Incidence of Occult Lymph Node Metastasis in Primary Larynx Squamous Cell Carcinoma, by Subsite, T Classification and Neck Level: A Systematic Review
Abstract
1. Introduction
2. Results
2.1. Description of Patients Included in the Studies
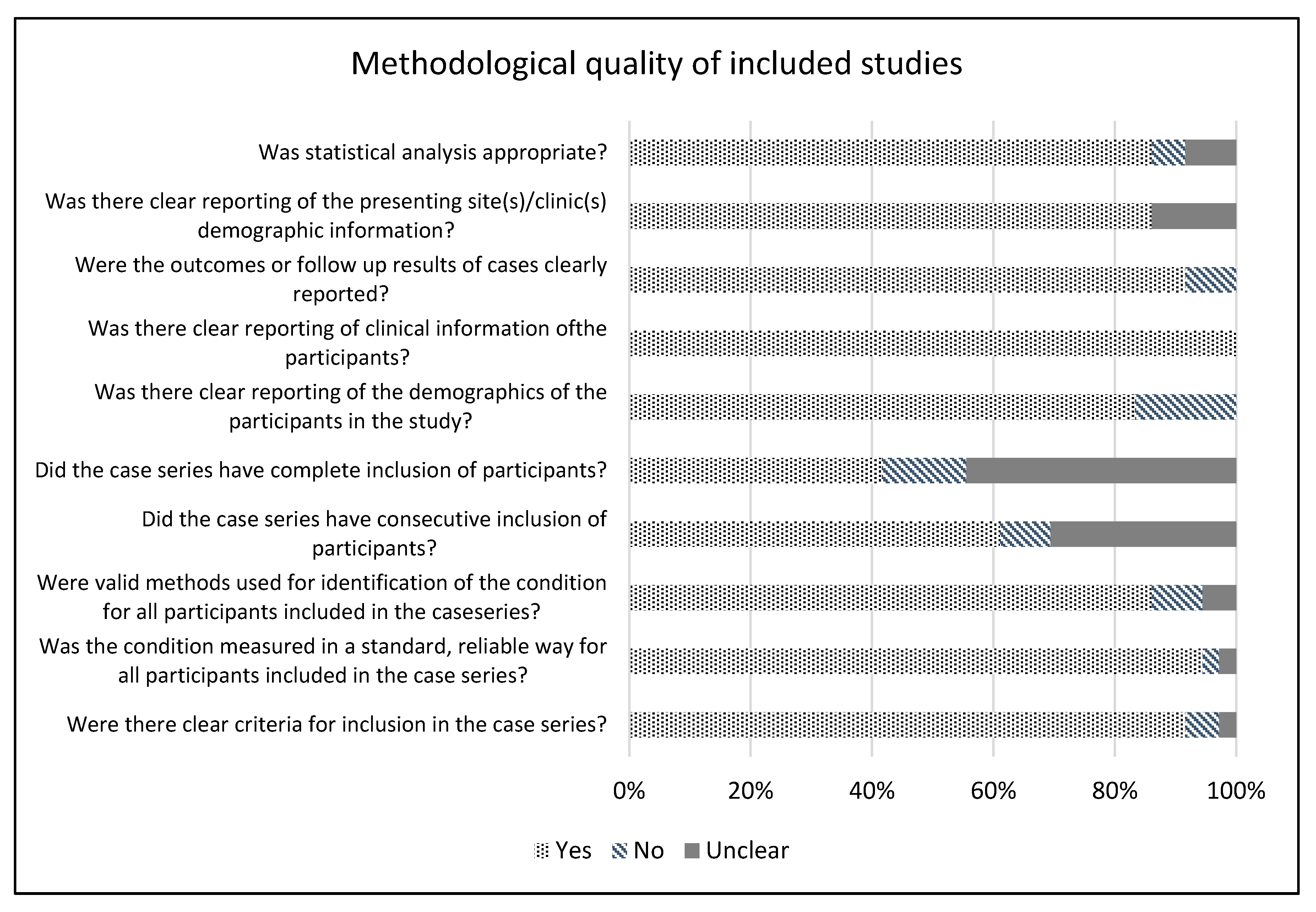
2.2. Methodological Quality
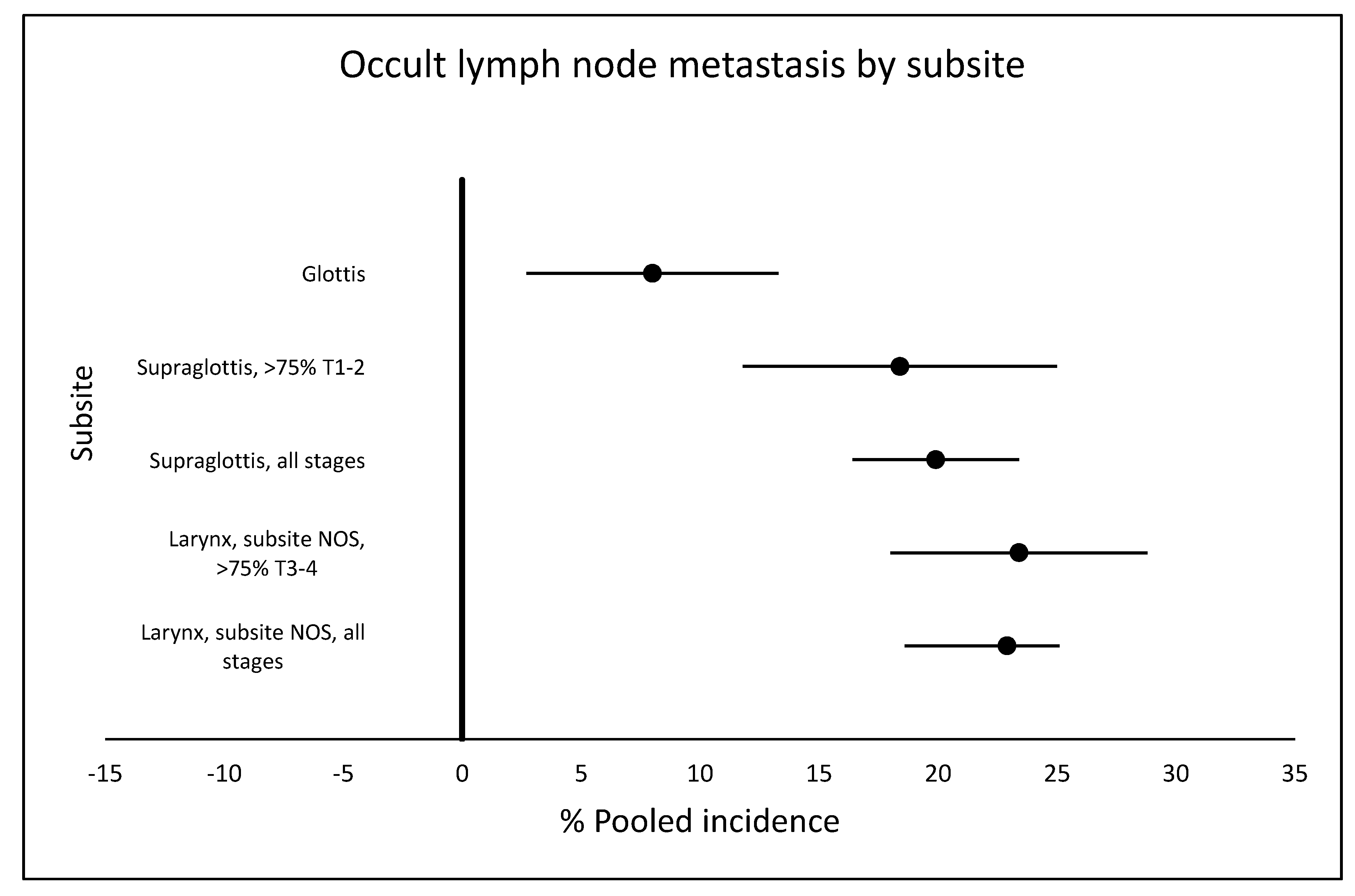
2.3. Rate of Occult Metastasis
2.3.1. Supraglottic Tumors
2.3.2. Glottic Tumors
2.3.3. Larynx Tumors without Specification of Subsite
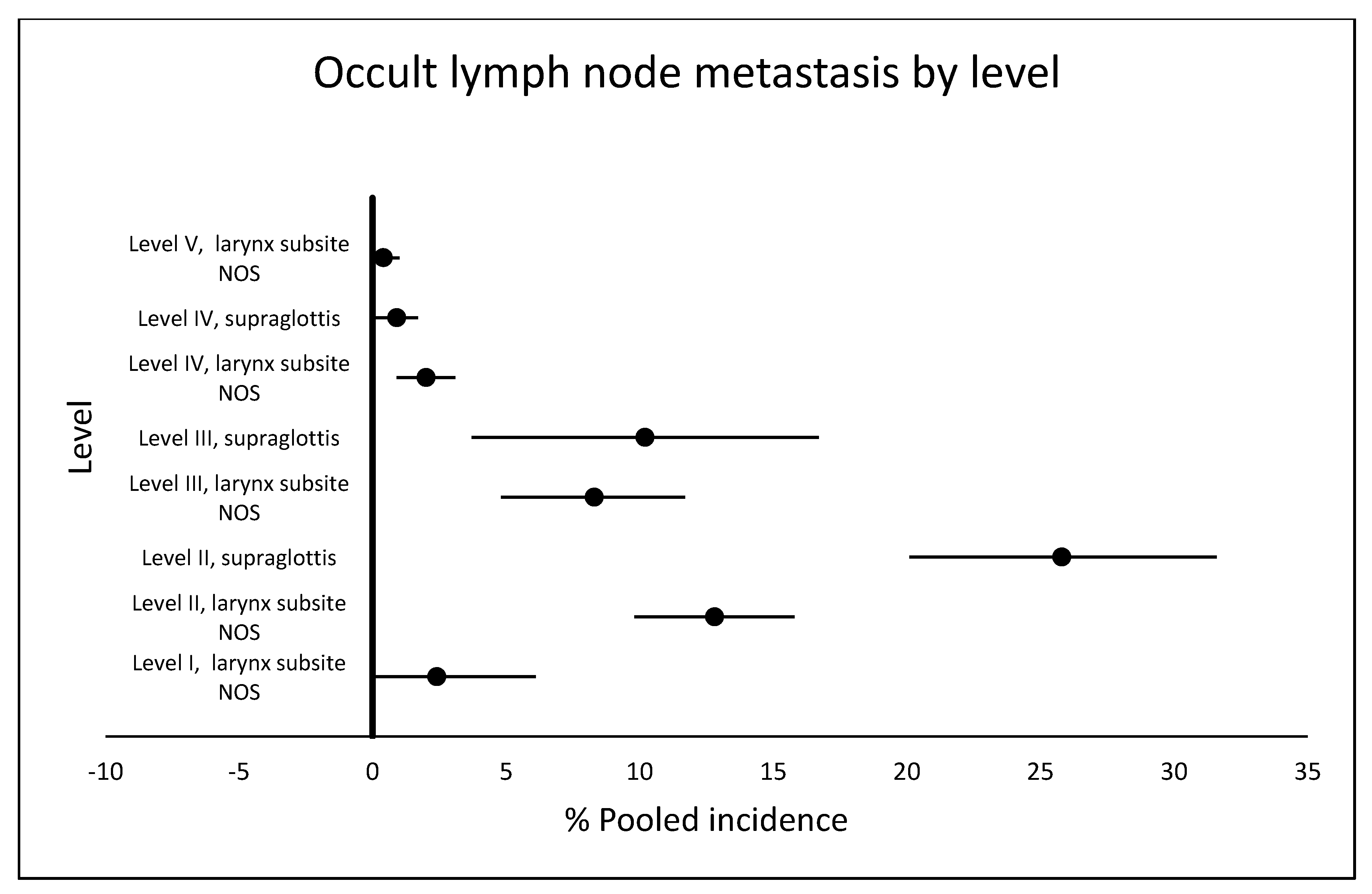
2.4. Occult Metastasis by Lymph Node Level
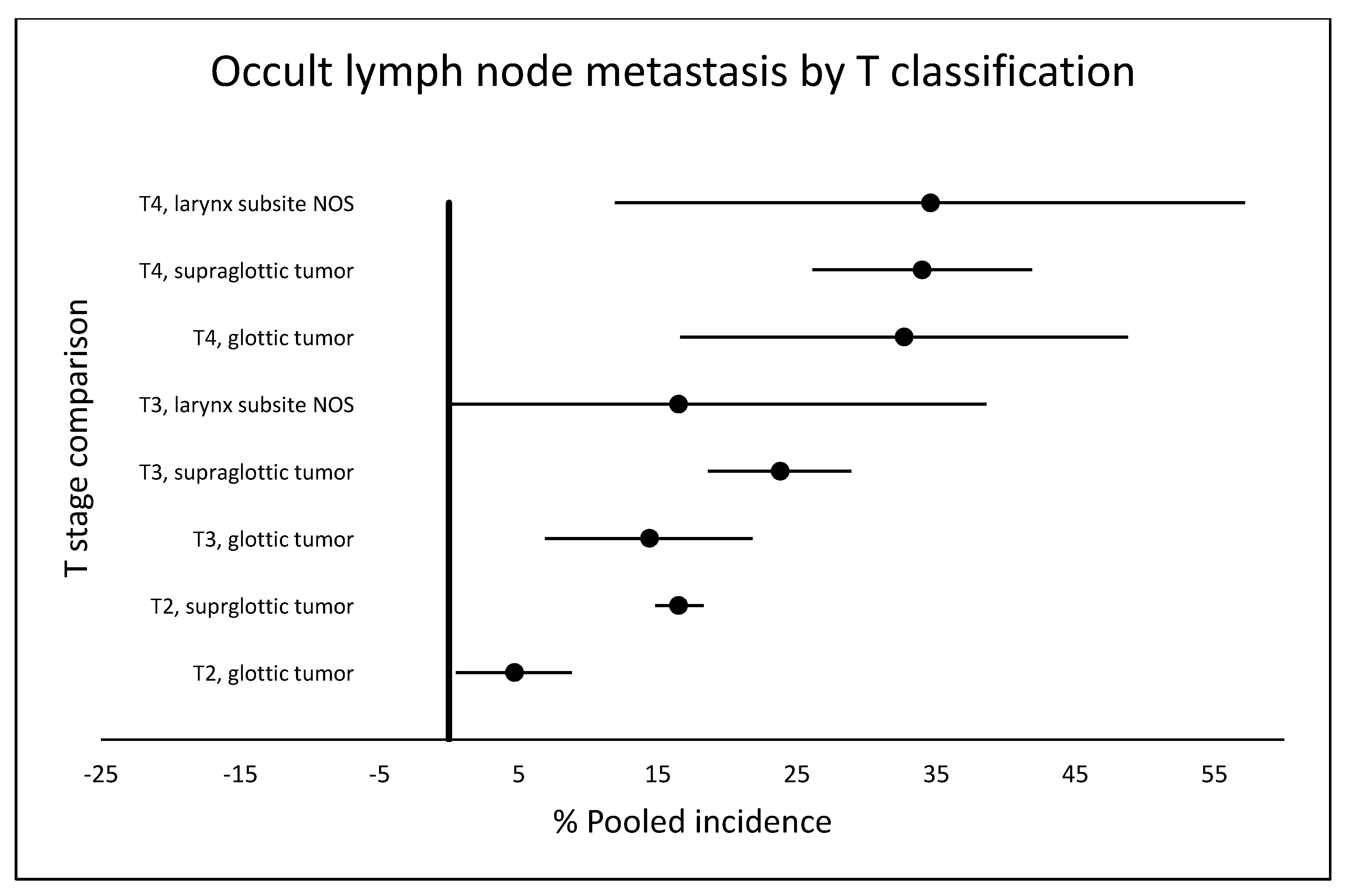
2.5. Occult Metastasis by T Classification
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Karim-Kos, H.E.; de Vries, E.; Soerjomataram, I.; Lemmens, V.; Siesling, S.; Coebergh, J.W. Recent trends of cancer in Europe: A combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur. J. Cancer 2008, 44, 1345–1389. [Google Scholar] [CrossRef] [PubMed]
- Pagedar, N.A.; Kahl, A.R.; Tasche, K.K.; Seaman, A.T.; Christensen, A.J.; Howren, M.B.; Charlton, M.E. Incidence trends for upper aerodigestive tract cancers in rural United States counties. Head Neck 2019, 41, 2619–2624. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, A.; Buckley, J.G.; Shaha, A.R.; Silver, C.E.; Rinaldo, A.; Kowalski, L. The role of neck dissection in the treatment of supraglottic laryngeal cancer. Acta Otolaryngol. 2001, 121, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, A.; Kowalski, L.P.; Silver, C.E.; Shaha, A.R.; Rinaldo, A.; Byers, R.M. The use and misuse of level IA dissection for head and neck cancer. Acta Otolaryngol. 2002, 122, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, A.; Rinaldo, A. Controversies in the treatment of N(0) neck in laryngeal cancer: Neck dissection, no surgery or sentinel lymph node biopsy? ORL J. Otorhinolaryngol. Relat. Spec. 2000, 62, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, A.; Silver, C.E.; Rinaldo, A. Selective neck dissection (IIA, III): A rational replacement for complete functional neck dissection in patients with N0 supraglottic and glottic squamous carcinoma. Laryngoscope 2008, 118, 676–679. [Google Scholar] [CrossRef]
- Rinaldo, A.; Ferlito, A.; Kowalski, L.P.; Quer, M.; Suárez, C.; León, X.; Robbins, K.T. Is dissection of level V necessary in patients with T(2)-T(4)N(0) supraglottic cancer? J. Laryngol. Otol. 2004, 118, 175–178. [Google Scholar] [CrossRef][Green Version]
- Rodrigo, J.P.; Shah, J.P.; Silver, C.E.; Medina, J.E.; Takes, R.P.; Robbins, K.T.; Rinaldo, A.; Werner, J.A.; Ferlito, A. Management of the clinically negative neck in early-stage head and neck cancers after transoral resection. Head Neck 2011, 33, 1210–1219. [Google Scholar] [CrossRef]
- Pantel, M.; Wittekindt, C.; Altendorf-Hofmann, A.; Boeger, D.; Buentzel, J.; Esser, D.; Mueller, A.; Wendt, T.G.; Guntinas-Lichius, O. Diversity of treatment of T2N0 glottic cancer of the larynx: Lessons to learn from epidemiological cancer registry data. Acta Otolaryngol. 2011, 131, 1205–1213. [Google Scholar] [CrossRef]
- Deganello, A.; Gitti, G.; Meccariello, G.; Parrinello, G.; Mannelli, G.; Gallo, O. Effectiveness and pitfalls of elective neck dissection in N0 laryngeal cancer. Acta Otorhinolaryngol. Ital. 2011, 31, 216–221. [Google Scholar]
