Evaluation of Two EGFR Mutation Tests on Tumor and Plasma from Patients with Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. EGFR Mutation Tests
2.3. Data Analyses
3. Results
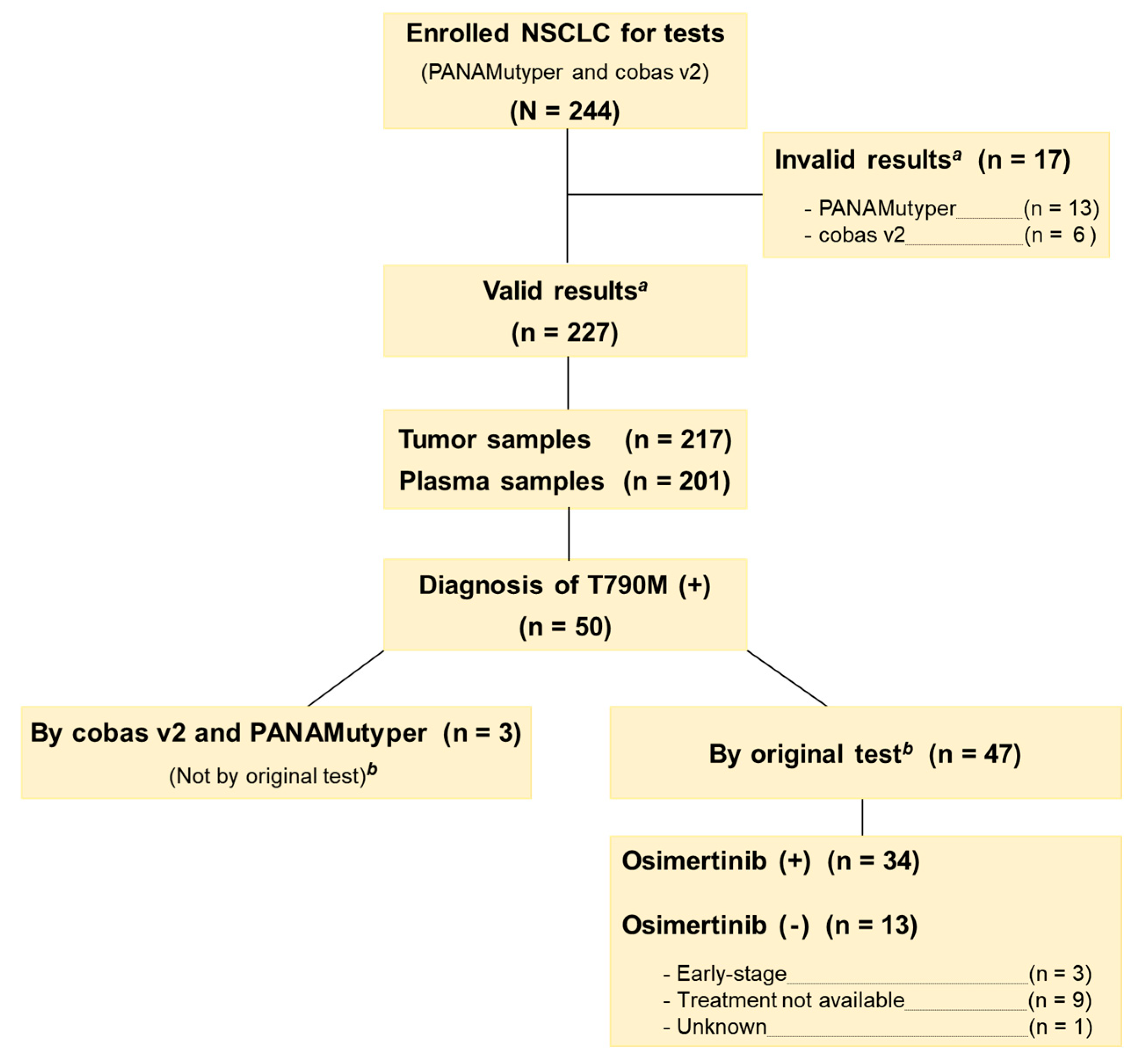
3.1. Patients
3.2. Concordance between Two Tests in Tumor and in Plasma
3.3. Concordance of Test Results between Tumor Tissue and Plasma
3.4. Re-Analyses of Discordant Results
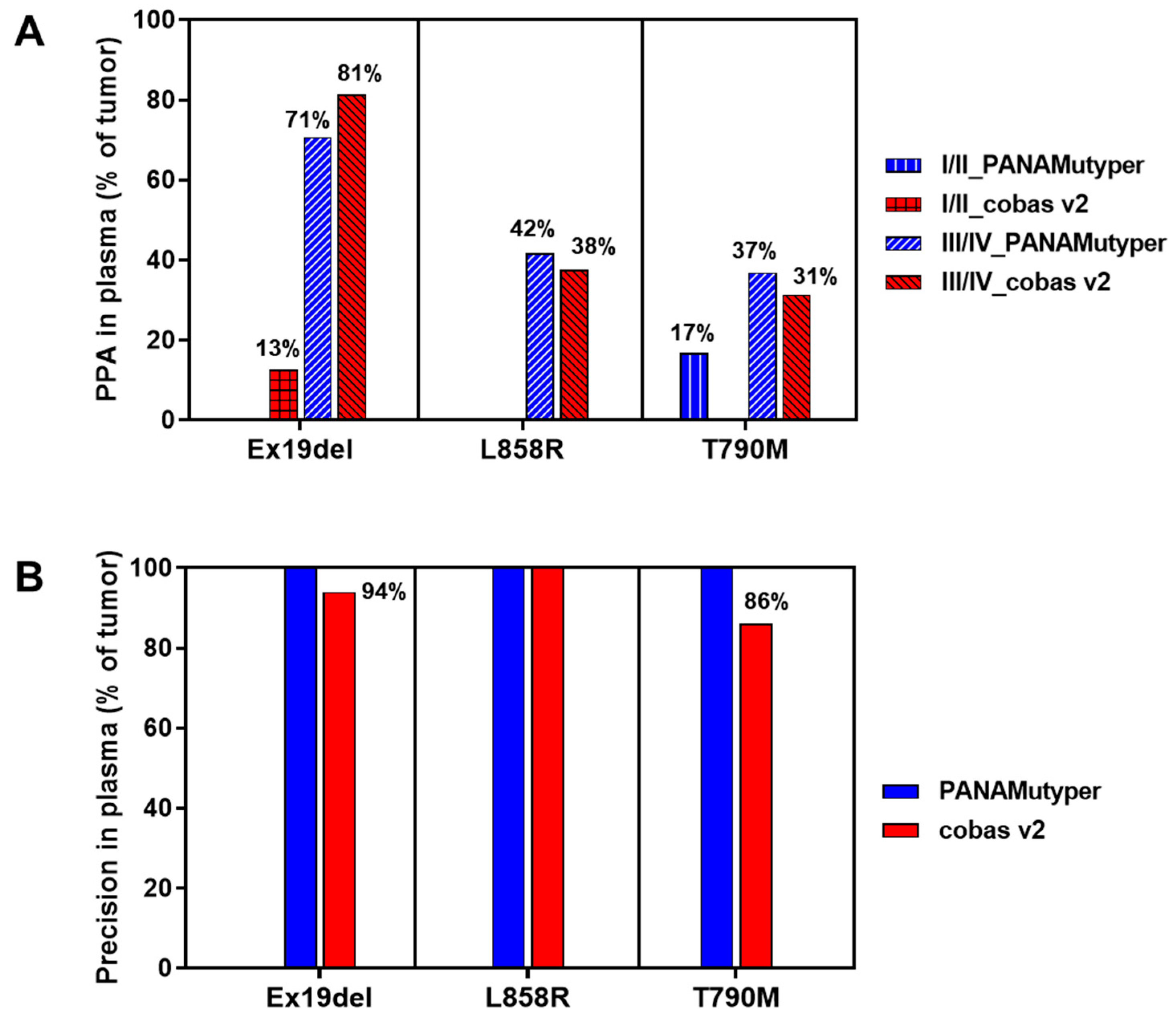
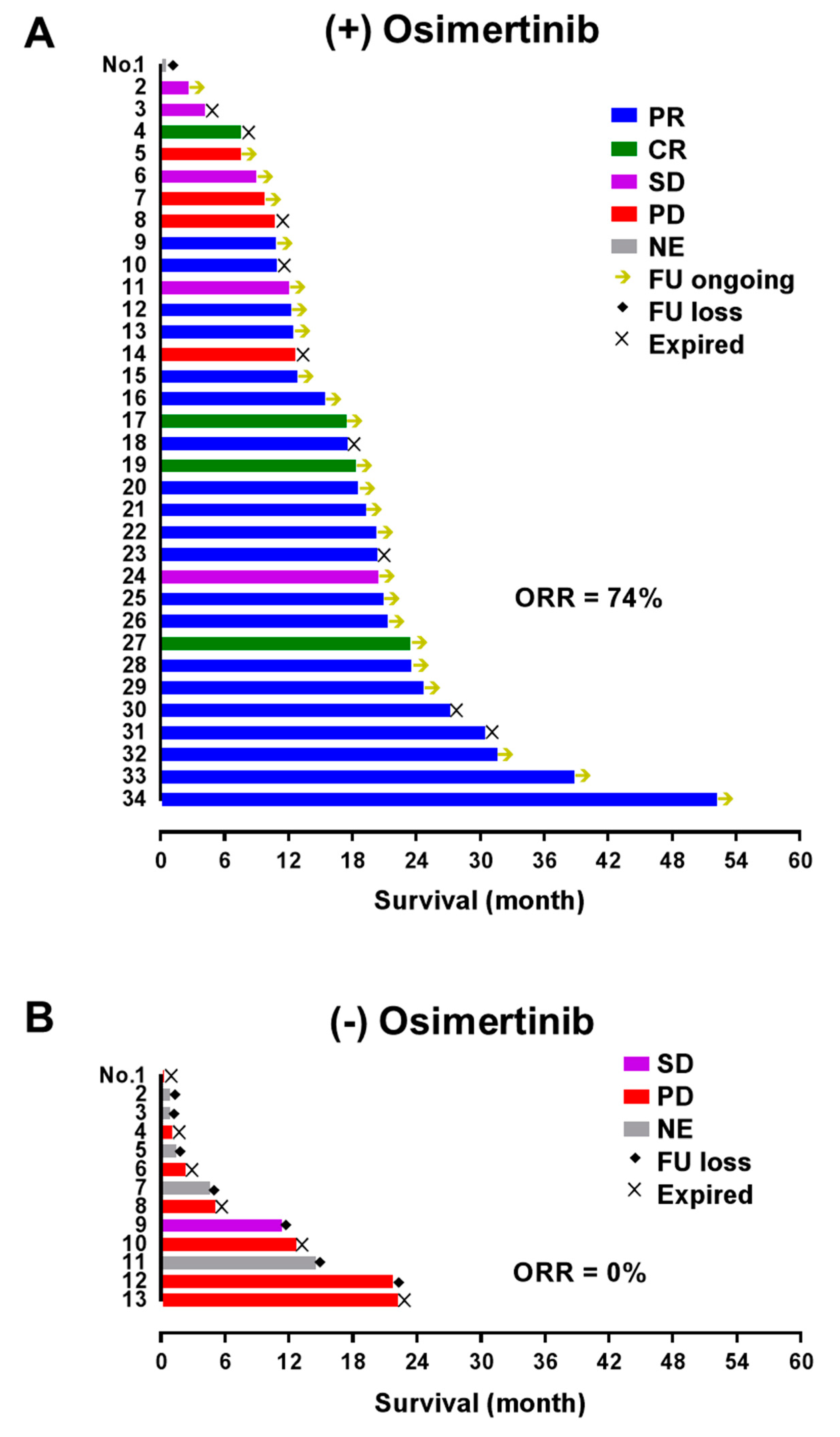
3.5. Test Results in Patients with T790M Mutation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hsu, W.H.; Yang, J.C.; Mok, T.S.; Loong, H.H. Overview of current systemic management of EGFR-mutant NSCLC. Ann. Oncol. 2018, 29, i3–i9. [Google Scholar] [CrossRef]
- Mok, T.S.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Lee, M.; Linke, R.; Rosell, R.; Corral, J.; et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. J. Clin. Oncol. 2018, 36, 2244–2250. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Pennell, N.A.; Arcila, M.E.; Gandara, D.R.; West, H. Biomarker Testing for Patients With Advanced Non-Small Cell Lung Cancer: Real-World Issues and Tough Choices. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 531–542. [Google Scholar] [CrossRef]
- Lim, E.A.; Lee, H.; Bae, E.; Lim, J.; Shin, Y.K.; Choi, S.E. Economic Evaluation of Companion Diagnostic Testing for EGFR Mutations and First-Line Targeted Therapy in Advanced Non-Small Cell Lung Cancer Patients in South Korea. PLoS ONE 2016, 11, e0160155. [Google Scholar] [CrossRef]
