Safety of Aflibercept in Metastatic Colorectal Cancer: A Literature Review and Expert Perspective on Clinical and Real-World Data
Abstract
:1. Introduction
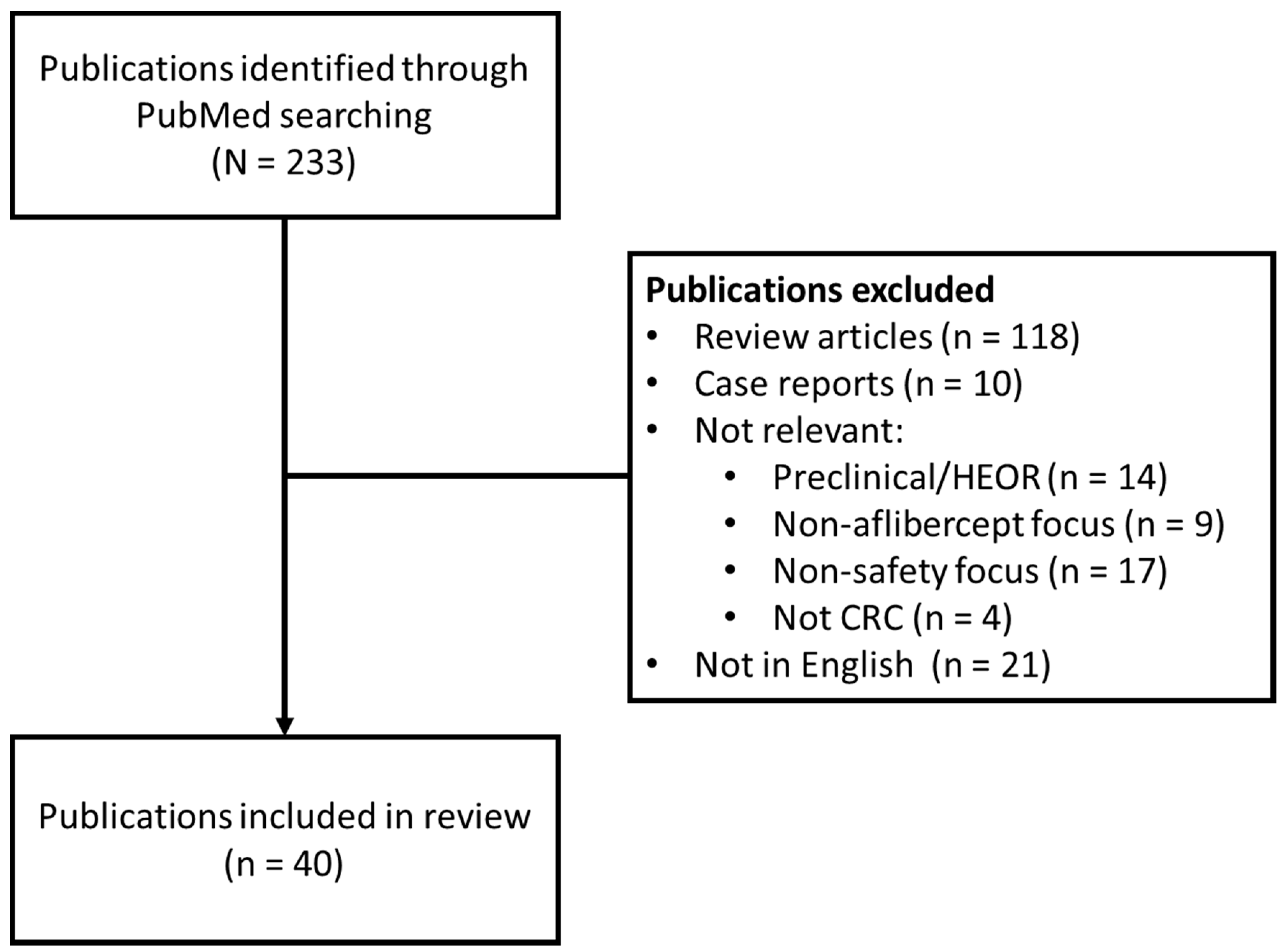
2. Methods
3. Results
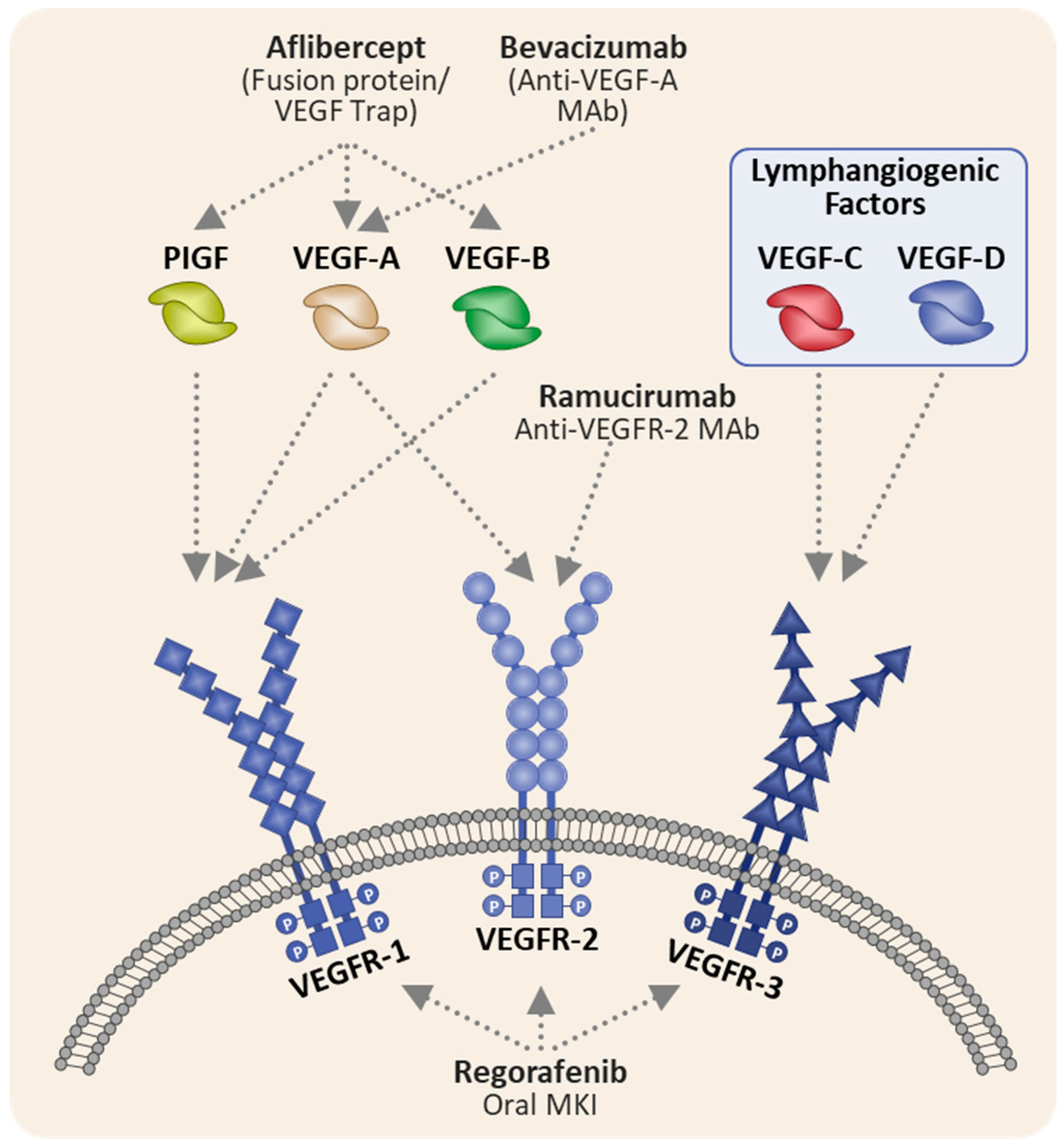
Aflibercept Mechanism of Action and Relevance To Safety Profile
4. Safety Profile of Aflibercept-Containing Therapy in mCRC
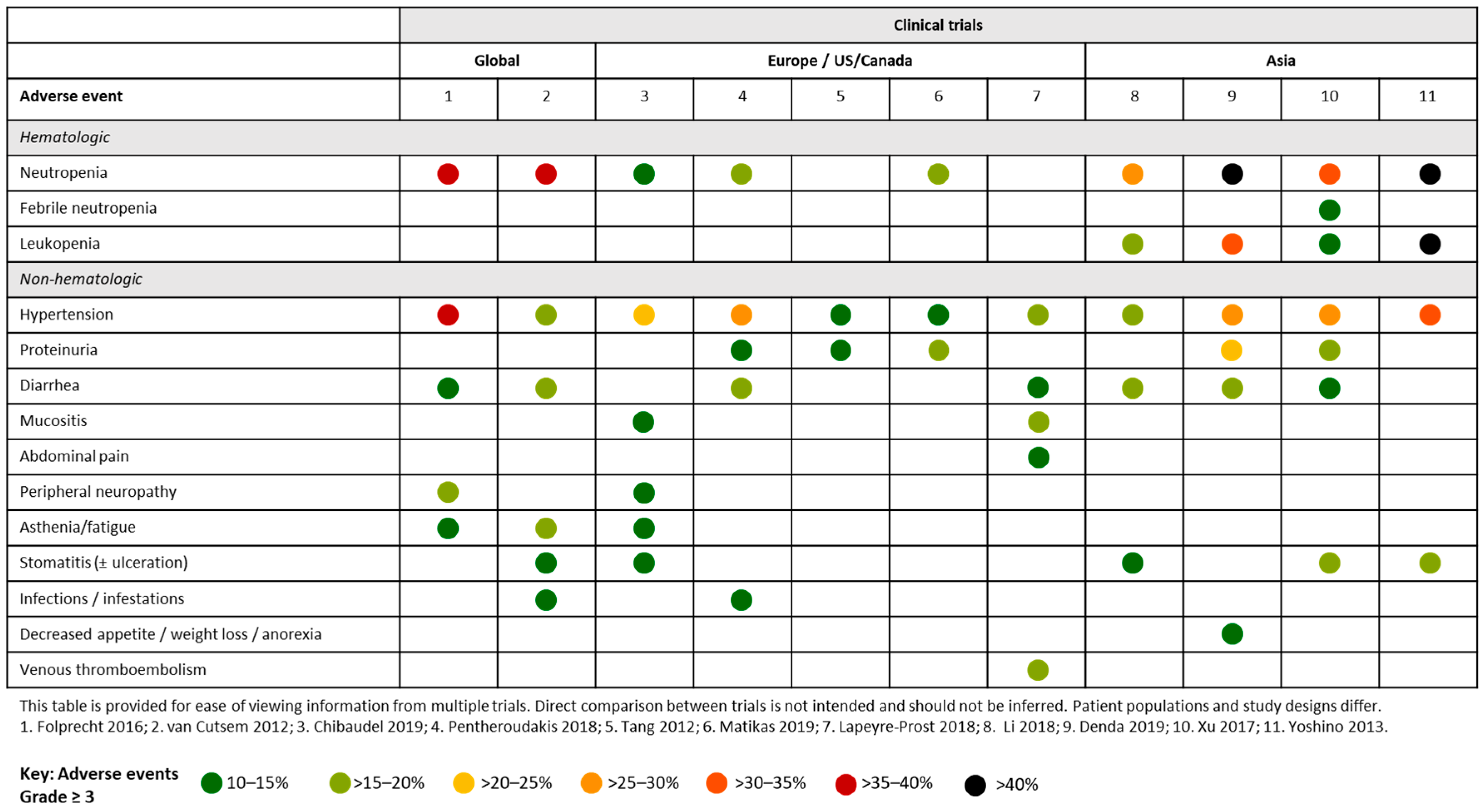
4.1. Aflibercept and FOLFOX/FOLFIRI in Clinical Trials
4.1.1. Global Studies
4.1.2. European and US Studies
4.1.3. Asian Studies
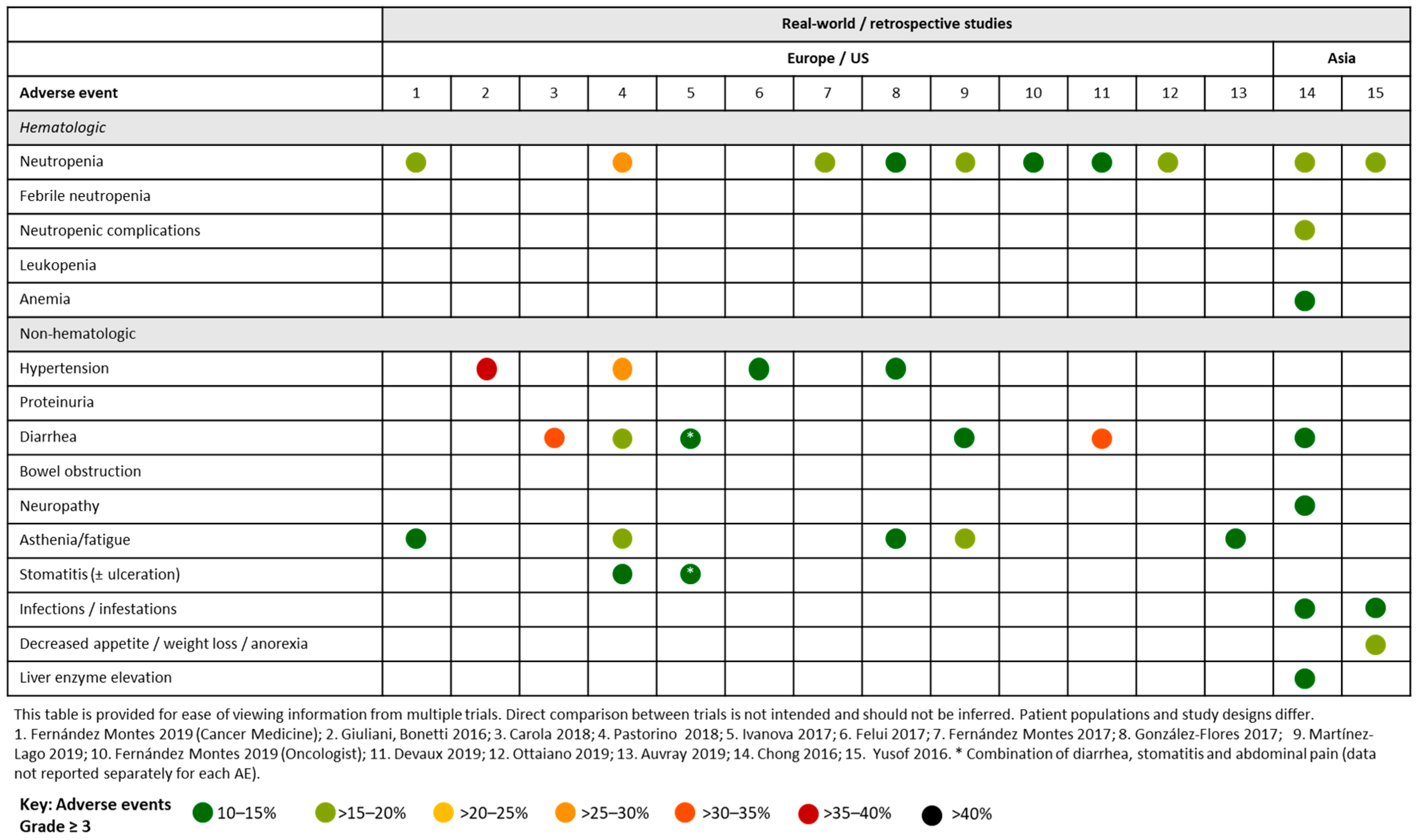
4.2. Aflibercept and FOLFOX/FOLFIRI in Real-World and Retrospective Studies
4.2.1. European and the US Studies
4.2.2. Asian studies
5. Management of aflibercept-Associated Adverse Events in mCRC
Management of hypertension and proteinuria
6. Management of Aflibercept-Related Adverse Events in My mCRC Patients—A European Perspective
7. Management of Aflibercept-Related Adverse Events in My mCRC Patients—An Asian Perspective
8. Discussion
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Pourhoseingholi, M.A. Increased burden of colorectal cancer in Asia. World J. Gastrointest. Oncol. 2012, 4, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Muro, K.; Ajioka, Y.; Hashiguchi, Y.; Ito, Y.; Saito, Y.; Hamaguchi, T.; Ishida, H.; Ishiguro, M.; Ishihara, S. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2018, 23, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, J.J.Y.; Chiu, H.M.; Jung, K.W.; Jun, J.K.; Sekiguchi, M.; Matsuda, T.; Kyaw, M.H. Increasing trend in young-onset colorectal cancer in Asia: More cancers in men and more rectal cancers. Am. J. Gastroenterol. 2019, 114, 322–329. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Joulain, F.; Hoff, P.M.; Mitchell, E.; Ruff, P.; Lakomý, R.; Prausová, J.; Moiseyenko, V.M.; van Hazel, G.; Cunningham, D.; et al. Aflibercept plus FOLFIRI vs. PLACEBO PLUS FOLFIRI in second-line metastatic colorectal cancer: A post hoc analysis of survival from the phase III VELOUR study subsequent to exclusion of patients who had recurrence during or within 6 months of completing adjuvant oxaliplatin-based therapy. Target. Oncol. 2016, 11, 383–400. [Google Scholar]
- Clarke, J.M.; Hurwitz, H.I.; Rangwala, F. Understanding the mechanisms of action of antiangiogenic agents in metastatic colorectal cancer: A clinician’s perspective. Cancer Treat. Rev. 2014, 40, 1065–1072. [Google Scholar] [CrossRef]