- Flach, G.B.; Bloemena, E.; van Schie, A.; Hoekstra, O.S.; van Weert, S.; Leemans, C.R.; de Bree, R. Sentinel node identification in laryngeal cancer: Feasible in primary cancer with previously untreated neck. Oral Oncol. 2013, 49, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Levendag, P.; Vikram, B. The problem of neck relapse in early stage supraglottic cancer--results of different treatment modalities for the clinically negative neck. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 1621–1624. [Google Scholar] [CrossRef]
- Candela, F.C.; Shah, J.; Jaques, D.P.; Shah, J.P. Patterns of cervical node metastases from squamous carcinoma of the larynx. Arch. Otolaryngol. 1990, 116, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, H.H.; Allen, G.C. The influence of elective neck dissection on neck relapse in NO supraglottic carcinoma. Am. J. Otolaryngol. 1993, 14, 278–281. [Google Scholar] [CrossRef]
- Kligerman, J.; Olivatto, L.O.; Lima, R.A.; Freitas, E.Q.; Soares, J.R.; Dias, F.L.; Melo, L.E.; Sa, G.M.; Duccini, E. Elective neck dissection in the treatment of T3/T4 N0 squamous cell carcinoma of the larynx. Am. J. Surg. 1995, 170, 436–439. [Google Scholar] [CrossRef]
- Petrovic, Z.; Krejovic, B.; Janosevic, S. Occult metastases from supraglottic laryngeal carcinoma. Clin. Otolaryngol. Allied Sci. 1997, 22, 522–524. [Google Scholar] [CrossRef]
- Yang, C.Y.; Andersen, P.E.; Everts, E.C.; Cohen, J.I. Nodal disease in purely glottic carcinoma: Is elective neck treatment worthwhile? Laryngoscope 1998, 108, 1006–1008. [Google Scholar] [CrossRef]
- Güney, E.; Yigitbasi, O.G. Management of No neck in T1–T2 unilateral supraglottic cancer. Ann. Otol. Rhinol. Laryngol. 1999, 108, 998–1003. [Google Scholar] [CrossRef]
- Tu, G.Y. Upper neck (level II) dissection for N0 neck supraglottic carcinoma. Laryngoscope 1999, 109, 467–470. [Google Scholar] [CrossRef]
- León, X.; Quer, M.; Orús, C.; Sancho, F.J.; Bagué, S.; Burgués, J. Selective dissection of levels II-III with intraoperative control of the upper and middle jugular nodes: A therapeutic option for the N0 neck. Head Neck 2001, 23, 441–446. [Google Scholar] [CrossRef]
- Elo, J.; Balatoni, Z.; Kotai, Z.; Bartfai, R. Considerations in the treatment of the node-negative (N0) neck in glottic carcinomas. Pathol. Oncol. Res. 2002, 8, 5. [Google Scholar] [CrossRef]
- Amoros, L.; Carrasco, M.; Lopez, C.; Pla, A.; Ferrer, M.; Estelles, J.; Lopez, R. Tratamiento del cuello N0 en el cáncer supraglótico. Acta Otorrinolaringol. Esp. 2003, 54, 7. [Google Scholar]
- Pinilla, M.; Gonzalez, F.M.; Lopez-Cortijo, C.; Arellano, B.; Herrero, J.; Trinidad, A.; Vergara, J. Management of N0 neck in laryngeal carcinoma. Impact on patient’s survival. J. Laryngol. Otol. 2003, 117, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Spriano, G.; Piantanida, R.; Pellini, R.; Muscatello, L. Elective treatment of the neck in squamous cell carcinoma of the larynx: Clinical experience. Head Neck 2003, 25, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Coskun, H.H.; Erisen, L.; Basut, O. Selective neck dissection for clinically N0 neck in laryngeal cancer: Is dissection of level IIb necessary? Otolaryngol. Head Neck Surg. 2004, 131, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Khafif, A.; Fliss, D.M.; Gil, Z.; Medina, J.E. Routine