Combination Treatment with Cold Physical Plasma and Pulsed Electric Fields Augments ROS Production and Cytotoxicity in Lymphoma
Abstract
1. Introduction
2. Results
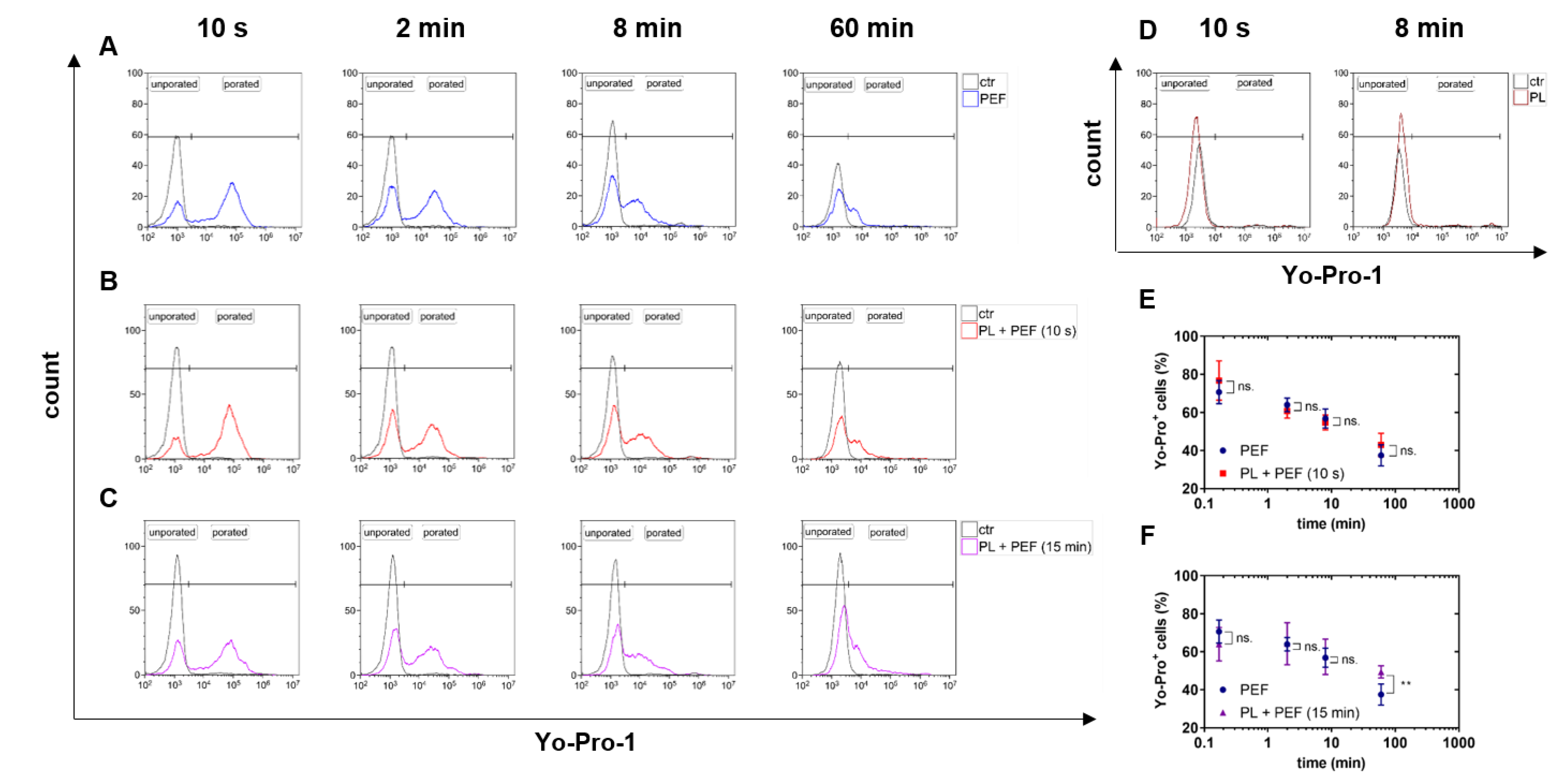
2.1. PEF but not Plasma Treatment Led to Cell Membrane Permeabilization
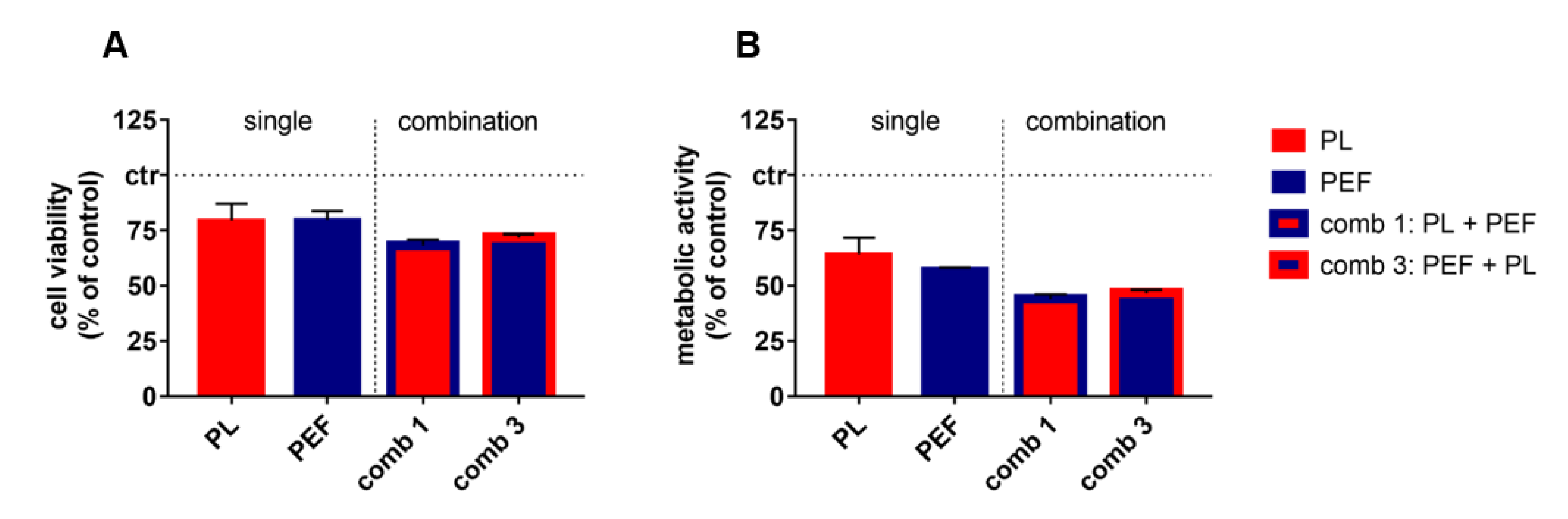
2.2. Combination of Plasma and PEF Treatment Showed Additive Toxicity
2.3. The Cell Death Kinetics of the Mono and Combination Treatments were Similar
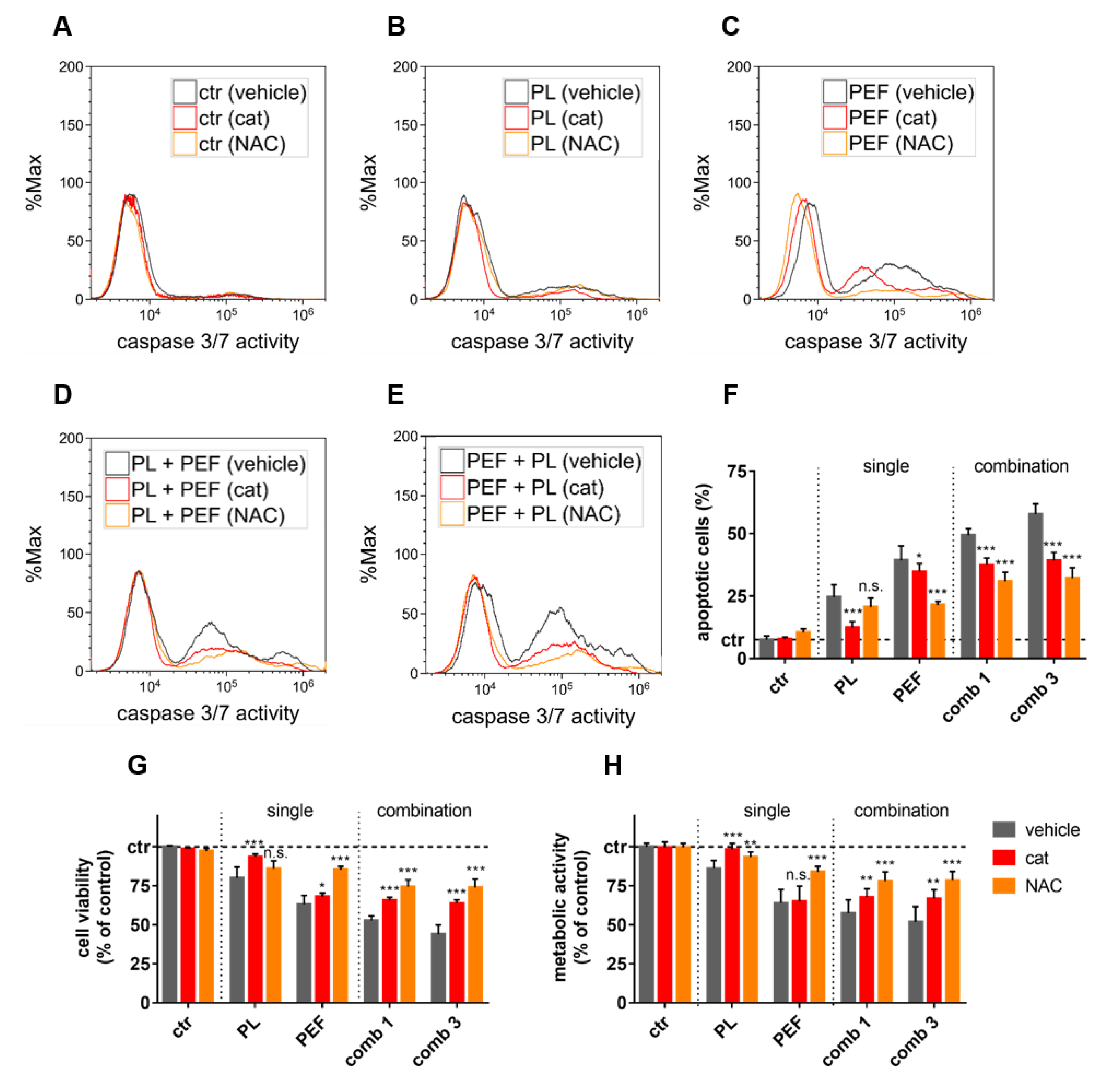
2.4. ROS and Lipid Peroxidation Contributed to Plasma and PEF Combination Treatment
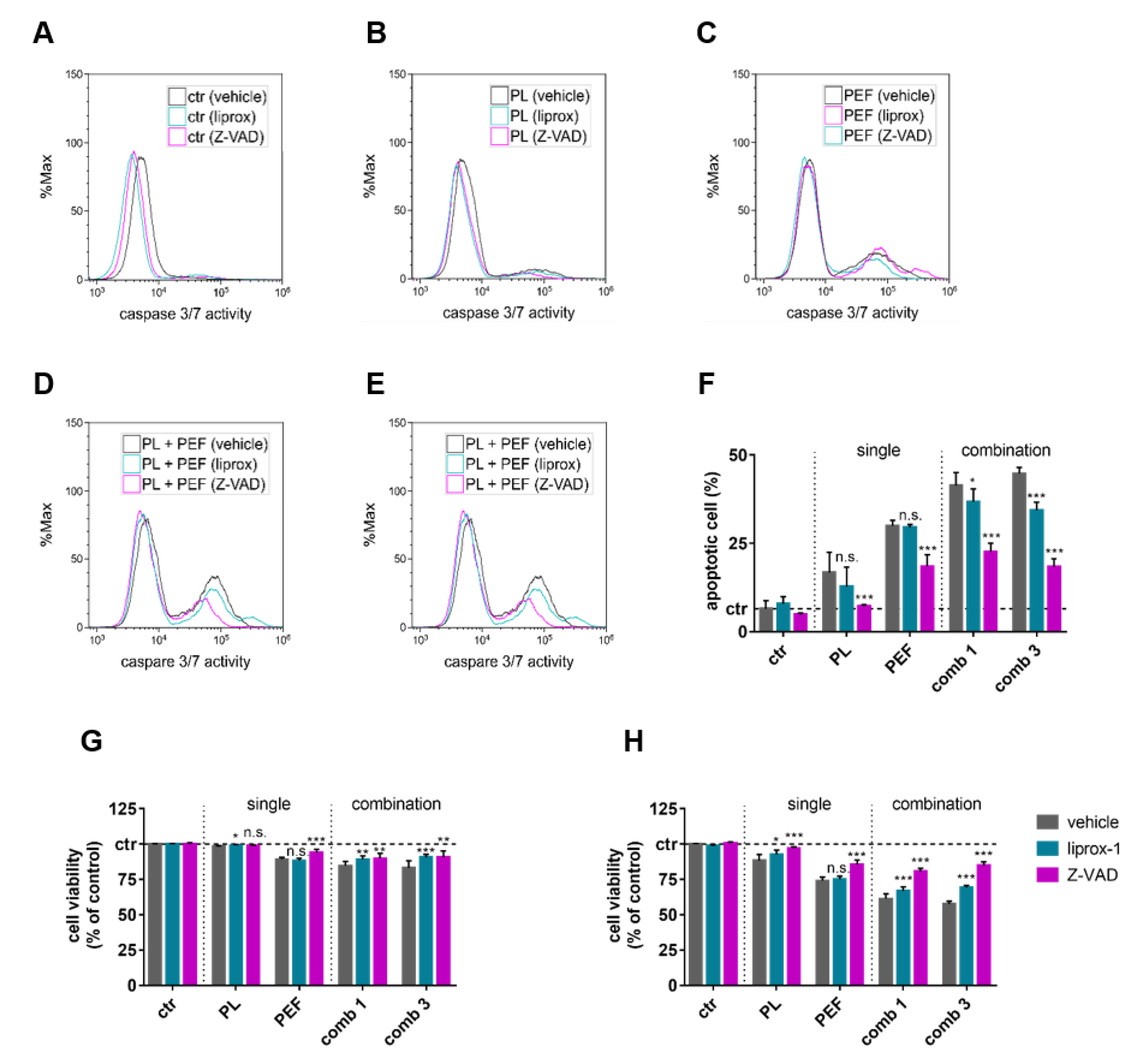
2.5. Apoptosis and Ferroptosis Contributed to Plasma and PEF Combination Treatment
2.6. Validation of Additive Cytotoxicity in a Second Lymphoma Cell Line
3. Discussion
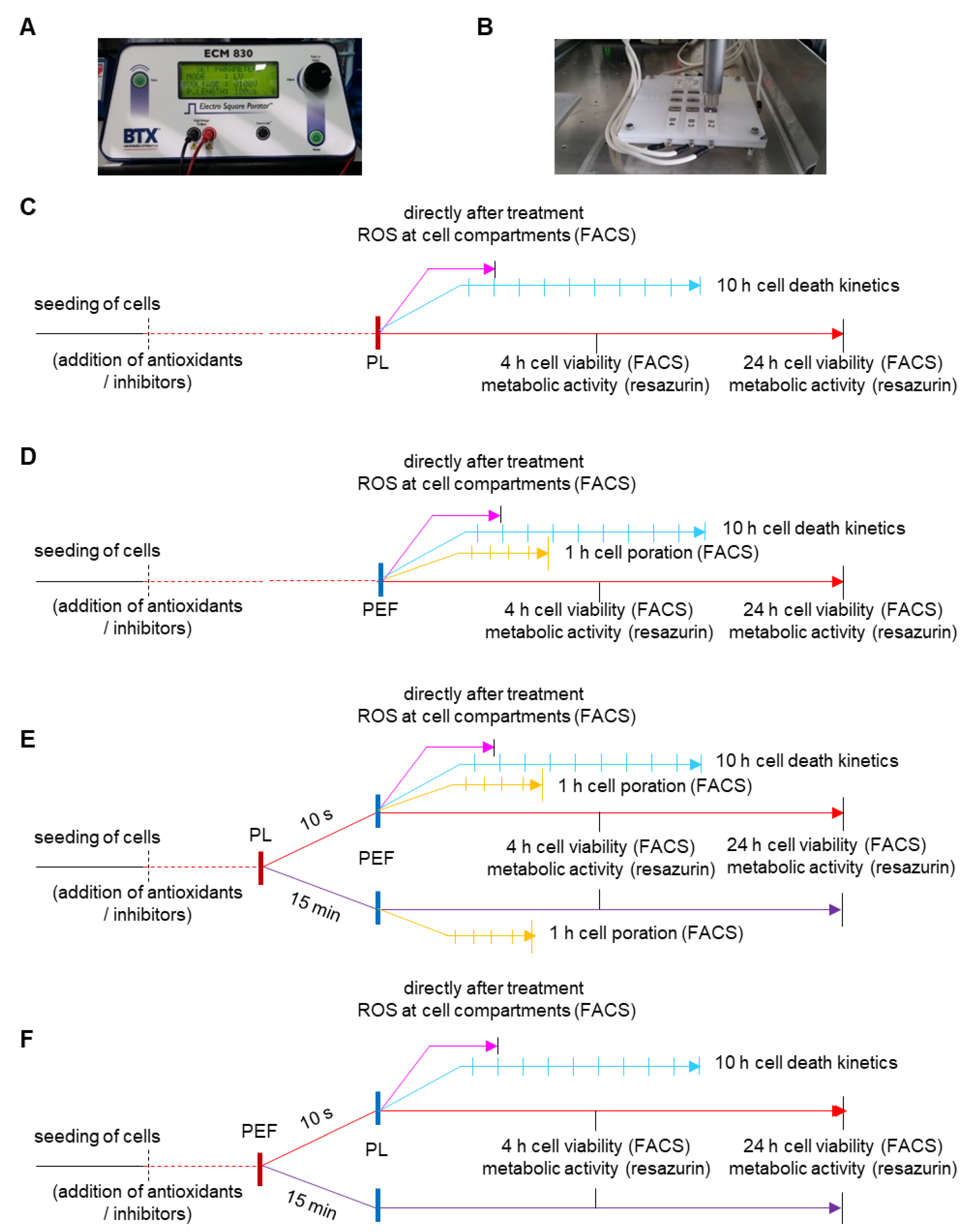
4. Material and Methods
4.1. Cell Culture
4.2. Exposure to Cold Physical Plasma and Pulsed Electric Fields
4.3. Analysis of Electropermeabilization
4.4. Metabolic Activity and Cell Viability
4.5. Cell Death Kinetics Using Live-Cell Microscopy
4.6. Oxidation of Cell Membrane and Mitochondria
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Weltmann, K.D.; von Woedtke, T. Plasma medicine-current state of research and medical application. Plasma Phys. Control. Fusion 2017, 59, 014031. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.S. Targeting cancer cells with reactive oxygen and nitrogen species generated by atmospheric-pressure air plasma. PLoS ONE 2014, 9, e86173. [Google Scholar] [CrossRef]
- Jablonowski, H.; von Woedtke, T. Research on plasma medicine-relevant plasma–liquid interaction: What happened in the past five years? Clin. Plasma Med. 2015, 3, 42–52. [Google Scholar] [CrossRef]
- Bekeschus, S.; Wende, K.; Hefny, M.M.; Rodder, K.; Jablonowski, H.; Schmidt, A.; Woedtke, T.V.; Weltmann, K.D.; Benedikt, J. Oxygen atoms are critical in rendering thp-1 leukaemia cells susceptible to cold physical plasma-induced apoptosis. Sci. Rep. 2017, 7, 2791. [Google Scholar] [CrossRef] [PubMed]
- Suda, Y.; Tero, R.; Yamashita, R.; Yusa, K.; Takikawa, H. Reduction in lateral lipid mobility of lipid bilayer membrane by atmospheric pressure plasma irradiation. Jpn. J. Appl. Phys. 2016, 55, 03df05. [Google Scholar] [CrossRef]
- Furuta, R.; Kurake, N.; Ishikawa, K.; Takeda, K.; Hashizume, H.; Tanaka, H.; Kondo, H.; Sekine, M.; Hori, M. Intracellular responses to reactive oxygen and nitrogen species, and lipid peroxidation in apoptotic cells cultivated in plasma-activated medium. Plasma Process. Polym. 2017, 14, 1700123. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.D.; Wende, K.; Bogaerts, A.; Bekeschus, S. Ros from physical plasmas: Redox chemistry for biomedical therapy. Oxidative Med. Cell. Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef]