- Hayman, S.R.; Leung, N.; Grande, J.P.; Garovic, V.D. VEGF inhibition, hypertension, and renal toxicity. Curr. Oncol. Rep. 2012, 14, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Rajabi, M.; Mousa, S.A. The role of angiogenesis in cancer treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Van Cutsem, E.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausová, J.; Macarulla, T.; Ruff, P.; van Hazel, G.A.; Moiseyenko, V.; Ferry, D.; et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 2012, 30, 3499–3506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruff, P.; Van Cutsem, E.; Lakomy, R.; Prausova, J.; van Hazel, G.A.; Moiseyenko, V.M.; Soussan-Lazard, K.; Dochy, E.; Magherini, E.; Macarulla, T.; et al. Observed benefit and safety of aflibercept in elderly patients with metastatic colorectal cancer: An age-based analysis from the randomized placebo-controlled phase III VELOUR trial. J. Geriatr. Oncol. 2018, 9, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Chau., I.; Joulain., F.; Iqbal, S.U.; Bridgewater, J. A VELOUR post hoc subset analysis: Prognostic groups and treatment outcomes in patients with metastatic colorectal cancer treated with aflibercept and FOLFIRI. BMC Cancer 2014, 14, 605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruff, P.; Ferry, D.R.; Lakomỳ, R.; Prausová, J.; Van Hazel, G.A.; Hoff, P.M.; Cunningham, D.; Arnold, D.; Schmoll, H.J.; Moiseyenko, V.M.; et al. Time course of safety and efficacy of aflibercept in combination with FOLFIRI in patients with metastatic colorectal cancer who progressed on previous oxaliplatin-based therapy. Eur. J. Cancer 2015, 51, 18–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riechelmann, R.P.; Srimuninnimit, V.; Bordonaro, R.; Kavan, P.; Di Bartolomeo, M.; Maiello, E.; Cicin, I.; García-Alfonso, P.; Chau, I.; Fedyanin, M.Y.; et al. Aflibercept plus FOLFIRI for second-line treatment of metastatic colorectal cancer: Observations from the global Aflibercept Safety and Health-Related Quality-of-Life Program (ASQoP). Clin. Colorectal Cancer 2019, 18, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Riechelmann, R.; Srimuninnimit, V.; Kavan, P.; Di Bartolomeo, M.; Maiello, E.; Cicin, I.; Kröning, H.; Garcia-Alfonso, P.; Chau, I.; Fernández-Martos, C.; et al. Aflibercept plus FOLFIRI for 2nd line treatment of metastatic colorectal cancer (mCRC): Long-term safety observation from the glo;al aflibercept safety and quality-of-life (QoL) program (ASQoP). Ann. Oncol. 2016, 27, 552. [Google Scholar] [CrossRef]
- Folprecht, G.; Pericay, C.; Saunders, M.P.; Thomas, A.; Lopez Lopez, R.; Roh, J.K.; Chistyakov, V.; Höhler, T.; Kim, J.S.; Hofheinz, R.D.; et al. Oxaliplatin and 5-FU/folinic acid (modified FOLFOX6) with or without aflibercept in first-line treatment of patients with metastatic colorectal cancer: The AFFIRM study. Ann. Oncol. 2016, 27, 1273–1279. [Google Scholar] [CrossRef]
