Optimal Body Mass Index Cut-off Point for Predicting Colorectal Cancer Survival in an Asian Population: A National Health Information Database Analysis
Abstract
1. Introduction
2. Results
2.1. Characteristics of Colorectal Cancer Patients and Associations with Overall Survival
2.2. Distribution of BMI at Diagnosis and Relationship with Overall Survival
2.3. Determination of the Optimal BMI Cut-Off Point for Overall Survival
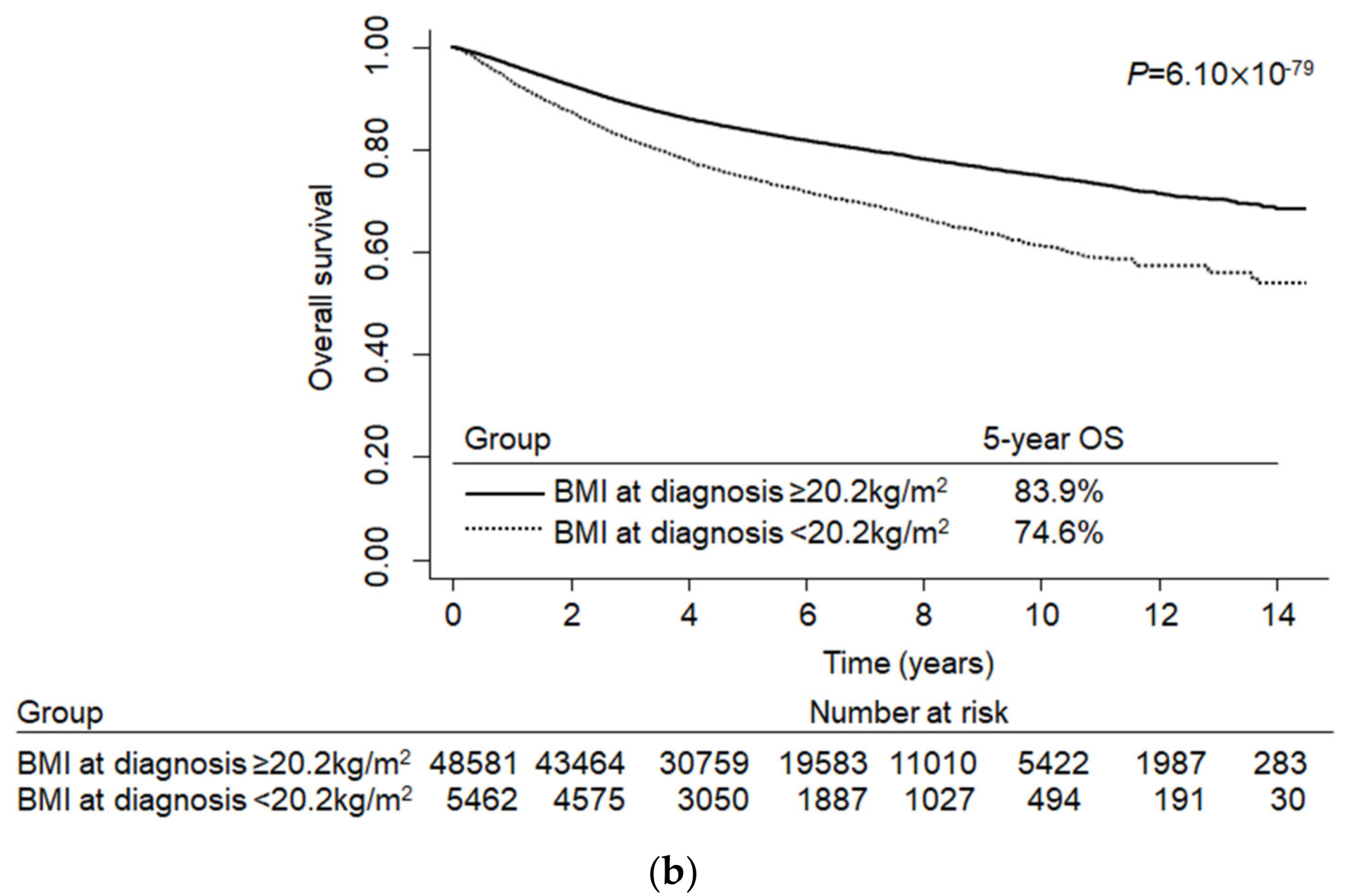
2.4. Characteristics and Survival Curves of Colorectal Cancer Patients by the Optimal BMI Cut-Off Point
2.5. Multivariable Analysis of the Association between the Optimal BMI Cut-Off Point and Overall Survival
3. Discussion
4. Materials and Methods
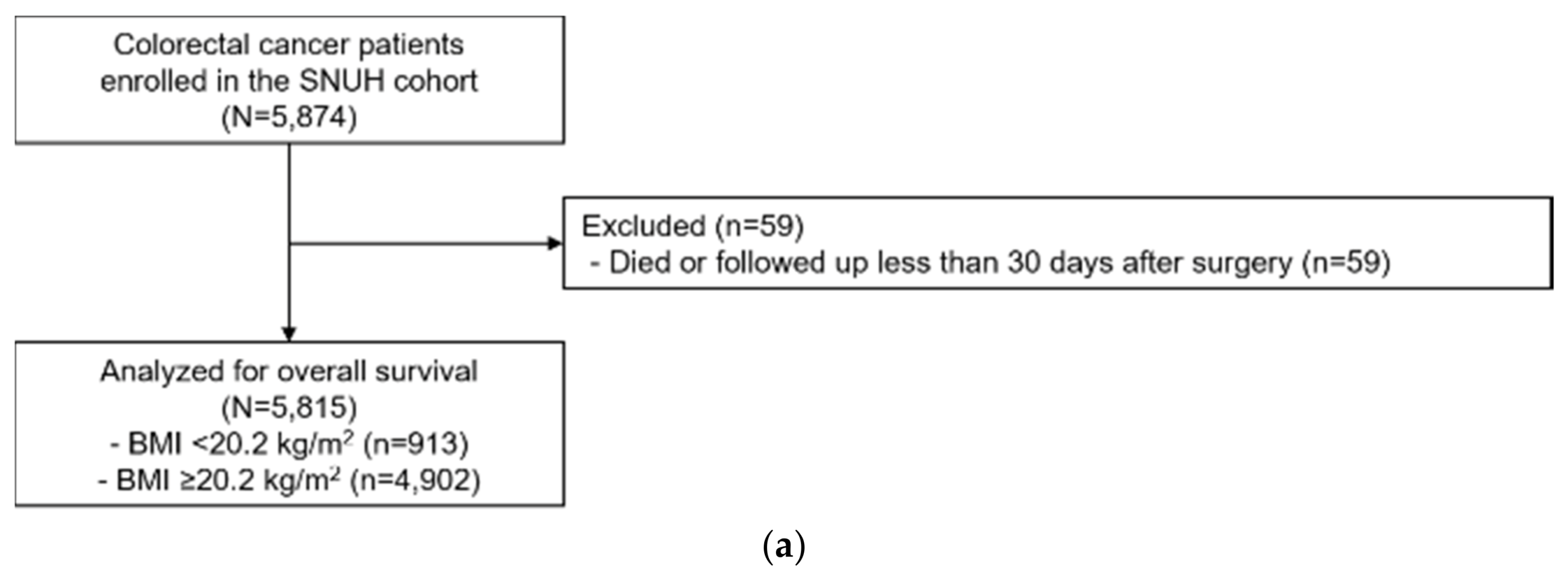
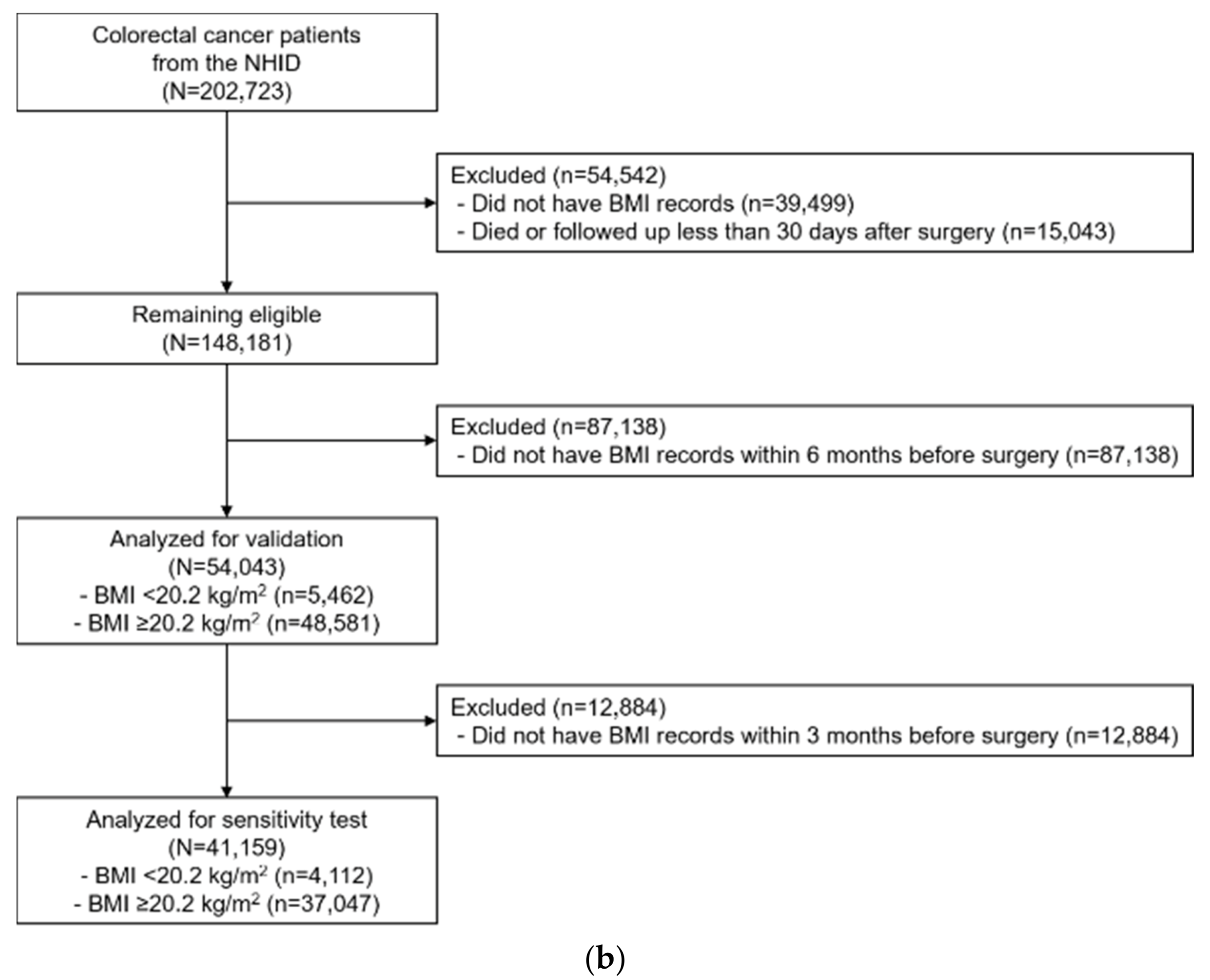
4.1. Study Population
4.2. Data Collection
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abar, L.; Vieira, A.R.; Aune, D.; Sobiecki, J.G.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.C.; Chan, D.S.M.; Schlesinger, S.; et al. Height and body fatness and colorectal cancer risk: An update of the WCRF–AICR systematic review of published prospective studies. Eur. J. Nutr. 2018, 57, 1701–1720. [Google Scholar] [CrossRef] [PubMed]
- Jochem, C.; Leitzmann, M. Obesity and Colorectal Cancer. In Obesity and Cancer; Pischon, T., Nimptsch, K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 17–41. [Google Scholar] [CrossRef]
- Renehan, A.G.; Sperrin, M. The Obesity Paradox and Mortality After Colorectal Cancer: A Causal Conundrum. JAMA Oncol. 2016, 2, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, S.; Siegert, S.; Koch, M.; Walter, J.; Heits, N.; Hinz, S.; Jacobs, G.; Hampe, J.; Schafmayer, C.; Nothlings, U. Postdiagnosis body mass index and risk of mortality in colorectal cancer survivors: A prospective study and meta-analysis. Cancer Causes Control 2014, 25, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Meyerhardt, J.A.; Giovannucci, E.; Jeon, J.Y. Association between body mass index and prognosis of colorectal cancer: A meta-analysis of prospective cohort studies. PLoS ONE 2015, 10, e0120706. [Google Scholar] [CrossRef] [PubMed]