- Schmidt, A.; Rodder, K.; Hasse, S.; Masur, K.; Toups, L.; Lillig, C.H.; von Woedtke, T.; Wende, K.; Bekeschus, S. Redox-regulation of activator protein 1 family members in blood cancer cell lines exposed to cold physical plasma-treated medium. Plasma Process. Polym. 2016, 13, 1179–1188. [Google Scholar] [CrossRef]
- Ishaq, M.; Kumar, S.; Varinli, H.; Han, Z.J.; Rider, A.E.; Evans, M.D.; Murphy, A.B.; Ostrikov, K. Atmospheric gas plasma-induced ros production activates tnf-ask1 pathway for the induction of melanoma cancer cell apoptosis. Mol. Biol. Cell 2014, 25, 1523–1531. [Google Scholar] [CrossRef]
- Bekeschus, S.; Freund, E.; Wende, K.; Gandhirajan, R.K.; Schmidt, A. Hmox1 upregulation is a mutual marker in human tumor cells exposed to physical plasma-derived oxidants. Antioxidants 2018, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Stolz, W.; Shimizu, T.; Monetti, R.; Bunk, W.; Schmidt, H.U.; Morfill, G.E.; Klämpfl, T.G.; Steffes, B.; Thomas, H.M.; et al. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo. Clin. Plasma Med. 2013, 1, 25–30. [Google Scholar] [CrossRef]
- Ulrich, C.; Kluschke, F.; Patzelt, A.; Vandersee, S.; Czaika, V.A.; Richter, H.; Bob, A.; Hutten, J.; Painsi, C.; Huge, R.; et al. Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: A pilot study. J. Wound Care 2015, 24, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; von Woedtke, T.; Vollmar, B.; Hasse, S.; Bekeschus, S. Nrf2 signaling and inflammation are key events in physical plasma-spurred wound healing. Theranostics 2019, 9, 1066–1084. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Eisenmann, S.; Sagwal, S.K.; Bodnar, Y.; Moritz, J.; Poschkamp, B.; Stoffels, I.; Emmert, S.; Madesh, M.; Weltmann, K.-D.; et al. Xct (slc7a11) expression confers intrinsic resistance to physical plasma treatment in tumor cells. Redox Biol. 2020, 30. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, H.; Utsumi, F.; Nakamura, K.; Tanaka, H.; Toyokuni, S.; Hori, M.; Kikkawa, F. Future perspective of strategic non-thermal plasma therapy for cancer treatment. J. Clin. Biochem. Nutr. 2017, 60, 33–38. [Google Scholar] [CrossRef]
- Semmler, M.L.; Bekeschus, S.; Schäfer, M.; Bernhardt, T.; Fischer, T.; Witzke, K.; Seebauer, C.; Rebl, H.; Grambow, E.; Vollmar, B.; et al. Molecular mechanisms of the efficacy of cold atmospheric pressure plasma (cap) in cancer treatment. Cancers 2020, 12, 269. [Google Scholar] [CrossRef]
- Volotskova, O.; Hawley, T.S.; Stepp, M.A.; Keidar, M. Targeting the cancer cell cycle by cold atmospheric plasma. Sci. Rep. 2012, 2, 636. [Google Scholar] [CrossRef]
