The Role of CCL21/CCR7 Chemokine Axis in Breast Cancer Progression
Abstract
1. Introduction
2. Chemokine Receptors in Cancer
3. C-C Chemokine Receptor 7 (CCR7) in Cancer
3.1. Mode of Action of C-C Chemokine Receptor 7 (CCR7) in Migration and Adhesion
3.2. C-C Chemokine Receptor 7 (CCR7) and Angiogenesis
4. C-C Chemokine Receptor 7 (CCR7) and Breast Cancer
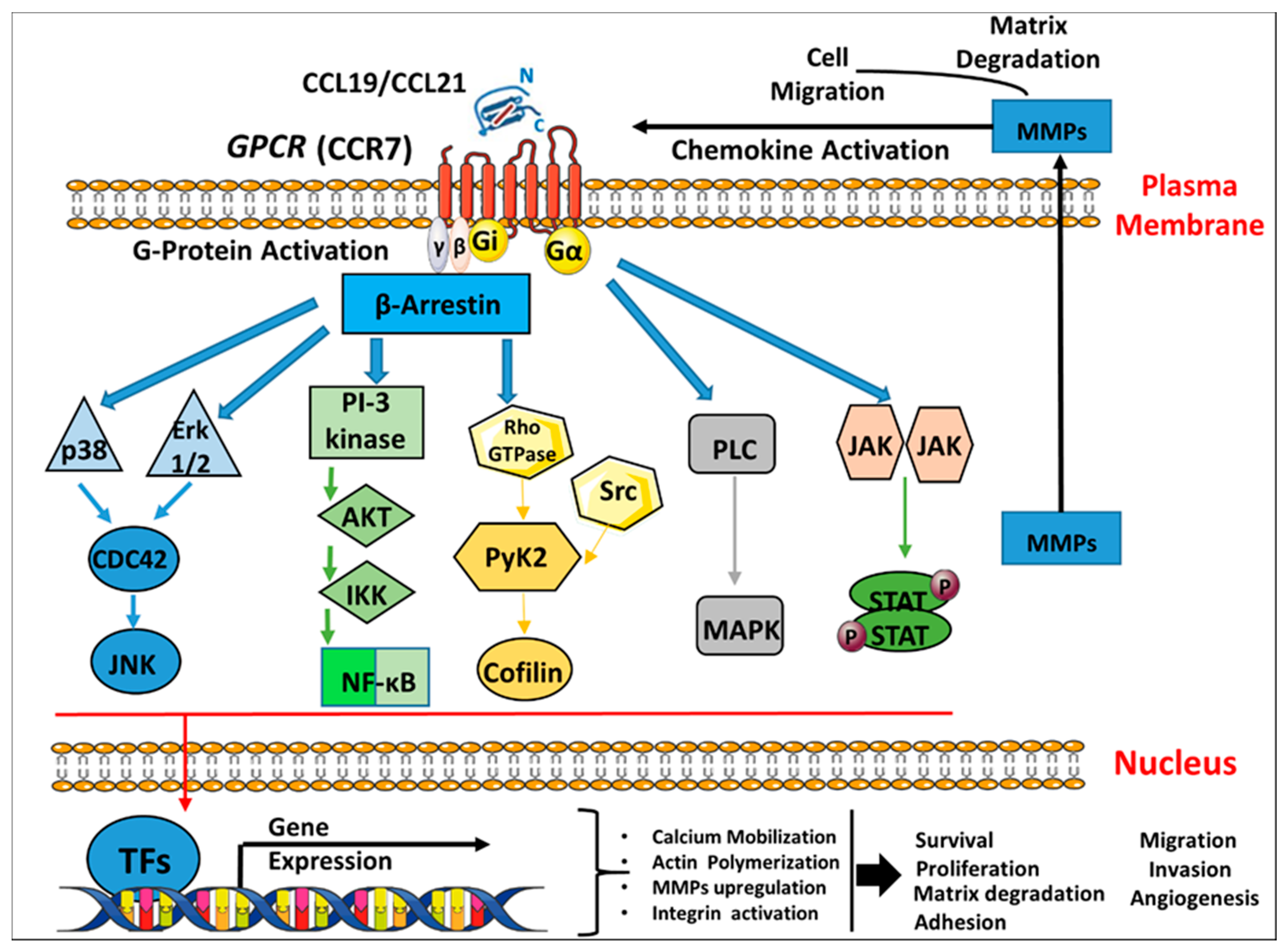
4.1. Molecular Aspects of C-C Chemokine Receptor 7 (CCR7) Signaling Cascades in Breast Cancer
4.2. The Expression and Functional Role of C-C Chemokine Receptor 7 (CCR7) in Breast Cancer Cells in Vitro
4.3. The Role of C-C Chemokine Receptor 7 (CCR7) on Breast Cancer Metastasis in Vivo
5. Therapy and Future Directions
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baggiolini, M. Chemokines and leukocyte traffic. Nature 1998, 392, 565–568. [Google Scholar] [CrossRef]
- Rossi, D.; Zlotnik, A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 2000, 18, 217–242. [Google Scholar] [CrossRef]
- Bachelerie, F.; Graham, G.J.; Locati, M.; Mantovani, A.; Murphy, P.M.; Nibbs, R.; Rot, A.; Sozzani, S.; Thelen, M. An atypical addition to the chemokine receptor nomenclature: IUPHAR Review 15. Br. J. Pharmacol. 2015, 172, 3945–3949. [Google Scholar] [CrossRef]
- Sánchez-Madrid, F.; Del Pozo, M.A. Leukocyte polarization in cell migration and immune interactions. EMBO 1999, 18, 501–511. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. BBA 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Sozzani, S.; Allavena, P.; D’Amico, G.; Luini, W.; Bianchi, G.; Kataura, M.; Imai, T.; Yoshie, O.; Bonecchi, R.; Mantovani, A. Cutting edge: Differential regulation of chemokine receptors during dendritic cell maturation: A model for their trafficking properties. Immunology 1998, 161, 1083–1086. [Google Scholar]
- Forster, R.; Emrich, T.; Kremmer, E.; Lipp, M. Expression of the G-protein--coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood 1994, 84, 830–840. [Google Scholar] [CrossRef]
- Heydtmann, M.; Adams, D. Understanding selective trafficking of lymphocyte subsets. Gut 2002, 50, 150–152. [Google Scholar] [CrossRef]
- Geissmann, F.; Jung, S.; Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003, 19, 71–82. [Google Scholar] [CrossRef]
- Zlotnik, A.; Burkhardt, A.M.; Homey, B. Homeostatic chemokine receptors and organ-specific metastasis. Nat. Rev. Immunol. 2011, 11, 597–606. [Google Scholar] [CrossRef]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, X.; Langer, M.; Weber, C.; Koenen, R.R.; von Hundelshausen, P. Touch of chemokines. Front. Immunol. 2012, 3, 175. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.J.; Hayward, J.A.; Huang, C.E.; Huma, Z.; Sanchez, J. Mechanisms of regulation of the chemokine-receptor network. Int. J. Mol. Med. 2017, 18, 342. [Google Scholar]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559. [Google Scholar] [CrossRef]
- Tanaka, T.; Bai, Z.; Srinoulprasert, Y.; Yang, B.; Hayasaka, H.; Miyasaka, M. Chemokines in tumor progression and metastasis. Cancer Sci. 2005, 96, 317–322. [Google Scholar] [CrossRef]
- Zhou, J.; Xiang, Y.; Yoshimura, T.; Chen, K.; Gong, W.; Huang, J.; Zhou, Y.; Yao, X.; Bian, X.; Wang, J.M. The role of chemoattractant receptors in shaping the tumor microenvironment. BioMed Res. Int. 2014, 2014, 751392. [Google Scholar] [CrossRef]
- Sarvaiya, P.J.; Guo, D.; Ulasov, I.; Gabikian, P.; Lesniak, M.S. Chemokines in tumor progression and metastasis. Oncotarget 2013, 4, 2171. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.L.; MacDonald, I.C.; Koop, S.; Schmidt, E.E.; Chambers, A.F.; Groom, A.C. Early interactions of cancer cells with the microvasculature in mouse liver and muscle during hematogenous metastasis: Videomicroscopic analysis. Clin. Exp. Metastasis 1993, 11, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, G.; Richmond, A. Chemokines and chemokine receptors: New insights into cancer-related inflammation. Trends Mol. Med. 2010, 16, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Kruizinga, R.C.; Bestebroer, J.; Berghuis, P.; de Haas, C.J.; Links, T.P.; de Vries, E.G.; Walenkamp, A.M. Role of chemokines and their receptors in cancer. Curr. Pharm. Des. 2009, 15, 3396–3416. [Google Scholar] [CrossRef]