- Doleman, B.; Mills, K.T.; Lim, S.; Zelhart, M.D.; Gagliardi, G. Body mass index and colorectal cancer prognosis: A systematic review and meta-analysis. Tech. Coloproctol. 2016, 20, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, J.; Wang, X.; Li, M.; Gan, Y.; Tang, Y. Association of obesity and overweight with overall survival in colorectal cancer patients: A meta-analysis of 29 studies. Cancer Causes Control 2014, 25, 1489–1502. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, C.H.; Neugebauer, R.; Meyerhardt, J.; Prado, C.M.; Weltzien, E.; Kwan, M.L.; Xiao, J.; Caan, B.J. Analysis of Body Mass Index and Mortality in Patients With Colorectal Cancer Using Causal Diagrams. JAMA Oncol. 2016, 2, 1137–1145. [Google Scholar] [CrossRef]
- Min, Y.W.; Kim, S.A.; Lee, J.H.; Kim, J.Y.; Chang, D.K.; Rhee, P.L.; Kim, J.J.; Rhee, J.C.; Kim, Y.H. Overweight is associated with a favorable survival in patients with colorectal cancer: A prospective cohort study in an Asian population. Ann. Surg. Oncol. 2012, 19, 3460–3464. [Google Scholar] [CrossRef]
- Wang, N.; Khankari, N.K.; Cai, H.; Li, H.L.; Yang, G.; Gao, Y.T.; Xiang, Y.B.; Shu, X.O.; Zheng, W. Prediagnosis body mass index and waist-hip circumference ratio in association with colorectal cancer survival. Int. J. Cancer 2017, 140, 292–301. [Google Scholar] [CrossRef]
- Walter, V.; Jansen, L.; Hoffmeister, M.; Ulrich, A.; Roth, W.; Blaker, H.; Chang-Claude, J.; Brenner, H. Prognostic relevance of prediagnostic weight loss and overweight at diagnosis in patients with colorectal cancer. Am. J. Clin. Nutr. 2016, 104, 1110–1120. [Google Scholar] [CrossRef]
