Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects
Abstract
1. Introduction
2. Properties of AgNPs
2.1. Shape and Size
2.2. Optical Properties
2.3. Electrical Properties
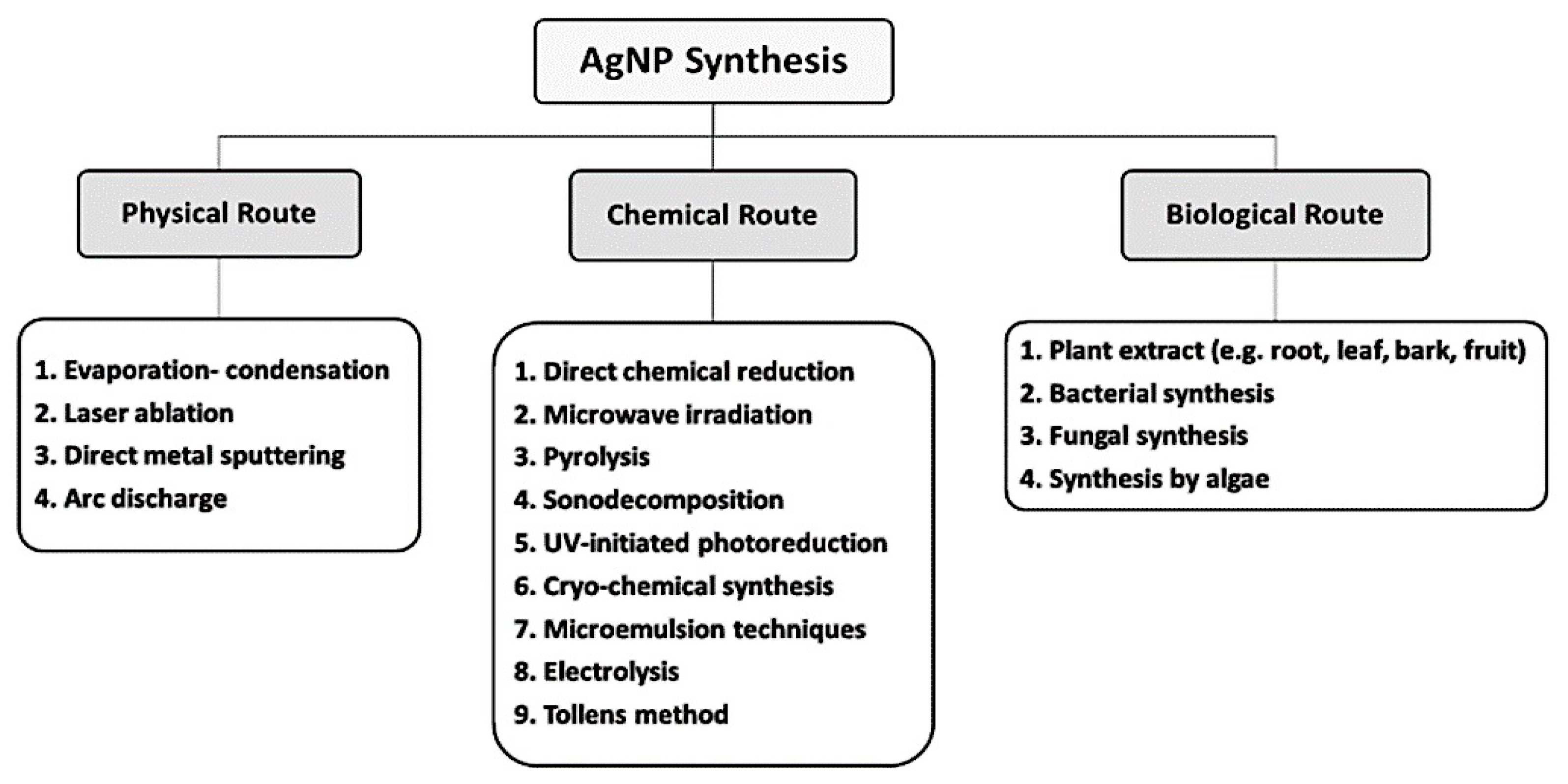
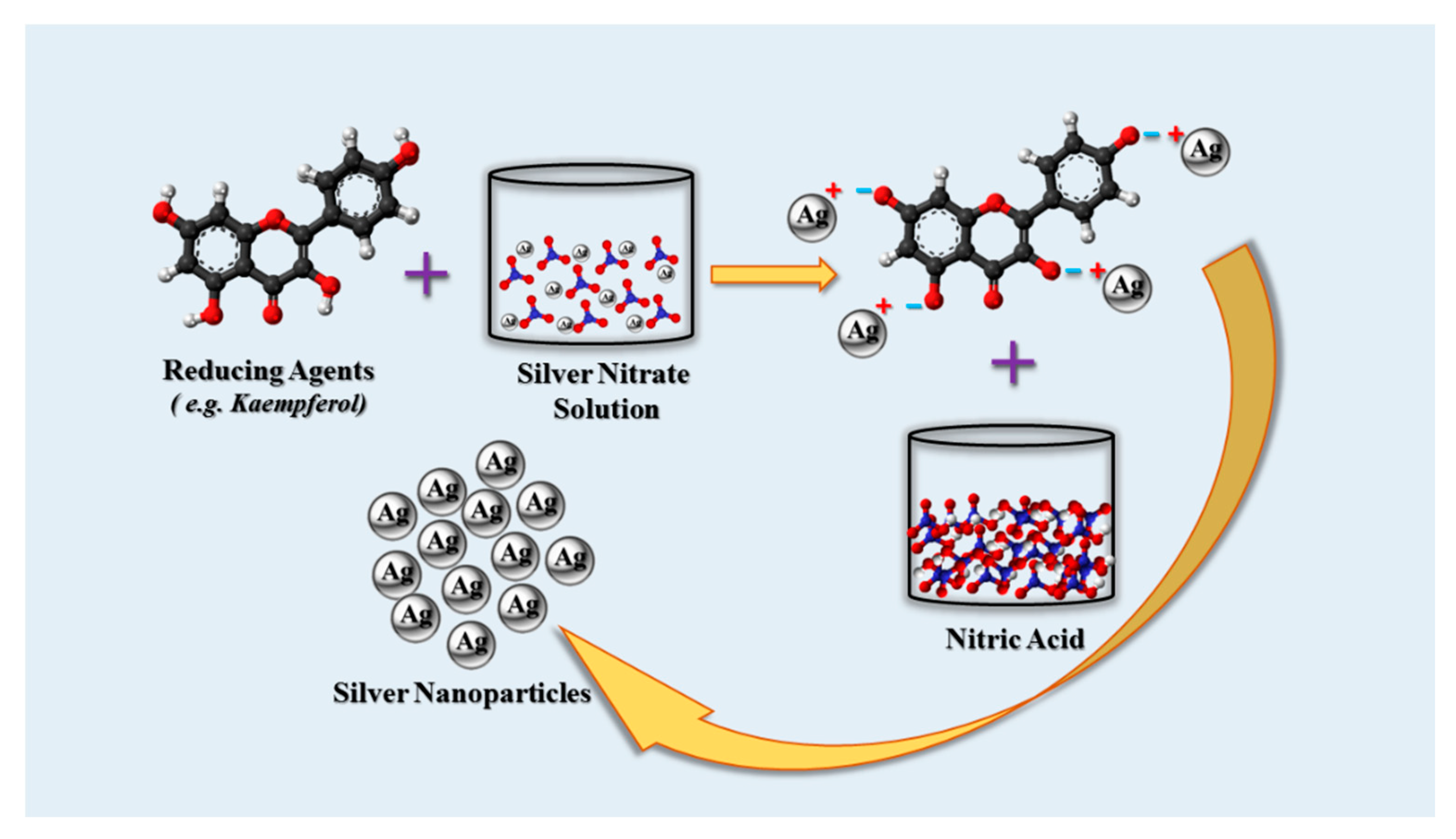
3. Biologic Synthesis of AgNPs
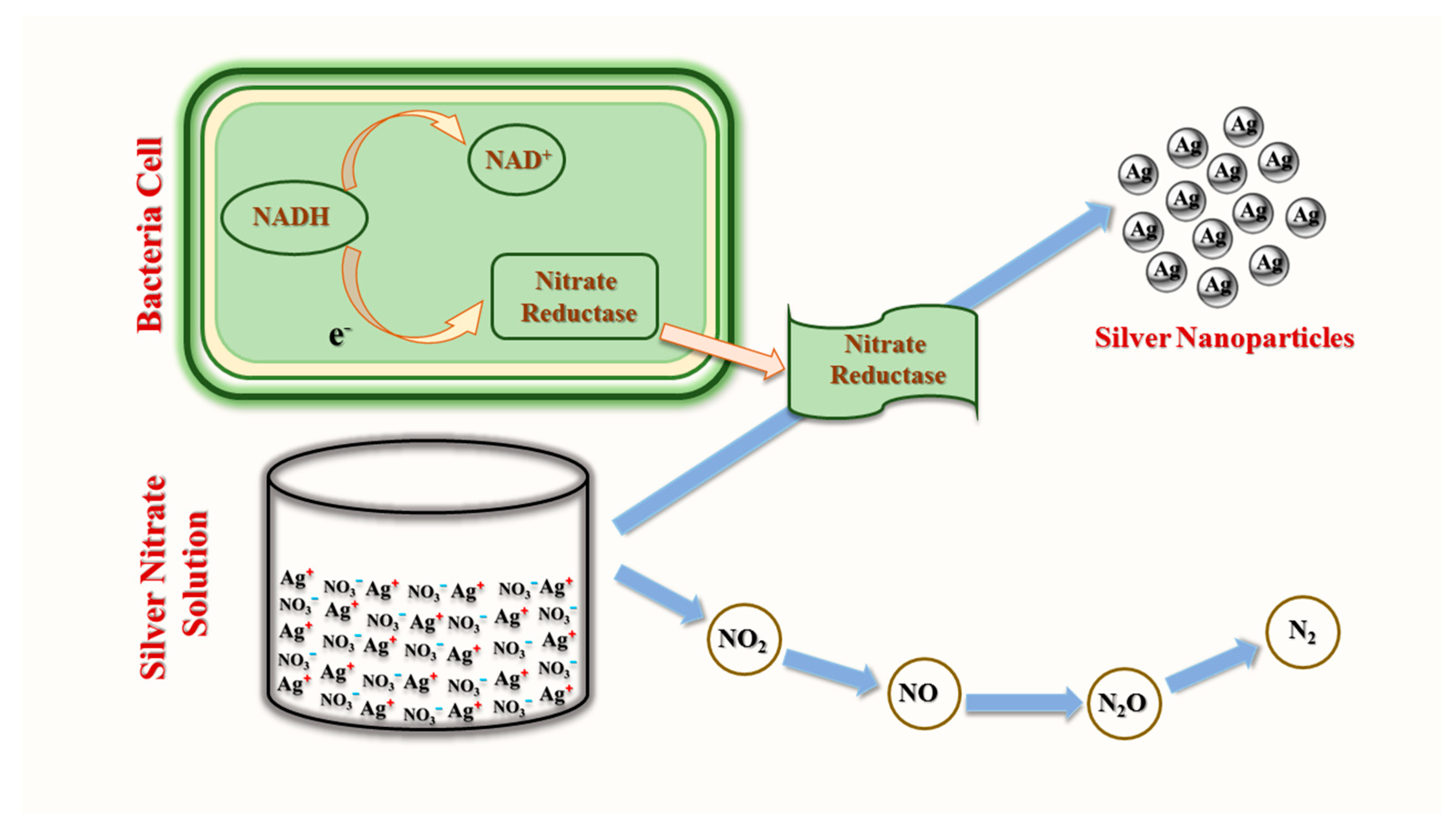
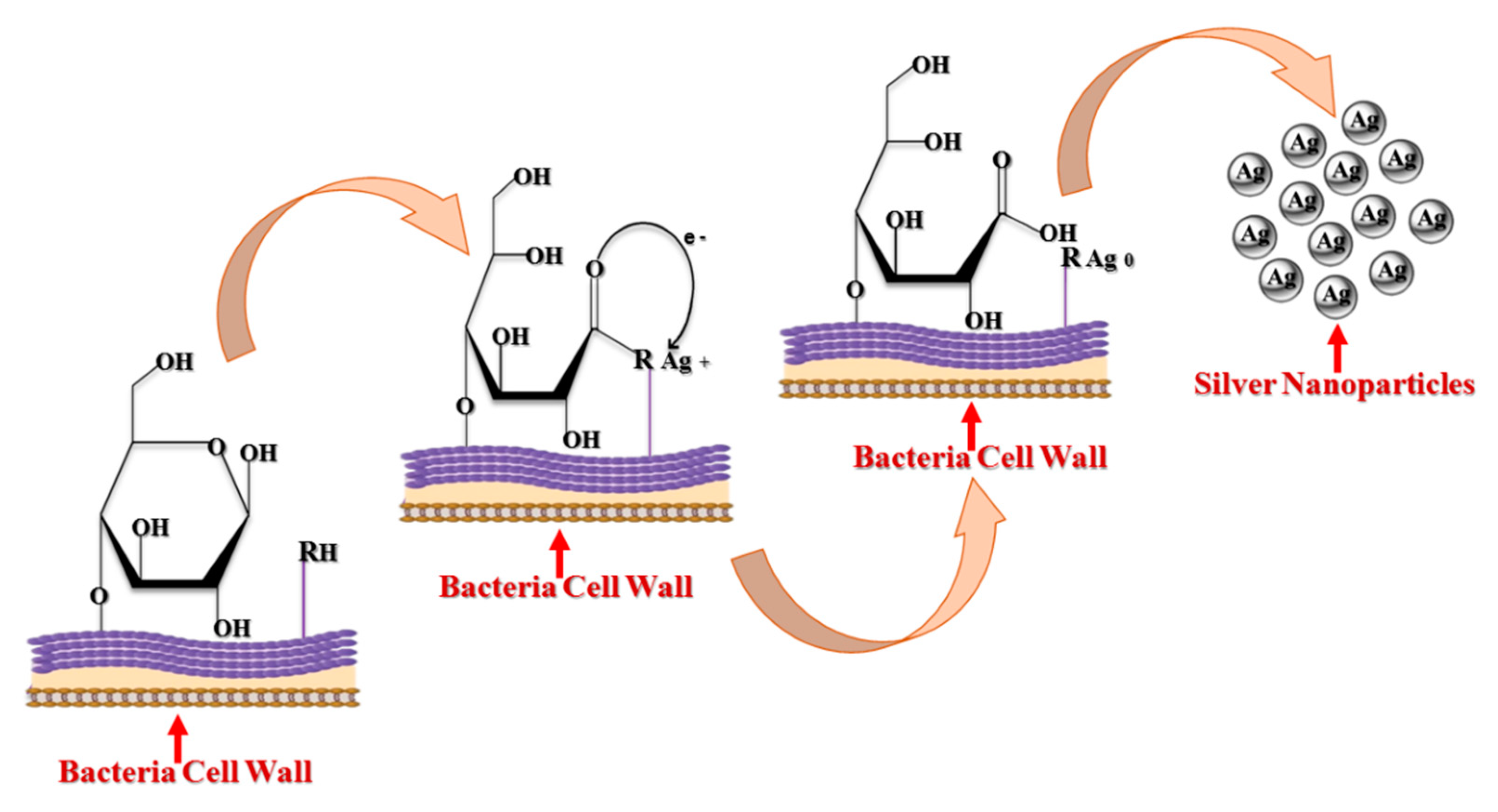
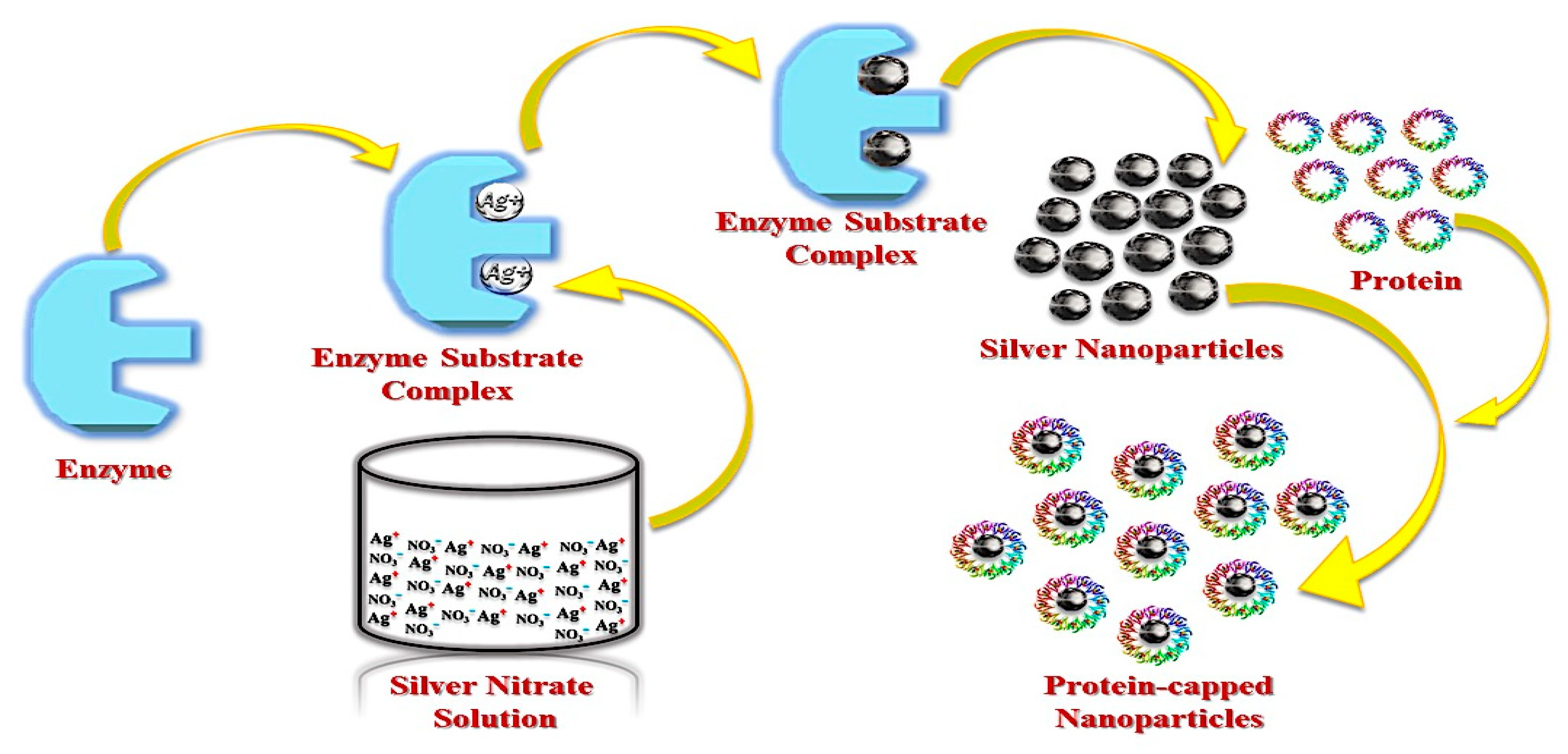
3.1. Bacterial Synthesis of AgNPs
3.2. Fungal Synthesis of AgNPs
3.3. Plant Synthesis of AgNPs
4. AgNPs Characterization
5. Biosynthesized AgNPs as Anticancer Agents in Vitro
6. Anticancer Mechanism of Biosynthesized AgNPs
7. Toxicity
8. Inter-Connection between Antimicrobial Property and Anticancer Activity of AgNPs
9. Clinical Application
10. Future of AgNPs
- These methods are inexpensive, eco-friendly and non-toxic.