- Arrieta, O.; Anaya, P.; Morales-Oyarvide, V.; Ramirez-Tirado, L.A.; Polanco, A.C. Cost-effectiveness analysis of EGFR mutation testing in patients with non-small cell lung cancer (NSCLC) with gefitinib or carboplatin-paclitaxel. Eur. J. Health Econ. 2016, 17, 855–863. [Google Scholar] [CrossRef]
- Narita, Y.; Matsushima, Y.; Shiroiwa, T.; Chiba, K.; Nakanishi, Y.; Kurokawa, T.; Urushihara, H. Cost-effectiveness analysis of EGFR mutation testing and gefitinib as first-line therapy for non-small cell lung cancer. Lung Cancer 2015, 90, 71–77. [Google Scholar] [CrossRef]
- Cheng, M.M.; Palma, J.F.; Scudder, S.; Poulios, N.; Liesenfeld, O. The Clinical and Economic Impact of Inaccurate EGFR Mutation Tests in the Treatment of Metastatic Non-Small Cell Lung Cancer. J. Pers. Med. 2017, 7, 5. [Google Scholar] [CrossRef]
- Thress, K.S.; Brant, R.; Carr, T.H.; Dearden, S.; Jenkins, S.; Brown, H.; Hammett, T.; Cantarini, M.; Barrett, J.C. EGFR mutation detection in ctDNA from NSCLC patient plasma: A cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer 2015, 90, 509–515. [Google Scholar] [CrossRef]
- Han, J.Y.; Choi, J.J.; Kim, J.Y.; Han, Y.L.; Lee, G.K. PNA clamping-assisted fluorescence melting curve analysis for detecting EGFR and KRAS mutations in the circulating tumor DNA of patients with advanced non-small cell lung cancer. BMC Cancer 2016, 16, 627. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, H.W.; Jeon, S.H.; Kim, B.N.; Kang, N.; Yeo, C.D.; Park, C.K.; Kim, Y.K.; Lee, Y.H.; Lee, K.Y.; et al. Comparison of PANAMutyper and PNAClamp for Detecting KRAS Mutations from Patients With