Abstract
Chemokines are chemotactic cytokines that mediate immune cell chemotaxis and lymphoid tissue development. Recent advances have indicated that chemokines and their cognate receptors play critical roles in cancer-related inflammation and cancer progression. On the basis of these findings, the chemokine system has become a new potential drug target for cancer immunotherapy. In this review, we summarize the essential roles of the complex network of chemokines and their receptors in cancer progression. Furthermore, we discuss the potential value of the chemokine system as a cancer prognostic marker. The chemokine system regulates the infiltration of immune cells into the tumor microenvironment, which induces both pro- and anti-immunity and promotes or suppresses tumor growth and proliferation, angiogenesis, and metastasis. Increasing evidence indicates the promising prognostic value of the chemokine system in cancer patients. While CCL2, CXCL10, and CX3CL1/CX3CR1 can serve as favorable or unfavorable prognostic factors depending on the cancer types, CCL14 and XCL1 possess good prognostic value. Other chemokines such as CXCL1, CXCL8, and CXCL12 are poor prognostic markers. Despite vast advances in our understanding of the complex nature of the chemokine system in tumor biology, knowledge about the multifaceted roles of the chemokine system in different types of cancers is still limited. Further studies are necessary to decipher distinct roles within the chemokine system in terms of cancer progression and to validate their potential value in cancer prognosis.
1. Introduction
The immune system interacts closely with tumor cells over entire stages of cancer progression from tumor initiation and development to metastasis, facilitating either cancer inhibition or promotion. The balance of these actions determines the eventual outcomes, which, in cases of clinically poor outcomes, are the consequences of immune evasion by tumors [1]. The tumor microenvironment (TME) comprises not only tumor cells, but also immune cells and the surrounding stromal cells. Interestingly, cancer cells can take advantage of these immune cells to help them escape the host’s immune system. In addition, the movement of different subsets of immune cells into the TME is orchestrated by the chemokine system, followed by the regulation of tumor immune responses in a spatiotemporal manner [2,3], and cancer-related inflammation [4].
Chemokines are a large family of low-molecular-weight (8 to 14 kDa) chemotactic cytokines [5], which are well-known for their roles in mediating immune cell recruitment [6] and lymphoid tissue development [7]. Chemokines can also directly impact tumor cells and endothelial cells in the TME to regulate tumor cell growth and proliferation, angiogenesis, cancer stem-like cell properties, invasiveness, and metastasis. Hence, chemokines directly and indirectly influence tumor immunity and cancer progression, resulting in a substantial impact on cancer therapy and outcomes [8]. Cancer immunotherapy targeting the chemokine system was recently introduced with several achievements. Mogamulizumab, an anti-CCR4 antibody, was clinically approved for the treatment of relapsed and refractory adult T cell leukemia-lymphoma [9]. Additionally, plerixafor (also known as AMD3100), a CXCR4 antagonist, was approved for the mobilization of hematopoietic stem cells for transplantation in patients with non-Hodgkin’s lymphoma (NHL) or multiple myeloma (MM) [10]. These advances have led to the recognition of chemokines and chemokine receptors as promising targets for cancer immunotherapy, and therefore, in-depth knowledge about the roles and mechanisms of the chemokine system in cancer is crucial for the development of drugs for cancer treatment.
Current standard therapies for most cancers do not benefit all patients. Therefore, the identification of applicable prognostic biomarkers is of great clinical value, not only to improve the therapeutic outcomes but also to provide novel therapeutic targets. Because of its powerful effects on cancer progression, the chemokine system is a potential marker that could predict outcomes for cancer patients. The present review summarizes the essential roles of the complex network of chemokines and their receptors in cancer progression. Furthermore, we discuss the prognostic value of the chemokine system, which has been investigated in diverse cancer types.
2. Chemokines and Chemokine Receptors
The chemokine family is divided into four major subfamilies (CC, CXC, CX3C, and C) based on the number and location of the highly conserved cysteine residues at the N-terminus of the chemokine ligands and the presence or absence of intervening amino acids. Whereas the CC, CXC, and CX3C chemokines have zero, one, and three non-conserved amino-acid residues between the first two cysteine residues, respectively, the C chemokines lack the first and third of the four conserved cysteine residues [11,12]. A nomenclature system has been developed, in which the chemokine ligands in the CC, CXC, CX3C, and C subfamilies are named CCL, CXCL, CX3CL, and XCL, respectively. These chemokines are recognized by seven transmembrane-domain G protein-coupled receptors (GPCRs), which are categorized and named CCR, CXCR, CX3CR, and XCR, respectively, based on their chemokine ligand sources [11,12]. Notably, some chemokines are ligands of more than one GPCR, and conversely, some GPCRs bind to more than one type of chemokines, inducing diverse effects. The chemokine receptors and their ligand pairings known in humans and mice are listed in Table 1.