- Van Loenhout, J.; Flieswasser, T.; Freire Boullosa, L.; De Waele, J.; Van Audenaerde, J.; Marcq, E.; Jacobs, J.; Lin, A.; Lion, E.; Dewitte, H.; et al. Cold atmospheric plasma-treated pbs eliminates immunosuppressive pancreatic stellate cells and induces immunogenic cell death of pancreatic cancer cells. Cancers 2019, 11, 1597. [Google Scholar] [CrossRef]
- Gandhirajan, R.K.; Rodder, K.; Bodnar, Y.; Pasqual-Melo, G.; Emmert, S.; Griguer, C.E.; Weltmann, K.D.; Bekeschus, S. Cytochrome c oxidase inhibition and cold plasma-derived oxidants synergize in melanoma cell death induction. Sci. Rep. 2018, 8, 12734. [Google Scholar] [CrossRef]
- Adachi, T.; Tanaka, H.; Nonomura, S.; Hara, H.; Kondo, S.; Hori, M. Plasma-activated medium induces a549 cell injury via a spiral apoptotic cascade involving the mitochondrial-nuclear network. Free Radic. Biol. Med. 2015, 79, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Turrini, E.; Laurita, R.; Stancampiano, A.; Catanzaro, E.; Calcabrini, C.; Maffei, F.; Gherardi, M.; Colombo, V.; Fimognari, C. Cold atmospheric plasma induces apoptosis and oxidative stress pathway regulation in t-lymphoblastoid leukemia cells. Oxidative Med. Cell. Longev. 2017, 2017, 4271065. [Google Scholar] [CrossRef] [PubMed]
- Bundscherer, L.; Wende, K.; Ottmuller, K.; Barton, A.; Schmidt, A.; Bekeschus, S.; Hasse, S.; Weltmann, K.D.; Masur, K.; Lindequist, U. Impact of non-thermal plasma treatment on mapk signaling pathways of human immune cell lines. Immunobiology 2013, 218, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.G.; Garbay, J.R.; Billard, V.; Geertsen, P.F.; Rudolf, Z.; O’Sullivan, G.C.; Marty, M. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the cliniporator (tm) by means of invasive or non-invasive electrodes. EJC Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Wolff, C.M.; Steuer, A.; Stoffels, I.; von Woedtke, T.; Weltmann, K.-D.; Bekeschus, S.; Kolb, J.F. Combination of cold plasma and pulsed electric fields—A rationale for cancer patients in palliative care. Clin. Plasma Med. 2019, 16. [Google Scholar] [CrossRef]
- Hu, Q.; Joshi, R.P.; Schoenbach, K.H. Simulations of nanopore formation and phosphatidylserine externalization in lipid membranes subjected to a high-intensity, ultrashort electric pulse. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2005, 72, 031902. [Google Scholar] [CrossRef] [PubMed]
- Breton, M.; Mir, L.M. Investigation of the chemical mechanisms involved in the electropulsation of membranes at the molecular level. Bioelectrochemistry 2018, 119, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Sersa, G.; Stabuc, B.; Cemazar, M.; Miklavcic, D.; Rudolf, Z. Electrochemotherapy with cisplatin: Clinical experience in malignant melanoma patients. Clin. Cancer Res. 2000, 6, 863–867. [Google Scholar]