inclusion of level IV in neck dissection for squamous cell carcinoma of the larynx: Is it justified? Head Neck 2004, 26, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, M.N.; Mahfouz, M.E.; Salim, E.I.; Elsheikh, E.A. Molecular assessment of neck dissections supports preserving level IIB lymph nodes in selective neck dissection for laryngeal squamous cell carcinoma with a clinically negative neck. ORL J. Otorhinolaryngol. Relat. Spec. 2006, 68, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Fiorella, R.; Di Nicola, V.; Fiorella, M.L.; Russo, C. “Conditional” neck dissection in management of laryngeal carcinoma. Acta Otorhinolaryngol. Ital. 2006, 26, 356–359. [Google Scholar]
- Lim, Y.C.; Choi, E.C.; Lee, J.S.; Koo, B.S.; Song, M.H.; Shin, H.A. Is dissection of level IV absolutely necessary in elective lateral neck dissection for clinically N0 laryngeal carcinoma? Oral Oncol. 2006, 42, 102–107. [Google Scholar] [CrossRef]
- Rodrigo, J.P.; Cabanillas, R.; Franco, V.; Suarez, C. Efficacy of routine bilateral neck dissection in the management of the N0 neck in T1-T2 unilateral supraglottic cancer. Head Neck 2006, 28, 534–539. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Z.G.; Tang, P.Z. Elective lateral neck dissection for laryngeal cancer in the clinically negative neck. J. Surg. Oncol. 2006, 93, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Cağli, S.; Yüce, I.; Güney, E. Is routine inclusion of level IV necessary in neck dissection for clinically N0 supraglottic carcinoma? Otolaryngol. Head Neck Surg. 2007, 136, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Cağli, S.; Yüce, I.; Yiğitbaşi, O.G.; Güney, E. Is routine bilateral neck dissection absolutely necessary in the management of N0 neck in patients with supraglottic carcinoma? Eur. Arch. Otorhinolaryngol. 2007, 264, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Katilmis, H.; Ozturkcan, S.; Ozdemir, I.; Tuna, B.; Guvenc, I.A.; Ozkul, Y. Is dissection of levels 4 and 5 justified for cN0 laryngeal and hypopharyngeal cancer? Acta Otolaryngol. 2007, 127, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Yüce, I.; Cağli, S.; Bayram, A.; Güney, E. Occult metastases from T1-T2 supraglottic carcinoma: Role of primary tumor localization. Eur. Arch. Otorhinolaryngol. 2009, 266, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Lawson, G.; Matar, N.; Nollevaux, M.C.; Jamart, J.; Krug, B.; Delos, M.; Remacle, M.; Borght, T.V. Reliability of sentinel node technique in the treatment of N0 supraglottic laryngeal cancer. Laryngoscope 2010, 120, 2213–2217. [Google Scholar] [CrossRef] [PubMed]
- Mnejja, M.; Hammami, B.; Bougacha, L.; Chakroun, A.; Charfeddine, I.; Khabir, A.; Boudaoura, T.; Ghorbel, A. Occult lymph node metastasis in laryngeal squamous cell carcinoma: Therapeutic and prognostic impact. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2010, 127, 173–176. [Google Scholar] [CrossRef]
- Csanady, M.; Czigner, J.; Vass, G.; Jori, J. Transoral CO2 laser management for selected supraglottic tumors and neck dissection. Eur. Arch. Otorhinolaryngol. 2011, 268, 1181–1186. [Google Scholar] [CrossRef]