- Schneider, M.A.; Meingassner, J.G.; Lipp, M.; Moore, H.D.; Rot, A. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J. Exp. Med. 2007, 204, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.J.; Murphy, K.E.; Kunkel, E.J.; Brightling, C.E.; Soler, D.; Shen, Z.; Boisvert, J.; Greenberg, H.B.; Vierra, M.A.; Goodman, S.B. CCR7 expression and memory T cell diversity in humans. Immunology 2001, 166, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.P.; Kelly, L.M.; Cyster, J.G. Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int. Immunol. 2010, 22, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, N.; Riol-Blanco, L.; de la Rosa, G.; Puig-Kröger, A.; García-Bordas, J.; Martín, D.; Longo, N.; Cuadrado, A.; Cabanas, C.; Corbí, A.L. Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood 2004, 104, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Nagira, M.; Kitaura, M.; Imagawa, N.; Imai, T.; Yoshie, O. Secondary lymphoid-tissue chemokine is a functional ligand for the CC chemokine receptor CCR7. J. Biol.Chem. 1998, 273, 7118–7122. [Google Scholar] [CrossRef] [PubMed]
- Britschgi, M.R.; Favre, S.; Luther, S.A. CCL21 is sufficient to mediate DC migration, maturation and function in the absence of CCL19. Eur. J. Immunol. 2010, 40, 1266–1271. [Google Scholar] [CrossRef]
- Otero, C.; Groettrup, M.; Legler, D.F. Opposite fate of endocytosed CCR7 and its ligands: Recycling versus degradation. Immunology 2006, 177, 2314–2323. [Google Scholar] [CrossRef]
- Kohout, T.A.; Nicholas, S.L.; Perry, S.J.; Reinhart, G.; Junger, S.; Struthers, R.S. Differential desensitization, receptor phosphorylation, β-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J. Biol.Chem. 2004, 279, 23214–23222. [Google Scholar] [CrossRef] [PubMed]
- Schaeuble, K.; Hauser, M.A.; Rippl, A.V.; Bruderer, R.; Otero, C.; Groettrup, M.; Legler, D.F. Ubiquitylation of the chemokine receptor CCR7 enables efficient receptor recycling and cell migration. J. Cell Sci. 2012, 125, 4463–4474. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, H.D.; Shannon, L.A.; Calloway, P.A.; Fassold, B.C.; Dunwiddie, I.; Vielhauer, G.; Zhang, M.; Vines, C.M. Expression of the CC chemokine receptor 7 mediates metastasis of breast cancer to the lymph nodes in mice. Transl. Oncol. 2010, 3, 354–361. [Google Scholar] [CrossRef]
- Mashino, K.; Sadanaga, N.; Yamaguchi, H.; Tanaka, F.; Ohta, M.; Shibuta, K.; Inoue, H.; Mori, M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002, 62, 2937–2941. [Google Scholar] [PubMed]
- Shields, J.; Emmett, M.; Dunn, D.; Joory, K.; Sage, L.; Rigby, H.; Mortimer, P.; Orlando, A.; Levick, J.; Bates, D. Chemokine-mediated migration of melanoma cells towards lymphatics–a mechanism contributing to metastasis. Oncogene 2007, 26, 2997–3005. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Thomas, S.M.; Grandis, J.R.; Wells, A.; Ferris, R.L. Chemokine receptor 7 activates phosphoinositide-3 kinase-mediated invasive and prosurvival pathways in head and neck cancer cells independent of EGFR. Oncogene 2005, 24, 5897–5904. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, K.; Kozawa, Y.; Ohashi, Y.; Nakamura, E.S.; Aozuka, Y.; Sakurai, H.; Ichiki, K.; Doki, Y.; Misaki, T.; Saiki, I. CCL21 promotes the migration and adhesion of highly lymph node metastatic human non-small cell lung cancer Lu-99 in vitro. Oncol. Rep. 2007, 17, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shimada, Y.; Maeda, M.; Kawabe, A.; Kaganoi, J.; Komoto, I.; Hashimoto, Y.; Miyake, M.; Hashida, H.; Imamura, M. Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clinical Cancer Res. 2003, 9, 3406–3412. [Google Scholar]
- Schimanski, C.C.; Bahre, R.; Gockel, I.; Junginger, T.; Simiantonaki, N.; Biesterfeld, S.; Achenbach, T.; Wehler, T.; Galle, P.R.; Moehler, M. Chemokine receptor CCR7 enhances intrahepatic and lymphatic dissemination of human hepatocellular cancer. Oncol. Rep. 2006, 16, 109–113. [Google Scholar] [CrossRef]
- Kodama, J.; Kusumoto, T.; Seki, N.; Matsuo, T.; Ojima, Y.; Nakamura, K.; Hongo, A.; Hiramatsu, Y. Association of CXCR4 and CCR7 chemokine receptor expression and lymph node metastasis in human cervical cancer. Ann. Oncol. 2007, 18, 70–76. [Google Scholar] [CrossRef]
- Sancho, M.; Vieira, J.M.; Casalou, C.; Mesquita, M.; Pereira, T.; Cavaco, B.M.; Dias, S.; Leite, V. Expression and function of the chemokine receptor CCR7 in thyroid carcinomas. Endocrinology 2006, 191, 229–238. [Google Scholar] [CrossRef]
- Pitkin, L.; Luangdilok, S.; Corbishley, C.; Wilson, P.; Dalton, P.; Bray, D.; Mady, S.; Williamson, P.; Odutoye, T.; Evans, P.R. Expression of CC chemokine receptor 7 in tonsillar cancer predicts cervical nodal metastasis, systemic relapse and survival. Br. J. Cancer 2007, 97, 670–677. [Google Scholar] [CrossRef]
