Molecular and Clinical Relevance of ZBTB38 Expression Levels in Prostate Cancer
Abstract
1. Introduction
2. Results
2.1. ZBTB38 Expression Is Lower in Prostate Cancer Compared to Non-Cancerous Prostate Tissues
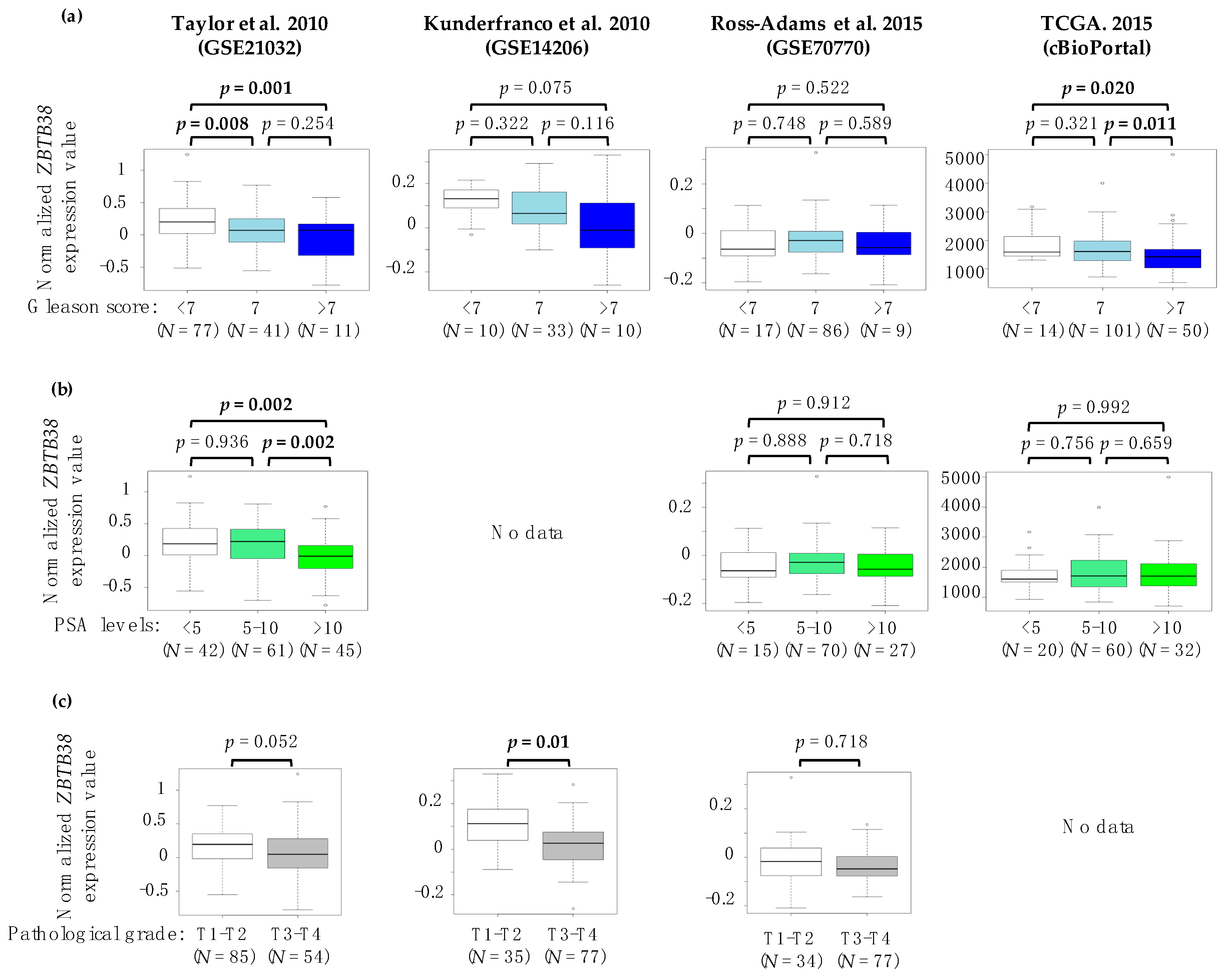
2.2. Association between ZBTB38 Expression and Clinico-Pathological Features of Prostate Cancer
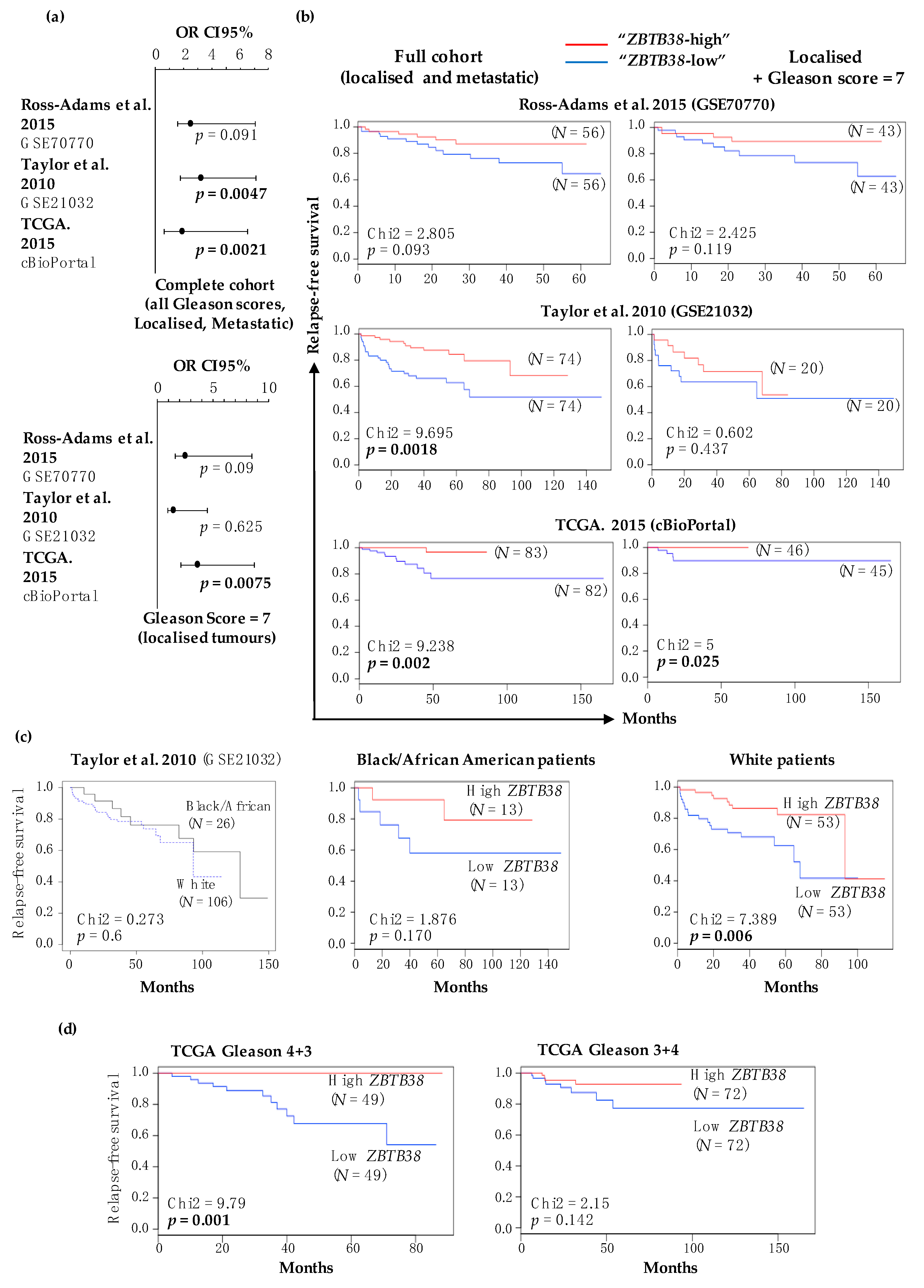
2.3. Low Expression of ZBTB38 Correlates with Poor Outcome
2.4. ZBTB38 Expression Level Is an Independent Prognostic Factor of Disease-Free Survival
2.5. Low Expression of ZBTB38 Associates with Genomic Instability in Localised Prostate Cancer
2.6. ZBTB38 Expression Is Not a Marker of Chromosomal Instability in Metastatic Prostate Cancer
2.7. Gene Expression Profiling Reveals a Gene Signature Distinguishing Tumours Expressing Low and High Levels of ZBTB38, and with Differential Sensitivity to Doxorubicin
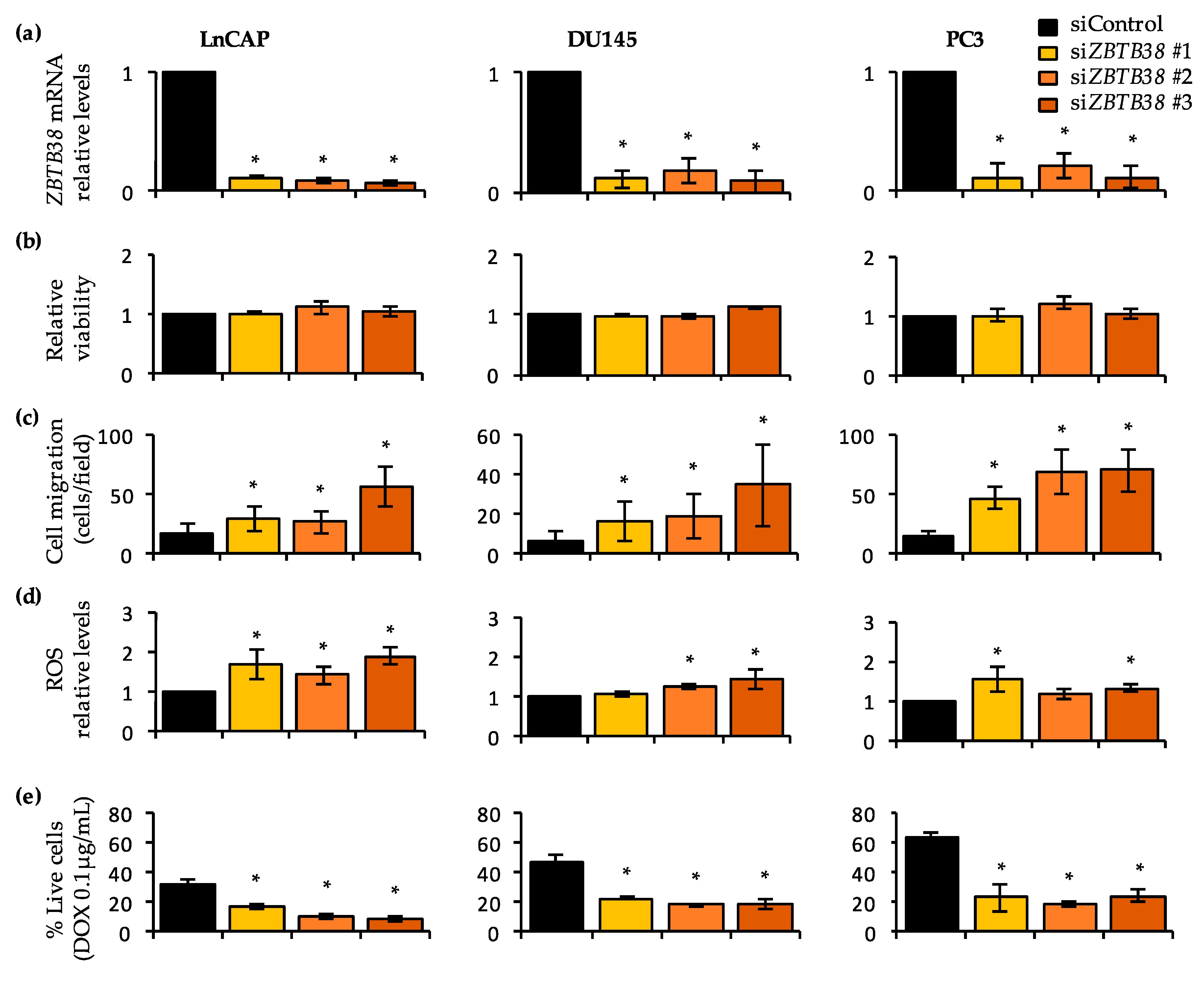
2.8. Knock-Down of ZBTB38 Promotes ROS Accumulation, Cell Migration and Doxorubicin Toxicity in Prostate Cancer Cells
3. Discussion
3.1. ZBTB38 Expression Correlates with Prostate Cancer Progression and Is an Independent Prognostic Marker
3.2. ZBTB38 and Its Paralogs ZBTB4 and KAISO/ZBTB33 Are Involved in Prostate Cancer
3.3. ZBTB38 Levels Are Correlated with SPOP Alterations and a Transcriptomic Signature Associated with Doxorubicin Sensitivity
3.4. ZBTB38 Expression Correlates with Genomic Instability in Prostate Cancer
4. Materials and Methods
4.1. Microarray Databases and Selection of Datasets
4.2. Clinical and Genomic Data Correlations
4.3. Gene Expression Analysis of Localised Tumours and Functional Annotation of Differentially Expressed Genes
4.4. Prostate Cancer Cell Lines: Maintenance and Treatments
4.5. Doxorubicin Sensitivity Tests
4.6. Gene Expression Analysis
4.7. Cell Parameters Analysis by Fluorescent Activated Cell Sorting (FACS)
4.8. Cell Migration Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hulsen, T. An overview of publicly available patient-centered prostate cancer datasets. Transl. Androl. Urol. 2019, 8, S64–S77. [Google Scholar] [CrossRef] [PubMed]
- Penney, K.L.; Stampfer, M.J.; Jahn, J.L.; Sinnott, J.A.; Flavin, R.; Rider, J.R.; Finn, S.; Giovannucci, E.; Sesso, H.D.; Loda, M.; et al. Gleason grade progression is uncommon. Cancer Res. 2013, 73, 5163–5168. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhao, D.; Spring, D.J.; DePinho, R.A. Genetics and biology of prostate cancer. Genes Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Allsbrook, W.C.; Amin, M.B.; Egevad, L.L. ISUP Grading Committee The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2005, 29, 1228–1242. [Google Scholar] [CrossRef]
- Wilkinson, S.; Harmon, S.A.; Terrigino, N.T.; Karzai, F.; Pinto, P.A.; Madan, R.A.; VanderWeele, D.J.; Lake, R.; Atway, R.; Bright, J.R.; et al. A case report of multiple primary prostate tumors with differential drug sensitivity. Nat. Commun. 2020, 11, 837. [Google Scholar] [CrossRef]