- Chone, C.T.; Kohler, H.F.; Magalhaes, R.; Navarro, M.; Altemani, A.; Crespo, A.N. Levels II and III neck dissection for larynx cancer with N0 neck. Braz. J. Otorhinolaryngol. 2012, 78, 59–63. [Google Scholar] [CrossRef]
- Xu, Y.; Fei, M.; Wang, J.; Zheng, L.; Chen, Y.; Liu, Q. Clinical significance of micrometastases in lymph nodes from laryngeal squamous cell carcinoma. Am. J. Otolaryngol. 2012, 33, 402–407. [Google Scholar] [CrossRef]
- Erdag, T.K.; Guneri, E.A.; Avincsal, O.; Sarioglu, S.; Ecevit, M.C.; Guneri, A.; Ikiz, A.O. Is elective neck dissection necessary for the surgical management of T2N0 glottic carcinoma? Auris Nasus Larynx 2013, 40, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Wang, Y.; He, H.; Xiang, C. Incidence of level IIB lymph node metastasis in supraglottic laryngeal squamous cell carcinoma with clinically negative neck--a prospective study. Head Neck 2013, 35, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Furtado de Araújo Neto, V.J.; Cernea, C.R.; Aparecido Dedivitis, R.; Furtado de Araujo Filho, V.J.; Fabiano Palazzo, J.; Garcia Brandao, L. Cervical metastasis on level IV in laryngeal cancer. Acta Otorhinolaryngol. Ital. 2014, 34, 15–18. [Google Scholar] [PubMed]
- Ma, H.; Lian, M.; Feng, L.; Li, P.; Hou, L.; Chen, X.; Huang, Z.; Fang, J. Factors contributing to lymph node occult metastasis in supraglottic laryngeal carcinoma cT2-T4 N0M0 and metastasis predictive equation. Chin. J. Cancer Res. 2014, 26, 7. [Google Scholar]
- Djordjevic, V.; Bukurov, B.; Arsovic, N.; Dimitrijevic, M.; Jesic, S.; Nesic, V.; Petrovic, Z. Prospective case-control study of efficacy of bilateral selective neck dissection in primary surgical treatment of supraglottic laryngeal cancers with clinically negative cervical findings (N0). Clin. Otolaryngol. 2016, 41, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, N.; Hayashi, R.; Shinozaki, T.; Tomioka, T.; Okano, W.; Ikeda, M. The role of elective neck dissection for cT4aN0 glottic squamous cell carcinoma. Jpn. J. Clin. Oncol. 2019, 49, 525–528. [Google Scholar] [CrossRef]
- Bottcher, A.; Dommerich, S.; Sander, S.; Olze, H.; Stromberger, C.; Coordes, A.; Jowett, N.; Knopke, S. Nodal yield of neck dissections and influence on outcome in laryngectomized patients. Eur. Arch. Otorhinolaryngol. 2016, 273, 3321–3329. [Google Scholar] [CrossRef]
- Dundar, R.; Aslan, H.; Ozbay, C.; Basoglu, S.; Guvenc, I.A.; Ogredik, E.A.; Ozturkcan, S.; Tayfun, M.A.; Katilmis, H. The necessity of dissection of level IIb in laryngeal squamous cell carcinoma: A clinical study. Otolaryngol. Head Neck Surg. 2012, 146, 390–394. [Google Scholar] [CrossRef]
- Gross, B.C.; Olsen, S.M.; Lewis, J.E.; Kasperbauer, J.L.; Moore, E.J.; Olsen, K.D.; Price, D.L. Level IIB lymph node metastasis in laryngeal and hypopharyngeal squamous cell carcinoma: Single-institution case series and review of the literature. Laryngoscope 2013, 123, 3032–3036. [Google Scholar] [CrossRef]
- Hicks, W.L., Jr.; Kollmorgen, D.R.; Kuriakose, M.A.; Orner, J.; Bakamjian, V.Y.; Winston, J.; Loree, T.R. Patterns of nodal metastasis and surgical management of the neck in supraglottic laryngeal carcinoma. Otolaryngol. Head Neck Surg. 1999, 121, 57–61. [Google Scholar] [CrossRef]