- Günther, K.; Leier, J.; Henning, G.; Dimmler, A.; Weißbach, R.; Hohenberger, W.; Förster, R. Prediction of lymph node metastasis in colorectal carcinoma by expressionof chemokine receptor CCR7. Int. J. Cancer 2005, 116, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Heresi, G.A.; Wang, J.; Taichman, R.; Chirinos, J.A.; Regalado, J.J.; Lichtstein, D.M.; Rosenblatt, J.D. Expression of the chemokine receptor CCR7 in prostate cancer presenting with generalized lymphadenopathy: Report of a case, review of the literature, and analysis of chemokine receptor expression. Urol. Oncol. 2005, 23, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Huang, F.; Li, X.; Chen, Z.; Feng, D.; Jiang, H.; Chen, W.; Zhang, X. CCL21/CCR7 interaction promotes cellular migration and invasion via modulation of the MEK/ERK1/2 signaling pathway and correlates with lymphatic metastatic spread and poor prognosis in urinary bladder cancer. Int. J. Oncol. 2017, 51, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Tutunea-Fatan, E.; Majumder, M.; Xin, X.; Lala, P.K. The role of CCL21/CCR7 chemokine axis in breast cancer-induced lymphangiogenesis. Mol. Cancer 2015, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhou, M.; Qiu, W.; Ye, J.; Feng, Q. CCR7 mediates human breast cancer cell invasion, migration by inducing epithelial–mesenchymal transition and suppressing apoptosis through AKT pathway. Cancer Med. 2017, 6, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Weitzenfeld, P.; Kossover, O.; Körner, C.; Meshel, T.; Wiemann, S.; Seliktar, D.; Legler, D.F.; Ben-Baruch, A. Chemokine axes in breast cancer: Factors of the tumor microenvironment reshape the CCR7-driven metastatic spread of luminal-A breast tumors. J. Leukoc. Biol. 2016, 99, 1009–1025. [Google Scholar] [CrossRef]
- Mo, M.; Zhou, M.; Wang, L.; Qi, L.; Zhou, K.; Liu, L.-F.; Chen, Z.; Zu, X.-B. CCL21/CCR7 enhances the proliferation, migration, and invasion of human bladder cancer T24 cells. PLoS ONE 2015, 10, e0119506. [Google Scholar] [CrossRef]
- Li, J.; Sun, R.; Tao, K.; Wang, G. The CCL21/CCR7 pathway plays a key role in human colon cancer metastasis through regulation of matrix metalloproteinase-9. Dig. Liver Dis. 2011, 43, 40–47. [Google Scholar] [CrossRef]
- Sun, R.H.; Wang, G.B.; Li, J.; Cui, J. Role of CCL21/CCR7 in invasion of colorectal carcinoma cell line SW480. CJC 2009, 28, 708–713. [Google Scholar] [CrossRef]
- Schimanski, C.C.; Schwald, S.; Simiantonaki, N.; Jayasinghe, C.; Gönner, U.; Wilsberg, V.; Junginger, T.; Berger, M.R.; Galle, P.R.; Moehler, M. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clinical Cancer Res. 2005, 11, 1743–1750. [Google Scholar] [CrossRef]
- Ma, H.; Gao, L.; Li, S.; Qin, J.; Chen, L.; Liu, X.; Xu, P.; Wang, F.; Xiao, H.; Zhou, S. CCR7 enhances TGF-β1-induced epithelial-mesenchymal transition and is associated with lymph node metastasis and poor overall survival in gastric cancer. Oncotarget 2015, 6, 24348. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Baek, S.W.; Moon, J.Y.; Jo, I.S.; Kim, N.; Lee, H.J. C-C motif chemokine receptors in gastric cancer. Mol. Clin. Oncol. 2018, 8, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Al-Jokhadar, M.; Al-Mandily, A.; Zaid, K.; Maalouf, E.A. CCR7 and CXCR4 Expression in Primary Head and Neck Squamous Cell Carcinomas and Nodal Metastases–a Clinical and Immunohistochemical Study. APJCP 2017, 18, 1093. [Google Scholar]
- González-Arriagada, W.A.; Lozano-Burgos, C.; Zúñiga-Moreta, R.; González-Díaz, P.; Coletta, R.D. Clinicopathological significance of chemokine receptor (CCR 1, CCR 3, CCR 4, CCR 5, CCR 7 and CXCR 4) expression in head and neck squamous cell carcinomas. J. Oral Pathol. Med. 2018, 47, 755–763. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Q.; Li, Y.; Tang, N.; Qiu, X. CCL21/CCR7 up-regulate vascular endothelial growth factor-D expression via ERK pathway in human non-small cell lung cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 15729. [Google Scholar] [PubMed]
- Zhong, G.; Chen, L.; Yin, R.; Qu, Y.; Bao, Y.; Xiao, Q.; Zhang, Z.; Shen, Y.; Li, C.; Xu, Y. Chemokine (C-C motif) ligand 21/C-C chemokine receptor type 7 triggers migration and invasion of human lung cancer cells by epithelial-mesenchymal transition via the extracellular signal-regulated kinase signaling pathway. Mol. Med. Rep. 2017, 15, 4100–4108. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, S.; Zhao, G.; Sun, B. Effect of chemokine receptors CCR7 on disseminated behavior of human T cell lymphoma: Clinical and experimental study. J. Exp. Clin. Cancer Res. 2011, 30, 51. [Google Scholar] [CrossRef] [PubMed]