- Kensler, K.H.; Rebbeck, T.R. Cancer Progress and Priorities: Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 267–277. [Google Scholar] [CrossRef]
- Stark, J.R.; Perner, S.; Stampfer, M.J.; Sinnott, J.A.; Finn, S.; Eisenstein, A.S.; Ma, J.; Fiorentino, M.; Kurth, T.; Loda, M.; et al. Gleason score and lethal prostate cancer: Does 3 + 4 = 4 + 3? J. Clin. Oncol. 2009, 27, 3459–3464. [Google Scholar] [CrossRef]
- Karakas, C.; Wang, C.; Deng, F.; Huang, H.; Wang, D.; Lee, P. Molecular mechanisms involving prostate cancer racial disparity. Am. J. Clin. Exp. Urol. 2017, 5, 34–48. [Google Scholar] [PubMed]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network the Molecular Taxonomy of Primary Prostate Cancer. Cell 2015, 163, 1011–1025. [CrossRef] [PubMed]
- Grasso, C.S.; Wu, Y.-M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, C.E.; Baca, S.C.; Lawrence, M.S.; Demichelis, F.; Blattner, M.; Theurillat, J.-P.; White, T.A.; Stojanov, P.; Van Allen, E.; Stransky, N.; et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat. Genet. 2012, 44, 685–689. [Google Scholar] [CrossRef]
- Fraser, M.; Sabelnykova, V.Y.; Yamaguchi, T.N.; Heisler, L.E.; Livingstone, J.; Huang, V.; Shiah, Y.-J.; Yousif, F.; Lin, X.; Masella, A.P.; et al. Genomic hallmarks of localized, non-indolent prostate cancer. Nature 2017, 541, 359–364. [Google Scholar] [CrossRef]
- Kumar, A.; Coleman, I.; Morrissey, C.; Zhang, X.; True, L.D.; Gulati, R.; Etzioni, R.; Bolouri, H.; Montgomery, B.; White, T.; et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat. Med. 2016, 22, 369–378. [Google Scholar] [CrossRef]
- Hieronymus, H.; Schultz, N.; Gopalan, A.; Carver, B.S.; Chang, M.T.; Xiao, Y.; Heguy, A.; Huberman, K.; Bernstein, M.; Assel, M.; et al. Copy number alteration burden predicts prostate cancer relapse. Proc. Natl. Acad. Sci. USA 2014, 111, 11139–11144. [Google Scholar] [CrossRef]
- Mazrooei, P.; Kron, K.J.; Zhu, Y.; Zhou, S.; Grillo, G.; Mehdi, T.; Ahmed, M.; Severson, T.M.; Guilhamon, P.; Armstrong, N.S.; et al. Cistrome Partitioning Reveals Convergence of Somatic Mutations and Risk Variants on Master Transcription Regulators in Primary Prostate Tumors. Cancer Cell 2019, 36, 674–689. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Laxman, B.; Dhanasekaran, S.M.; Helgeson, B.E.; Cao, X.; Morris, D.S.; Menon, A.; Jing, X.; Cao, Q.; Han, B.; et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature 2007, 448, 595–599. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.-W.; Varambally, S.; Cao, X.; Tchinda, J.; Kuefer, R.; et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005, 310, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Armenia, J.; Wankowicz, S.A.M.; Liu, D.; Gao, J.; Kundra, R.; Reznik, E.; Chatila, W.K.; Chakravarty, D.; Han, G.C.; Coleman, I.; et al. The long tail of oncogenic drivers in prostate cancer. Nat. Genet. 