Liposomal TLR9 Agonist Combined with TLR2 Agonist-Fused Antigen Can Modulate Tumor Microenvironment through Dendritic Cells
Abstract
1. Introduction
2. Results
2.1. Formulation of Lipoprotein and Phosphodiester CpG within Cationic Liposomes
2.2. The Combination of rlipoE7m and POCpG/DOTAP Enhanced CTL Responses
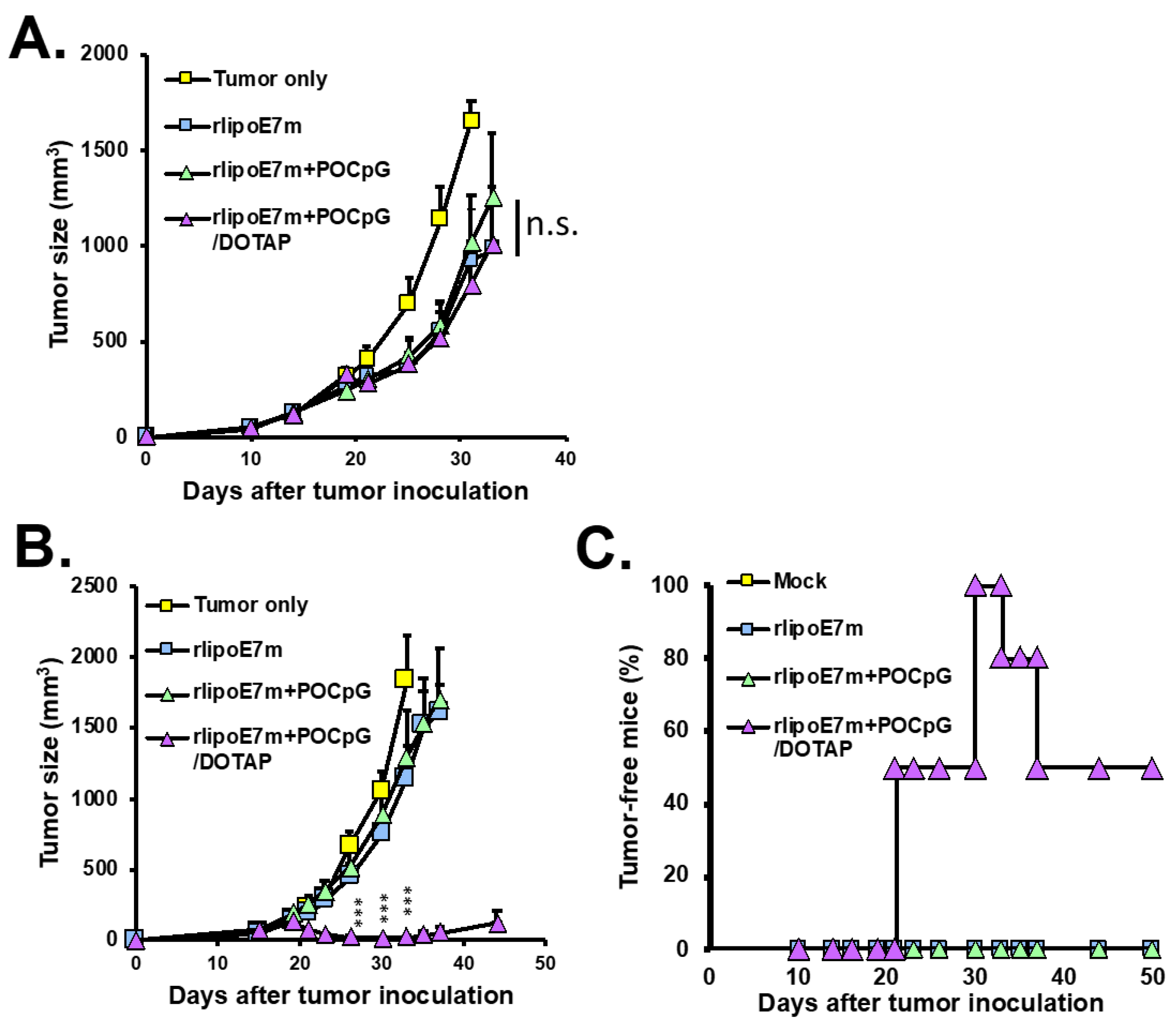
2.3. The Combination of rlipoE7m and POCpG/DOTAP Could Induce Tumor Regression
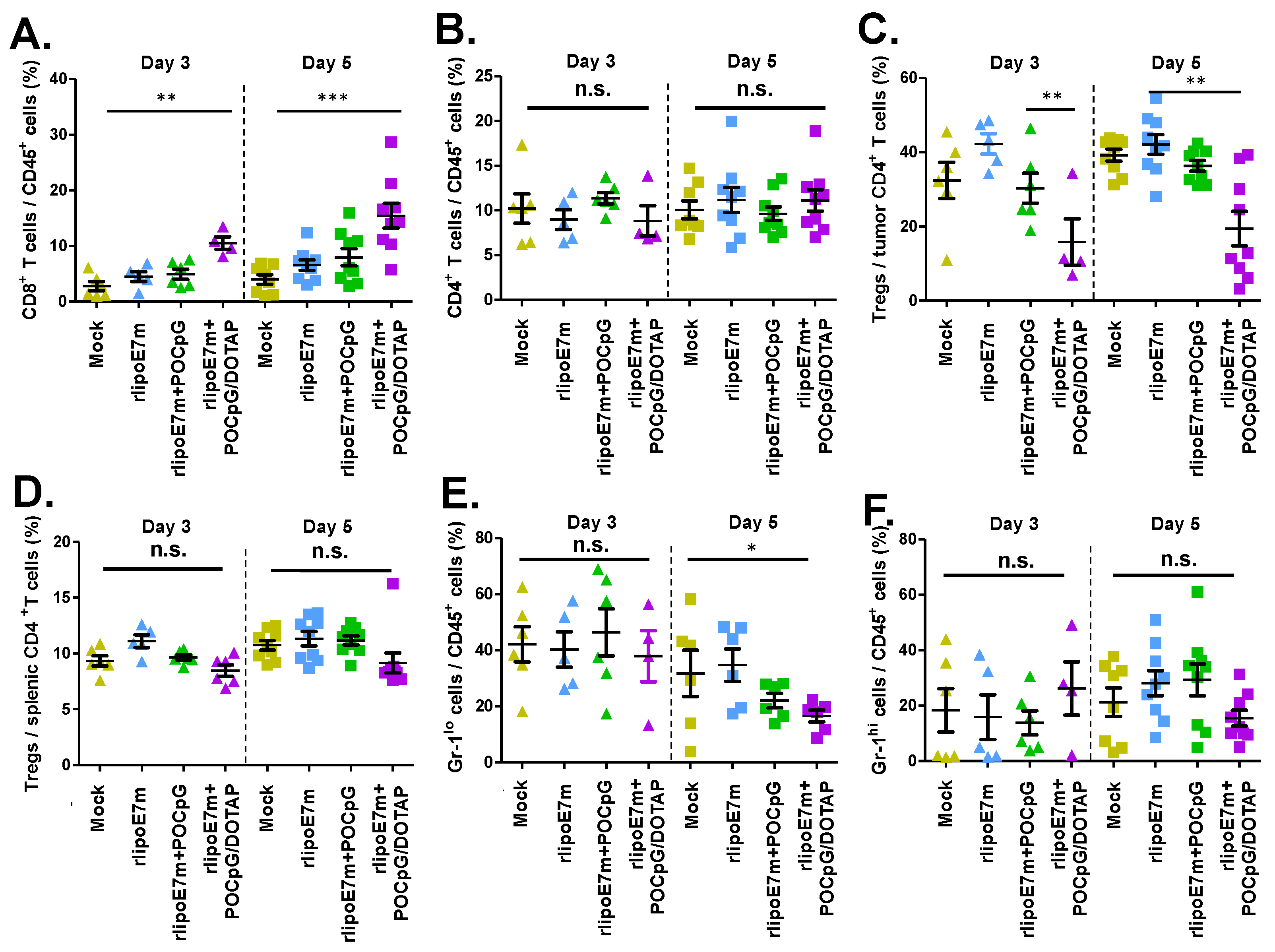
2.4. The Combination of rlipoE7m and POCpG/DOTAP Could Reduce the Number of regulatory T Cells in Tumors
3. Discussion
4. Materials and Methods
4.1. Recombinant Lipoprotein Preparation
4.2. Liposome Preparation
4.3. Encapsulation Rate and Adsorption Rate Determination
4.4. Analysis of the Expression of Costimulatory Molecules on BMDCs and Plasmacytoid DCs
4.5. Analysis of IFN-γ-Producing CTLs
4.6. Animal and Tumor Model
4.7. Analysis of Tumor-Infiltrating Cells
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DCs | Dendritic cells |
| TLR2 | Toll-like receptor 2 |
| HPV | Human papillomavirus |
| ODN | Oligodeoxynucleotides |
| cDCs | Conventional DCs |
| pDCs | Plasmacytoid DCs |
| CTL | Cytotoxic T lymphocyte |
| PAMP | Pathogen-associated molecular pattern |
| NOD | Nucleotide-binding oligomerization domain |
| Tregs | Regulatory T cells |
| TAMs | Tumor-associated macrophages |
| MDSCs | Myeloid-derived suppressor cells |
| DOTAP | 1, 2-Dioleoyloxy-3-trimethylammonium propane |
| TNF-α | Tumor necrosis factor-α |
| IL | Interleukin |
| PSCpG | Phosphorothioate CpG ODN |
| POCpG | Phosphodiester CpG ODN |
| BMDCs | Bone marrow-derived dendritic cells |
| CCL2 | C-C motif chemokine ligand 2 |
| ROS | Reactive oxygen species |
| MAPK | Mitogen-activated protein kinase |
References
- Looi, C.K.; Chung, F.F.; Leong, C.O.; Wong, S.F.; Rosli, R.; Mai, C.W. Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. J. Exp. Clin. Cancer Res. 2019, 38, e162. [Google Scholar] [CrossRef]
- Pitt, J.M.; Marabelle, A.; Eggermont, A.; Soria, J.C.; Kroemer, G.; Zitvogel, L. Targeting the tumor microenvironment: Removing obstruction to anticancer immune responses and immunotherapy. Ann. Oncol. 2016, 27, 1482–1492. [Google Scholar] [CrossRef]