- Hanna, H.; Denzi, A.; Liberti, M.; Andre, F.M.; Mir, L.M. Electropermeabilization of inner and outer cell membranes with microsecond pulsed electric fields: Quantitative study with calcium ions. Sci. Rep. 2017, 7, 13079. [Google Scholar] [CrossRef]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [CrossRef]
- Zafarullah, M.; Li, W.Q.; Sylvester, J.; Ahmad, M. Molecular mechanisms of n-acetylcysteine actions. Cell. Mol. Life Sci. 2003, 60, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Vernier, P.T.; Levine, Z.A.; Wu, Y.H.; Joubert, V.; Ziegler, M.J.; Mir, L.M.; Tieleman, D.P. Electroporating fields target oxidatively damaged areas in the cell membrane. PLoS ONE 2009, 4, e7966. [Google Scholar] [CrossRef] [PubMed]
- Yusupov, M.; Wende, K.; Kupsch, S.; Neyts, E.C.; Reuter, S.; Bogaerts, A. Effect of head group and lipid tail oxidation in the cell membrane revealed through integrated simulations and experiments. Sci. Rep. 2017, 7, 5761. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Uddin, N.; Sim, G.B.; Hong, Y.J.; Baik, K.Y.; Kim, C.H.; Lee, S.J.; Kaushik, N.K.; Choi, E.H. Responses of solid tumor cells in dmem to reactive oxygen species generated by non-thermal plasma and chemically induced ros systems. Sci. Rep. 2015, 5, 8587. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yao, C.; Sun, C.; Guo, F.; Zhou, W.; Xiong, Z. Dependence on electric field intensities of cell biological effects induced by microsecond pulsed electric fields. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 2083–2088. [Google Scholar] [CrossRef]
- Steuer, A.; Wolff, C.M.; von Woedtke, T.; Weltmann, K.D.; Kolb, J.F. Cell stimulation versus cell death induced by sequential treatments with pulsed electric fields and cold atmospheric pressure plasma. PLoS ONE 2018, 13, e0204916. [Google Scholar] [CrossRef]
- Griseti, E.; Kolosnjaj-Tabi, J.; Gibot, L.; Fourquaux, I.; Rols, M.P.; Yousfi, M.; Merbahi, N.; Golzio, M. Pulsed electric field treatment enhances the cytotoxicity of plasma-activated liquids in a three-dimensional human colorectal cancer cell model. Sci. Rep. 2019, 9, 7583. [Google Scholar] [CrossRef]
- Chung, T.H.; Stancampiano, A.; Sklias, K.; Gazeli, K.; Andre, F.M.; Dozias, S.; Douat, C.; Pouvesle, J.M.; Santos Sousa, J.; Robert, E.; et al. Cell electropermeabilisation enhancement by non-thermal-plasma-treated pbs. Cancers 2020, 12, 219. [Google Scholar] [CrossRef]
- Estlack, L.E.; Roth, C.C.; Thompson, G.L., 3rd; Lambert, W.A., 3rd; Ibey, B.L. Nanosecond pulsed electric fields modulate the expression of fas/cd95 death receptor pathway regulators in u937 and jurkat cells. Apoptosis Int. J. Program. Cell Death 2014, 19, 1755–1768. [Google Scholar] [CrossRef]