2018, 50, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Eklund, M.; Nordström, T.; Aly, M.; Adolfsson, J.; Wiklund, P.; Brandberg, Y.; Thompson, J.; Wiklund, F.; Lindberg, J.; Presti, J.C.; et al. The Stockholm-3 (STHLM3) Model can Improve Prostate Cancer Diagnostics in Men Aged 50–69 yr Compared with Current Prostate Cancer Testing. Eur. Urol. Focus 2018, 4, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Boysen, G.; Rodrigues, D.N.; Rescigno, P.; Seed, G.; Dolling, D.; Riisnaes, R.; Crespo, M.; Zafeiriou, Z.; Sumanasuriya, S.; Bianchini, D.; et al. SPOP-Mutated/CHD1-Deleted Lethal Prostate Cancer and Abiraterone Sensitivity. Clin. Cancer Res. 2018, 24, 5585–5593. [Google Scholar] [CrossRef] [PubMed]
- Hieronymus, H.; Murali, R.; Tin, A.; Yadav, K.; Abida, W.; Moller, H.; Berney, D.; Scher, H.; Carver, B.; Scardino, P.; et al. Tumor copy number alteration burden is a pan-cancer prognostic factor associated with recurrence and death. Elife 2018, 7, e37294. [Google Scholar] [CrossRef]
- Abida, W.; Cyrta, J.; Heller, G.; Prandi, D.; Armenia, J.; Coleman, I.; Cieslik, M.; Benelli, M.; Robinson, D.; Van Allen, E.M.; et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 11428–11436. [Google Scholar] [CrossRef]
- Kote-Jarai, Z.; Olama, A.A.A.; Giles, G.G.; Severi, G.; Schleutker, J.; Weischer, M.; Campa, D.; Riboli, E.; Key, T.; Gronberg, H.; et al. Seven prostate cancer susceptibility loci identified by a multi-stage genome-wide association study. Nat. Genet. 2011, 43, 785–791. [Google Scholar] [CrossRef]
- Filion, G.J.P.; Zhenilo, S.; Salozhin, S.; Yamada, D.; Prokhortchouk, E.; Defossez, P.-A. A family of human zinc finger proteins that bind methylated DNA and repress transcription. Mol. Cell. Biol. 2006, 26, 169–181. [Google Scholar] [CrossRef]
- Hudson, N.O.; Whitby, F.G.; Buck-Koehntop, B.A. Structural insights into methylated DNA recognition by the C-terminal zinc fingers of the DNA reader protein ZBTB38. J. Biol. Chem. 2018, 293, 19835–19843. [Google Scholar] [CrossRef]
- Pozner, A.; Hudson, N.O.; Trewhella, J.; Terooatea, T.W.; Miller, S.A.; Buck-Koehntop, B.A. The C-Terminal Zinc Fingers of ZBTB38 are Novel Selective Readers of DNA Methylation. J. Mol. Biol. 2018, 430, 258–271. [Google Scholar] [CrossRef]
- Miotto, B.; Chibi, M.; Xie, P.; Koundrioukoff, S.; Moolman-Smook, H.; Pugh, D.; Debatisse, M.; He, F.; Zhang, L.; Defossez, P.-A. The RBBP6/ZBTB38/MCM10 axis regulates DNA replication and common fragile site stability. Cell Rep. 2014, 7, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Miotto, B.; Marchal, C.; Adelmant, G.; Guinot, N.; Xie, P.; Marto, J.A.; Zhang, L.; Defossez, P.-A. Stabilization of the methyl-CpG binding protein ZBTB38 by the deubiquitinase USP9X limits the occurrence and toxicity of oxidative stress in human cells. Nucleic Acids Res. 2018, 46, 4392–4404. [Google Scholar] [CrossRef] [PubMed]