- Fleige, H.; Bosnjak, B.; Permanyer, M.; Ristenpart, J.; Bubke, A.; Willenzon, S.; Sutter, G.; Luther, S.A.; Förster, R. Manifold roles of CCR7 and its ligands in the induction and maintenance of bronchus-associated lymphoid tissue. Cell Rep. 2018, 23, 783–795. [Google Scholar] [CrossRef]
- Cristiani, C.; Tallerico, R.; Ventura, V.; Capone, M.; Madonna, G.; Mallardo, D.; Selinger, E.; Garofalo, C.; Staaf, E.; Simeone, E. Monitoring of melanoma clinical progression by circulating NK and T cells immunoprofiling: A potential role for CCR7 in metastatic spread. J. Transl. Med. 2018. [Google Scholar] [CrossRef]
- Cai, Q.-Y.; Liang, G.-Y.; Zheng, Y.-F.; Tan, Q.-Y.; Wang, R.-W.; Li, K. CCR7 enhances the angiogenic capacity of esophageal squamous carcinoma cells in vitro via activation of the NF-κB/VEGF signaling pathway. Am. J. Transl. Res. 2017, 9, 3282. [Google Scholar]
- Goto, M.; Liu, M. Chemokines and their receptors as biomarkers in esophageal cancer. Esophagus 2019, 1–9. [Google Scholar] [CrossRef]
- Shi, M.; Chen, D.; Yang, D.; Liu, X.-y. CCL21-CCR7 promotes the lymph node metastasis of esophageal squamous cell carcinoma by up-regulating MUC1. J. Exp. Clin. Cancer Res. 2015, 34, 149. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Izumi, K.; Maolake, A.; Natsagdorj, A.; Iwamoto, H.; Kadomoto, S.; Naito, R.; Hiratsuka, K.; Kadono, Y.; Mizokami, A. Tumor necrosis factor-α upregulation of CCR7 induces prostate cancer cell migration in lymphatic metastasis. AACR 2019. [Google Scholar] [CrossRef]
- Maolake, A.; Izumi, K.; Natsagdorj, A.; Iwamoto, H.; Kadomoto, S.; Makino, T.; Naito, R.; Shigehara, K.; Kadono, Y.; Hiratsuka, K. Tumor necrosis factor-α induces prostate cancer cell migration in lymphatic metastasis through CCR 7 upregulation. Cancer Sci. 2018, 109, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Even-Ram, S.; Yamada, K.M. Cell migration in 3D matrix. Curr. Opin. Cell Biol. 2005, 17, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.E.; Odde, D.J. Traction dynamics of filopodia on compliant substrates. Science 2008, 322, 1687–1691. [Google Scholar] [CrossRef]
- Proudfoot, A.E.; Handel, T.M.; Johnson, Z.; Lau, E.K.; LiWang, P.; Clark-Lewis, I.; Borlat, F.; Wells, T.N.; Kosco-Vilbois, M.H. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc. Nat. Acad. Sci. 2003, 100, 1885–1890. [Google Scholar] [CrossRef]
- Schumann, K.; Lämmermann, T.; Bruckner, M.; Legler, D.F.; Polleux, J.; Spatz, J.P.; Schuler, G.; Förster, R.; Lutz, M.B.; Sorokin, L. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 2010, 32, 703–713. [Google Scholar] [CrossRef]
- Nandagopal, S.; Wu, D.; Lin, F. Combinatorial guidance by CCR7 ligands for T lymphocytes migration in co-existing chemokine fields. PLoS ONE 2011, 6, e18183. [Google Scholar] [CrossRef]
- Legler, D.F.; Uetz-von Allmen, E.; Hauser, M.A. CCR7: Roles in cancer cell dissemination, migration and metastasis formation. Int. J. Biochem. Cell Biol. 2014, 54, 78–82. [Google Scholar]
- He, Y.; Karpanen, T.; Alitalo, K. Role of lymphangiogenic factors in tumor metastasis. BBA-REV. CANCER 2004, 1654, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.D.; Fleury, M.E.; Yong, C.; Tomei, A.A.; Randolph, G.J.; Swartz, M.A. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 2007, 11, 526–538. [Google Scholar] [CrossRef]
- Shields, J.D.; Kourtis, I.C.; Tomei, A.A.; Roberts, J.M.; Swartz, M.A. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 2010, 328, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Raman, D.; Baugher, P.J.; Thu, Y.M.; Richmond, A. Role of chemokines in tumor growth. Cancer Lett. 2007, 256, 137–165. [Google Scholar] [CrossRef] [PubMed]
- Correale, P.; Rotundo, M.S.; Botta, C.; Del Vecchio, M.T.; Tassone, P.; Tagliaferri, P. Tumor infiltration by chemokine receptor 7 (CCR7)+ T-lymphocytes is a favorable prognostic factor in metastatic colorectal cancer. Oncoimmunology 2012, 1, 531–532. [Google Scholar] [CrossRef][Green Version]
- Sperveslage, J.; Frank, S.; Heneweer, C.; Egberts, J.; Schniewind, B.; Buchholz, M.; Bergmann, F.; Giese, N.; Munding, J.; Hahn, S.A. Lack of CCR7 expression is rate limiting for lymphatic spread of pancreatic ductal adenocarcinoma. Int. J. Cancer 2012, 131, E371–E381. [Google Scholar] [CrossRef]