- Lambrechts, D.; Thienpont, B.; Thuillier, V.; Sagaert, X.; Moisse, M.; Peuteman, G.; Pericay, C.; Folprecht, G.; Zalcberg, J.; Zilocchi, C.; et al. Evaluation of efficacy and safety markers in a phase II study of metastatic colorectal cancer treated with aflibercept in the first-line setting. Br. J. Cancer 2015, 113, 1027–1034. [Google Scholar] [CrossRef]
- Chibaudel, B.; Bachet, J.B.; Andre, T.; Auby, D.; Desrame, J.; Deplanque, G.; Lecaille, C.; Louvet, C.; Tournigand, C.; Lebrun-Ly, V.; et al. Efficacy of aflibercept with FOLFOX and maintenance with fluoropyrimidine as firstline therapy for metastatic colorectal cancer: GERCOR VELVET phase II study. Int. J. Oncol. 2019, 54, 1433–1445. [Google Scholar]
- Pentheroudakis, G.; Kotoula, V.; Koliou, G.-A.; Karavasilis, V.; Samantas, E.; Aravantinos, G.; Kalogeropoulou, L.; Souglakos, I.; Kentepozidis, N.; Koumakis, G.; et al. AMALTHEA: Prospective, single-arm study of the Hellenic Cooperative Oncology Group (HeCOG) evaluating efficacy and safety of first-line FOLFIRI+ aflibercept for 6 months followed by aflibercept maintenance in patients with metastatic colorectal cancer. Clin. Colorectal Cancer 2018, 17, e631–e637. [Google Scholar] [CrossRef]
- Matikas, A.; Souglakos, J.; Katsaounis, P.; Kotsakis, A.; Kouroupakis, P.; Pantazopoulos, N.; Kentepozidis, N.; Nikolaidi, A.; Messaritakis, I.; Tzovara, I.; et al. MINOAS: A Single-arm Translational Phase II Trial of FOLFIRI Plus Aflibercept as First-line Therapy in Unresectable, Metastatic Colorectal Cancer. Target. Oncol. 2019, 14, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Lapeyre-Prost, A.; Pernot, S.; Sigrand, J.; Mary, F.; Le Malicot, K.; Aparicio, T.; Dahan, L.; Caroli-Bosc, F.X.; Lecomte, T.; Racine Doat, S.; et al. Aflibercept in combination with irinotecan, fluorouracil and leucovorin (FOLFIRI) as first-line chemotherapy in metastatic colorectal cancer (mCRC) patients: A phase II multicentric study. Ann. Oncol. 2018, 29, viii160. [Google Scholar] [CrossRef]
- Tang, P.A.; Cohen, S.J.; Kollmannsberger, C.; Bjarnason, G.; Virik, K.; MacKenzie, M.J.; Lourenco, L.; Wang, L.; Chen, A.; Moore, M.J. Phase II clinical and pharmacokinetic study of aflibercept in patients with previously treated metastatic colorectal cancer. Clin. Cancer Res. 2012, 18, 6023–6031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Xu, R.; Qin, S.; Liu, T.; Pan, H.; Xu, J.; Bi, F.; Lim, R.; Zhang, S.; Ba, Y.; et al. Aflibercept plus FOLFIRI in Asian patients with pretreated metastatic colorectal cancer: A randomized Phase III study. Future Oncol. 2018, 14, 2031–2044. [Google Scholar] [CrossRef] [PubMed]