- Marchal, C.; de Dieuleveult, M.; Saint-Ruf, C.; Guinot, N.; Ferry, L.; Olalla Saad, S.T.; Lazarini, M.; Defossez, P.-A.; Miotto, B. Depletion of ZBTB38 potentiates the effects of DNA demethylating agents in cancer cells via CDKN1C mRNA up-regulation. Oncogenesis 2018, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Kotoku, T.; Kosaka, K.; Nishio, M.; Ishida, Y.; Kawaichi, M.; Matsuda, E. CIBZ Regulates Mesodermal and Cardiac Differentiation of by Suppressing T and Mesp1 Expression in Mouse Embryonic Stem Cells. Sci. Rep. 2016, 6, 34188. [Google Scholar] [CrossRef] [PubMed]
- Nishii, T.; Oikawa, Y.; Ishida, Y.; Kawaichi, M.; Matsuda, E. CtBP-interacting BTB zinc finger protein (CIBZ) promotes proliferation and G1/S transition in embryonic stem cells via Nanog. J. Biol. Chem. 2012, 287, 12417–12424. [Google Scholar] [CrossRef]
- Oikawa, Y.; Omori, R.; Nishii, T.; Ishida, Y.; Kawaichi, M.; Matsuda, E. The methyl-CpG-binding protein CIBZ suppresses myogenic differentiation by directly inhibiting myogenin expression. Cell Res. 2011, 21, 1578–1590. [Google Scholar] [CrossRef]
- Chen, J.; Xing, C.; Yan, L.; Wang, Y.; Wang, H.; Zhang, Z.; Yu, D.; Li, J.; Li, H.; Li, J.; et al. Transcriptome profiling reveals the role of ZBTB38 knock-down in human neuroblastoma. Peer J. 2019, 7, e6352. [Google Scholar] [CrossRef]
- Jing, J.; Liu, J.; Wang, Y.; Zhang, M.; Yang, L.; Shi, F.; Liu, P.; She, J. The role of ZBTB38 in promoting migration and invasive growth of bladder cancer cells. Oncol. Rep. 2019, 41, 1980–1990. [Google Scholar] [CrossRef]
- Wu, J.; Mamidi, T.K.K.; Zhang, L.; Hicks, C. Deconvolution of the Genomic and Epigenomic Interaction Landscape of Triple-Negative Breast Cancer. Cancers 2019, 11, 1692. [Google Scholar] [CrossRef]
- De Dieuleveult, M.; Miotto, B. DNA Methylation and Chromatin: Role(s) of Methyl-CpG-Binding Protein ZBTB38. Epigenet. Insights 2018, 11. [Google Scholar] [CrossRef]
- Oh, B.; Figtree, G.; Costa, D.; Eade, T.; Hruby, G.; Lim, S.; Elfiky, A.; Martine, N.; Rosenthal, D.; Clarke, S.; et al. Oxidative stress in prostate cancer patients: A systematic review of case control studies. Prostate Int. 2016, 4, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Udensi, U.K.; Tchounwou, P.B. Oxidative stress in prostate hyperplasia and carcinogenesis. J. Exp. Clin. Cancer Res. 2016, 35, 139. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Chuffa, L.G.; Seiva, F.R.F.; Cucielo, M.S.; Silveira, H.S.; Reiter, R.J.; Lupi, L.A. Mitochondrial functions and melatonin: A tour of the reproductive cancers. Cell. Mol. Life Sci. 2019, 76, 837–863. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 2011, 32, 491–509. [Google Scholar] [CrossRef]
- Lapointe, J.; Li, C.; Higgins, J.P.; van de Rijn, M.; Bair, E.; Montgomery, K.; Ferrari, M.; Egevad, L.; Rayford, W.; Bergerheim, U.; et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc. Natl. Acad. Sci. USA. 2004, 101, 811–816. [Google Scholar] [CrossRef]
- Luo, J.-H.; Yu, Y.P.; Cieply, K.; Lin, F.; Deflavia, P.; Dhir, R.; Finkelstein, S.; Michalopoulos, G.; Becich, M. Gene expression analysis of prostate cancers. Mol. Carcinog. 2002, 33, 25–35. [Google Scholar] [CrossRef]