- Van der Jeught, K.; Bialkowski, L.; Daszkiewicz, L.; Broos, K.; Goyvaerts, C.; Renmans, D.; Van Lint, S.; Heirman, C.; Thielemans, K.; Breckpot, K. Targeting the tumor microenvironment to enhance antitumor immune responses. Oncotarget 2015, 6, 1359–1381. [Google Scholar] [CrossRef]
- Marabelle, A.; Kohrt, H.; Levy, R. New insights into the mechanism of action of immune checkpoint antibodies. Oncoimmunology 2014, 3, e954869. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.V.; Nino-Castro, A.C.; Schultze, J.L. Regulatory dendritic cells: There is more than just immune activation. Front. Immunol. 2012, 3, e274. [Google Scholar] [CrossRef] [PubMed]
- Wylie, B.; Macri, C.; Mintern, J.D.; Waithman, J. Dendritic Cells and Cancer: From Biology to Therapeutic Intervention. Cancers (Basel) 2019, 11, 521. [Google Scholar] [CrossRef]
- Escors, D. Tumour immunogenicity, antigen presentation and immunological barriers in cancer immunotherapy. New J. Sci. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef] [PubMed]
- Koucky, V.; Boucek, J.; Fialova, A. Immunology of Plasmacytoid Dendritic Cells in Solid Tumors: A Brief Review. Cancers (Basel) 2019, 11, 470. [Google Scholar] [CrossRef]
- Temizoz, B.; Kuroda, E.; Ishii, K.J. Vaccine adjuvants as potential cancer immunotherapeutics. Int. Immunol. 2016, 28, 329–338. [Google Scholar] [CrossRef]
- Berk, E.; Xu, S.; Czerniecki, B.J. Dendritic cells matured in the presence of TLR ligands overcome the immunosuppressive functions of regulatory T cells. Oncoimmunology 2014, 3, e27617. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.S.; Leng, C.H.; Yeh, Y.C.; Wu, C.C.; Chen, H.W.; Huang, H.M.; Liu, S.J. Toll-like receptor 9 agonist enhances anti-tumor immunity and inhibits tumor-associated immunosuppressive cells numbers in a mouse cervical cancer model following recombinant lipoprotein therapy. Mol. Cancer 2014, 13, e60. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.Y.; Liu, H.Y.; Li, H.J.; Wu, C.C.; Liou, G.G.; Chang, Y.C.; Leng, C.H.; Liu, S.J. A novel liposomal recombinant lipoimmunogen enhances anti-tumor immunity. J. Control. Release 2016, 233, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.Y.; Chang, L.S.; Leng, C.H.; Liu, S.J. Self-adjuvanting lipoimmunogens for therapeutic HPV vaccine development: Potential clinical impact. Expert Rev. Vaccines 2015, 14, 383–394. [Google Scholar] [CrossRef]
