) | 0.903 | 8.8 (4.8–12.9) | 9.3 (7.1–11.6) | 0.889 |
| Peritoneum | 2.8 (2.3–3.5) | 2.9 (2.6–3.4) | 0.554 | 6.6 (5.0–8.2) | 7.7 (4.7–10.9) | 0.676 |
| Bone | 1.7 (-) | 2.1 (1.0–3.4) | 0.321 | 2.6 (-) | 7.7 (2.2–13.4) | 0.145 |
| BMI | ||||||
| ≤25 kg/m2 | 3.5 (1.9–5.1) | 3.2 (2.9–3.6) | 0.751 | 9.1 (5.8–12.5) | 8.0 (6.8–9.2) | 0.792 |
| >25 kg/m2 | 4.0 (1.8–6.3) | 3.7 (0.6–7.0) | 0.415 | 10.3 (4.1–16.7) | 10.0 (7.0–13.1) | 0.639 |
| CA19.9 | ||||||
| <40 U/mL | 6.5 (5.5–7.5) | 4.0 (2.8–5.2) | 0.857 | 14.8 (13.0–16.7) | 7.4 (4.9–9.9) | 0.959 |
| ≥40 U/mL | 3.5 (2.4–4.6) | 3.2 (3.0–3.5) | 0.482 | 9.6 (8.0–11.3) | 8.6 (7.4–9.9) | 0.827 |
| N/L ratio | ||||||
| ≤5 | 3.8 (2.6–5.1) | 3.3 (3.2–3.6) | 0.364 | 10.3 (7.8–12.9) | 8.0 (6.4–9.7) | 0.564 |
| >5 | 6.4 (6.3–6.6) | 2.8 (2.6–3.2) | 0.384 | 12.2 (7.0–17.5) | 7.7 (3.6–11.8) | 0.816 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merz, V.; Cavaliere, A.; Messina, C.; Salati, M.; Zecchetto, C.; Casalino, S.; Milella, M.; Caffo, O.; Melisi, D. Multicenter Retrospective Analysis of Second-Line Therapy after Gemcitabine Plus Nab-Paclitaxel in Advanced Pancreatic Cancer Patients. Cancers 2020, 12, 1131. https://doi.org/10.3390/cancers12051131
Merz V, Cavaliere A, Messina C, Salati M, Zecchetto C, Casalino S, Milella M, Caffo O, Melisi D. Multicenter Retrospective Analysis of Second-Line Therapy after Gemcitabine Plus Nab-Paclitaxel in Advanced Pancreatic Cancer Patients. Cancers. 2020; 12(5):1131. https://doi.org/10.3390/cancers12051131
Chicago/Turabian StyleMerz, Valeria, Alessandro Cavaliere, Carlo Messina, Massimiliano Salati, Camilla Zecchetto, Simona Casalino, Michele Milella, Orazio Caffo, and Davide Melisi. 2020. "Multicenter Retrospective Analysis of Second-Line Therapy after Gemcitabine Plus Nab-Paclitaxel in Advanced Pancreatic Cancer Patients" Cancers 12, no. 5: 1131. https://doi.org/10.3390/cancers12051131
APA StyleMerz, V., Cavaliere, A., Messina, C., Salati, M., Zecchetto, C., Casalino, S., Milella, M., Caffo, O., & Melisi, D. (2020). Multicenter Retrospective Analysis of Second-Line Therapy after Gemcitabine Plus Nab-Paclitaxel in Advanced Pancreatic Cancer Patients. Cancers, 12(5), 1131. https://doi.org/10.3390/cancers12051131