- Ibey, B.L.; Pakhomov, A.G.; Gregory, B.W.; Khorokhorina, V.A.; Roth, C.C.; Rassokhin, M.A.; Bernhard, J.A.; Wilmink, G.J.; Pakhomova, O.N. Selective cytotoxicity of intense nanosecond-duration electric pulses in mammalian cells. Biochim. Biophys. Acta 2010, 1800, 1210–1219. [Google Scholar] [CrossRef]
- Pakhomova, O.N.; Khorokhorina, V.A.; Bowman, A.M.; Rodaite-Riseviciene, R.; Saulis, G.; Xiao, S.; Pakhomov, A.G. Oxidative effects of nanosecond pulsed electric field exposure in cells and cell-free media. Arch. Biochem. Biophys. 2012, 527, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Kolata, J.; Winterbourn, C.; Kramer, A.; Turner, R.; Weltmann, K.D.; Broker, B.; Masur, K. Hydrogen peroxide: A central player in physical plasma-induced oxidative stress in human blood cells. Free Radic. Res. 2014, 48, 542–549. [Google Scholar] [CrossRef]
- Bauer, G. The synergistic effect between hydrogen peroxide and nitrite, two long-lived molecular species from cold atmospheric plasma, triggers tumor cells to induce their own cell death. Redox Biol. 2019, 26, 101291. [Google Scholar] [CrossRef] [PubMed]
- Fleury, C.; Mignotte, B.; Vayssiere, J.L. Mitochondrial reactive oxygen species in cell death signaling. Biochimie 2002, 84, 131–141. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Shi, L.; Toyokuni, S. Non-thermal plasma as a simple ferroptosis inducer in cancer cells: A possible role of ferritin. Pathol. Int. 2018, 68, 442–443. [Google Scholar] [CrossRef]
- Basit, F.; van Oppen, L.M.; Schockel, L.; Bossenbroek, H.M.; van Emst-de Vries, S.E.; Hermeling, J.C.; Grefte, S.; Kopitz, C.; Heroult, M.; Hgm Willems, P.; et al. Mitochondrial complex i inhibition triggers a mitophagy-dependent ros increase leading to necroptosis and ferroptosis in melanoma cells. Cell Death Dis. 2017, 8, e2716. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, D.H.; Lee, Y.S.; Jo, M.J.; Jeong, Y.A.; Kwon, W.T.; Choudry, H.A.; Bartlett, D.L.; Lee, Y.J. Molecular crosstalk between ferroptosis and apoptosis: Emerging role of er stress-induced p53-independent puma expression. Oncotarget 2017, 8, 115164–115178. [Google Scholar] [CrossRef]
- Zilka, O.; Shah, R.; Li, B.; Friedmann Angeli, J.P.; Griesser, M.; Conrad, M.; Pratt, D.A. On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent. Sci. 2017, 3, 232–243. [Google Scholar] [CrossRef]
- Choudhary, S.; Zhang, W.; Zhou, F.; Campbell, G.A.; Chan, L.L.; Thompson, E.B.; Ansari, N.H. Cellular lipid peroxidation end-products induce apoptosis in human lens epithelial cells. Free Radic. Biol. Med. 2002, 32, 360–369. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.D. The kinpen-a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51. [Google Scholar] [CrossRef]
- Wende, K.; Reuter, S.; von Woedtke, T.; Weltmann, K.D.; Masur, K. Redox-based assay for assessment of biological impact of plasma treatment. Plasma Process. Polym. 2014, 11, 655–663. [Google Scholar] [CrossRef]