- Shen, K.Y.; Song, Y.C.; Chen, I.H.; Chong, P.; Liu, S.J. Depletion of tumor-associated macrophages enhances the anti-tumor immunity induced by a Toll-like receptor agonist-conjugated peptide. Human Vaccines Immunother. 2014, 10, 3241–3250. [Google Scholar] [CrossRef][Green Version]
- Huang, C.Y.; Chen, J.J.; Shen, K.Y.; Chang, L.S.; Yeh, Y.C.; Chen, I.H.; Chong, P.; Liu, S.J.; Leng, C.H. Recombinant lipidated HPV E7 induces a Th-1-biased immune response and protective immunity against cervical cancer in a mouse model. PLoS ONE 2012, 7, e40970. [Google Scholar] [CrossRef]
- Chen, H.W.; Liu, S.J.; Liu, H.H.; Kwok, Y.; Lin, C.L.; Lin, L.H.; Chen, M.Y.; Tsai, J.P.; Chang, L.S.; Chiu, F.F.; et al. A novel technology for the production of a heterologous lipoprotein immunogen in high yield has implications for the field of vaccine design. Vaccine 2009, 27, 1400–1409. [Google Scholar] [CrossRef]
- Basto, A.P.; Badenes, M.; Almeida, S.C.; Martins, C.; Duarte, A.; Santos, D.M.; Leitao, A. Immune response profile elicited by the model antigen ovalbumin expressed in fusion with the bacterial OprI lipoprotein. Mol. Immunol. 2015, 64, 36–45. [Google Scholar] [CrossRef]
- Zoglmeier, C.; Bauer, H.; Noerenberg, D.; Wedekind, G.; Bittner, P.; Sandholzer, N.; Rapp, M.; Anz, D.; Endres, S.; Bourquin, C. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin. Cancer Res. 2011, 17, 1765–1775. [Google Scholar] [CrossRef]
- Song, Y.C.; Liu, S.J. A TLR9 agonist enhances the anti-tumor immunity of peptide and lipopeptide vaccines via different mechanisms. Sci. Rep. 2015, 5, e12578. [Google Scholar] [CrossRef]
- Sparwasser, T.; Hultner, L.; Koch, E.S.; Luz, A.; Lipford, G.B.; Wagner, H. Immunostimulatory CpG-oligodeoxynucleotides cause extramedullary murine hemopoiesis. J. Immunol. 1999, 162, 2368–2374. [Google Scholar] [PubMed]
- Deng, G.M.; Nilsson, I.M.; Verdrengh, M.; Collins, L.V.; Tarkowski, A. Intra-articularly localized bacterial DNA containing CpG motifs induces arthritis. Nat. Med. 1999, 5, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Moon, C.; Oh, S.S.; Park, S.; Jeong, J.W.; Kim, S.; Lee, H.G.; Kwon, H.J.; Kim, K.D. Liposome-encapsulated CpG enhances antitumor activity accompanying the changing of lymphocyte populations in tumor via intratumoral administration. Nucleic Acid Ther. 2015, 25, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Shargh, V.H.; Jaafari, M.R.; Khamesipour, A.; Jaafari, I.; Jalali, S.A.; Abbasi, A.; Badiee, A. Liposomal SLA co-incorporated with PO CpG ODNs or PS CpG ODNs induce the same protection against the murine model of leishmaniasis. Vaccine 2012, 30, 3957–3964. [Google Scholar] [CrossRef]