- No complex setup is required to conduct the synthesis process.
- It is not necessary to use stabilizing agents to prevent agglomeration of the NPs.
- Since these processes are carried out in ambient conditions, they are not energy intensive.
- These methods offer finer tuned control of the size and shape of the NPs compared to chemical and physical methods.
- Synthesis by biologic methods is not as fast as synthesis by chemical methods.
- Because of the presence of numerous biomolecules present in the biologic sources, it is difficult to pinpoint the exact biomolecules responsible for the synthesis of the NPs.
- If the biologic synthesis is conducted on a large scale, an ecological imbalance may kick in from overuse of different biologic species.
- Microbe toxins may be brought together by biologic synthesis.
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A. Alternative cancer cures:“Unproven” or “disproven”? CA A Cancer J. Clin. 2004, 54, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, N. Silver nanoparticles: Synthesis, characterization and applications. In Silver Nanoparticles-Fabrication, Characterization and Applications; IntechOpen: London, UK, 2018; pp. 21–57. [Google Scholar]
- Nguyen, K.T. Targeted nanoparticles for cancer therapy: Promises and challenge. Nanomed. Nanotechnol. 2011. [Google Scholar] [CrossRef]
- Li, X.; Cui, R.; Liu, W.; Sun, L.; Yu, B.; Fan, Y.; Feng, Q.; Cui, F.; Watari, F. The use of nanoscaled fibers or tubes to improve biocompatibility and bioactivity of biomedical materials. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- Russell, A.; Hugo, W. 7 antimicrobial activity and action of silver. Prog. Med. Chem. 1994, 31, 351–370. [Google Scholar]
- Lee, S.H.; Jun, B.-H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; de Lima, R. Synthesis of silver nanoparticles mediated by fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef]
- Ratika, K.; Vedpriya, A. Biosynthesis and characterization of silver nanoparticles from aqueous leaf extracts of Carica papaya and its antibacterial activity. Int. J. Nanomater. Biostructures 2013, 3, 17–20. [Google Scholar]
- Paulkumar, K.; Gnanajobitha, G.; Vanaja, M.; Rajeshkumar, S.; Malarkodi, C.; Pandian, K.; Annadurai, G. Piper nigrum leaf and stem assisted green synthesis of silver nanoparticles and evaluation of its antibacterial activity against agricultural plant pathogens. Sci. World J. 2014, 2014, 829894. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.; Pradeep, T. Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst. Growth Des. 2002, 2, 293–298. [Google Scholar] [CrossRef]
- Makarov, V.; Love, A.; Sinitsyna, O.; Makarova, S.; Yaminsky, I.; Taliansky, M.; Kalinina, N. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Kalaichelvan, P.; Venkatesan, R. Fungal based synthesis of silver nanoparticles—An effect of temperature on the size of particles. Colloids Surf. B Biointerfaces 2009, 74, 123–126. [Google Scholar] [CrossRef]
- Dhanalakshmi, P.; Azeez, R.; Rekha, R.; Poonkodi, S.; Nallamuthu, T. Synthesis of silver nanoparticles using green and brown seaweeds. Phykos 2012, 42, 39–45. [Google Scholar]
- Shiny, P.; Mukherjee, A.; Chandrasekaran, N. Marine algae mediated synthesis of the silver nanoparticles and its antibacterial efficiency. Int. J. Pharm. Pharm. Sci 2013, 5, 239–241. [Google Scholar]
- Mie, R.; Samsudin, M.W.; Din, L.B.; Ahmad, A.; Ibrahim, N.; Adnan, S.N.A. Synthesis of silver nanoparticles with antibacterial activity using the lichen Parmotrema praesorediosum. Int. J. Nanomed. 2014, 9, 121. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, N.; Singh, A.; Singh, H.; Singh, S. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef]
- Yoon, K.-Y.; Byeon, J.H.; Park, J.-H.; Hwang, J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total Environ. 2007, 373, 572–575. [Google Scholar] [CrossRef]
- Aziz, N.; Sherwani, A.; Faraz, M.; Fatma, T.; Prasad, R. Illuminating the anticancerous efficacy of a new fungal chassis for silver nanoparticle synthesis. Front. Chem. 2019, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Sukirtha, R.; Priyanka, K.M.; Antony, J.J.; Kamalakkannan, S.; Thangam, R.; Gunasekaran, P.; Krishnan, M.; Achiraman, S. Cytotoxic effect of green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochem. 2012, 47, 273–279. [Google Scholar] [CrossRef]
- Boca-Farcau, S.; Potara, M.; Simon, T.; Juhem, A.; Baldeck, P.; Astilean, S. Folic acid-conjugated, SERS-labeled silver nanotriangles for multimodal detection and targeted photothermal treatment on human ovarian cancer cells. Mol. Pharm. 2014, 11, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Vasanth, K.; Ilango, K.; MohanKumar, R.; Agrawal, A.; Dubey, G.P. Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf. B Biointerfaces 2014, 117, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Woo, H.J.; Kim, Y.H.; Lee, H.J.; Park, K.H.; Park, S.; Youn, B. Optimizing hemocompatibility of surfactant-coated silver nanoparticles in human erythrocytes. J. Nanosci. Nanotechnol. 2012, 12, 6168–6175. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.; Hande, M.P.; Valiyaveettil, S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009, 10, 65. [Google Scholar] [CrossRef]
- Wiley, B.; Sun, Y.; Mayers, B.; Xia, Y. Shape-controlled synthesis of metal nanostructures: The case of silver. Chem. A Eur. J. 2005, 11, 454–463. [Google Scholar] [CrossRef]
- Yang, Y.; Matsubara, S.; Xiong, L.; Hayakawa, T.; Nogami, M. Solvothermal synthesis of multiple shapes of silver nanoparticles and their SERS properties. J. Phys. Chem. C 2007, 111, 9095–9104. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Biosynthesis of silver nanoparticles by phytopathogen Xanthomonas oryzae pv. oryzae strain BXO8. J. Microbiol. Biotechnol. 2013, 23, 1287–1292. [Google Scholar] [CrossRef]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.-G. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef]
- Ingle, A.; Gade, A.; Pierrat, S.; Sonnichsen, C.; Rai, M. Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr. Nanosci. 2008, 4, 141–144. [Google Scholar] [CrossRef]
- Chen, S.; Carroll, D.L. Silver nanoplates: Size control in two dimensions and formation mechanisms. J. Phys. Chem. B 2004, 108, 5500–5506. [Google Scholar] [CrossRef]
- Santhoshkumar, T.; Rahuman, A.A.; Rajakumar, G.; Marimuthu, S.; Bagavan, A.; Jayaseelan, C.; Zahir, A.A.; Elango, G.; Kamaraj, C. Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol. Res. 2011, 108, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Vilchis-Nestor, A.R.; Sánchez-Mendieta, V.; Camacho-López, M.A.; Gómez-Espinosa, R.M.; Camacho-López, M.A.; Arenas-Alatorre, J.A. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater. Lett. 2008, 62, 3103–3105. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment; ACS Publications: Washington, DC, USA, 2003. [Google Scholar]
- Evanoff, D.D.; Chumanov, G. Size-controlled synthesis of nanoparticles. 2. Measurement of extinction, scattering, and absorption cross sections. J. Phys. Chem. B 2004, 108, 13957–13962. [Google Scholar] [CrossRef]
- Kajani, A.A.; Zarkesh-Esfahani, S.H.; Bordbar, A.-K.; Khosropour, A.R.; Razmjou, A.; Kardi, M. Anticancer effects of silver nanoparticles encapsulated by Taxus baccata extracts. J. Mol. Liq. 2016, 223, 549–556. [Google Scholar] [CrossRef]