- Varambally, S.; Yu, J.; Laxman, B.; Rhodes, D.R.; Mehra, R.; Tomlins, S.A.; Shah, R.B.; Chandran, U.; Monzon, F.A.; Becich, M.J.; et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell 2005, 8, 393–406. [Google Scholar] [CrossRef]
- Ross-Adams, H.; Lamb, A.D.; Dunning, M.J.; Halim, S.; Lindberg, J.; Massie, C.M.; Egevad, L.A.; Russell, R.; Ramos-Montoya, A.; Vowler, S.L.; et al. Integration of copy number and transcriptomics provides risk stratification in prostate cancer: A discovery and validation cohort study. EBioMedicine 2015, 2, 1133–1144. [Google Scholar] [CrossRef]
- Arredouani, M.S.; Lu, B.; Bhasin, M.; Eljanne, M.; Yue, W.; Mosquera, J.-M.; Bubley, G.J.; Li, V.; Rubin, M.A.; Libermann, T.A.; et al. Identification of the transcription factor single-minded homologue 2 as a potential biomarker and immunotherapy target in prostate cancer. Clin. Cancer Res. 2009, 15, 5794–5802. [Google Scholar] [CrossRef]
- Kunderfranco, P.; Mello-Grand, M.; Cangemi, R.; Pellini, S.; Mensah, A.; Albertini, V.; Malek, A.; Chiorino, G.; Catapano, C.V.; Carbone, G.M. ETS transcription factors control transcription of EZH2 and epigenetic silencing of the tumor suppressor gene Nkx3.1 in prostate cancer. PLoS ONE 2010, 5, e10547. [Google Scholar] [CrossRef]
- Vanaja, D.K.; Cheville, J.C.; Iturria, S.J.; Young, C.Y.F. Transcriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progression. Cancer Res. 2003, 63, 3877–3882. [Google Scholar] [PubMed]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Van Allen, E.M.; Wu, Y.-M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.-M.; Montgomery, B.; Taplin, M.-E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Cappetta, D.; Rossi, F.; Piegari, E.; Quaini, F.; Berrino, L.; Urbanek, K.; De Angelis, A. Doxorubicin targets multiple players: A new view of an old problem. Pharmacol. Res. 2018, 127, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Karasaki, Y.; Fujiwara, K.; Saito, K.; Suzuki-Karasaki, M.; Ochiai, T.; Soma, M. Distinct effects of TRAIL on the mitochondrial network in human cancer cells and normal cells: Role of plasma membrane depolarization. Oncotarget 2015, 6, 21572–21588. [Google Scholar] [CrossRef]
- Kelly, M.M.; Hoel, B.D.; Voelkel-Johnson, C. Doxorubicin pretreatment sensitizes prostate cancer cell lines to TRAIL induced apoptosis which correlates with the loss of c-FLIP expression. Cancer Biol. Ther. 2002, 1, 520–527. [Google Scholar] [CrossRef]
- Jones, J.; Wang, H.; Zhou, J.; Hardy, S.; Turner, T.; Austin, D.; He, Q.; Wells, A.; Grizzle, W.E.; Yates, C. Nuclear Kaiso indicates aggressive prostate cancers and promotes migration and invasiveness of prostate cancer cells. Am. J. Pathol. 2012, 181, 1836–1846. [Google Scholar] [CrossRef]
- Kim, K.; Chadalapaka, G.; Pathi, S.S.; Jin, U.-H.; Lee, J.-S.; Park, Y.-Y.; Cho, S.-G.; Chintharlapalli, S.; Safe, S. Induction of the transcriptional repressor ZBTB4 in prostate cancer cells by drug-induced targeting of microRNA-17-92/106b-25 clusters. Mol. Cancer Ther. 2012, 11, 1852–1862. [Google Scholar] [CrossRef]