- Yan, W.; Chen, W.; Huang, L. Reactive oxygen species play a central role in the activity of cationic liposome based cancer vaccine. J. Control. Release 2008, 130, 22–28. [Google Scholar] [CrossRef]
- Coch, C.; Busch, N.; Wimmenauer, V.; Hartmann, E.; Janke, M.; Abdel-Mottaleb, M.M.; Lamprecht, A.; Ludwig, J.; Barchet, W.; Schlee, M.; et al. Higher activation of TLR9 in plasmacytoid dendritic cells by microbial DNA compared with self-DNA based on CpG-specific recognition of phosphodiester DNA. J. Leukoc. Biol. 2009, 86, 663–670. [Google Scholar] [CrossRef]
- De Castro, L.F.; Longhi, L.N.A.; Paiao, M.R.; Justo-Junior, A.D.S.; de Jesus, M.B.; Blotta, M.; Mamoni, R.L. NLRP3 inflammasome is involved in the recognition of Paracoccidioides brasiliensis by human dendritic cells and in the induction of Th17 cells. J. Infect. 2018, 77, 137–144. [Google Scholar] [CrossRef]
- Wesa, A.K.; Galy, A. IL-1 beta induces dendritic cells to produce IL-12. Int. Immunol. 2001, 13, 1053–1061. [Google Scholar] [CrossRef]
- Luft, T.; Jefford, M.; Luetjens, P.; Hochrein, H.; Masterman, K.A.; Maliszewski, C.; Shortman, K.; Cebon, J.; Maraskovsky, E. IL-1 beta enhances CD40 ligand-mediated cytokine secretion by human dendritic cells (DC): A mechanism for T cell-independent DC activation. J. Immunol. 2002, 168, 713–722. [Google Scholar] [CrossRef]
- Shen, K.Y.; Song, Y.C.; Chen, I.H.; Leng, C.H.; Chen, H.W.; Li, H.J.; Chong, P.; Liu, S.J. Molecular mechanisms of TLR2-mediated antigen cross-presentation in dendritic cells. J. Immunol. 2014, 192, 4233–4241. [Google Scholar] [CrossRef]
- Bevaart, L.; Van Ojik, H.H.; Sun, A.W.; Sulahian, T.H.; Leusen, J.H.; Weiner, G.J.; Van De Winkel, J.G.; Van Vugt, M.J. CpG oligodeoxynucleotides enhance FcgammaRI-mediated cross presentation by dendritic cells. Int. Immunol. 2004, 16, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Kuchtey, J.; Chefalo, P.J.; Gray, R.C.; Ramachandra, L.; Harding, C.V. Enhancement of dendritic cell antigen cross-presentation by CpG DNA involves type I IFN and stabilization of class I MHC mRNA. J. Immunol. 