- Kajani, A.A.; Bordbar, A.-K.; Esfahani, S.H.Z.; Khosropour, A.R.; Razmjou, A. Green synthesis of anisotropic silver nanoparticles with potent anticancer activity using Taxus baccata extract. RSC Adv. 2014, 4, 61394–61403. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, P.; Cao, X.; Jin, L. Colloidal silver nanoparticles modified electrode and its application to the electroanalysis of Cytochrome c. Electrochim. Acta 2008, 53, 5368–5372. [Google Scholar] [CrossRef]
- Sankar, R.; Karthik, A.; Prabu, A.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf. B Biointerfaces 2013, 108, 80–84. [Google Scholar] [CrossRef]
- Sepeur, S. Nanotechnology: Technical Basics and Applications; Vincentz Network GmbH & Co KG: Hanover, Germany, 2008. [Google Scholar]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.; Alam, M.; Kumar, R. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: A novel biological approach to nanoparticle synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Deepak, V.; Ramkumarpandian, S.; Nellaiah, H.; Sangiliyandi, G. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater. Lett. 2008, 62, 4411–4413. [Google Scholar] [CrossRef]
- Sintubin, L.; Verstraete, W.; Boon, N. Biologically produced nanosilver: Current state and future perspectives. Biotechnol. Bioeng. 2012, 109, 2422–2436. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Karthik, L.; Kumar, G.; Kirthi, A.V.; Rahuman, A.; Rao, K.B. Streptomyces sp. LK3 mediated synthesis of silver nanoparticles and its biomedical application. Bioprocess Biosyst. Eng. 2014, 37, 261–267. [Google Scholar] [CrossRef]
- Vaidyanathan, R.; Gopalram, S.; Kalishwaralal, K.; Deepak, V.; Pandian, S.R.K.; Gurunathan, S. Enhanced silver nanoparticle synthesis by optimization of nitrate reductase activity. Colloids Surf. B Biointerfaces 2010, 75, 335–341. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Babu, R.S.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B Biointerfaces 2008, 65, 150–153. [Google Scholar] [CrossRef]
- Golinska, P.; Wypij, M.; Ingle, A.P.; Gupta, I.; Dahm, H.; Rai, M. Biogenic synthesis of metal nanoparticles from actinomycetes: Biomedical applications and cytotoxicity. Appl. Microbiol. Biotechnol. 2014, 98, 8083–8097. [Google Scholar] [CrossRef]
- Van Hullebusch, E.D.; Zandvoort, M.H.; Lens, P.N. Metal immobilisation by biofilms: Mechanisms and analytical tools. Rev. Environ. Sci. Biotechnol. 2003, 2, 9–33. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, C.; Wu, J.; Zhou, J.; Wang, L. A further insight into the mechanism of Ag+ biosorption by Lactobacillus sp. strain A09. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Sintubin, L.; De Windt, W.; Dick, J.; Mast, J.; van der Ha, D.; Verstraete, W.; Boon, N. Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl. Microbiol. Biotechnol. 2009, 84, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanoparticle Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Green approach for nanoparticle biosynthesis by fungi: Current trends and applications. Crit. Rev. Biotechnol. 2012, 32, 49–73. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Bhargava, A.; Majumdar, S.; Tarafdar, J.; Panwar, J. Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: A mechanism perspective. Nanoscale 2011, 3, 635–641. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Ichwan, S.J.; Al-Zikri, P.N.H.; Suriyah, W.H.; Soundharrajan, I.; Govindan, N.; Maniam, G.P.; Yusoff, M.M. In vitro anticancer activity of Au, Ag nanoparticles synthesized using Commelina nudiflora L. aqueous extract against HCT-116 colon cancer cells. Biol. Trace Elem. Res. 2016, 173, 297–305. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J.-H.; Lee, J. Direct one-pot synthesis of cinnamaldehyde immobilized on gold nanoparticles and their antibiofilm properties. Colloids Surf. B Biointerfaces 2017, 160, 639–648. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009, 84, 151–157. [Google Scholar] [CrossRef]
- Mukunthan, K.; Balaji, S. Cashew apple juice (Anacardium occidentale L.) speeds up the synthesis of silver nanoparticles. Int. J. Green Nanotechnol. 2012, 4, 71–79. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Vennila, K.; Jayakumar, R.; Yusoff, A.R.M.; Hadibarata, T.; Palvannan, T. Phyto-synthesis of silver nanoparticles using Alternanthera tenella leaf extract: An effective inhibitor for the migration of human breast adenocarcinoma (MCF-7) cells. Bioprocess Biosyst. Eng. 2016, 39, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Nalini, S.K.; Prakash, N.U.; Madhankumar, D. Biomimetic synthesis of silver nanoparticles by aqueous extract of Syzygium aromaticum. Mater. Lett. 2012, 75, 33–35. [Google Scholar] [CrossRef]
- Jasuja, N.D.; Gupta, D.K.; Reza, M.; Joshi, S.C. Green Synthesis of AgNPs Stabilized with biowaste and their antimicrobial activities. Braz. J. Microbiol. 2014, 45, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Mariselvam, R.; Ranjitsingh, A.; Nanthini, A.U.R.; Kalirajan, K.; Padmalatha, C.; Selvakumar, P.M. Green synthesis of silver nanoparticles from the extract of the inflorescence of Cocos nucifera (Family: Arecaceae) for enhanced antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Controlling the optical properties of lemongrass extract synthesized gold nanotriangles and potential application in infrared-absorbing optical coatings. Chem. Mater. 2005, 17, 566–572. [Google Scholar] [CrossRef]
- Ramteke, C.; Chakrabarti, T.; Sarangi, B.K.; Pandey, R.-A. Synthesis of silver nanoparticles from the aqueous extract of leaves of Ocimum sanctum for enhanced antibacterial activity. J. Chem. 2012, 2013, 278925. [Google Scholar]
- Arokiyaraj, S.; Arasu, M.V.; Vincent, S.; Prakash, N.U.; Choi, S.H.; Oh, Y.-K.; Choi, K.C.; Kim, K.H. Rapid green synthesis of silver nanoparticles from Chrysanthemum indicum L and its antibacterial and cytotoxic effects: An in vitro study. Int. J. Nanomed. 2014, 9, 379. [Google Scholar] [CrossRef]
- Muniyappan, N.; Nagarajan, N. Green synthesis of silver nanoparticles with Dalbergia spinosa leaves and their applications in biological and catalytic activities. Process Biochem. 2014, 49, 1054–1061. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Nalini, S.K.; Prakash, N.U.; Madhankumar, D. One step green synthesis of silver nano/microparticles using extracts of Trachyspermum ammi and Papaver somniferum. Colloids Surf. B Biointerfaces 2012, 94, 114–117. [Google Scholar] [CrossRef]
- Dubey, M.; Bhadauria, S.; Kushwah, B. Green synthesis of nanosilver particles from extract of Eucalyptus hybrida (safeda) leaf. Dig. J. Nanomater. Biostructures 2009, 4, 537–543. [Google Scholar]
- Sre, P.R.; Reka, M.; Poovazhagi, R.; Kumar, M.A.; Murugesan, K. Antibacterial and cytotoxic effect of biologically synthesized silver nanoparticles using aqueous root extract of Erythrina indica lam. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 1137–1144. [Google Scholar]
- Firdhouse, M.J.; Lalitha, P. Biosynthesis of silver nanoparticles using the extract of Alternanthera sessilis—Antiproliferative effect against prostate cancer cells. Cancer Nanotechnol. 2013, 4, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Flores, J.C.; Torres, V.; Popa, M.; Crespo, D.; Calderón-Moreno, J.M. Variations in morphologies of silver nanoshells on silica spheres. Colloids Surf. A Physicochem. Eng. Asp. 2008, 330, 86–90. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanoparticle Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Shenashen, M.A.; El-Safty, S.A.; Elshehy, E.A. Synthesis, morphological control, and properties of silver nanoparticles in potential applications. Part. Part. Syst. Charact. 2014, 31, 293–316. [Google Scholar] [CrossRef]