- Denda, T.; Sakai, D.; Hamaguchi, T.; Sugimoto, N.; Ura, T.; Yamazaki, K.; Fujii, H.; Kajiwara, T.; Nakajima, T.E.; Takahashi, S.; et al. Phase II trial of aflibercept with FOLFIRI as a second-line treatment for Japanese patients with metastatic colorectal cancer. Cancer Sci. 2019, 110, 1032–1043. [Google Scholar] [CrossRef]
- Yoshino, T.; Yamazaki, K.; Yamaguchi, K.; Doi, T.; Boku, N.; Machida, N.; Onozawa, Y.; Asayama, M.; Fujino, T.; Ohtsu, A. A phase I study of intravenous aflibercept with FOLFIRI in Japanese patients with previously treated metastatic colorectal cancer. Investig. New Drugs 2013, 31, 910–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Li, Y.; Sun, X.; Zhang, D.; Liu, R.; Ziti-Ljajic, S.; Shi, D.; Xue, F.; Le bail, N.; Xu, R. A phase I and pharmacokinetic study of afilbercept with FOLFIRI: Comparison of Chinese and Caucasian populations. Investig. New Drugs 2017, 35, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Montes, A.; Carlos Lopez, L.; Carmona, A.; Paez, D.; Lopez Munoz Ana, M.; Gutierrez Abad, D.; Carmen Castanon, L.; Maria, D.; Eva Martinez, C.; Manuel Sanchez, C.; et al. Efficacy and toxicity results of FOLFIRI-Aflibercept in advanced colorectal cancer (CRCm) in “real life”, retrospective study. Prognostic factors and specific populations. Ann. Oncol 2017, 28, iii103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Flores, E.; Gonzalez-Astorga, B.; Delgado Urena Ma, T.; Gonzalez Cebrian, I.; Sanchez-Toro, C.; Garcia-Garcia, J.; Conde, V. Experience with aflibercept as a second line chemotherapy in metastatic colorectal cancer: Safety and efficacy in a real-life population. Ann. Oncol. 2017, 28, iii110–iii111. [Google Scholar] [CrossRef]
- Fernández Montes, A.; Martínez Lago, N.; Covela Rúa, M.; de la Cámara Gómez, J.; González Villaroel, P.; Méndez Méndez, J.C.; Jorge Fernández, M.; Salgado Fernández, M.; Reboredo López, M.; Quintero Aldana, G.; et al. Efficacy and safety of FOLFIRI/aflibercept in second-line treatment of metastatic colorectal cancer in a real-world population: Prognostic and predictive markers. Cancer Med. 2019, 8, 882–889. [Google Scholar] [CrossRef]
- Salgado Fernández, M.; Pérez Hoyos, M.T.; Díaz de Corcuera, I.; Vidal Arbués, A.; García de la Torre, M. Aflibercept for metastatic colorectal cancer: Safety data from the Spanish named patient program. Expert Opin. Drug Saf. 2015, 14, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Feliu, J.; Díez de Corcuera, I.; Manzano, J.L.; Valladares-Ayerbes, M.; Alcaide, J.; García García, T.; Vera, R.; Sastre, J. Effectiveness and safety of aflibercept for metastatic colorectal cancer: Retrospective review within an early access program in Spain. Clin. Transl. Oncol. 