2005, 175, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhao, Y. Toll-like receptors and immune regulation: Their direct and indirect modulation on regulatory CD4+ CD25+ T cells. Immunology 2007, 122, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Sutmuller, R.P.; den Brok, M.H.; Kramer, M.; Bennink, E.J.; Toonen, L.W.; Kullberg, B.J.; Joosten, L.A.; Akira, S.; Netea, M.G.; Adema, G.J. Toll-like receptor 2 controls expansion and function of regulatory T cells. J. Clin. Invest. 2006, 116, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Komai-Koma, M.; Xu, D.; Liew, F.Y. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2006, 103, 7048–7053. [Google Scholar] [CrossRef]
- Zheng, Y.; Manzotti, C.N.; Liu, M.; Burke, F.; Mead, K.I.; Sansom, D.M. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J. Immunol. 2004, 172, 2778–2784. [Google Scholar] [CrossRef]
- Kashimura, S.; Saze, Z.; Terashima, M.; Soeta, N.; Ohtani, S.; Osuka, F.; Kogure, M.; Gotoh, M. CD83(+) dendritic cells and Foxp3(+) regulatory T cells in primary lesions and regional lymph nodes are inversely correlated with prognosis of gastric cancer. Gastric Cancer 2012, 15, 144–153. [Google Scholar] [CrossRef]
- Engblom, C.; Pfirschke, C.; Pittet, M.J. The role of myeloid cells in cancer therapies. Nat. Rev. Cancer 2016, 16, 447–462. [Google Scholar] [CrossRef]
- Mittal, S.K.; Roche, P.A. Suppression of antigen presentation by IL-10. Curr. Opin. Immunol. 2015, 34, 22–27. [Google Scholar] [CrossRef]
- Mittal, S.K.; Cho, K.J.; Ishido, S.; Roche, P.A. Interleukin 10 (IL-10)-mediated Immunosuppression: MARCH-I INDUCTION REGULATES ANTIGEN PRESENTATION BY MACROPHAGES BUT NOT DENDRITIC CELLS. J. Biol. Chem. 2015, 290, 27158–27167. [Google Scholar] [CrossRef]
- Chang, J.; Kunkel, S.L.; Chang, C.H. Negative regulation of MyD88-dependent signaling by IL-10 in dendritic cells. Proc. Natl. Acad. Sci. USA 2009, 106, 18327–18332. [Google Scholar] [CrossRef] [PubMed]
- Ewert, K.K.; Zidovska, A.; Ahmad, A.; Bouxsein, N.F.; Evans, H.M.; McAllister, C.S.; Samuel, C.E.; Safinya, C.R. Cationic liposome-nucleic acid complexes for gene delivery and silencing: Pathways and mechanisms for plasmid DNA and siRNA. Top. Curr. Chem. 2010, 296, 191–226. [Google Scholar] [CrossRef] [PubMed]