- Schaffer, B.; Hohenester, U.; Trügler, A.; Hofer, F. High-resolution surface plasmon imaging of gold nanoparticles by energy-filtered transmission electron microscopy. Phys. Rev. B 2009, 79, 041401. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 2009, 32, 79. [Google Scholar] [CrossRef]
- Eppler, A.S.; Rupprechter, G.; Anderson, E.A.; Somorjai, G.A. Thermal and chemical stability and adhesion strength of Pt nanoparticle arrays supported on silica studied by transmission electron microscopy and atomic force microscopy. J. Phys. Chem. B 2000, 104, 7286–7292. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Gnanadhas, D.P.; Thomas, M.B.; Thomas, R.; Raichur, A.M.; Chakravortty, D. Interaction of silver nanoparticles with serum proteins affects their antimicrobial activity in vivo. Antimicrob. Agents Chemother. 2013, 57, 4945–4955. [Google Scholar] [CrossRef]
- Kathiravan, V.; Ravi, S.; Ashokkumar, S. Synthesis of silver nanoparticles from Melia dubia leaf extract and their in vitro anticancer activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Baharara, J.; Namvar, F.; Ramezani, T.; Mousavi, M.; Mohamad, R. Silver nanoparticles biosynthesized using Achillea biebersteinii flower extract: Apoptosis induction in MCF-7 cells via caspase activation and regulation of Bax and Bcl-2 gene expression. Molecules 2015, 20, 2693–2706. [Google Scholar] [CrossRef] [PubMed]
- Castro-Aceituno, V.; Ahn, S.; Simu, S.Y.; Singh, P.; Mathiyalagan, R.; Lee, H.A.; Yang, D.C. Anticancer activity of silver nanoparticles from Panax ginseng fresh leaves in human cancer cells. Biomed. Pharmacother. 2016, 84, 158–165. [Google Scholar] [CrossRef]
- Devaraj, P.; Aarti, C.; Kumari, P. Synthesis and characterization of silver nanoparticles using Tabernae montana divaricata and its cytotoxic activity against MCF7 cell line. Int. J. Pharm. Pharm. Sci. 2014, 6, 86–90. [Google Scholar]
- Elangovan, K.; Elumalai, D.; Anupriya, S.; Shenbhagaraman, R.; Kaleena, P.; Murugesan, K. Phyto mediated biogenic synthesis of silver nanoparticles using leaf extract of Andrographis echioides and its bio-efficacy on anticancer and antibacterial activities. J. Photochem. Photobiol. B Biol. 2015, 151, 118–124. [Google Scholar] [CrossRef]
- Heydari, R.; Rashidipour, M. Green synthesis of silver nanoparticles using extract of oak fruit hull (Jaft): Synthesis and in vitro cytotoxic effect on MCF-7 cells. Int. J. Breast Cancer 2015, 2015, 846743. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Sathishkumar, G.; Sivanandhan, G.; MubarakAli, D.; Rajesh, M.; Arun, R.; Kapildev, G.; Manickavasagam, M.; Thajuddin, N.; Premkumar, K. Biogenic silver nanoparticles for cancer treatment: An experimental report. Colloids Surf. B Biointerfaces 2013, 106, 86–92. [Google Scholar] [CrossRef]
- Lalitha, P. Apoptotic efficacy of biogenic silver nanoparticles on human breast cancer MCF-7 cell lines. Prog. Biomater. 2015, 4, 113–121. [Google Scholar]
- Mittal, A.K.; Thanki, K.; Jain, S.; Banerjee, U.C. Comparative studies of anticancer and antimicrobial potential of bioinspired silver and silver-selenium nanoparticles. Appl. Nanomed. 2016, 1, 1–6. [Google Scholar]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Ramar, M.; Manikandan, B.; Marimuthu, P.N.; Raman, T.; Mahalingam, A.; Subramanian, P.; Karthick, S.; Munusamy, A. Synthesis of silver nanoparticles using Solanum trilobatum fruits extract and its antibacterial, cytotoxic activity against human breast cancer cell line MCF 7. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 140, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.J.; Vali, D.N.; Rani, M.; Rani, S.S. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater. Sci. Eng. C 2014, 34, 115–122. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Gobinath, C.; Wilson, A.; Sivaramakrishnan, S. Dendrophthoe falcata (Lf) Ettingsh (Neem mistletoe): A potent bioresource to fabricate silver nanoparticles for anticancer effect against human breast cancer cells (MCF-7). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 285–290. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Preethi, J.; Vijayan, R.; Yusoff, A.R.M.; Ameen, F.; Suresh, S.; Balagurunathan, R.; Palvannan, T. Anti-acne, anti-dandruff and anti-breast cancer efficacy of green synthesised silver nanoparticles using Coriandrum sativum leaf extract. J. Photochem. Photobiol. B Biol. 2016, 163, 69–76. [Google Scholar] [CrossRef]
- Sharma, D.; Ledwani, L.; Bhatnagar, N. Antimicrobial and cytotoxic potential of silver nanoparticles synthesized using Rheum emodi roots extract. Ann. West Univ. Timis. Ser. Chem. 2015, 24, 121–135. [Google Scholar]
- Shawkey, A.M.; Rabeh, M.A.; Abdulall, A.K.; Abdellatif, A.O. Green nanotechnology: Anticancer activity of silver nanoparticles using Citrullus colocynthis aqueous extracts. Adv. Life Sci. Technol. 2013, 13, 60–70. [Google Scholar]
- Venugopal, K.; Rather, H.; Rajagopal, K.; Shanthi, M.; Sheriff, K.; Illiyas, M.; Rather, R.; Manikandan, E.; Uvarajan, S.; Bhaskar, M. Synthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. J. Photochem. Photobiol. B Biol. 2017, 167, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Devi, J.; Bhimba, B. Anticancer activity of silver nanoparticles synthesized by the seaweed Ulva lactuca in vitro. Open Access Sci. Rep. 2012, 1, 242. [Google Scholar] [CrossRef]
- Mishra, A.; Mehdi, S.J.; Irshad, M.; Ali, A.; Sardar, M.; Moshahid, M.; Rizvi, A. Effect of biologically synthesized silver nanoparticles on human cancer cells. Sci. Adv. Mater. 2012, 4, 1200–1206. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Mata, R.; Gupta, A.K.; Sadras, S.R. Biological activities of green silver nanoparticles synthesized with Acorous calamus rhizome extract. Eur. J. Med. Chem. 2014, 85, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Rajkuberan, C.; Sudha, K.; Sathishkumar, G.; Sivaramakrishnan, S. Antibacterial and cytotoxic potential of silver nanoparticles synthesized using latex of Calotropis gigantea L. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 924–930. [Google Scholar] [CrossRef]
- Chanthini, A.B.; Balasubramani, G.; Ramkumar, R.; Sowmiya, R.; Balakumaran, M.D.; Kalaichelvan, P.T.; Perumal, P. Structural characterization, antioxidant and in vitro cytotoxic properties of seagrass, Cymodocea serrulata (R. Br.) Asch. & Magnus mediated silver nanoparticles. J. Photochem. Photobiol. B Biol. 2015, 153, 145–152. [Google Scholar]
- Vijistella Bai, G. Green synthesis of silver nanostructures against human cancer cell lines and certain pathogens. Int. J. Pharm. Chem. Biol. Sci. 2014, 4, 101–111. [Google Scholar]
- Jeyaraj, M.; Rajesh, M.; Arun, R.; MubarakAli, D.; Sathishkumar, G.; Sivanandhan, G.; Dev, G.K.; Manickavasagam, M.; Premkumar, K.; Thajuddin, N. An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized silver nanoparticles using Podophyllum hexandrum on human cervical carcinoma cells. Colloids Surf. B Biointerfaces 2013, 102, 708–717. [Google Scholar] [CrossRef]
- Govindaraju, K.; Krishnamoorthy, K.; Alsagaby, S.A.; Singaravelu, G.; Premanathan, M. Green synthesis of silver nanoparticles for selective toxicity towards cancer cells. IET Nanobiotechnology 2015, 9, 325–330. [Google Scholar] [CrossRef]