- Koybasioglu, A.; Uslu, S.; Yilmaz, M.; Inal, E.; Ileri, F.; Asal, K. Lymphatic metastasis to the supraretrospinal recess in laryngeal squamous cell carcinoma. Ann. Otol. Rhinol. Laryngol. 2002, 111, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Lee, J.S.; Koo, B.S.; Choi, E.C. Level IIb lymph node metastasis in laryngeal squamous cell carcinoma. Laryngoscope 2006, 116, 268–272. [Google Scholar] [CrossRef]
- Redaelli de Zinis, L.O.; Nicolai, P.; Tomenzoli, D.; Ghizzardi, D.; Trimarchi, M.; Cappiello, J.; Peretti, G.; Antonelli, A.R. The distribution of lymph node metastases in supraglottic squamous cell carcinoma: Therapeutic implications. Head Neck 2002, 24, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Sezen, O.S.; Kubilay, U.; Haytoglu, S.; Unver, S. Frequency of metastases at the area of the supraretrospinal (level IIB) lymph node in laryngeal cancer. Head Neck 2007, 29, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Villaret, A.B.; Piazza, C.; Peretti, G.; Calabrese, L.; Ansarin, M.; Chiesa, F.; Pellini, R.; Spriano, G.; Nicolai, P. Multicentric prospective study on the prevalence of sublevel IIb metastases in head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 897–903. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wiegand, S.; Esters, J.; Muller, H.H.; Jacker, T.; Roessler, M.; Fasunla, J.A.; Werner, J.A.; Sesterhenn, A.M. Relevance of level I and IIB neck dissection in laryngeal cancer. J. Laryngol. Otol. 2012, 126, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.X.; Li, Y.S.; Wang, Z.H.; Liu, C.; Lu, T.; Zeng, Q.; Wang, X.Q.; Zhu, J.; Cao, Y.D.; Hu, G.H. Characteristics of cervical lymph node metastasis of cN0 laryngeal carcinoma. Chin. J. Otorhinolaryngol. Head Neck Surg. 2019, 54, 343–348. [Google Scholar] [CrossRef]
- Lansaat, L.; van der Noort, V.; Bernard, S.E.; Eerenstein, S.E.J.; Plaat, B.E.C.; Langeveld, T.; Lacko, M.; Hilgers, F.J.M.; de Bree, R.; Takes, R.P.; et al. Predictive factors for pharyngocutaneous fistulization after total laryngectomy: A Dutch Head and Neck Society audit. Eur. Arch. Otorhinolaryngol. 2018, 275, 783–794. [Google Scholar] [CrossRef]
- Strojan, P.; Hutcheson, K.A.; Eisbruch, A.; Beitler, J.J.; Langendijk, J.A.; Lee, A.W.M.; Corry, J.; Mendenhall, W.M.; Smee, R.; Rinaldo, A.; et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat. Rev. 2017, 59, 79–92. [Google Scholar] [CrossRef]
- Welsh, L.W. The normal human laryngeal lymphatics. Ann. Otol. Rhinol. Laryngol. 1964, 73, 569–582. [Google Scholar] [CrossRef]
- Welsh, L.W.; Welsh, J.J. Laryngeal lymphatics, human in vivo studies. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1963, 67, 524–529. [Google Scholar] [PubMed]
- Welsh, L.W.; Welsh, J.J.; Rizzo, T.A., Jr. Laryngeal spaces and lymphatics: Current anatomic concepts. Ann. Otol. Rhinol. Laryngol. Suppl. 1983, 105, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Xu, S.C.; Tu, L.L.; Zhang, K.L.; Lu, D.H.; Zhang, M. A rich lymphatic network exists in the inferior surface of the vocal cord. Surg. Radiol. Anat. 2006, 28, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, A.; Devaney, K.O.; Ferlito, A. Immunohistochemical studies in the identification of lymph node micrometastases in patients with squamous cell carcinoma of the head and neck. ORL J. Otorhinolaryngol. Relat. Spec. 2004, 66, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Brazilian Head and Neck Cancer Study Group. End results of a prospective trial on elective lateral neck dissection vs. type III modified radical neck dissection in the management of supraglottic and transglottic carcinomas. Head Neck 1999, 21, 694–702. [Google Scholar] [CrossRef]