- Who, E.C. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Caan, B.J.; Meyerhardt, J.A.; Kroenke, C.H.; Alexeeff, S.; Xiao, J.; Weltzien, E.; Feliciano, E.C.; Castillo, A.L.; Quesenberry, C.P.; Kwan, M.L.; et al. Explaining the Obesity Paradox: The Association between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer Epidemiol. Biomark. Prev. 2017, 26, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.; Mandili, G.; Witzmann, F.A.; Novelli, F.; Zimmers, T.A.; Bonetto, A. Cancer and Chemotherapy Contribute to Muscle Loss by Activating Common Signaling Pathways. Front. Physio. 2016, 7, 472. [Google Scholar] [CrossRef] [PubMed]
- Uzogara, S.G. Underweight, the less discussed type of unhealthy weight and its implications: A review. Am. J. Food Sci. Nutr. Res. 2016, 3, 126–142. [Google Scholar]
- Chang, H.-C.; Yang, H.-C.; Chang, H.-Y.; Yeh, C.-J.; Chen, H.-H.; Huang, K.-C.; Pan, W.-H. Morbid obesity in Taiwan: Prevalence, trends, associated social demographics, and lifestyle factors. PLoS ONE 2017, 12, e0169577. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Y.; Liu, Y.; Cui, C.; Wu, L.; Wang, Y.; Wang, H.; Zhang, P.; Guo, X. Prevalence of metabolic syndrome and optimal waist circumference cut-off points for adults in Beijing. Diabetes Res. Clin. Pract. 2010, 88, 209–216. [Google Scholar] [CrossRef]
- Qiao, Q.; Nyamdorj, R. The optimal cutoff values and their performance of waist circumference and waist-to-hip ratio for diagnosing type II diabetes. Eur. J. Clin. Nutr. 2009, 64, 23. [Google Scholar] [CrossRef]
- Majed, B.; Moreau, T.; Asselain, B.; Curie Institute Breast Cancer, G. Overweight, obesity and breast cancer prognosis: Optimal body size indicator cut-points. Breast Cancer Res. Treat 2009, 115, 193–203. [Google Scholar] [CrossRef]