- Abisoye-Ogunniyan, A.; Lin, H.; Ghebremedhin, A.; Salam, A.B.; Karanam, B.; Theodore, S.; Jones-Trich, J.; Davis, M.; Grizzle, W.; Wang, H.; et al. Transcriptional repressor Kaiso promotes epithelial to mesenchymal transition and metastasis in prostate cancer through direct regulation of miR-200c. Cancer Lett. 2018, 431, 1–10. [Google Scholar] [CrossRef]
- Boysen, G.; Barbieri, C.E.; Prandi, D.; Blattner, M.; Chae, S.-S.; Dahija, A.; Nataraj, S.; Huang, D.; Marotz, C.; Xu, L.; et al. SPOP mutation leads to genomic instability in prostate cancer. Elife 2015, 4, e09207. [Google Scholar] [CrossRef]
- Geng, C.; Rajapakshe, K.; Shah, S.S.; Shou, J.; Eedunuri, V.K.; Foley, C.; Fiskus, W.; Rajendran, M.; Chew, S.A.; Zimmermann, M.; et al. Androgen receptor is the key transcriptional mediator of the tumor suppressor SPOP in prostate cancer. Cancer Res. 2014, 74, 5631–5643. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Wang, C.; Deng, Y.; Yu, L.; Huang, H. Destruction of full-length androgen receptor by wild-type SPOP, but not prostate-cancer-associated mutants. Cell Rep. 2014, 6, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, M.; Zhu, Y.; Dai, X.; Dang, F.; Ren, J.; Ren, S.; Shulga, Y.V.; Beca, F.; Gan, W.; et al. SPOP Promotes Nanog Destruction to Suppress Stem Cell Traits and Prostate Cancer Progression. Dev. Cell 2019, 48, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Arima, T.; Enokida, H.; Kubo, H.; Kagara, I.; Matsuda, R.; Toki, K.; Nishimura, H.; Chiyomaru, T.; Tatarano, S.; Idesako, T.; et al. Nuclear translocation of ADAM-10 contributes to the pathogenesis and progression of human prostate cancer. Cancer Sci. 2007, 98, 1720–1726. [Google Scholar] [CrossRef]
- Sung, S.-Y.; Kubo, H.; Shigemura, K.; Arnold, R.S.; Logani, S.; Wang, R.; Konaka, H.; Nakagawa, M.; Mousses, S.; Amin, M.; et al. Oxidative stress induces ADAM9 protein expression in human prostate cancer cells. Cancer Res. 2006, 66, 9519–9526. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 6. [Google Scholar] [CrossRef]
- Rhodes, D.R.; Kalyana-Sundaram, S.; Mahavisno, V.; Varambally, R.; Yu, J.; Briggs, B.B.; Barrette, T.R.; Anstet, M.J.; Kincead-Beal, C.; Kulkarni, P.; et al. Oncomine 3.0: Genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 2007, 9, 166–180. [Google Scholar] [CrossRef]
- NCBI Resource Coordinators Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 46, D8–D13. [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. G: Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
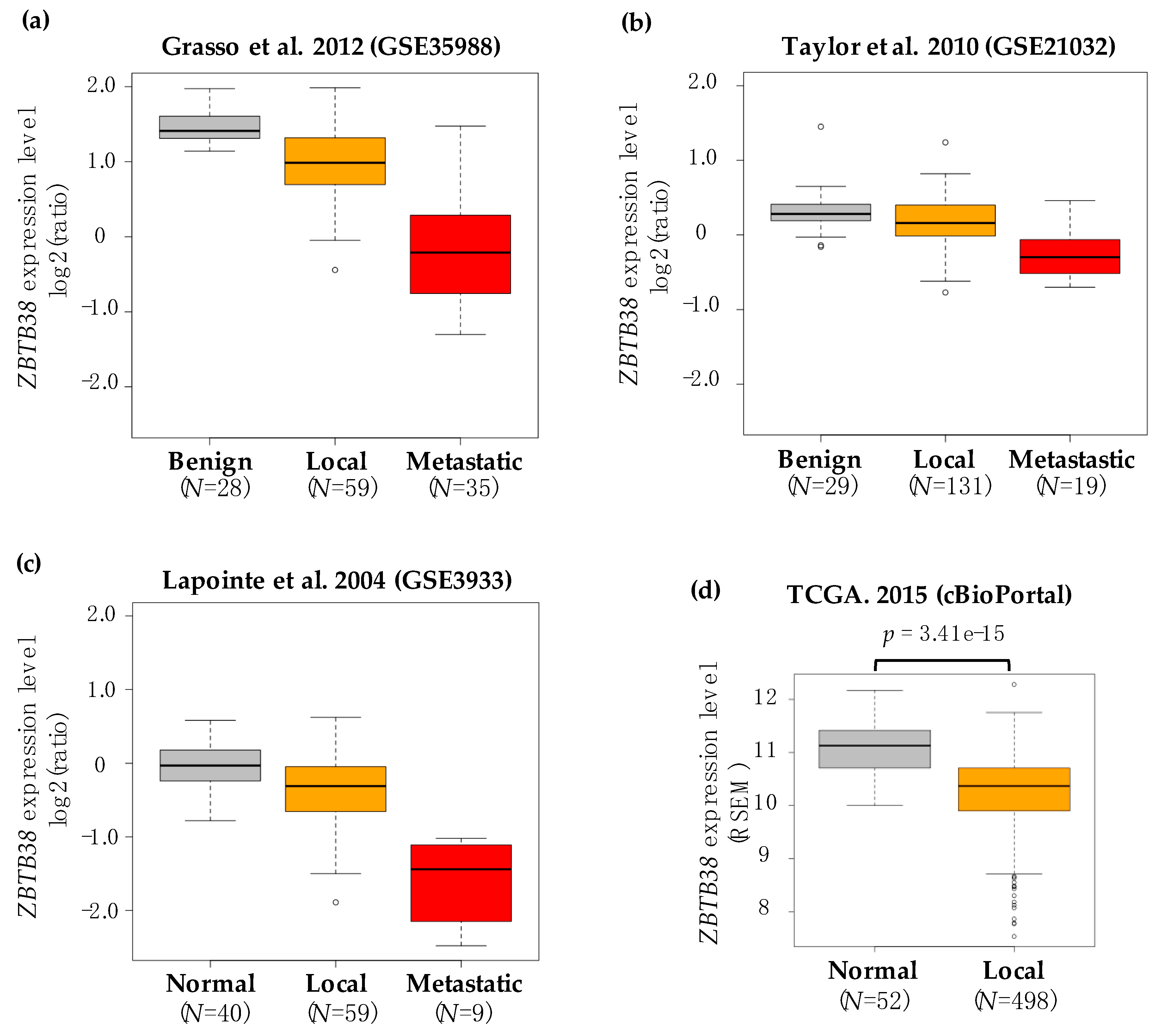

| Dataset | Specimen (N) | ZBTB38 Expression | ZBTB38 ID | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Normal/Benign vs. Local | Normal/Benign vs. Metastatic | ||||||||
| Normal/ Benign | Local | Metastasis | Fold Change | p-Value | Fold Change | p-Value | |||
| GSE68545 | 15 | 15 | - | −1.188 | 0.039 | - | - | RC_AA465527 | [46] |
| GSE70770 | 73 | 113 | - | −1.134 | 0.01 | - | - | ILMN_2248843 | [48] |