| Abbreviation | Combination Treatment |
|---|---|
| comb 1 | combination of first plasma and second PEF waiting for 10 s in between |
| comb 2 | combination of first plasma and second PEF waiting 15 min in between |
| comb 3 | combination of first PEF and second plasma waiting for 10 s in between |
| comb 4 | combination of first PEF and second plasma waiting 15 min in between |
| comb 5 | pretreatment with catalase before combination of first plasma and second PEF waiting for 10 s in between |
| comb 6 | pretreatment with catalase before combination of first PEF and second plasma waiting for 10 s in between |
| Treatment Regimen | 4 h | 24 h | ||||||
|---|---|---|---|---|---|---|---|---|
| Significance | p-Value | Difference | S.E. | Significance | p-Value | Difference | S.E. | |
| ctr / PL | n.s. | 0.400 | 1.82 | 2.14 | *** | <0.001 | 23.01 | 3.53 |
| ctr / PEF | *** | <0.001 | 15.66 | 1.17 | *** | <0.001 | 26.29 | 1.90 |
| ctr / comb 1 | *** | <0.001 | 31.03 | 1.67 | *** | <0.001 | 66.23 | 1.55 |
| ctr / comb 3 | *** | <0.001 | 27.88 | 2.59 | *** | <0.001 | 61.37 | 1.10 |
| ctr / comb 2 | *** | <0.001 | 18.66 | 1.14 | *** | <0.001 | 49.45 | 1.19 |
| ctr / comb 4 | *** | <0.001 | 24.55 | 1.51 | *** | <0.001 | 53.75 | 1.36 |
| PL / PEF | *** | <0.001 | 13.84 | 2.35 | n.s. | 0.420 | 3.29 | 3.99 |
| PL / comb 1 | *** | <0.001 | 29.21 | 2.64 | *** | <0.001 | 43.22 | 3.84 |
| PL / comb 3 | *** | <0.001 | 26.05 | 3.30 | *** | <0.001 | 38.36 | 3.68 |
| PL / comb 2 | *** | <0.001 | 16.83 | 2.63 | *** | <0.001 | 26.44 | 4.25 |
| PL / comb 4 | *** | <0.001 | 22.72 | 2.81 | *** | <0.001 | 30.74 | 4.30 |
| PEF / comb 1 | *** | <0.001 | 15.37 | 1.93 | *** | <0.001 | 39.94 | 2.42 |
| PEF / comb 3 | *** | <0.001 | 12.21 | 2.77 | *** | <0.001 | 35.07 | 2.16 |
| PEF / comb 2 | n.s. | 0.080 | 3.00 | 1.61 | *** | <0.001 | 23.16 | 2.46 |
| PEF / comb 4 | *** | <0.001 | 8.89 | 1.89 | *** | <0.001 | 27.45 | 2.55 |
| comb 1 / comb 3 | n.s. | 0.310 | −3.157 | 3.02 | * | 0.020 | −4.87 | 1.86 |
| comb 1 / comb 2 | *** | <0.001 | −12.38 | 2.12 | *** | <0.001 | −16.78 | 2.11 |
| comb 1 / comb 4 | * | 0.010 | −6.49 | 2.34 | *** | <0.001 | −12.48 | 2.21 |
| comb 3 / comb 2 | ** | 0.008 | −9.22 | 3.13 | *** | <0.001 | −11.91 | 1.68 |
| comb 3 / comb 4 | n.s. | 0.320 | −3.33 | 3.29 | *** | <0.001 | −7.62 | 1.81 |
| comb 2 / comb 4 | * | 0.010 | 5.89 | 2.02 | n.s. | 0.050 | 4.30 | 2.04 |
| Treatment Regimen | 4 h | 24 h | ||||||
|---|---|---|---|---|---|---|---|---|
| Significance | p-Value | Difference | S.E. | Significance | p-Value | Difference | S.E. | |
| ctr / PL | n.s. | 0.190 | 4.72 | 3.52 | *** | <0.001 | 18.12 | 2.01 |
| ctr / PEF | *** | <0.001 | 3.80 | 0.89 | *** | <0.001 | 30.75 | 2.63 |