2017, 19, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lago, N.; Carmona Campos, M.; Gonzalez Villarroel, P.; Salgado Fernandez, M.; De la Camara Gomez, J.; Romero Reinoso, C.; Cousillas Castineiras, A.; Mendez Mendez, J.; Vidal Insua, Y.; Reboredo Rendo, C.; et al. Efficacy and safety of FOLFIRI/Aflibercept (FA) in an elderly population with metastatic colorectal cancer (mCRC) after the failure of an oxaliplatin-based regimen. Ann. Oncol. 2019, 30, iv42–iv43. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Montes, A.; Lopez Lopez, C.; Argiles Martinez, G.; Paez Lopez, D.; Lopez Munoz, A.M.; Garcia Paredes, B.; Gutierrez Abad, D.; Castanon Lopez, C.; Jimenez Fonseca, P.; Gallego Plazas, J.; et al. Prognostic Nomogram and Patterns of Use of FOLFIRI-Aflibercept in Advanced Colorectal Cancer: A Real-World Data Analysis. Oncologist 2019, 24, e687–e695. [Google Scholar] [CrossRef] [Green Version]
- Vera, R.; Mata, E.; Gonzalez, E.; Juez, I.; Alonso, V.; Iranzo, P.; Martinez, N.P.; Lopez, C.; Cabrera, J.M.; Safont, M.J.; et al. Is aflibercept an optimal treatment for wt RAS mCRC patients after progression to first line containing anti-EGFR? Int J. Colorectal Dis. 2020, 35, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Carola, C.; Ghiringhelli, F.; Kim, S.; André, T.; Barlet, J.; Bengrine-Lefevre, L.; Marijon, H.; Garcia-Larnicol, M.-L.; Borg, C.; Dainese, L.; et al. FOLFIRI3-aflibercept in previously treated patients with metastatic colorectal cancer. World J. Clin. Oncol. 2018, 9, 110–118. [Google Scholar] [CrossRef]
- Devaux, M.; Gerard, L.; Richard, C.; Bengrine-Lefevre, L.; Vincent, J.; Schmitt, A.; Ghiringhelli, F. Retrospective evaluation of FOLFIRI3 alone or in combination with bevacizumab or aflibercept in metastatic colorectal cancer. World J. Clin. Oncol. 2019, 10, 75–85. [Google Scholar] [CrossRef]
- Auvray, M.; Tougeron, D.; Auclin, E.; Moulin, V.; Artru, P.; Hautefeuille, V.; Hammel, P.; Lecomte, T.; Locher, C.; Sickersen, G.; et al. Efficacy and Safety of Aflibercept in Combination With Chemotherapy Beyond Second-Line Therapy in Metastatic Colorectal Carcinoma Patients: An AGEO Multicenter Study. Clin. Colorectal Cancer 2020, 19, 39–47.e5. [Google Scholar] [CrossRef]
- Pastorino, A.; Di Bartolomeo, M.; Maiello, E.; Iaffaioli, V.; Ciuffreda, L.; Fasola, G.; Di Costanzo, F.; Frassineti, G.L.; Marchetti, P.; Antoniotti, C.; et al. Aflibercept plus FOLFIRI in the real-life setting: Safety and quality of life data from the Italian patient cohort of the Aflibercept Aafety and Quality-of-Life Program study. Clin. Colorectal Cancer 2018, 17, e457–e470. [Google Scholar] [CrossRef] [Green Version]