- Ferlito, A.; Silver, C.E.; Suarez, C.; Rinaldo, A. Preliminary multi-institutional prospective pathologic and molecular studies support preservation of sublevel IIB and level IV for laryngeal squamous carcinoma with clinically negative neck. Eur. Arch. Otorhinolaryngol. 2007, 264, 111–114; discussion 109. [Google Scholar] [CrossRef]
- Rinaldo, A.; Elsheikh, M.N.; Ferlito, A.; Chone, C.T.; Coskun, H.H.; Koybasioglu, A.; Esclamado, R.M.; Corlette, T.H.; Talmi, Y.P. Prospective studies of neck dissection specimens support preservation of sublevel IIB for laryngeal squamous carcinoma with clinically negative neck. J. Am. Coll. Surg. 2006, 202, 967–970. [Google Scholar] [CrossRef]
- Ferlito, A.; Robbins, K.T.; Shah, J.P.; Medina, J.E.; Silver, C.E.; Al-Tamimi, S.; Fagan, J.J.; Paleri, V.; Takes, R.P.; Bradford, C.R.; et al. Proposal for a rational classification of neck dissections. Head Neck 2011, 33, 445–450. [Google Scholar] [CrossRef]
- Antonelli, A.R.; Nicolai, P.; Cappiello, J.; Peretti, G.; Molinari Tosatti, M.P.; Rosa, D.; Grigolato, P.G.; Favret, M.; Maroccolo, D. Basement membrane components in normal, dysplastic, neoplastic laryngeal tissue and metastatic lymph nodes. Acta Otolaryngol. 1991, 111, 437–443. [Google Scholar] [CrossRef]
- Ozdek, A.; Sarac, S.; Akyol, M.U.; Unal, O.F.; Sungur, A. Histopathological predictors of occult lymph node metastases in supraglottic squamous cell carcinomas. Eur. Arch. Otorhinolaryngol. 2000, 257, 389–392. [Google Scholar] [CrossRef]
- Kaur, K.; Sonkhya, N.; Bapna, A.S. Nodal metastases from laryngeal carcinoma and their correlation with certain characteristics of the primary tumor. Indian J. Otolaryngol. Head Neck Surg. 2002, 54, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Makitie, A.A.; Almangush, A.; Rodrigo, J.P.; Ferlito, A.; Leivo, I. Hallmarks of cancer: Tumor budding as a sign of invasion and metastasis in head and neck cancer. Head Neck 2019, 41, 3712–3718. [Google Scholar] [CrossRef] [PubMed]
- van Hooff, S.R.; Leusink, F.K.; Roepman, P.; Baatenburg de Jong, R.J.; Speel, E.J.; van den Brekel, M.W.; van Velthuysen, M.L.; van Diest, P.J.; van Es, R.J.; Merkx, M.A.; et al. Validation of a gene expression signature for assessment of lymph node metastasis in oral squamous cell carcinoma. J. Clin. Oncol. 2012, 30, 4104–4110. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.G.; Crispim, J.C.; Miranda, F.A.; Hassumi, M.K.; de Mello, J.M.; Simoes, R.T.; Souto, F.; Soares, E.G.; Donadi, E.A.; Soares, C.P. Expression of the nonclassical HLA-G and HLA-E molecules in laryngeal lesions as biomarkers of tumor invasiveness. Histol. Histopathol. 2011, 26, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Neyeloff, J.L.; Fuchs, S.C.; Moreira, L.B. Meta-analyses and Forest plots using a microsoft excel spreadsheet: Step-by-step guide focusing on descriptive data analysis. BMC Res. Notes 2012, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual; Aromataris, E., Munn, Z., Eds.; The Joanna Briggs Institute: Adelaide, Australia, 2017. [Google Scholar]