Malignant Pleural Effusion. In Vivo 2019, 33, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Midha, A.; Dearden, S.; McCormack, R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: A systematic review and global map by ethnicity (mutMapII). Am. J. Cancer Res. 2015, 5, 2892–2911. [Google Scholar]
- Jazieh, A.R.; Jaafar, H.; Jaloudi, M.; Mustafa, R.S.; Rasul, K.; Zekri, J.; Bamefleh, H.; Gasmelseed, A. Patterns of epidermal growth factor receptor mutation in non-small-cell lung cancers in the Gulf region. Mol. Clin. Oncol. 2015, 3, 1371–1374. [Google Scholar] [CrossRef]
- Chang, Q.; Zhang, Y.; Xu, J.; Zhong, R.; Qiang, H.; Zhang, B.; Han, B.; Qian, J.; Chu, T. First-line pemetrexed/carboplatin or cisplatin/bevacizumab compared with paclitaxel/carboplatin/bevacizumab in patients with advanced non-squamous non-small cell lung cancer with wild-type driver genes: A real-world study in China. Thorac. Cancer 2019, 10, 1043–1050. [Google Scholar] [CrossRef]
- Chang, W.Y.; Wu, Y.L.; Su, P.L.; Yang, S.C.; Lin, C.C.; Su, W.C. The impact of EGFR mutations on the incidence and survival of stages I to III NSCLC patients with subsequent brain metastasis. PLoS ONE 2018, 13, e0192161. [Google Scholar] [CrossRef]
- Tas, F.; Ciftci, R.; Kilic, L.; Karabulut, S. Age is a prognostic factor affecting survival in lung cancer patients. Oncol. Lett. 2013, 6, 1507–1513. [Google Scholar] [CrossRef]
- Takai, E.; Totoki, Y.; Nakamura, H.; Morizane, C.; Nara, S.; Hama, N.; Suzuki, M.; Furukawa, E.; Kato, M.; Hayashi, H.; et al. Clinical utility of circulating tumor DNA for molecular assessment in pancreatic cancer. Sci. Rep. 2015, 5, 18425. [Google Scholar] [CrossRef]