- Ottaiano, A.; Capozzi, M.; Tafuto, S.; De Stefano, A.; De Divitiis, C.; Romano, C.; Avallone, A.; Nasti, G. Folfiri-Aflibercept vs. Folfiri-Bevacizumab as Second Line Treatment of RAS Mutated Metastatic Colorectal Cancer in Real Practice. Front. Oncol. 2019, 9, 766. [Google Scholar] [CrossRef] [Green Version]
- Hofheinz, R.; Scholten, F.; Derigs, H.; Thaler, J.; von Moos, R. Quality of life in patients treated with aflibercept and FOLFIRI for metastatic colorectal cancer: Interim analysis with focus on therapy lines of the non-interventional study QoLiTrap (AIO-LQ-0113). Ann. Oncol. 2019, 30, iv118. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, J.I.; Saverno, K.R.; Sung, J.; Duh, M.S.; Zhao, C.; Cai, S.; Vekeman, F.; Peevyhouse, A.; Dhawan, R.; Fuchs, C.S. Real-world treatment patterns and effectiveness among patients with metastatic colorectal cancer treated with ziv-aflibercept in community oncology practices in the USA. Med. Oncol. 2017, 34, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, D.Q.; Manalo, M.; Imperial, M.; Teo, P.; Yong, G.; Ng, M.; Tan, I.B.H.; Choo, S.P.; Chua, C. Safety and efficacy of aflibercept in combination with fluorouracil, leucovorin and irinotecan in the treatment of Asian patients with metastatic colorectal cancer. Asia-Pac. J. Clin. Oncol. 2016, 12, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Yusof, M.M.; Abdullah, N.; Sharial, M.S.N.; Zaatar, A. Safety and management of toxicity related to aflibercept in combination with fluorouracil, leucovorin and irinotecan in Malaysian patients with metastatic colorectal cancer. Asian Pac. J. Cancer Prev. 2016, 17, 973–978. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.-S.; Lin, J.-K.; Chen, W.-S.; Lin, T.-C.; Jiang, J.-K.; Yang, S.-H.; Wang, H.-S.; Chang, S.-C.; Lan, Y.-T.; Lin, C.-C.; et al. Intraperitoneal ziv-aflibercept effectively manages refractory ascites in colorectal cancer patients. Oncotarget 2017, 8, 36707–36715. [Google Scholar] [CrossRef] [Green Version]
- Geng, F.; Wang, Z.; Yin, H.; Yu, J.; Cao, B. Molecular targeted drugs and treatment of colorectal cancer: Recent progress and future perspectives. Cancer Biother. Radiopharm. 2017, 32, 149–160. [Google Scholar] [CrossRef]
- Zhang, B.; Fang, C.; Deng, D.; Xia, L. Research progress on common adverse events caused by targeted therapy for colorectal cancer. Oncol. Lett. 2018, 16, 27–33. [Google Scholar] [CrossRef]