| Author | Year | Country | Time Period | Study Type | Design | N | Subsite | cN+ | T1–2/T3–4 | Partial/Total Laryngectomy | % Occult Metastasis | Neck Level | T Classification | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | IIB | T1 | T1t | T2 | T2t | T3 | T3t | T4 | T4t | ||||||||||||
| Levendag and Vikram [12] | 1987 | USA | 1965–1979 | CS | R | 79 | S | 79/0 | 31/48 | 10 | |||||||||||||||
| Candela et al. [13] | 1990 | USA | 1965–1986 | CS | R | 78 | LNOS | 36/42 | NR | 29 | 4 | 15 | 16 | 7 | 2 | ||||||||||
| Ramadan and Allen [14] | 1993 | USA | 1975–1986 | CS | R | 63 | S | 16/49 | NR | 7 | 1 | 4 | 3 | 8 | 3 | 9 | |||||||||
| Kligerman et al. [15] | 1995 | Brazil | 1981–1989 | CS | R | 76 | LNOS | 0/76 | 1/75 | 23 | 16 | 56 | 7 | 20 | |||||||||||
| Petrovic et al. [16] | 1997 | Yugoslavia | 1976–1990 | CS | R | 161 | S | 90/71 | NR | 29 | 4 | 22 | 9 | 68 | 6 | 45 | 10 | 26 | |||||||
| Yang et al. [17] | 1998 | USA | 1984–1994 | CS | R | 92 | G | 71/21 | NR | 4 | 0 | 0 | 0 | 0 | 3 | 14 | 1 | 7 | |||||||
| Güney and Yigitbasi [18] | 1999 | Turkey | 1991–1996 | CS | R | 39 | S | 39/0 | NR | 9 | |||||||||||||||
| Tu [19] | 1999 | China | 1976–1990 | CS | R | 155 | S | 51/91 | 128/34 | 13 | |||||||||||||||
| León et al. [20] | 2001 | Spain | 1991–1997 | CS | P | 79 | LNOS | 23/56 | NR | 23 | 2 | 0 | |||||||||||||
| Elo et al. [21] | 2002 | Hungary | 1989–1999 | CS | R | 206 | G | 133/73 | 133/73 | 24 | 0 | 61 | 6 | 72 | 9 | 54 | 9 | 19 | |||||||
| Amoros et al. [22] | 2003 | Spain | 1977–1999 | CS | R | 164 | S | 103/61 | NR | 40 | |||||||||||||||
| Pinilla et al. [23] | 2003 | Spain | 1983–1993 | CS | R R | 124 | S | 56/68 | NR | 34 | 0 | 6 | 9 | 50 | 9 | 42 | 16 | 26 | |||||||
| 66 | G | 44/22 | NR | 9 | 0 | 1 | 1 | 43 | 6 | 18 | 2 | 4 | |||||||||||||
| Spriano et al. [24] | 2003 | Italy | 1980–1997 | CS | R | 346 | LNOS | NR | NR | 59 | 56 | 33 | 5 | 10 | |||||||||||
| Coskun et al. [25] | 2004 | Turkey | 1999–2002 | CS | P | 71 | LNOS | 12/59 | NR | 14 | 0 | 0 | |||||||||||||
| Khafif et al. [26] | 2004 | Israel USA | CS | R | 43 | LNOS | 0/43 | 0/100 | 9 | 1 | |||||||||||||||
| Elsheikh et al. [27] | 2006 | Egypt | 2001–2004 | CS | P | 31 | LNOS | 19/12 | NR | 6 | 2 | 3 | 1 | 0 | |||||||||||
| Fiorella et al. [28] | 2006 | Italy | CS | R | 106 | S | 50/56 | NR | 29 | 0, 16 | 19 | 53 | 2 | 3 | |||||||||||
| Lim et al. [29] | 2006 | Korea | 1997–2002 | CS | P | 73 | LNOS | 33/40 | 16/53 | 21 | 12 | 9 | 5 | 0 | 3 | 1 | 30 | 2 | 34 | 2 | 6 | ||||
| Rodrigo et al. [30] | 2006 | Spain | 1975–1998 | CS | R | 108 | S | 108/0 | 108/0 | 16 | |||||||||||||||
| Zhang et al. [31] | 2006 | China | 1997–2002 | CS | R | 72 | S | 36/36 | NR | 15 | 1 | 12 | 6 | 24 | 7 | 30 | 1 | 6 | |||||||
| 38 | G | 13/25 | NR | 7 | 1 | 13 | 2 | 14 | 4 | 11 | |||||||||||||||
| Cağli et al. [33] | 2007 | Turkey | 1998–2006 | CS | R | 72 | S | 12/60 | NR | 16 | 16 | 7 | 1 | 1 | 12 | 10 | 44 | 5 | 16 | ||||||
| Cağli et al. [32] | 2007 | Turkey | CS | R | 58 | S | 22/36 | NR | 14 | 11 | 7 | 1 | 3 | 22 | 7 | 28 | 4 | 8 | |||||||
| Katilmis et al. [34] | 2007 | Turkey | 1998–2003 | CS | R | 224 | LNOS | 79/145 | 60/164 | 24 | 12 | 7 | 0 | ||||||||||||
| Yüce et al. [35] | 2009 | Turkey | 1991–2005 | CC | R | 71 | S | 71/0 | 67/4 | 9 | |||||||||||||||