- Lichtenstein, P.; Holm, N.V.; Verkasalo, P.K.; Iliadou, A.; Kaprio, J.; Koskenvuo, M.; Pukkala, E.; Skytthe, A.; Hemminki, K. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, D.-W.; Park, J.W.; Ryoo, S.-B.; Shin, R.; Jeong, S.-Y.; Park, K.J. Impact of body mass index on overall survival after surgery for colorectal cancer. Korean J. Clin. Oncol. 2016, 12, 91–96. [Google Scholar] [CrossRef]
- Cheol Seong, S.; Kim, Y.-Y.; Khang, Y.-H.; Heon Park, J.; Kang, H.-J.; Lee, H.; Do, C.-H.; Song, J.-S.; Hyon Bang, J.; Ha, S.; et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017, 46, 799–800. [Google Scholar] [CrossRef] [PubMed]
- Kamila, C.; Paul, L.; Kari, H. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish family-cancer database. Int. J. Cancer 2002, 99, 260–266. [Google Scholar] [CrossRef]
- Contal, C.; O’Quigley, J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput. Stat. Data Anal. 1999, 30, 253–270. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]


| Characteristics | NHID | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Event | pa | All | Event | pa | |||||
| (N = 1,180, 20.3%) | (N = 54,043) | (N = 10,244, 19.0%) | |||||||
| (%) | N | (%) | N | (%) | N | (%) | |||
| Follow-up (years), median (range) | 5.1 (0.1–14.5) | ||||||||
| Person-time (years) | 300,395.8 | ||||||||
| Age at diagnosis (years), mean (SD) | (11.2) | 66.3 | (11.8) | <0.01 | 62.7 | (10.3) | 66.1 | (10.8) | <0.01 |
| BMI at diagnosis (kg/m2), mean (SD) | (3.1) | 22.8 | (3.4) | <0.01 | 23.9 | (3.1) | |||
| Sex | <0.01 | <0.01 | |||||||
| Men | (61.5) | 799 | (67.7) | 34,109 | (63.1) | 6916 | (67.5) | ||
| Women | (38.5) | 381 | (32.3) | 19,934 | (36.9) | 3328 | (32.5) | ||
| Alcohol drinking status | 0.20 | 0.02 | |||||||
| Never | (67.8) | 795 | (67.4) | 22,114 | (40.9) | 4459 | (43.5) | ||
| Ever | (30.6) | 343 | (29.1) | 20,384 | (37.7) | 3862 | (37.7) | ||
| Unknown | (1.6) | 42 | (3.6) | 11,545 | (21.4) | 1923 | (18.8) | ||
| Smoking status | <0.01 | <0.01 | |||||||