- Saif, M.W.; Relias, V.; Syrigos, K.; Gunturu, K.S. Incidence and management of ZIv-aflibercept related toxicities in colorectal cancer. World J. Clin. Oncol 2014, 5, 1028–1035. [Google Scholar] [CrossRef] [Green Version]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Yoshino, T.; Arnold, D.; Taniguchi, H.; Pentheroudakis, G.; Yamazaki, K.; Xu, R.H.; Kim, T.W.; Ismail, F.; Tan, I.B.; Yeh, K.H.; et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol. 2018, 29, 44–70. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.R. Adverse drug event monitoring at the Food and Drug Administration. J. Gen. Intern. Med. 2003, 18, 57–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Adverse Event | Placebo Plus FOLFIRI (n = 605) | Aflibercept Plus FOLFIRI (n = 611) | ||||
|---|---|---|---|---|---|---|
| All grades (%) * | Grade 3 (%) * | Grade 4 (%) * | All Grades (%) * | Grade 3 (%) * | Grade 4 (%) * | |
| Any | 97.9 | 45.1 | 17.4 | 99.2 | 62.0 | 21.4 |
| Diarrhea | 56.5 | 7.6 | 0.2 | 69.2 | 19.0 | 0.3 |
| Asthenic conditions | 50.2 | 10.4 | 0.2 | 60.4 | 16.0 | 0.8 |
| Stomatitis and ulceration | 34.9 | 5.0 | — | 54.8 | 13.6 | 0.2 |
| Nausea | 54 | 3.0 | — | 53.4 | 1.8 | — |
| Infections and infestations | 32.7 | 6.1 | 0.8 | 46.2 | 11.0 | 1.3 |
| Hypertension | 10.7 | 1.5 | — | 41.4 | 19.1 | 0.2 |
| Hemorrhage | 19 | 1.7 | — | 37.8 | 2.8 | 0.2 |
| Epistaxis | 7.4 | — | — | 27.7 | 0.2 | — |
| GI and abdominal pains | 29.1 | 3.1 | 0.2 | 34 | 5.1 | 0.3 |
| Vomiting | 33.4 | 3.5 | — | 32.9 | 2.6 | 0.2 |
| Decreased appetite | 23.8 | 1.7 | 0.2 | 31.9 | 3.4 | — |
| Weight decreased | 14.4 | 0.8 | — | 31.9 | 2.6 | — |
| Alopecia | 30.1 | — | — | 26.8 | — | — |
| Dysphonia | 3.3 | — | — | 25.4 | 0.5 | — |
| Constipation | 24.6 | 1.0 | — | 22.4 | 0.8 | — |
| Headache | 8.8 | 0.3 | — | 22.3 | 1.6 | — |
| PPES | 4.3 | 0.5 | — | 11.0 | 2.8 | — |
| VTE | 7.3 | 2.6 | 3.6 | 9.3 | 3.1 | 4.7 |
| Anemia | 91.1 | 3.5 | 0.8 | 82.3 | 3.3 | 0.5 |
| Neutropenia | 56.3 | 19.1 | 10.4 | 67.8 | 23.1 | 13.6 |
| Thrombocytopenia | 33.8 | 0.8 | 0.8 | 47.4 | 1.7 | 1.7 |
| Proteinuria | 40.7 | 1.2 | — | 62.2 | 7.5 | 0.3 |
| ALT increased | 37.1 | 2.2 | — | 47.3 | 2.5 | 0.2 |
| Adverse Events | ASQoP Aflibercept + FOLFIRI (n = 779) | VELOUR Aflibercept + FOLFIRI (n = 611) | ||||
|---|---|---|---|---|---|---|
| All Grades (%) [15] | Grade 3–4 (%) [15] | Grade 4 (%) [16] | All grades (%) | Grade 3–4 (%) | Grade 4 (%) | |
| Any treatment-emergent adverse event | 98.7 | 78.2 | 18.0 | 99.2 | 83.5 | 21.4 |
| Selected treatment-emergent adverse events of any grade in ≥ 20% of patients | ||||||
| Diarrhea | 61.6 | 15.3 | 0.3 | 69.2 | 19.3 | 0.3 |
| Asthenic conditions | 57.8 | 13.6 | 0 | 60.4 | 16.9 | 0.8 |
| Hypertension | 48.4 | 24.1 | 0 | 41.4 | 19.3 | 0.2 |
| Stomatitis 1 (and ulcerations) 2 | 42.9 | 10.5 | 0.1 | 54.8 | 13.7 | 0.2 |
| Infections and infestations 2 | 31.3 | 11.7 | 2.1 | 46.2 | 12.3 | 1.3 |
| Venous thromboembolic events 2 | 6.2 | 4.1 | 0.9 | 9.3 | 7.9 | 4.7 |