- Chen, K.Z.; Lou, F.; Yang, F.; Zhang, J.B.; Ye, H.; Chen, W.; Guan, T.; Zhao, M.Y.; Su, X.X.; Shi, R.; et al. Circulating Tumor DNA Detection in Early-Stage Non-Small Cell Lung Cancer Patients by Targeted Sequencing. Sci. Rep. 2016, 6, 31985. [Google Scholar] [CrossRef]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J. Thorac. Oncol. 2018, 13, 323–358. [Google Scholar] [CrossRef] [PubMed]
| Patient Demographics (Wild vs. Mutant) | PANAMutyper Test Result | cobas v2 Test Result | ||||
|---|---|---|---|---|---|---|
| Wild | Mutant | p value | Wild | Mutant | p Value | |
| (n = 130) | (n = 97) | (n = 143) | (n = 84) | |||
| Age a | ||||||
| Median y (range) | 68 (44–85) | 63 (35–83) | p < 0.001 | 68 (44–85) | 63 (35–83) | p < 0.001 |
| Sex b | ||||||
| Male | 89 (39%) | 36 (16%) | p < 0.001 | 99 (44%) | 26 (12%) | p < 0.001 |
| Female | 41 (18%) | 61 (27%) | 44 (19%) | 58 (26%) | ||
| Smoking history b | ||||||
| Never | 57 (25%) | 68 (30%) | p < 0.001 | 61 (27%) | 64 (28%) | p < 0.001 |
| Former | 57 (25%) | 24 (11%) | 66 (29%) | 15 (6.6%) | ||
| Current | 16 (7.0%) | 5 (2.2%) | 16 (7.0%) | 5 (2.2%) | ||
| Histology b | ||||||
| Adenocarcinoma | 83 (37%) | 91 (40%) | p < 0.001 | 90 (40%) | 84 (37%) | p < 0.001 |
| Squamous | 45 (20%) | 6 (2.6%) | 51 (23%) | 0 | ||
| Large cell | 2 (0.9%) | 0 | 2 (0.9%) | 0 | ||
| TNM stage b,c | ||||||
| I | 48 (21%) | 33 (15%) | p = 0.644 | 53 (23%) | 28 (12%) | p = 0.491 |
| II | 22 (9.7%) | 9 (4.0%) | 23 (10%) | 8 (3.5%) | ||