| GSE55945 | 8 | 13 | - | −1.482 | 0.015 | - | - | 1558733_at | [49] |
| GSE14206 | 14 | 68 | - | −1.062 | 0.021 | - | - | 21694 | [50] |
| Oncomine | 8 | 27 | - | −1.079 | 0.313 | - | - | 225512_at | [51] |
| GSE21032 | 29 | 131 | 19 | −1.072 | 0.018 | −1.47 | 4.52 × 10−6 | 4299 | [12] |
| GSE35988 | 28 | 59 | 35 | −1.261 | 3.27 × 10−4 | −3.01 | 1.31 × 10−12 | A_23_P380076 | [14] |
| GSE3933 | 40 | 59 | 9 | −1.258 | 8.65 × 10−5 | −3.28 | 2.37 × 10−17 | IMAGE:50920 | [45] |
| GSE3325 | 6 | 7 | 6 | −1.264 | 0.03 | −1.84 | 5.1 × 10−3 | 1558733_at | [47] |
| Characteristics | Taylor et al. 2010 (GSE21032) | Kunderfranco et al. 2010 (GSE14206) | Ross-Adams et al. 2015 (GSE70770) | TCGA. 2015 (cBioPortal) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZBTB38 Expression (N) | Chi2 | p- Value | ZBTB38 Expression (N) | Chi2 | p- Value | ZBTB38 Expression (N) | Chi2 | p- Value | ZBTB38 Expression (N) | Chi2 | p- Value | |||||
| Low | High | Low | High | Low | High | Low | High | |||||||||
| Age (year) at diagnostics | ||||||||||||||||
| <50 | 4 | 11 | 3 | 3 | 2 | 4 | 6 | 10 | ||||||||
| 50–60 | 35 | 30 | 22 | 14 | 24 | 17 | 50 | 48 | ||||||||
| >60 | 25 | 24 | 3.66 | 0.160 | 2 | 9 | 6.21 | 0.044 | 30 | 33 | 1.96 | 0.373 | 62 | 60 | 1.07 | 0.584 |
| Seminal Vesicle Invasion (SVI) | ||||||||||||||||
| Negative | 56 | 59 | - | - | - | - | - | - | ||||||||
| Positive | 7 | 6 | 0.12 | 0.725 | - | - | - | - | - | - | - | - | - | - | - | - |
| Lymph Node Involvement (LNI) | ||||||||||||||||
| Normal | 46 | 54 | - | - | - | - | - | - | ||||||||
| Abnormal | 4 | 2 | 0.97 | 0.324 | - | - | - | - | - | - | - | - | - | - | - | - |
| Surgical Margin Status (SMS) | ||||||||||||||||
| Negative | 51 | 48 | - | - | 35 | 51 | 70 | 87 | ||||||||
| Positive | 13 | 17 | 0.61 | 0.432 | - | - | - | - | 21 | 5 | 12.8 | 0.0003 | 43 | 26 | 6.02 | 0.014 |
| Extra-Capsular Extension (ECE) | ||||||||||||||||
| None | 18 | 23 | - | - | 16 | 19 | - | - | ||||||||
| Yes | 46 | 41 | 0.89 | 0.343 | - | - | - | - | 40 | 37 | 0.37 | 0.54 | - | - | - | - |
| Gleason Score | ||||||||||||||||
| <7 | 32 | 45 | 2 | 8 | 8 | 9 | 11 | 8 | ||||||||
| 7 | 24 | 17 | 18 | 15 | 43 | 43 | 49 | 68 | ||||||||
| >7 | 8 | 3 | 5.65 | 0.059 | 7 | 3 | 5.45 | 0.065 | 5 | 4 | 0.16 | 0.918 | 58 | 42 | 6.11 | 0.046 |
| Clinical grade | ||||||||||||||||
| T1 | 35 | 37 | - | - | 32 | 29 | - | - | ||||||||
| T2 | 22 | 24 | - | - | 18 | 17 | - | - | ||||||||
| T3 | 2 | 2 | 0.01 | 0.994 | - | - | - | - | 6 | 9 | 0.76 | 0.681 | - | - | - | - |
| Pathological grade | ||||||||||||||||
| T1-T2 | 38 | 46 | 13 | 22 | 14 | 20 | - | - | ||||||||
| T3-T4 | 26 | 19 | 5.45 | 0.019 | 12 | 5 | 5.12 | 0.023 | 42 | 35 | 1.68 | 0.194 | - | - | - | - |
| PSA levels (in ng/mL) at diagnosis | ||||||||||||||||
| <5 | 20 | 26 | - | - | 8 | 5 | 17 | 11 | ||||||||
| 5–10 | 28 | 33 | - | - | 31 | 39 | 33 | 37 | ||||||||
| >10 | 16 | 5 | 6.95 | 0.03 | - | - | - | - | 16 | 11 | 2.53 | 0.289 | 23 | 25 | 1.59 | 0.449 |