| Arterial thromboembolic events 2 | 2.3 | 0.8 | 0.3 | 2.6 | 1.8 | 1.0 |
| Neutropenia | 60.5 * | 30.5 * | 9.7 * | 67.8 ** | 36.7 ** | 13.6 ** |
| Proteinuria | 60.1 | 7.6 | 0.6 | 62.2 | 7.9 | 0.3 |
| Event | Action to Be Taken |
|---|---|
| Hypertension Grade 3 Grade 4 | If not controlled with medication, discontinue aflibercept Discontinue aflibercept |
| Proteinuria > 2 g protein/24 h Grade 4 proteinuria (nephrotic syndrome) | Hold aflibercept until proteinuria improves to < 2 g of protein/24 h Discontinue aflibercept in patients with > 2 g proteinuria/24 h that does not resolve within 3 months after holding aflibercept. Work-up for proteinuria such as renal biopsy should be considered Discontinue aflibercept |
| Gastrointestinal perforation Gastrointestinal perforation or dehiscence | Discontinue aflibercept |
| Thromboembolic events Grade 3 venous thromboembolic event or incidentally discovered pulmonary embolus first occurrence Any grade arterial thromboembolic event or symptomatic Grade 4 venous thromboembolic event first occurrence | Hold aflibercept treatment If the planned duration of therapeutic-dose anticoagulant therapy is < 2 weeks, aflibercept should be held until the period of therapeutic-dose anticoagulant therapy is over If the planned duration of therapeutic-dose anticoagulant therapy is > 2 weeks, aflibercept should be held for 2 weeks and then may be resumed during the period of therapeutic-dose anticoagulant therapy as soon as all of the following criteria are met:
|
| Hemorrhage Grade 1 and 2 Grade 3 or 4 (first occurrence) | No dose modification Discontinue aflibercept |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muro, K.; Salinardi, T.; Singh, A.R.; Macarulla, T. Safety of Aflibercept in Metastatic Colorectal Cancer: A Literature Review and Expert Perspective on Clinical and Real-World Data. Cancers 2020, 12, 844. https://doi.org/10.3390/cancers12040844
Muro K, Salinardi T, Singh AR, Macarulla T. Safety of Aflibercept in Metastatic Colorectal Cancer: A Literature Review and Expert Perspective on Clinical and Real-World Data. Cancers. 2020; 12(4):844. https://doi.org/10.3390/cancers12040844
Chicago/Turabian StyleMuro, Kei, Taylor Salinardi, Arvind Rup Singh, and Teresa Macarulla. 2020. "Safety of Aflibercept in Metastatic Colorectal Cancer: A Literature Review and Expert Perspective on Clinical and Real-World Data" Cancers 12, no. 4: 844. https://doi.org/10.3390/cancers12040844
APA StyleMuro, K., Salinardi, T., Singh, A. R., & Macarulla, T. (2020). Safety of Aflibercept in Metastatic Colorectal Cancer: A Literature Review and Expert Perspective on Clinical and Real-World Data. Cancers, 12(4), 844. https://doi.org/10.3390/cancers12040844