| Never | (78.9) | 884 | (74.9) | 31,802 | (58.9) | 5944 | (58.0) | ||
| Ever | (19.5) | 256 | (21.7) | 20,676 | (38.3) | 4002 | (39.1) | ||
| Unknown | (1.5) | 40 | (3.4) | 1565 | (2.9) | 298 | (2.9) | ||
| Diabetes mellitus | 0.09 | <0.01 | |||||||
| No | (85.6) | 994 | (84.2) | 44,036 | (81.5) | 7817 | (76.3) | ||
| Yes | (14.3) | 185 | (15.7) | 10,007 | (18.5) | 2427 | (23.7) | ||
| Unknown | (0.0) | 1 | (0.0) | - | - | ||||
| Hypertension | <0.01 | <0.01 | |||||||
| No | (64.3) | 725 | (61.4) | 34,976 | (64.7) | 6198 | (60.5) | ||
| Yes | (35.7) | 454 | (38.5) | 19,067 | (35.3) | 4046 | (39.5) | ||
| Unknown | (0.0) | 1 | (0.0) | - | - | ||||
| Tumor site | <0.01 | 0.06 | |||||||
| Colon | (64.7) | 487 | (41.3) | 38,685 | (71.6) | 6998 | (68.3) | ||
| Rectum | (35.3) | 693 | (58.7) | 13,771 | (25.5) | 2750 | (26.8) | ||
| Unknown | - | 1587 | (2.9) | 496 | (4.8) | ||||
| TNM stage | <0.01 | ||||||||
| I | (23.0) | 148 | (12.5) | - | - | ||||
| II | (36.3) | 388 | (32.9) | - | - | ||||
| III | (40.7) | 644 | (54.6) | - | - | ||||
| Perioperative chemotherapy | <0.01 | <0.01 | |||||||
| No | (29.2) | 408 | (34.6) | 41,742 | (77.2) | 5867 | (57.3) | ||
| Yes | (59.8) | 709 | (60.1) | 12,301 | (22.8) | 4377 | (42.7) | ||
| Unknown | (11.0) | 63 | (1.1) | - | - | ||||
| Perioperative radiotherapy | <0.01 | <0.01 | |||||||
| No | (68.7) | 822 | (69.7) | 48,334 | (89.4) | 7891 | (77.0) | ||
| Yes | (19.5) | 294 | (24.9) | 5709 | (10.6) | 2353 | (23.0) | ||
| Unknown | (11.8) | 64 | (5.4) | - | - | ||||
| BMI | Total | Event | Age and Sex-Adjusted Model a | Mutivariable-Adjusted Model b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | HR | (95% CI) | p | HR | (95% CI) | p | |
| Development | ||||||||||
| BMI at diagnosis (kg/m2), SNUH | ||||||||||
| <20.2 | 913 | (15.7) | 265 | (22.5) | 1.00 | (ref.) | 1.00 | (ref.) | ||