- He, Y.; Rajantie, I.; Pajusola, K.; Jeltsch, M.; Holopainen, T.; Yla-Herttuala, S.; Harding, T.; Jooss, K.; Takahashi, T.; Alitalo, K. Vascular endothelial cell growth factor receptor 3–mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005, 65, 4739–4746. [Google Scholar] [CrossRef]
- Vicari, A.P.; Ait-Yahia, S.; Chemin, K.; Mueller, A.; Zlotnik, A.; Caux, C. Antitumor effects of the mouse chemokine 6Ckine/SLC through angiostatic and immunological mechanisms. Immunology 2000, 165, 1992–2000. [Google Scholar] [CrossRef]
- Luker, K.E.; Luker, G.D. Functions of CXCL12 and CXCR4 in breast cancer. Cancer Lett. 2006, 238, 30–41. [Google Scholar] [CrossRef]
- Cabioglu, N.; Yazici, M.S.; Arun, B.; Broglio, K.R.; Hortobagyi, G.N.; Price, J.E.; Sahin, A. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin. Cancer Res. 2005, 11, 5686–5693. [Google Scholar] [CrossRef] [PubMed]
- Kochetkova, M.; Kumar, S.; McColl, S. Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell Death Differ. 2009, 16, 664–673. [Google Scholar] [CrossRef]
- Wilson, J.L.; Burchell, J.; Grimshaw, M.J. Endothelins induce CCR7 expression by breast tumor cells via endothelin receptor A and hypoxia-inducible factor-1. Cancer Res. 2006, 66, 11802–11807. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Kim, J.; Yamano, T.; Takeuchi, H.; Huang, S.; Umetani, N.; Koyanagi, K.; Hoon, D.S. Epigenetic up-regulation of CC chemokine receptor 7 and CXC chemokine receptor 4 expression in melanoma cells. Cancer Res. 2005, 65, 1800–1807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, S.; Lu, X.; Zhang, Z.; Lei, P.; Hu, P.; Wang, M.; Huang, B.; Xing, W.; Jiang, X.; Liu, H. CC chemokine ligand 21 enhances the immunogenicity of the breast cancer cell line MCF-7 upon assistance of TLR2. Carcinogenesis 2011, 32, 296–304. [Google Scholar] [CrossRef]
- Chen, Y.; Stamatoyannopoulos, G.; Song, C.-Z. Down-regulation of CXCR4 by inducible small interfering RNA inhibits breast cancer cell invasion in vitro. Cancer Res. 2003, 63, 4801–4804. [Google Scholar]
- Hattermann, K.; Gebhardt, H.; Krossa, S.; Ludwig, A.; Lucius, R.; Held-Feindt, J.; Mentlein, R. Transmembrane chemokines act as receptors in a novel mechanism termed inverse signaling. Elife 2016, 5, e10820. [Google Scholar] [CrossRef]
- Lacalle, R.A.; Blanco, R.; Carmona-Rodriguez, L.; Martin-Leal, A.; Mira, E.; Manes, S. Chemokine receptor signaling and the hallmarks of cancer. Int. Rev. Cell Mol. Biol. 2017, 331, 181–244. [Google Scholar]
- Wang, J.; Knaut, H. Chemokine signaling in development and disease. Development 2014, 141, 4199–4205. [Google Scholar] [CrossRef]
- Curnock, A.P.; Logan, M.K.; Ward, S.G. Chemokine signalling: Pivoting around multiple phosphoinositide 3-kinases. Immunology 2002, 105, 125–136. [Google Scholar] [CrossRef]
- Bonecchi, R.; Mollica Poeta, V.; Capucetti, A.; Massara, M. Chemokines and chemokine receptors: New targets for cancer immunotherapy. Front. Immunol. 2019, 10, 379. [Google Scholar]
- Kehrl, J.H. Chemoattractant receptor signaling and the control of lymphocyte migration. Immunol. Res. 2006, 34, 211–227. [Google Scholar] [CrossRef]
- Legler, D.F.; Thelen, M. New insights in chemokine signaling. F1000 Res. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Endo, M.; Ochi, H.; Hojo, H.; Miyasaka, M.; Hayasaka, H. Regulation of CCR7-dependent cell migration through CCR7 homodimer formation. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, H.; Mills, G.B. Targeting PI3K-AKT pathway for cancer therapy. Rev Clin Exp Hematol. 2003, 7, 205–228. [Google Scholar] [PubMed]
- Sotsios, Y.; Ward, S.G. Phosphoinositide 3-kinase: A key biochemical signal for cell migration in response to chemokines. Immunol. Rev. 2000, 177, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Rolin, J.; Maghazachi, A.A. Implications of chemokine receptors and inflammatory lipids in cancer. ImmunoTargets Ther. 2014, 3, 9. [Google Scholar]
- Wong, M.; Fish, E.N. RANTES and MIP-1α activate stats in T cells. J. Biol. Chem. 1998, 273, 309–314. [Google Scholar] [CrossRef]
- Miyagaki, T.; Sugaya, M.; Murakami, T.; Asano, Y.; Tada, Y.; Kadono, T.; Okochi, H.; Tamaki, K.; Sato, S. CCL11–CCR3 interactions promote survival of anaplastic large cell lymphoma cells via ERK1/2 activation. Cancer Res. 2011, 71, 2056–2065. [Google Scholar] [CrossRef]