- Mata, R.; Nakkala, J.R.; Sadras, S.R. Catalytic and biological activities of green silver nanoparticles synthesized from Plumeria alba (frangipani) flower extract. Mater. Sci. Eng. C 2015, 51, 216–225. [Google Scholar] [CrossRef]
- Manikandan, R.; Manikandan, B.; Raman, T.; Arunagirinathan, K.; Prabhu, N.M.; Basu, M.J.; Perumal, M.; Palanisamy, S.; Munusamy, A. Biosynthesis of silver nanoparticles using ethanolic petals extract of Rosa indica and characterization of its antibacterial, anticancer and anti-inflammatory activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 120–129. [Google Scholar] [CrossRef]
- Prabhu, D.; Arulvasu, C.; Babu, G.; Manikandan, R.; Srinivasan, P. Biologically synthesized green silver nanoparticles from leaf extract of Vitex negundo L. induce growth-inhibitory effect on human colon cancer cell line HCT15. Process Biochem. 2013, 48, 317–324. [Google Scholar] [CrossRef]
- Arunachalam, K.D.; Arun, L.B.; Annamalai, S.K.; Arunachalam, A.M. Potential anticancer properties of bioactive compounds of Gymnema sylvestre and its biofunctionalized silver nanoparticles. Int. J. Nanomed. 2015, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Pradhan, S.; Ashe, S.; Rauta, P.R.; Nayak, B. Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J. Colloid Interface Sci. 2015, 457, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Shandiz, S.A.S.; Ghanbar, F.; Darvish, M.R.; Ardestani, M.S.; Mirzaie, A.; Jafari, M. Phytosynthesis of silver nanoparticles using Artemisia marschalliana Sprengel aerial part extract and assessment of their antioxidant, anticancer, and antibacterial properties. Int. J. Nanomed. 2016, 11, 1835. [Google Scholar]
- Pandian, A.M.K.; Karthikeyan, C.; Rajasimman, M.; Dinesh, M. Synthesis of silver nanoparticle and its application. Ecotoxicol. Environ. Saf. 2015, 121, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Smita, K.; Seqqat, R.; Benalcazar, K.; Grijalva, M.; Cumbal, L. In vitro evaluation of silver nanoparticles cytotoxicity on Hepatic cancer (Hep-G2) cell line and their antioxidant activity: Green approach for fabrication and application. J. Photochem. Photobiol. B Biol. 2016, 159, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Balasubramanian, T. Biomedical potential of silver nanoparticles synthesized from calli cells of Citrullus colocynthis (L.) Schrad. J. Nanobiotechnology 2011, 9, 43. [Google Scholar]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Balasubramanian, T. Toxicity study of silver nanoparticles synthesized from Suaeda monoica on Hep-2 cell line. Avicenna J. Med Biotechnol. 2012, 4, 35. [Google Scholar]
- He, Y.; Du, Z.; Ma, S.; Liu, Y.; Li, D.; Huang, H.; Jiang, S.; Cheng, S.; Wu, W.; Zhang, K. Effects of green-synthesized silver nanoparticles on lung cancer cells in vitro and grown as xenograft tumors in vivo. Int. J. Nanomed. 2016, 11, 1879. [Google Scholar] [CrossRef]
- Xia, Q.H.; Ma, Y.J.; Wang, J.W. Biosynthesis of silver nanoparticles using Taxus yunnanensis callus and their antibacterial activity and cytotoxicity in human cancer cells. Nanomaterials 2016, 6, 160. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeong, J.-K.; Han, J.W.; Zhang, X.-F.; Park, J.H.; Kim, J.-H. Multidimensional effects of biologically synthesized silver nanoparticles in Helicobacter pylori, Helicobacter felis, and human lung (L132) and lung carcinoma A549 cells. Nanoscale Res. Lett. 2015, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Kanipandian, N.; Thirumurugan, R. A feasible approach to phyto-mediated synthesis of silver nanoparticles using industrial crop Gossypium hirsutum (cotton) extract as stabilizing agent and assessment of its in vitro biomedical potential. Ind. Crop. Prod. 2014, 55, 1–10. [Google Scholar] [CrossRef]
- Khanra, K.; Panja, S.; Choudhuri, I.; Chakraborty, A.; Bhattacharyya, N. Evaluation of antibacterial activity and cytotoxicity of green synthesized silver nanoparticles using Scoparia dulcis. Nano Biomed Eng 2015, 7, 128–133. [Google Scholar] [CrossRef]
- Khanra, K.; Panja, S.; Choudhuri, I.; Chakraborty, A.; Bhattacharyya, N. Antimicrobial and cytotoxicity effect of silver nanoparticle synthesized by Croton bonplandianum Baill. leaves. Nanomed. J. 2016, 3, 15–22. [Google Scholar]
- Majeed, S.; bin Abdullah, M.S.; Dash, G.K.; Ansari, M.T.; Nanda, A. Biochemical synthesis of silver nanoprticles using filamentous fungi Penicillium decumbens (MTCC-2494) and its efficacy against A-549 lung cancer cell line. Chin. J. Nat. Med. 2016, 14, 615–620. [Google Scholar] [CrossRef]
- Palaniappan, P.; Sathishkumar, G.; Sankar, R. Fabrication of nano-silver particles using Cymodocea serrulata and its cytotoxicity effect against human lung cancer A549 cells line. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 885–890. [Google Scholar] [CrossRef]
- Venkatesan, B.; Subramanian, V.; Tumala, A.; Vellaichamy, E. Rapid synthesis of biocompatible silver nanoparticles using aqueous extract of Rosa damascena petals and evaluation of their anticancer activity. Asian Pac. J. Trop. Med. 2014, 7, S294–S300. [Google Scholar] [CrossRef]
- Mollick, M.M.R.; Rana, D.; Dash, S.K.; Chattopadhyay, S.; Bhowmick, B.; Maity, D.; Mondal, D.; Pattanayak, S.; Roy, S.; Chakraborty, M. Studies on green synthesized silver nanoparticles using Abelmoschus esculentus (L.) pulp extract having anticancer (in vitro) and antimicrobial applications. Arab. J. Chem. 2015, 12, 2572–2584. [Google Scholar] [CrossRef]
- Banerjee, K.; Das, S.; Choudhury, P.; Ghosh, S.; Baral, R.; Choudhuri, S.K. A novel approach of synthesizing and evaluating the anticancer potential of silver oxide nanoparticles in vitro. Chemotherapy 2017, 62, 279–289. [Google Scholar] [CrossRef]
- Inbakandan, D.; Kumar, C.; Bavanilatha, M.; Ravindra, D.N.; Kirubagaran, R.; Khan, S.A. Ultrasonic-assisted green synthesis of flower like silver nanocolloids using marine sponge extract and its effect on oral biofilm bacteria and oral cancer cell lines. Microb. Pathog. 2016, 99, 135–141. [Google Scholar] [CrossRef]
- Priyadharshini, R.I.; Prasannaraj, G.; Geetha, N.; Venkatachalam, P. Microwave-mediated extracellular synthesis of metallic silver and zinc oxide nanoparticles using macro-algae (Gracilaria edulis) extracts and its anticancer activity against human PC3 cell lines. Appl. Biochem. Biotechnol. 2014, 174, 2777–2790. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Du, Z.; Ma, S.; Cheng, S.; Jiang, S.; Liu, Y.; Li, D.; Huang, H.; Zhang, K.; Zheng, X. Biosynthesis, antibacterial activity and anticancer effects against prostate cancer (PC-3) cells of silver nanoparticles using Dimocarpus Longan Lour. Nanoscale Res. Lett. 2016, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Priyaragini, S.; Sathishkumar, S.; Bhaskararao, K. Biosynthesis of silver nanoparticles using actinobacteria and evaluating its antimicrobial and cytotoxicity activity. Int. J. Pharm. Pharm. Sci. 2013, 5, 709–712. [Google Scholar]
- Kim, J.-H.; Lee, Y.; Kim, E.-J.; Gu, S.; Sohn, E.J.; Seo, Y.S.; An, H.J.; Chang, Y.-S. Exposure of iron nanoparticles to Arabidopsis thaliana enhances root elongation by triggering cell wall loosening. Environ. Sci. Technol. 2014, 48, 3477–3485. [Google Scholar] [CrossRef]
- Birbrair, A.; Zhang, T.; Wang, Z.-M.; Messi, M.L.; Olson, J.D.; Mintz, A.; Delbono, O. Type-2 pericytes participate in normal and tumoral angiogenesis. Am. J. Physiol. Cell Physiol. 2014, 307, C25–C38. [Google Scholar] [CrossRef]