| ctr / comb 1 | *** | <0.001 | 22.15 | 1.42 | *** | <0.001 | 57.09 | 2.08 |
| ctr / comb 3 | *** | <0.001 | 17.50 | 1.89 | *** | <0.001 | 54.99 | 1.00 |
| ctr / comb 2 | *** | <0.001 | 11.46 | 1.49 | *** | <0.001 | 42.87 | 1.23 |
| ctr / comb 4 | *** | <0.001 | 10.54 | 1.22 | *** | <0.001 | 45.62 | 1.12 |
| PL / PEF | n.s. | 0.790 | −0.92 | 3.42 | *** | <0.001 | 12.63 | 3.28 |
| PL / comb 1 | *** | <0.001 | 17.43 | 3.58 | *** | <0.001 | 38.96 | 2.82 |
| PL / comb 3 | ** | 0.003 | 12.78 | 3.77 | *** | <0.001 | 36.87 | 2.07 |
| PL / comb 2 | n.s. | 0.120 | 6.74 | 4.10 | *** | <0.001 | 24.75 | 2.44 |
| PL / comb 4 | n.s. | 0.160 | 5.82 | 4.02 | *** | <0.001 | 27.50 | 2.38 |
| PEF / comb 1 | *** | <0.001 | 18.35 | 1.48 | *** | <0.001 | 26.33 | 3.23 |
| PEF / comb 3 | *** | <0.001 | 13.70 | 1.90 | *** | <0.001 | 24.24 | 2.67 |
| PEF / comb 2 | *** | <0.001 | 7.66 | 1.58 | ** | 0.001 | 12.12 | 3.13 |
| PEF / comb 4 | *** | <0.001 | 6.45 | 1.36 | *** | <0.001 | 14.87 | 3.08 |
| comb 1 / comb 3 | * | 0.040 | −4.65 | 2.18 | n.s. | 0.340 | −2.10 | 2.14 |
| comb 1 / comb 2 | *** | <0.001 | −10.69 | 2.00 | *** | <0.001 | −14.21 | 2.51 |
| comb 1 / comb 4 | *** | <0.001 | −11.61 | 1.83 | *** | <0.001 | −11.46 | 2.46 |
| comb 3 / comb 2 | * | 0.020 | −6.04 | 2.44 | *** | <0.001 | −12.12 | 1.34 |
| comb 3 / comb 4 | ** | 0.007 | −6.96 | 2.30 | *** | <0.001 | −9.37 | 1.24 |
| comb 2 / comb 4 | n.s. | 0.650 | −0.92 | 1.98 | n.s. | 0.090 | 2.75 | 1.52 |
| Treatment Regimen | Immediately after Treatment | 1 h after Treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| Significance | p-Value | Difference | S.E. | Significance | p-Value | Difference | S.E. | |
| ctr / PL | *** | <0.001 | −14.52 | 2.22 | *** | <0.001 | −7.86 | 0.93 |
| ctr / PEF | *** | <0.001 | −17.32 | 2.46 | n.s. | 0.300 | −1.12 | 1.05 |
| ctr / comb 1 | *** | <0.001 | −32.59 | 3.56 | *** | <0.001 | −16.30 | 1.49 |
| ctr / comb 1 + cat | *** | <0.001 | −7.58 | 1.21 | *** | <0.001 | −5.62 | 1.03 |
| ctr / comb 3 | *** | <0.001 | −36.21 | 3.76 | *** | <0.001 | −15.95 | 1.24 |
| ctr / comb 3 + cat | *** | <0.001 | −10.65 | 1.29 | *** | <0.001 | −7.01 | 1.25 |
| PL / PEF | n.s. | 0.400 | −2.80 | 3.26 | *** | <0.001 | 6.74 | 1.26 |
| PL / comb 1 | *** | <0.001 | −18.07 | 4.15 | *** | <0.001 | −8.44 | 1.63 |
| PL / comb 1 + cat | * | 0.030 | 6.94 | 3.04 | n.s. | 0.110 | 2.24 | 1.35 |
| PL / comb 3 | *** | <0.001 | −21.69 | 4.32 | *** | <0.001 | −8.09 | 1.41 |
| PL / comb 3 + cat | n.s. | 0.220 | 3.87 | 3.07 | n.s. | 0.580 | 0.85 | 1.52 |
| PEF / comb 1 | ** | 0.001 | −15.28 | 4.28 | *** | <0.001 | −15.18 | 1.74 |
| PEF / comb 1 + cat | ** | 0.008 | 9.74 | 3.33 | **s | 0.007 | −4.50 | 1.50 |
| PEF / comb 3 | *** | <0.001 | −18.90 | 4.45 | *** | <0.001 | −14.83 | 1.52 |