| ≥20.2 | 4902 | (84.3) | 915 | (77.5) | 0.61 | (0.53–0.70) | 1.6 × 10−12 | 0.62 | (0.54–0.72) | 1.1 × 10−10 |
| Validation | ||||||||||
| BMI recorded less than 6 months prior to surgery (kg/m2), NHID | ||||||||||
| <20.2 | 5462 | (10.1) | 1514 | (14.8) | 1.00 | (ref.) | 1.00 | (ref.) | ||
| ≥20.2 | 48,581 | (89.9) | 8730 | (85.2) | 0.64 | (0.56–0.63) | <0.001 | 0.63 | (0.60–0.67) c | <0.001 |
| BMI recorded less than 3 months prior to surgery (kg/m2), NHID | ||||||||||
| <20.2 | 4112 | (10.0) | 1077 | (15.2) | 1.00 | (ref.) | 1.00 | (ref.) | ||
| ≥20.2 | 37,047 | (90.0) | 6022 | (84.8) | 0.60 | (0.56–0.64) | <0.001 | 0.61 | (0.57–0.65) c | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, N.; Huang, D.; Jang, D.; Kim, M.J.; Jeong, S.-Y.; Shin, A.; Park, J.W. Optimal Body Mass Index Cut-off Point for Predicting Colorectal Cancer Survival in an Asian Population: A National Health Information Database Analysis. Cancers 2020, 12, 830. https://doi.org/10.3390/cancers12040830
Song N, Huang D, Jang D, Kim MJ, Jeong S-Y, Shin A, Park JW. Optimal Body Mass Index Cut-off Point for Predicting Colorectal Cancer Survival in an Asian Population: A National Health Information Database Analysis. Cancers. 2020; 12(4):830. https://doi.org/10.3390/cancers12040830
Chicago/Turabian StyleSong, Nan, Dan Huang, Doeun Jang, Min Jung Kim, Seung-Yong Jeong, Aesun Shin, and Ji Won Park. 2020. "Optimal Body Mass Index Cut-off Point for Predicting Colorectal Cancer Survival in an Asian Population: A National Health Information Database Analysis" Cancers 12, no. 4: 830. https://doi.org/10.3390/cancers12040830
APA StyleSong, N., Huang, D., Jang, D., Kim, M. J., Jeong, S.-Y., Shin, A., & Park, J. W. (2020). Optimal Body Mass Index Cut-off Point for Predicting Colorectal Cancer Survival in an Asian Population: A National Health Information Database Analysis. Cancers, 12(4), 830. https://doi.org/10.3390/cancers12040830