- Bhat, T.A.; Singh, R.P. Tumor angiogenesis—A potential target in cancer chemoprevention. Food Chem. Toxicol. 2008, 46, 1334–1345. [Google Scholar] [CrossRef]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. In Seminars in Oncology; Elsevier: Amsterdam, The Netherlands, 2002; pp. 15–18. [Google Scholar]
- Shen, H.-H.; Chan, E.C.; Lee, J.H.; Bee, Y.-S.; Lin, T.-W.; Dusting, G.J.; Liu, G.-S. Nanocarriers for treatment of ocular neovascularization in the back of the eye: New vehicles for ophthalmic drug delivery. Nanomedicine 2015, 10, 2093–2107. [Google Scholar] [CrossRef]
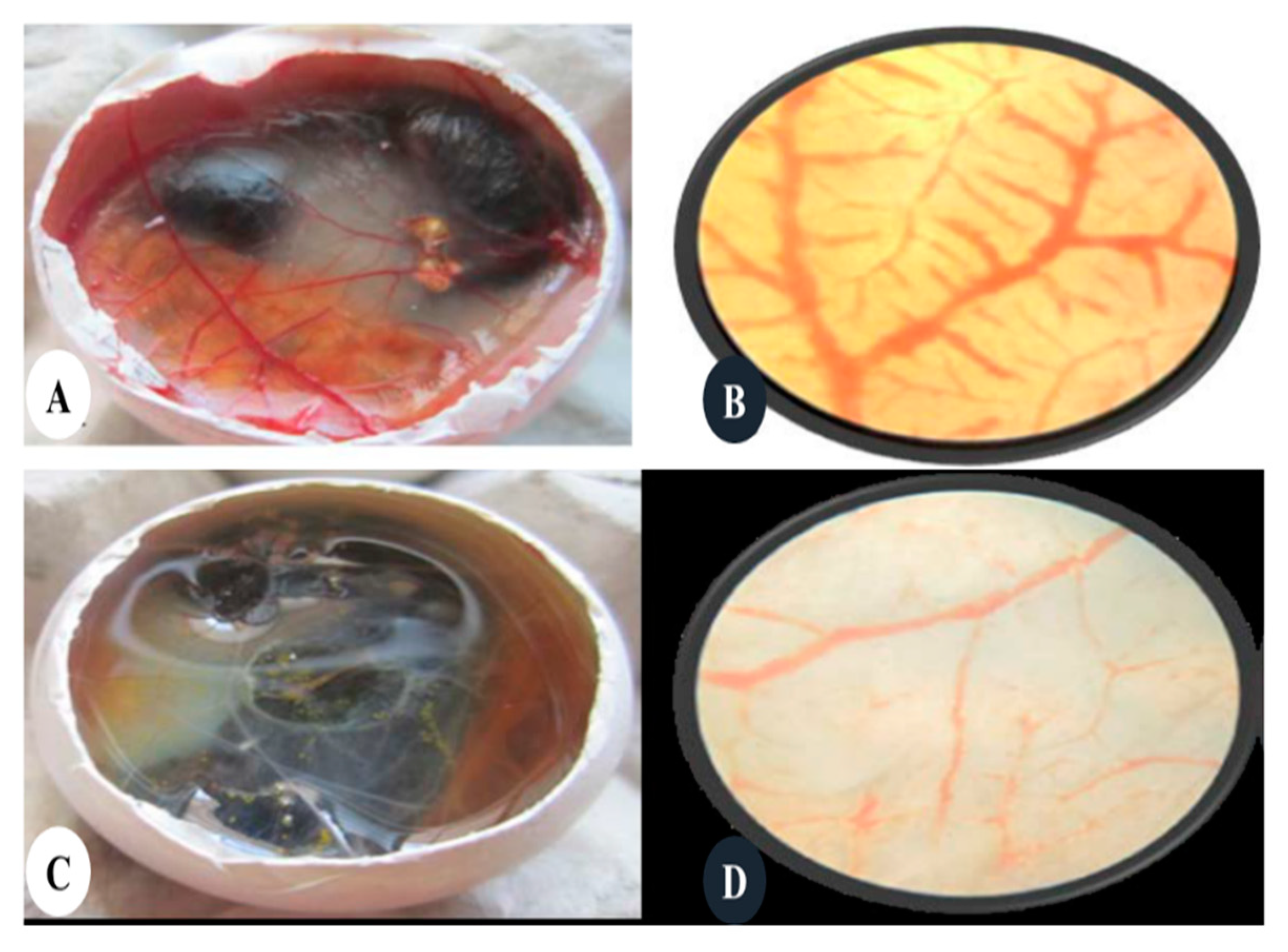
- Khandia, R.; Munjal, A.; Bangrey, R.; Mehra, R.; Dhama, K.; Sharma, N. Evaluation of silver nanoparticle mediated reduction of neovascularisation (angiogenesis) in chicken model. Adv. Anim. Vet. Sci 2015, 3, 372–376. [Google Scholar] [CrossRef]
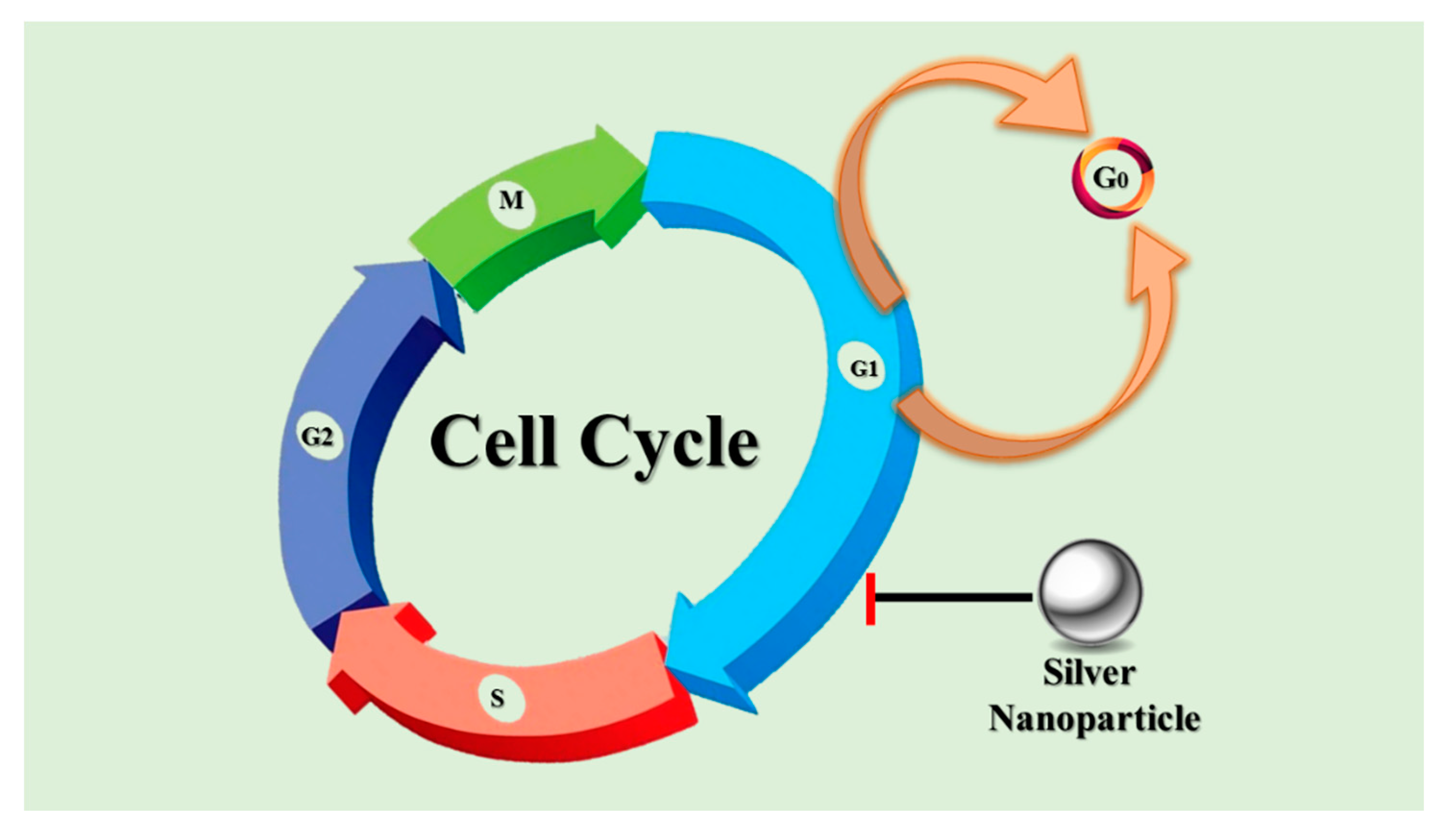
- Collins, K.; Jacks, T.; Pavletich, N.P. The cell cycle and cancer. Proc. Natl. Acad. Sci. USA 1997, 94, 2776–2778. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Tai, C.-J.; Kuo, L.-J.; Wei, P.-L.; Liang, H.-H.; Liu, T.-Z.; Wang, W.; Tai, C.-J.; Ho, Y.-S.; Wu, C.-H. Glucose-regulated protein 78 (GRP78) mediated the efficacy to curcumin treatment on hepatocellular carcinoma. Ann. Surg. Oncol. 2011, 18, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Seidlitz, E.; Truant, R.; Hitt, M.; Ghosh, H.P. Re-expression of TSLC1 in a non-small-cell lung cancer cell line induces apoptosis and inhibits tumor growth. Oncogene 2004, 23, 5632–5642. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj Punita, S. Silver or silver nanoparticle a safety or a risk. J. Environ. Res. Dev. 2012, 7, 452–456. [Google Scholar]
- Mahmoudi, M.; Serpooshan, V. Silver-coated engineered magnetic nanoparticles are promising for the success in the fight against antibacterial resistance threat. ACS Nano 2012, 6, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Ahmed, K.B.R.; Nagy, A.M.; Brown, R.P.; Zhang, Q.; Malghan, S.G.; Goering, P.L. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol. Vitr. 2017, 38, 179–192. [Google Scholar] [CrossRef]
- Dziedzic, A.; Kubina, R.; Bułdak, R.J.; Skonieczna, M.; Cholewa, K. Silver nanoparticles exhibit the dose-dependent anti-proliferative effect against human squamous carcinoma cells attenuated in the presence of berberine. Molecules 2016, 21, 365. [Google Scholar] [CrossRef]
- Skonieczna, M.; Hudy, D. Biological activity of silver nanoparticles and their applications in anticancer therapy. Silver Nanoparticles Fabr. Charact. Appl. 2018, 131. [Google Scholar] [CrossRef]
- Braydich-Stolle, L.K.; Lucas, B.; Schrand, A.; Murdock, R.C.; Lee, T.; Schlager, J.J.; Hussain, S.M.; Hofmann, M.-C. Silver nanoparticles disrupt GDNF/Fyn kinase signaling in spermatogonial stem cells. Toxicol. Sci. 2010, 116, 577–589. [Google Scholar] [CrossRef]
- Swanner, J.; Fahrenholtz, C.D.; Tenvooren, I.; Bernish, B.W.; Sears, J.J.; Hooker, A.; Furdui, C.M.; Alli, E.; Li, W.; Donati, G.L.; et al. Silver nanoparticles selectively treat triple-negative breast cancer cells without affecting non-malignant breast epithelial cells in vitro and in vivo. FASEB Bioadv. 2019, 1, 639–660. [Google Scholar] [CrossRef]
- Gurunathan, S.; Raman, J.; Malek, S.N.A.; John, P.A.; Vikineswary, S. Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: A potential cytotoxic agent against breast cancer cells. Int. J. Nanomed. 2013, 8, 4399. [Google Scholar]
- Madamsetty, V.S.; Mukherjee, A.; Mukherjee, S. Recent trends of the bio-inspired nanoparticles in cancer theranostics. Front. Pharmacol. 2019, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- García-Contreras, R.; Argueta-Figueroa, L.; Mejía-Rubalcava, C.; Jiménez-Martínez, R.; Cuevas-Guajardo, S.; Sánchez-Reyna, P.A.; Mendieta-Zeron, H. Perspectives for the use of silver nanoparticles in dental practice. Int. Dent. J. 2011, 61, 297–301. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Paralikar, P.; Gupta, I.; Medici, S.; Santos, C.A. Recent advances in use of silver nanoparticles as antimalarial agents. Int. J. Pharm. 2017, 526, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Park, E.-J.; Chun, I.K.; Choi, K.; Lee, S.H.; Yoon, J.; Lee, B.C. Bioavailability and toxicokinetics of citrate-coated silver nanoparticles in rats. Arch. Pharmacal Res. 2011, 34, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Samberg, M.E.; Lin, Z.; Monteiro-Riviere, N.A. In vitro and in vivo toxicity and pharmacokinetics of silver nanoparticles. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–14. [Google Scholar]
- Ravanshad, R.; Karimi Zadeh, A.; Amani, A.M.; Mousavi, S.M.; Hashemi, S.A.; Savar Dashtaki, A.; Mirzaei, E.; Zare, B. Application of nanoparticles in cancer detection by Raman scattering based techniques. Nano Rev. Exp. 2018, 9, 1373551. [Google Scholar] [CrossRef]
| Plant | Phytochemical | Reference |
|---|---|---|
| Alternanthera tenella | Flavonoids | [65] |
| Cocos nucifera | Carbohydrates, alkaloids, terpenoids, tannins, saponins, phenolics and reducing sugars | [68] |
| Lemongrass | Reducing sugars | [69] |
| Ocimum sanctum | Caffeine | [70] |
| Chrysanthemum indicum | Tannins, flavonoids and glycosides | [71] |
| Dalbergia spinose | Reducing sugars and flavonoids | [72] |
| Cinnamomum camphora | Phenolics, terpenoids, polysaccharides and flavones | [73] |
| Eucalyptus hybrid | Flavonoids and terpenoids | [74] |
| Plant | Part Used | NP Size/NP Shape | Incubation Time | IC50 | Ref. |
|---|---|---|---|---|---|
| Achillea biebersteinii | Flower | 12 nm/spherical, pentagonal | 3 h | 20 μg/mL | [88] |
| Alternanthera sessilis | Aerial parts | 10–30 nm/spherical | 6 h | 3.04 μg/mL | [94] |
| Alternanthera tenella | Leaf | About 48 nm/- | - | 42.5 μg/mL | [65] |
| Andrographis echioides | Leaf | 68.06 nm/cubic, pentagonal, hexagonal | 12 h | 31.5 μg/mL | [91] |
| Azadirachta indica | Leaf | <40 nm/spherical | 6 h | Dose dependent | [95] |
| Butea monosperma | Leaf | 20–80 nm/spherical | 2 h | Dose dependent | [101] |
| Coriandrum sativum | Leaf | About 37 nm/spherical, rod, triangular, hexagonal | - | 30.5 μg/mL | [97] |
| Citrullus colocynthis | Leaf | 13.37 nm/spherical | 24 h | >30 μg/mL | [103] |
| Roots | 7.39 nm/spherical | 24 h | 2.4 μg/mL | ||
| Seeds | 16.57 nm/spherical | 24 h | >30 μg/mL | ||
| Fruit | 19.26 nm/spherical | 24 h | >30 μg/mL | ||
| Dendrophthoe falcata (L.f) Ettingsh | Mistletoe, leaf | 5–45 nm/spherical | 24 h | Dose dependent | [100] |
| Erythrina indica | Root | 20–118 nm/spherical | Overnight | 23.89 (% viability at 25 μL) | [75] |
| Melia dubia | Leaf | 7.3 nm/irregular | 15 min | 31.2 μg/mL | [85] |
| Olax scandens | Leaf | 30–60 nm/spherical | 2 h | Dose dependent | [96] |
| Panax ginseng | Leaf | - | - | Dose dependent | [89] |
| Piper longum | Fruit | 46 nm/spherical | 24 h | 67 μg/mL | [99] |
| Quercus (genus) | Fruit hull | 40 nm/spherical | - | 50 μg/mL | [92] |
| Rheum emodi | Root | 27.5 nm/spherical | 24 h | Dose dependent | [102] |
| Sesbania grandiflora | Leaf | 22 nm/spherical | 24 h | 20 μg/mL | [93] |
| Solanum trilobatum | Fruit | 41.90 nm/spherical, polygonal | - | 30 μg/mL | [98] |
| Syzygium cumini | Flower | <40 nm/spherical | 6 h | Dose dependent | [95] |
| Syzygium aromaticum | Fruit | 5–20 nm/spherical | 20 min | 70 µg/mL | [104] |
| Tabernae montana divaricate | Leaf | Mean 22.85 nm/spherical | 24 h | 20 μg/mL | [90] |
| Taxus baccata | Needles | Mean 75.1 nm/spherical | 48 h | 0.25 mg/mL | [40] |
| Ulva lactuca | Whole | 56 nm/spherical | 10 min | 37 µg/mL | [105] |