| III | 29 (13%) | 11 (4.8%) | 34 (15%) | 6 (2.6%) | ||
| IV | 31 (14%) | 44 (19%) | 33 (15%) | 42 (19%) | ||
| Test Results | Tumor Tissue | Plasma |
|---|---|---|
| (Reference: cobas v2) | (n = 217) | (n = 201) |
| Concordant Results | ||
| Both Wild | 123 (57%) | 165 (82%) |
| Both Mutant | 75 (35%) | 21 (10%) |
| Ex19del | 17 (8.5%) | 11 (5.5%) |
| L858R | 14 (6.5%) | 3 (1.4%) |
| T790M | 1 (0.5%) | 2 (1.0%) |
| L858R+T790M | 30 (14%) | 3 (1.5%) |
| Ex19del+T790M | 13 (6.0%) | 2 (1.0%) |
| Discordant Result | ||
| P_Mutant/c_Wilda | 14 (6.5%) | 7 (3.5%) |
| c_Mutant/P_Wildb | 1 (0.5%) | 6 (3.0%) |
| Different Mutant | 4 (1.8%) | 2 (1.0%) |
| Test Evaluation | ||
| Sensitivity (PPA)c(95% CI) | 94% (86%–97%) | 72% (54%–85%) |
| Specificity (NPA)c(95% CI) | 90% (84%–94%) | 96% (92%–98%) |
| Accuracy (OPA)c (95% CI) | 91% (87%–94%) | 93% (88%–95%) |
| Kappa Coefficient(95% CI) | 0.82 (0.74–0.92) | 0.69 (0.54–0.84) |
| No | TNM Stage | PANAMutyper | Cobas v2 | PNA Clamping | dd-PCR |
|---|---|---|---|---|---|
| 1 | IIa | Ex19del | Wild | Ex19del | - |
| 2 | Ib | Ex19del | Wild | Wild | - |
| 3 | Ia | Ex20ins | Wild | Wild | - |
| 4 | IIIa | G719S | Wild | Wild | - |
| 5 | IIa | G719S | Wild | Wild | - |
| 6 | IIIb | G719S+T790M | Wild | Wild | - |
| 7 | IIIb | L858R | Wild | Wild | Wild |
| 8 | Ib | L858R | Wild | Wild | - |
| 9 | IIIb | L858R | Wild | Wild | L858R |
| 10 | Ia | L858R | Wild | L858R a | - |
| 11 | IV | L858R | Wild | L858R a | - |
| 12 | IV | L858R | Wild | L858R | L858R |
| 13 | IIIa | T790M | Wild | Ex20 Gln787Gln b | - |