- Brust, T.F.; Conley, J.M.; Watts, V.J. Gαi/o-coupled receptor-mediated sensitization of adenylyl cyclase: 40 years later. Eur. J. Pharmacol. 2015, 763, 223–232. [Google Scholar] [CrossRef]
- Neves, S.R.; Ram, P.T.; Iyengar, R. G protein pathways. Science 2002, 296, 1636–1639. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.O.; Verkaar, F.; van der Lee, M.M.; Timmerman, C.A.; Kuijer, M.; van Offenbeek, J.; Van Lith, L.H.; Smit, M.J.; Leurs, R.; Zaman, G.J. β-Arrestin recruitment and G protein signaling by the atypical human chemokine decoy receptor CCX-CKR. J. Biol. Chem. 2013, 288, 7169–7181. [Google Scholar] [CrossRef] [PubMed]
- López-Giral, S.; Quintana, N.E.; Cabrerizo, M.; Alfonso-Pérez, M.; Sala-Valdés, M.; de Soria, V.G.G.; Fernández-Rañada, J.M.; Fernández-Ruiz, E.; Muñoz, C. Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. J. Leukoc. Biol. 2004, 76, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-L.; Zhou, Z.-J.; Hu, Z.-Q.; Li, X.; Huang, X.-W.; Wang, Z.; Fan, J.; Dai, Z.; Zhou, J. CXCR2/CXCL5 axis contributes to epithelial–mesenchymal transition of HCC cells through activating PI3K/Akt/GSK-3β/Snail signaling. Cancer Lett. 2015, 358, 124–135. [Google Scholar] [CrossRef]
- Raju, R.; Gadakh, S.; Gopal, P.; George, B.; Advani, J.; Soman, S.; Prasad, T.K.; Girijadevi, R. Differential ligand-signaling network of CCL19/CCL21-CCR7 system. Database 2015, 2015, bav106. [Google Scholar] [CrossRef]
- Sonbul, S.N.; Gorringe, K.L.; Aleskandarany, M.A.; Mukherjee, A.; Green, A.R.; Ellis, I.O.; Rakha, E.A. Chemokine (C-C motif) receptor 7 (CCR7) associates with the tumour immune microenvironment but not progression in invasive breast carcinoma. J. Pathol. 2017, 3, 105–114. [Google Scholar] [CrossRef]
- Wu, J.; Li, L.; Liu, J.; Wang, Y.; Wang, Z.; Wang, Y.; Liu, W.; Zhou, Z.; Chen, C.; Liu, R. CC chemokine receptor 7 promotes triple-negative breast cancer growth and metastasis. ABBS 2018, 50, 835–842. [Google Scholar] [CrossRef]
- Al Akoum, C.; Akl, I.; Rouas, R.; Fayyad-Kazan, M.; Falha, L.; Renno, T.; Burny, A.; Lewalle, P.; Fayyad-Kazan, H.; Badran, B. NFAT-1, Sp-1, Sp-3, and miR-21: New regulators of chemokine C receptor 7 expression in mature human dendritic cells. Hum. Immunol. 2015, 76, 307–317. [Google Scholar] [CrossRef]
- Mburu, Y.K.; Egloff, A.M.; Walker, W.H.; Wang, L.; Seethala, R.R.; Van Waes, C.; Ferris, R.L. Chemokine receptor 7 (CCR7) gene expression is regulated by NF-κB and activator protein 1 (AP1) in metastatic squamous cell carcinoma of head and neck (SCCHN). J. Biol. Chem. 2012, 287, 3581–3590. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Chen, Y.; Zhou, M.; Zhang, H.; Chen, R.; Shi, F.; Wang, C.; Rui, Z. Transcriptional silencing of ETS-1 abrogates epithelial-mesenchymal transition resulting in reduced motility of pancreatic cancer cells. Oncol. Rep. 2015, 33, 559–565. [Google Scholar] [CrossRef]
- Fang, L.-W.; Kao, Y.-H.; Chuang, Y.-T.; Huang, H.-L.; Tai, T.-S. Ets-1 enhances tumor migration through regulation of CCR7 expression. BMB Rep. 2019, 52, 548. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, L.; Ghasemi, M.; Yazdani, J.; Ranjbar, S.; Nouri, B.; Alizadeh, A.; Afshar, P. Investigation of CCR7 Marker Expression Using Immunohistochemical Method and Its Association with Clinicopathologic Properties in Patients with Breast Cancer. Hematology 2018, 12, 103. [Google Scholar]
- Zabicki, K.; Colbert, J.A.; Dominguez, F.J.; Gadd, M.A.; Hughes, K.S.; Jones, J.L.; Specht, M.C.; Michaelson, J.S.; Smith, B.L. Breast cancer diagnosis in women≤ 40 versus 50 to 60 years: Increasing size and stage disparity compared with older women over time. Ann. Surg. Oncol. 2006, 13, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, S.; Li, N.; Gao, J.; Yu, J.; Zhao, J.; Li, M.; Zhao, Z. High expression of CCR7 predicts lymph node metastasis and good prognosis in triple negative breast cancer. Cell. Physiol. Biochem. 2017, 43, 531–539. [Google Scholar] [CrossRef]
- Gracio, F.; Burford, B.; Gazinska, P.; Mera, A.; Noor, A.M.; Marra, P.; Gillett, C.; Grigoriadis, A.; Pinder, S.; Tutt, A. Splicing imbalances in basal-like breast cancer underpin perturbation of cell surface and oncogenic pathways and are associated with patients’ survival. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Huang, H.; Ding, Q.; Liu, X.; Chen, L.; Zha, X.; Liang, M.; He, J.; Zhu, Q. Comparative profiling of triple-negative breast carcinomas tissue glycoproteome by sequential purification of glycoproteins and stable isotope labeling. Cell. Physiol. Biochem. 2016, 38, 110–121. [Google Scholar] [CrossRef]