| Lawson et al. [36] | 2010 | Belgium | 2001–2004 | CS | P | 29 | S | 25/6 | 29/0 | 14 | |||||||||||||||
| Mnejja et al. [37] | 2010 | Tunisia | 1990–2007 | CS | R | 164 | LNOS | 36/128 | NR | 32 | 9 | 8 | |||||||||||||
| Csanady et al. [38] | 2011 | Hungary | 1987–2006 | CS | R | 55 | S | 55/0 | 55/0 | 15 | |||||||||||||||
| Deganello et al. [10] | 2011 | Italy | 2000–2004 | CS | R | 96 | LNOS | 55/41 | 82/14 | 12 | 1 | 10 | 3 | 2 | 0 | ||||||||||
| 57 | S | 35/22 | NR | 9 | 4 | 24 | 3 | 10 | 2 | 12 | |||||||||||||||
| Deganello et al. [10] | 2011 | Italy | 2000–2004 | CS | R | 39 | G | 20/19 | NR | 3 | 1 | 17 | 2 | 2 | |||||||||||
| Chone et al. [39] | 2012 | Brazil | 2007–2011 | CS | P | 20 | LNOS | NR | NR | NR | 0 | ||||||||||||||
| Xu et al. [40] | 2012 | China | 1996–2009 | CS | R | 126 | LNOS | 15/111 | NR | 41 | |||||||||||||||
| Erdag et al. [41] | 2013 | Turkey | 1996–2009 | CS | R | 24 | G | 24/0 | 24/0 | 0 | |||||||||||||||
| Jia et al. [42] | 2013 | China | 2002–2010 | CS | P | 68 | S | 36/32 | 52/16 | 21 | 0 | 9 | 36 | 10 | 27 | 2 | 5 | ||||||||
| Furtado de Araújo Neto et al. [43] | 2014 | Brazil | 2007–2012 | CS | R | 77 | LNOS | 0/77 | NR | 12 | 3 | ||||||||||||||
| Ma et al. [44] | 2014 | China | 2002–2013 | CS | R | 121 | S | 39/82 | 66/55 | 34 | 22 | 21 | 4 | 2 | 6 | 39 | 13 | 40 | 15 | 42 | |||||
| Djordjevic et al. [45] | 2016 | Serbia | 1996–2005 | CC | R | 193 | S | 107/86 | NR | 35 | 31 | 8 | 1 | ||||||||||||
| Tsushima et al. [46] | 2019 | Japan | 1998–2014 | CS | R | 39 | LNOS | 0/39 | 0/39 | 14 | 4 | 3 | 0 | 0 | |||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanabria, A.; Shah, J.P.; Medina, J.E.; Olsen, K.D.; Robbins, K.T.; Silver, C.E.; Rodrigo, J.P.; Suárez, C.; Coca-Pelaz, A.; Shaha, A.R.; et al. Incidence of Occult Lymph Node Metastasis in Primary Larynx Squamous Cell Carcinoma, by Subsite, T Classification and Neck Level: A Systematic Review. Cancers 2020, 12, 1059. https://doi.org/10.3390/cancers12041059
Sanabria A, Shah JP, Medina JE, Olsen KD, Robbins KT, Silver CE, Rodrigo JP, Suárez C, Coca-Pelaz A, Shaha AR, et al. Incidence of Occult Lymph Node Metastasis in Primary Larynx Squamous Cell Carcinoma, by Subsite, T Classification and Neck Level: A Systematic Review. Cancers. 2020; 12(4):1059. https://doi.org/10.3390/cancers12041059
Chicago/Turabian StyleSanabria, Alvaro, Jatin P. Shah, Jesus E. Medina, Kerry D. Olsen, K. Thomas Robbins, Carl E. Silver, Juan P. Rodrigo, Carlos Suárez, Andrés Coca-Pelaz, Ashok R. Shaha, and et al. 2020. "Incidence of Occult Lymph Node Metastasis in Primary Larynx Squamous Cell Carcinoma, by Subsite, T Classification and Neck Level: A Systematic Review" Cancers 12, no. 4: 1059. https://doi.org/10.3390/cancers12041059
APA StyleSanabria, A., Shah, J. P., Medina, J. E., Olsen, K. D., Robbins, K. T., Silver, C. E., Rodrigo, J. P., Suárez, C., Coca-Pelaz, A., Shaha, A. R., Mäkitie, A. A., Rinaldo, A., de Bree, R., Strojan, P., Hamoir, M., Takes, R. P., Sjögren, E. V., Cannon, T., Kowalski, L. P., & Ferlito, A. (2020). Incidence of Occult Lymph Node Metastasis in Primary Larynx Squamous Cell Carcinoma, by Subsite, T Classification and Neck Level: A Systematic Review. Cancers, 12(4), 1059. https://doi.org/10.3390/cancers12041059