| PEF / comb 3 + cat | n.s. | 0.060 | 6.67 | 3.37 | ** | 0.002 | −5.89 | 1.66 |
| comb 1 / comb 1 + cat | *** | <0.001 | 25.01 | 4.73 | *** | <0.001 | 10.68 | 2.03 |
| comb 1 / comb 3 | n.s. | 0.490 | −3.62 | 5.14 | n.s. | 0.850 | 0.349 | 1.83 |
| comb 1 / comb 3 + cat | *** | <0.001 | 4.62 | 4.62 | *** | <0.001 | 9.29 | 2.14 |
| comb 1 + cat / comb 3 | *** | <0.001 | −28.63 | 4.98 | *** | <0.001 | −10.33 | 1.72 |
| comb 1 + cat / comb 3 + cat | n.s. | 0.160 | −3.07 | 2.09 | n.s. | 0.450 | −1.39 | 1.81 |
| comb 3 / comb 3 + cat | *** | <0.001 | 25.56 | 5.00 | *** | <0.001 | 8.94 | 1.86 |
| Treatment Regimen | Cell Viability | Metabolic Activity | ||||||
|---|---|---|---|---|---|---|---|---|
| Significance | p-Value | Difference | S.E. | Significance | p-Value | Difference | S.E. | |
| ctr / PL | *** | <0.001 | 20.50 | 3.09 | *** | <0.001 | 35.54 | 3.04 |
| ctr / PEF | *** | <0.001 | 20.15 | 1.60 | *** | <0.001 | 42.24 | 0.34 |
| ctr / comb 1 | *** | <0.001 | 31.77 | 1.08 | *** | <0.001 | 55.98 | 0.90 |
| ctr / comb 3 | *** | <0.001 | 28.14 | 0.61 | *** | <0.001 | 53.31 | 0.63 |
| PL / PEF | n.s. | 0.920 | 0.35 | 3.47 | n.s. | 0.050 | −6.69 | 3.03 |
| PL / comb 1 | ** | 0.006 | −11.27 | 3.27 | *** | <0.001 | −20.43 | 3.14 |
| PL / comb 3 | n.s. | 0.050 | −7.64 | 3.46 | *** | <0.001 | −17.76 | 3.08 |
| PEF / comb 1 | *** | <0.001 | 11.62 | 1.92 | *** | <0.001 | 13.74 | 0.88 |
| PEF / comb 3 | ** | 0.002 | 7.99 | 1.86 | *** | <0.001 | 11.07 | 0.59 |
| comb 1 / comb 3 | * | 0.020 | −3.63 | 1.33 | * | 0.020 | −2.67 | 1.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolff, C.M.; Kolb, J.F.; Weltmann, K.-D.; von Woedtke, T.; Bekeschus, S. Combination Treatment with Cold Physical Plasma and Pulsed Electric Fields Augments ROS Production and Cytotoxicity in Lymphoma. Cancers 2020, 12, 845. https://doi.org/10.3390/cancers12040845
Wolff CM, Kolb JF, Weltmann K-D, von Woedtke T, Bekeschus S. Combination Treatment with Cold Physical Plasma and Pulsed Electric Fields Augments ROS Production and Cytotoxicity in Lymphoma. Cancers. 2020; 12(4):845. https://doi.org/10.3390/cancers12040845
Chicago/Turabian StyleWolff, Christina M., Juergen F. Kolb, Klaus-Dieter Weltmann, Thomas von Woedtke, and Sander Bekeschus. 2020. "Combination Treatment with Cold Physical Plasma and Pulsed Electric Fields Augments ROS Production and Cytotoxicity in Lymphoma" Cancers 12, no. 4: 845. https://doi.org/10.3390/cancers12040845
APA StyleWolff, C. M., Kolb, J. F., Weltmann, K.-D., von Woedtke, T., & Bekeschus, S. (2020). Combination Treatment with Cold Physical Plasma and Pulsed Electric Fields Augments ROS Production and Cytotoxicity in Lymphoma. Cancers, 12(4), 845. https://doi.org/10.3390/cancers12040845