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef]
- Pan, M.-R.; Hou, M.-F.; Chang, H.-C.; Hung, W.-C. Cyclooxygenase-2 up-regulates CCR7 via EP2/EP4 receptor signaling pathways to enhance lymphatic invasion of breast cancer cells. J. Biol. Chem. 2008, 283, 11155–11163. [Google Scholar] [CrossRef]
- Tamamura, H.; Hori, A.; Kanzaki, N.; Hiramatsu, K.; Mizumoto, M.; Nakashima, H.; Yamamoto, N.; Otaka, A.; Fujii, N. T140 analogs as CXCR4 antagonists identified as anti-metastatic agents in the treatment of breast cancer. FEBS Lett. 2003, 550, 79–83. [Google Scholar] [CrossRef]
- Andre, F.; Cabioglu, N.; Assi, H.; Sabourin, J.; Delaloge, S.; Sahin, A.; Broglio, K.; Spano, J.; Combadiere, C.; Bucana, C. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann. Oncol. 2006, 17, 945–951. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, R.; Li, J.; Gu, Q.; Zhao, X.; Sun, T.; Wang, J.; Li, J.; Du, Q.; Sun, B. Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J. Exp. Clin. Cancer Res. 2010, 29, 16. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.-W.; Zhao, S.-M.; Yue, G.G.-L.; Lee, J.K.-M.; Fung, K.-P.; Leung, P.-C.; Tan, N.-H.; Bik-San Lau, C. RA-XII inhibits tumour growth and metastasis in breast tumour-bearing mice via reducing cell adhesion and invasion and promoting matrix degradation. Sci. Rep. 2015, 5, 16985. [Google Scholar] [CrossRef] [PubMed]
- Pearson, H.B.; Pouliot, N. Modeling metastasis in vivo. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Bähr, A.; Wolf, E. Domestic animal models for biomedical research. Reprod. Domest. Anim. 2012, 47, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; Muñoz, D.; Hoy, J.; Lokeshwar, B.L. Use of shRNA for stable suppression of chemokine receptor expression and function in human cancer cell lines. Methods Mol. Biol. 2014, 1172, 209–218. [Google Scholar]
- Li, F.; Zou, Z.; Suo, N.; Zhang, Z.; Wan, F.; Zhong, G.; Qu, Y.; Ntaka, K.S.; Tian, H. CCL21/CCR7 axis activating chemotaxis accompanied with epithelial–mesenchymal transition in human breast carcinoma. Med. Oncol. 2014, 31, 180. [Google Scholar] [CrossRef]
- Saur, D.; Seidler, B.; Schneider, G.; Algül, H.; Beck, R.; Senekowitsch–Schmidtke, R.; Schwaiger, M.; Schmid, R.M. CXCR4 expression increases liver and lung metastasis in a mouse model of pancreatic cancer. Gastroenterology 2005, 129, 1237–1250. [Google Scholar] [CrossRef]
- Takekoshi, T.; Fang, L.; Paragh, G.; Hwang, S.T. CCR7-expressing B16 melanoma cells downregulate interferon-γ-mediated inflammation and increase lymphangiogenesis in the tumor microenvironment. Oncogenesis 2012, 1, e9. [Google Scholar] [CrossRef]
- Wiley, H.E.; Gonzalez, E.B.; Maki, W.; Wu, M.-t.; Hwang, S.T. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J. Natl. Cancer Inst. 2001, 93, 1638–1643. [Google Scholar] [CrossRef]
- Hudis, C.A.; Gianni, L. Triple-negative breast cancer: An unmet medical need. Oncologist 2011, 16, 1–11. [Google Scholar] [CrossRef]
- Strien, L.; Joensuu, K.; HEIKKILÄ, P.; Leidenius, M.H. Different expression patterns of CXCR4, CCR7, maspin and FOXP3 in luminal breast cancers and their sentinel node metastases. Anticancer Res. 2017, 37, 175–182. [Google Scholar] [CrossRef]
- Power, C.A. Knock out models to dissect chemokine receptor function in vivo. J. Immunol. Methods 2003, 273, 73–82. [Google Scholar] [CrossRef]
- Houshmand, P.; Zlotnik, A. Therapeutic applications in the chemokine superfamily. Curr. Opin. Chem. Biol 2003, 7, 457–460. [Google Scholar] [CrossRef]
- Richardson, R.M.; Marjoram, R.J.; Barak, L.S.; Snyderman, R. Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. Immunology 2003, 170, 2904–2911. [Google Scholar] [CrossRef]
- Otero, C.; Eisele, P.S.; Schaeuble, K.; Groettrup, M.; Legler, D.F. Distinct motifs in the chemokine receptor CCR7 regulate signal transduction, receptor trafficking and chemotaxis. J. Cell Sci. 2008, 121, 2759–2767. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.V.; Rot, A.; Luo, Y.; Narasimhaswamy, M.; Nakano, H.; Gunn, M.D.; Matsuzawa, A.; Quackenbush, E.J.; Dorf, M.E.; von Andrian, U.H. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function–associated antigen 1–mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 2000, 191, 61–76. [Google Scholar] [CrossRef]