| 14 | Ia | T790M | Wild | Wild | - |
| 15 | IV | L858R+T790M | T790M | L858R+T790M | L858R+T790M |
| 16 | Ia | G719C+S768I+T790M | G719X+S768I | - | - |
| 17 | IV | Ex19del | Ex19del+T790M | Ex19del+T790M | Ex19del+T790M |
| 18 | IV | Ex19del+T790M | Ex19del | Ex19del+T790M | Ex19del+T790M |
| 19 | IV | Wild | Ex19del | T790M | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-O.; Shin, J.-Y.; Kim, S.R.; Shin, K.S.; Kim, J.; Kim, M.-Y.; Lee, M.-R.; Kim, Y.; Kim, M.; Hong, S.H.; et al. Evaluation of Two EGFR Mutation Tests on Tumor and Plasma from Patients with Non-Small Cell Lung Cancer. Cancers 2020, 12, 785. https://doi.org/10.3390/cancers12040785
Kim J-O, Shin J-Y, Kim SR, Shin KS, Kim J, Kim M-Y, Lee M-R, Kim Y, Kim M, Hong SH, et al. Evaluation of Two EGFR Mutation Tests on Tumor and Plasma from Patients with Non-Small Cell Lung Cancer. Cancers. 2020; 12(4):785. https://doi.org/10.3390/cancers12040785
Chicago/Turabian StyleKim, Jeong-Oh, Jung-Young Shin, Seo Ree Kim, Kab Soo Shin, Joori Kim, Min-Young Kim, Mi-Ran Lee, Yonggoo Kim, Myungshin Kim, Sook Hee Hong, and et al. 2020. "Evaluation of Two EGFR Mutation Tests on Tumor and Plasma from Patients with Non-Small Cell Lung Cancer" Cancers 12, no. 4: 785. https://doi.org/10.3390/cancers12040785
APA StyleKim, J.-O., Shin, J.-Y., Kim, S. R., Shin, K. S., Kim, J., Kim, M.-Y., Lee, M.-R., Kim, Y., Kim, M., Hong, S. H., & Kang, J. H. (2020). Evaluation of Two EGFR Mutation Tests on Tumor and Plasma from Patients with Non-Small Cell Lung Cancer. Cancers, 12(4), 785. https://doi.org/10.3390/cancers12040785