| Cancer | Plant | Part Used | NP Size/NP Shape | Incubation Time | Cell Line | IC50 | Ref. |
|---|---|---|---|---|---|---|---|
| Brain Cancer | Butea monosperma | Leaf | 20–80 nm/spherical | 2 h | HNGC2 | Dose dependent | [101] |
| Cervical Cancer | Azadirachta indica | Leaf | 2–18 nm/triangular, hexagonal | - | Siha | ≤4.25 μg/mL | [106] |
| Acorous calamus | Rhizome | 31.86 nm/spherical | 20 h | HeLa | Dose dependent | [107] | |
| Azadirachta indica | Leaf | <40 nm/spherical | 6 h | Dose dependent | [95] | ||
| Calotropis gigantea | Latex | 5–30 nm/spherical | 24 h | Dose dependent | [108] | ||
| Cymodocea serrulata | Whole | 17–29 nm/spherical | 2 h | 107.7 (GI50) | [109] | ||
| Heliotropium indicum | Leaf | 80–120 nm/spherical | 2 h | 20 μg/mL | [110] | ||
| Melia azedarach | Leaf | 78 nm/cubical, spherical | 10 min | 300 μg/mL (LD50) | [23] | ||
| Moringa olifera | Stem bark | 38–40 nm/spherical, pentagonal | 24 h | Dose dependent | [25] | ||
| Podophyllum hexandrum | Leaf | 14 nm/spherical | 30–150 min | 20 μg/mL | [111] | ||
| Sargassum vulgare (algae) | Whole | 10 nm/spherical | 3 h | Dose dependent | [112] | ||
| Syzygium cumini | Flower | <40 nm/spherical | 6 h | Dose dependent | [95] | ||
| Colon Cancer | Plumeria alba | Flower | 36.19 nm/spherical | 30 min | COLO 205 | 5.5 μg/mL | [113] |
| Rosa indica | Petal | 23.52–60.83 nm/spherical | 1 h | HCT 15 | 30 μg/mL | [114] | |
| Vitex nigundo | Leaf | 22 nm/spherical | 4 h | 20 μg/mL | [115] | ||
| Citrullus colocynthis | Leaf | 13.37 nm/spherical | 24 h | HCT-116 | >30 μg/mL | [103] | |
| Roots | 7.39 nm/spherical | 24 h | >30 μg/mL | ||||
| Seeds | 16.57 nm/Spherical | 24 h | >30 μg/mL | ||||
| Fruit | 19.26 nm/spherical | 24 h | 21.2 μg/mL | ||||
| Commelina nudiflora L. | Whole | 24–80 nm/spherical, triangular | 24 h | 100 μg/mL | [61] | ||
| Gymnema sylvestre | Leaf | -/spherical | 24 h | HT29 | 85 μg/mL | [116] | |
| Ulva lactuca (algae) | Whole | 56 nm/spherical | 10 min | 49 μg/mL | [105] |
| Cancer | Plant | Part Used | NP Size/NP Shape | Incubation Time | Cell Line | IC50 | Ref. |
|---|---|---|---|---|---|---|---|
| Epidermoid Cancer | Acorus calamus | Rhizome | 59 nm/cuboidal, spherical | 5–60 min | A431 | 78.58 μg/mL | [117] |
| Cucurbita maxima | Petal | 76 nm/cuboidal, spherical | 5–60 min | 82.39 μg/mL | |||
| Moringa oleifera | Leaf | 94 nm/cuboidal, spherical | 5–60 min | 83.57 μg/mL | |||
| Gastric Cancer | Artemisia marschalliana | Aerial Part | 5–50 nm/spherical | 5 min | AGS | 21.05 μg/mL | [118] |
| Hepatic Cancer | Allium sativum | Whole | 100–1200 nm/spherical | 48 h | HEP-G2 | 31.25 ng/mL (LD50) | [119] |
| Citrullus colocynthis | Leaf | 13.37 nm/spherical | 24 h | Hep-G2 | 10.02 μg/mL | [97] | |
| Root | 7.39 nm/spherical | 24 h | 17.2 μg/mL | ||||
| Seed | 16.57 nm/spherical | 24 h | >30 μg/mL | ||||
| Fruit | 19.26 nm/spherical | 24 h | 22.4 μg/mL | ||||
| Erythrina indica | Root | 20–118 nm/spherical | Overnight | 13.86 (% viability at 25 μL) | [75] | ||
| Panax ginseng | Leaf | - | - | Dose dependent | [89] | ||
| Rubus glaucus Benth | Leaf | 12–50 nm/Quasi-spherical | 48 h | Dose dependent | [120] | ||
| Intestinal Cancaer | Taxus yunnanensis | Callus | 6.4–27.2 nm/spherical | 10 min | SMMC-7721 | 27.75 μg/mL | [124] |
| Intestinal Cancaer | Citrullus colocynthis | Leaf | 13.37 nm/spherical | 24 h | Caco-2 | >30 μg/mL | [97] |
| Root | 7.39 nm/spherical | ||||||
| Seed | 16.57 nm/spherical | ||||||
| Fruit | 19.26 nm/spherical | ||||||
| Kidney Cancer | Azadirachta indica | Leaf | <40 nm/spherical | 6 h | Hek-293 | Dose dependent | [95] |
| Syzygium cumini | Flower | <40 nm/spherical | 6 h | Dose dependent | [98] | ||
| Laryngeal Cancer | Citrullus colocynthis | Callus | 31 nm/spherical | 24 h | Hep-2 | 3.42 μg/mL | [121] |
| Suaeda monoica | Leaf | 31 nm/spherical | 5 h | 500 nM, AgNPs conc. | [122] | ||
| Ulva lactuca (algae) | Whole | 56 nm/spherical | 10 min | 12.5 μg/mL | [105] | ||
| Leukemia Cancer | Dimocarpus longan | Peel | 8–22 nm/spherical | 2 h | H1299 | 5.33 μg/mL | [123] |
| Sargassum vulgare (algae) | Whole | 10 nm/spherical | 3 h | HL-60 | Dose dependent | [112] |
| Cancer | Plant | Part Used | NP Size/NP Shape | Incubation Time | Cell Line | IC50 | Ref. |
|---|---|---|---|---|---|---|---|
| Lung Cancer | Acorous calamus | Rhizome | 31.86 nm/spherical | 20 h | A549 | Dose dependent | [106] |
| Artemisia princeps | Leaf | 20 nm/spherical | 15 min | Time dependent | [125] | ||
| Butea Monosperma | Leaf | 20–80 nm/spherical | 2 h | Dose dependent | [101] | ||
| Croton bonplandianum | Leaf | 32 nm/spherical | 1 h | Dose dependent | [128] | ||
| Cymodocea serrulata | Leaf | 29.28 nm/spherical | 1 h | 100 μg/mL (LD50) | [130] | ||
| Gossypium hirsutum | Leaf | 13–40 nm/spherical | 3 min | 40 μg/mL | [126] | ||
| Olax scandens | Leaf | 30–60 nm/spherical | 2 h | Dose dependent | [75] | ||
| Origanum vulgare | Leaf | 136 ± 10.09 nm/spherical | Temp. dependent | 100 μg/mL (LD50) | [42] | ||
| Panax ginseng | Leaf | - | - | Dose dependent | [89] | ||
| Penicillium decumbens (MTCC 2494) | Whole | 30–60 nm/spherical | - | 80 μg/mL, 24 h, 60 μg/mL, 48 h | [129] | ||
| Rosa damascena | Petal | 15–27 nm/spherical | 0–25 min | 80 μg/mL | [131] | ||
| Scoparia dulcis | Leaf | 15–25 nm/spherical | 1 h | Dose dependent | [127] | ||
| Syzygium aromaticum | Fruit | 5–20 nm/spherical | 20 min | 70 µg/mL | [104] | ||
| Lymphoma Cancer | Abelmoschus esculentus | Pulp | ~6.7 nm/spherical | 27 h | Jurkat | 16.15 μg/mL | [132] |
| Melanoma Cancer | Excoecaria agallocha L. | Leaf | 228 nm/- | - | B16F10 | 7.6 ± 0.8 μg/mL | [133] |
| Butea monosperma | Leaf | 20–80 nm/spherical | 2 h | Dose dependent | [101] | ||
| Oral Cancer | Haliclona exigua | Whole | 100–120 nm/flower-like | 10 min | KB | 0.6 μg/mL | [134] |
| Ovarian Cancer | Croton bonplandianum | Leaf | 32 nm/spherical | 1 h | PA1 | 7.5 μg/mL | [125] |
| Scoparia dulcis | Leaf | 150–25 nm/spherical | 1 h | Dose dependent | [127] | ||
| Pancreatic Cancer | Dimocarpus longan | Peel | 8–22 nm/spherical | 2 h | BxPC 3 | 38.9 μg/mL | [123] |
| Prostate Cancer | Alternanthera sessilis | Leaf | 300–50 nm/spherical | 6 h | PC3 | 6.85 μg/mL | [76] |
| Dimocarpus longan Lour. | Peel | 9–32 nm/cubic | 5 h 20 min | Dose dependent | [136] | ||
| Gracilaria edulis | Whole | 55–99 nm/spherical | - | 53.99 μg/mL | [135] | ||
| Dimocarpus longan | Peel | 8–22 nm/spherical | Overnight | VCaP | 87.33 μg/mL | [123] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.J.S.; Shim, Y.Y.; Reaney, M.J.T.; Cho, J.Y. Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects. Cancers 2020, 12, 855. https://doi.org/10.3390/cancers12040855
Ratan ZA, Haidere MF, Nurunnabi M, Shahriar SM, Ahammad AJS, Shim YY, Reaney MJT, Cho JY. Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects. Cancers. 2020; 12(4):855. https://doi.org/10.3390/cancers12040855
Chicago/Turabian StyleRatan, Zubair Ahmed, Mohammad Faisal Haidere, Md. Nurunnabi, Sadi Md. Shahriar, A.J. Saleh Ahammad, Youn Young Shim, Martin J.T. Reaney, and Jae Youl Cho. 2020. "Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects" Cancers 12, no. 4: 855. https://doi.org/10.3390/cancers12040855
APA StyleRatan, Z. A., Haidere, M. F., Nurunnabi, M., Shahriar, S. M., Ahammad, A. J. S., Shim, Y. Y., Reaney, M. J. T., & Cho, J. Y. (2020). Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects. Cancers, 12(4), 855. https://doi.org/10.3390/cancers12040855