- Yang, S.-C.; Hillinger, S.; Riedl, K.; Zhang, L.; Zhu, L.; Huang, M.; Atianzar, K.; Kuo, B.Y.; Gardner, B.; Batra, R.K. Intratumoral administration of dendritic cells overexpressing CCL21 generates systemic antitumor responses and confers tumor immunity. Clinical Cancer Res. 2004, 10, 2891–2901. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Tao, H.; Chang, A.E.; Hu, Y.; Shu, G.; Chen, Q.; Egenti, M.; Owen, J.; Moyer, J.S.; Prince, M.E. Cancer stem cell vaccine inhibits metastases of primary tumors and induces humoral immune responses against cancer stem cells. Oncoimmunology 2015, 4, e990767. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Timmerman, J.M.; Levy, R. Dendritic cell vaccines for cancer immunotherapy. Annu. Rev. Med. 1999, 50, 507–529. [Google Scholar] [CrossRef]
- Warnock, R.; Campbell, J.; Dorf, M.; Matsuzawa, A.; McEvoy, L.; Butcher, E. The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer’s patch high endothelial venules. J. Exp. Med. 2000, 191, 77–88. [Google Scholar] [CrossRef]
- Hovden, A.O.; Appel, S. The first dendritic cell-based therapeutic cancer vaccine is approved by the FDA. Scand. J. Immunol. 2010, 72, 554. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Garon, E.; Lee, M.; Baratelli, F.; Wang, G.; Abtin, F.; Suh, R.; Wallace, W.; Zeng, G.; Sharma, S. Phase I trial of trans-thoracic injection of CCL21 gene modified dendritic cells in human non-small cell lung carcinoma. J. Surg. Res. 2014, 186, 558. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, M.-H.; Garon, E.; Goldman, J.W.; Salehi-Rad, R.; Baratelli, F.E.; Schaue, D.; Wang, G.; Rosen, F.; Yanagawa, J. Phase I trial of intratumoral injection of CCL21 gene–modified dendritic cells in lung cancer elicits tumor-specific immune responses and CD8+ T-cell infiltration. Clin. Cancer Res. 2017, 23, 4556–4568. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-m.; Zhong, C.-p.; Sun, R.-x.; Liu, B.-b.; Huang, C.; Qin, J.; Zhou, S.; Shan, J.; Liu, Y.-k.; Ye, S.-l. Local expression of secondary lymphoid tissue chemokine delivered by adeno-associated virus within the tumor bed stimulates strong anti-liver tumor immunity. Virology 2007, 81, 9502–9511. [Google Scholar] [CrossRef]
- Sharma, S.; Stolina, M.; Luo, J.; Strieter, R.M.; Burdick, M.; Zhu, L.X.; Batra, R.K.; Dubinett, S.M. Secondary lymphoid tissue chemokine mediates T cell-dependent antitumor responses in vivo. Immunology 2000, 164, 4558–4563. [Google Scholar] [CrossRef]
- Yang, S.-C.; Batra, R.K.; Hillinger, S.; Reckamp, K.L.; Strieter, R.M.; Dubinett, S.M.; Sharma, S. Intrapulmonary administration of CCL21 gene-modified dendritic cells reduces tumor burden in spontaneous murine bronchoalveolar cell carcinoma. Cancer Res. 2006, 66, 3205–3213. [Google Scholar] [CrossRef]

| Tumor Type | Observations | References |
|---|---|---|
| Breast Cancer | Correlates and promotes lymphogenesis and metastasis. CCL21 induces actin polymerization and migration. | [18,44,45,46] |
| Human Bladder Cancer | Enhances the migration, invasion and tumor proliferation/ correlates with poor prognosis. | [43,47] |
| Cervical Cancer | Metastasis and poor prognosis. | [38,40] |
| Colorectal Cancer | Poor prognosis and metastasis. | [48,49,50] |
| Gastric Cancer | Overall poor survival as well as metastasis and EMT. | [51,52] |
| Head and Neck Cell Carcinoma | CCR7/CCL21 correlates with metastasis. | [53,54] |
| Lung Cancer | Tumor dissemination and metastasis. | [55,56] |
| Lymphomas | Poor prognosis and tumor dissemination. | [57,58] |
| Melanomas | Metastasis and poor outcome. | [18,59] |
| Esophageal Cancer | Expression of CCR7 correlates with poor prognosis and metastasis. CCL21 induces pseudopodia formation, cell metastasis and angiogenesis. | [60,61,62] |
| Prostate Cancer | Tumor growth, metastasis and poor survival through lymphatic metastasis. | [63,64] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizeq, B.; Malki, M.I. The Role of CCL21/CCR7 Chemokine Axis in Breast Cancer Progression. Cancers 2020, 12, 1036. https://doi.org/10.3390/cancers12041036
Rizeq B, Malki MI. The Role of CCL21/CCR7 Chemokine Axis in Breast Cancer Progression. Cancers. 2020; 12(4):1036. https://doi.org/10.3390/cancers12041036
Chicago/Turabian StyleRizeq, Balsam, and Mohammed Imad Malki. 2020. "The Role of CCL21/CCR7 Chemokine Axis in Breast Cancer Progression" Cancers 12, no. 4: 1036. https://doi.org/10.3390/cancers12041036
APA StyleRizeq, B., & Malki, M. I. (2020). The Role of CCL21/CCR7 Chemokine Axis in Breast Cancer Progression. Cancers, 12(4), 1036. https://doi.org/10.3390/cancers12041036