- Reidel, I.G.; Camussone, C.; Suarez Archilla, G.A.; Calvinho, L.F.; Veaute, C. Liposomal and CpG-ODN formulation elicits strong humoral immune responses to recombinant Staphylococcus aureus antigens in heifer calves. Vet. Immunol. Immunopathol. 2019, 212, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mansury, D.; Ghazvini, K.; Amel Jamehdar, S.; Badiee, A.; Tafaghodi, M.; Nikpoor, A.R.; Amini, Y.; Jaafari, M.R. Increasing Cellular Immune Response in Liposomal Formulations of DOTAP Encapsulated by Fusion Protein Hspx, PPE44, And Esxv, as a Potential Tuberculosis Vaccine Candidate. Rep. Biochem. Mol. Biol. 2019, 7, 156–166. [Google Scholar]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Li, J.; Byrne, K.T.; Yan, F.; Yamazoe, T.; Chen, Z.; Baslan, T.; Richman, L.P.; Lin, J.H.; Sun, Y.H.; Rech, A.J.; et al. Tumor Cell-Intrinsic Factors Underlie Heterogeneity of Immune Cell Infiltration and Response to Immunotherapy. Immunity 2018, 49, 178–193. [Google Scholar] [CrossRef]
- Rodallec, A.; Sicard, G.; Fanciullino, R.; Benzekry, S.; Lacarelle, B.; Milano, G.; Ciccolini, J. Turning cold tumors into hot tumors: Harnessing the potential of tumor immunity using nanoparticles. Expert Opin. Drug Metab. Toxicol. 2018, 14, 1139–1147. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, K.-Y.; Liu, H.-Y.; Yan, W.-L.; Wu, C.-C.; Lee, M.-H.; Leng, C.-H.; Liu, S.-J. Liposomal TLR9 Agonist Combined with TLR2 Agonist-Fused Antigen Can Modulate Tumor Microenvironment through Dendritic Cells. Cancers 2020, 12, 810. https://doi.org/10.3390/cancers12040810
Shen K-Y, Liu H-Y, Yan W-L, Wu C-C, Lee M-H, Leng C-H, Liu S-J. Liposomal TLR9 Agonist Combined with TLR2 Agonist-Fused Antigen Can Modulate Tumor Microenvironment through Dendritic Cells. Cancers. 2020; 12(4):810. https://doi.org/10.3390/cancers12040810
Chicago/Turabian StyleShen, Kuan-Yin, Hsin-Yu Liu, Wan-Lun Yan, Chiao-Chieh Wu, Ming-Hui Lee, Chih-Hsing Leng, and Shih-Jen Liu. 2020. "Liposomal TLR9 Agonist Combined with TLR2 Agonist-Fused Antigen Can Modulate Tumor Microenvironment through Dendritic Cells" Cancers 12, no. 4: 810. https://doi.org/10.3390/cancers12040810
APA StyleShen, K.-Y., Liu, H.-Y., Yan, W.-L., Wu, C.-C., Lee, M.-H., Leng, C.-H., & Liu, S.-J. (2020). Liposomal TLR9 Agonist Combined with TLR2 Agonist-Fused Antigen Can Modulate Tumor Microenvironment through Dendritic Cells. Cancers, 12(4), 810. https://doi.org/10.3390/cancers12040810