| PSA levels (in ng/mL) pre-treatment | ||||||||||||||||
| <5 | 18 | 22 | - | - | - | - | - | - | ||||||||
| 5–10 | 24 | 34 | - | - | - | - | - | - | ||||||||
| >10 | 22 | 8 | 8.65 | 0.013 | - | - | - | - | - | - | - | - | - | - | - | - |
| Variable | Taylor et al. 2010 (GSE21032) | TCGA. 2015 (cBioPortal) | Ross-Adams et al. 2015 (GSE70770) |
|---|---|---|---|
| C-Index for recurrence | |||
| ZBTB38 expression | 0.76 | 0.791 | 0.716 |
| Gleason score | 0.842 | 0.932 | 0.797 |
| PSA levels | 0.680 | 0.838 | 0.632 |
| SMS | 0.723 | 0.764 | 0.628 |
| Patient age | 0.519 | 0.7 | 0.642 |
| Multivariate analysis of disease-free survival (HR, [95% CI], p-value) | |||
| ZBTB38 expression | 2.95, [1.44–6.03], 0.003 | 4.01, [1.20–13.36], 0.02 | 2.19, [0.83–5.79], 0.11 |
| ZBTB38 + Gleason score | 2.01, [0.94–4.01], 0.07 | 3.82, [1.11–13.15], 0.03 | 1.92, [0.72–5.12], 0.19 |
| ZBTB38 + PSA levels | 2.66, [1.28–5.50], 0.008 | 2.11, [0.33–13.50], 0.43 | 2.43, [0.92–6.44], 0.07 |
| ZBTB38 + SMS | 2.93, [1.43–5.98], 0.003 | 3.87, [1.15–13.00], 0.03 | 2.05, [0.75–5.59], 0.16 |
| ZBTB38 + Patient age | 2.91, [1.42–5.95], 0.003 | 3.96, [1.18–13.25], 0.02 | 2.19, [0.83–5.77], 0.11 |
| Characteristics | Taylor et al. 2010 (GSE21032) | TCGA. 2015 (cBioPortal) | ||||||
|---|---|---|---|---|---|---|---|---|
| ZBTB38 Expression (N) | Chi2 | p-Value | ZBTB38 Expression (N) | Chi2 | p-Value | |||
| Low | High | Low | High | |||||
| Copy Number Aberration Cluster | ||||||||
| Minimal CNA (Class 1–4) | 40 | 43 | - | - | ||||
| Substantial CNA (Class 5–6) | 17 | 6 | 4.793 | 0.028 | - | - | - | - |
| SCNA (Somatic Copy number Aberration) | ||||||||
| Quiet | - | - | 14 | 39 | ||||
| Some SCNA | - | - | 62 | 76 | ||||
| More SCNA | - | - | - | - | 66 | 31 | 25.791 | <0.00001 |
| Fraction Genome Altered | ||||||||
| 0 | - | - | 5 | 21 | ||||
| 0–0.1 | - | - | 58 | 36 | ||||
| >0.1 | - | - | - | - | 82 | 88 | 15.209 | 0.0004 |
| Mutations | ||||||||
| <20 | - | - | 18 | 28 | ||||
| >20 | - | - | - | - | 127 | 118 | 2.501 | 0.113 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Dieuleveult, M.; Marchal, C.; Jouinot, A.; Letessier, A.; Miotto, B. Molecular and Clinical Relevance of ZBTB38 Expression Levels in Prostate Cancer. Cancers 2020, 12, 1106. https://doi.org/10.3390/cancers12051106
de Dieuleveult M, Marchal C, Jouinot A, Letessier A, Miotto B. Molecular and Clinical Relevance of ZBTB38 Expression Levels in Prostate Cancer. Cancers. 2020; 12(5):1106. https://doi.org/10.3390/cancers12051106
Chicago/Turabian Stylede Dieuleveult, Maud, Claire Marchal, Anne Jouinot, Anne Letessier, and Benoit Miotto. 2020. "Molecular and Clinical Relevance of ZBTB38 Expression Levels in Prostate Cancer" Cancers 12, no. 5: 1106. https://doi.org/10.3390/cancers12051106
APA Stylede Dieuleveult, M., Marchal, C., Jouinot, A., Letessier, A., & Miotto, B. (2020). Molecular and Clinical Relevance of ZBTB38 Expression Levels in Prostate Cancer. Cancers, 12(5), 1106. https://doi.org/10.3390/cancers12051106