Table 1.
Chemokine receptors with their ligand pairings in humans and mice and various kinds of immune cells expressing chemokine receptors [8,13,14,15].
As described above, chemokines function as chemoattractants, orchestrating the infiltration of immune cells to the TME, influencing tumor cell growth and proliferation, angiogenesis, and metastasis, and therefore contributing to cancer initiation and development [16]. These multifaceted roles of chemokines and their receptors in cancer progression are discussed in the following section.
3. Roles of Chemokine System in Cancer Progression
A number of studies have demonstrated the roles of chemokines and chemokine receptors in cancer progression. The recent advances in our understanding of the various chemokine systems and their differential roles in the recruitments of key immune cells, tumor cell growth and proliferation, angiogenesis, and tumor metastasis are discussed.
3.1. Roles of Chemokine System in Immune Cell Recruitment
Chemokine receptors are expressed in various kinds of immune cells (Table 1). The trafficking of these cells is directed by chemokine gradients that guide the cells to migrate to locations with high concentrations of chemokines [16], inducing either pro- or antitumor immune responses in the primary tumors and metastatic sites [8]. We discuss the roles of chemokines and their receptor networks which control the recruitment of key immune cells into the TME and demonstrate how the infiltrated cells regulate the immune response and tumor development.
3.1.1. T Cells
T cells are leukocytes expressing T cell receptors (TCRs) that recognize antigens presented by the major histocompatibility complex (MHC). T cells are classically divided into CD8+ cytotoxic T lymphocytes (CTLs) and CD4+ T cells, which recognize peptides presented by MHC class I and MHC class II, respectively [17]. CD4+ T cells include several T helper (Th) cells, among which Th1 and Th2 are the most important, and other T cell types such as Treg.
CD8+ CTLs are considered critical mediators of the antitumor response [17]. As shown in Table 1, various chemokine receptors, including CCR4/5, CXCR3, and CX3CR1, are expressed on CD8+ CTLs. Thus, their corresponding ligands such as CCL5, CXCL9, CXCL10, and CX3CL1 effectively guide CD8+ CTL mobilization from regional lymph nodes to tumor tissues [18]. CD8+ CTLs have powerful cytotoxic abilities due to the secretion of effector cytokines or cytotoxic molecules such as perforin and granzyme B, or interactions of the CD95 (Fas) molecule and its ligand CD95L, ultimately resulting in apoptosis in tumor cells [19,20,21]. Due to these antitumor effects, CD8+ CTL expression was reported to be associated with a favorable prognosis in breast cancer (BC) patients [22].
Th1 cells also have potent antitumor effects in the TME. Chemokines such as CXCL9 and CXCL10 can orchestrate the migration of effector CXCR3+ immune cells such as Th1 cells into the tumor sites, subsequently shaping both the tumor immunity and therapeutic responses [23,24,25,26]. Importantly, interferon gamma (IFN-γ) produced by Th1 cells not only has direct effects on arresting cellular proliferation, promoting apoptosis, and reducing angiogenesis but also on improving CTL responses to robust antitumor immunity [17]. Notably, Th1 immune responses in lymph nodes signify a protective effect in colon cancer patients [27] and can be considered a marker for prolonged survival in pancreatic ductal adenocarcinoma (PDAC) patients [28].
By contrast with Th1 cells, Th2 cells have protumor functions. Interleukin (IL)-4 is the signature cytokine for Th2 cells and functions as either an inducer or an effector cytokine of the cells [29]. The chemokines CCL8, CCL17, and CCL22 have chemoattractions with Th2 cells expressing CCR8 and CCR4 (Table 1) [25,30,31]. Th2 lymphocytes help B cells produce antibodies and suppress the action of cytotoxic T cells [16]. Intriguingly, a low circulating level of IL-4 can identify resectable pancreatic adenocarcinoma patients with better prognosis [32].
Treg cells play an essential role in maintaining self-tolerance and immune homeostasis [33]. The recruitment of Treg cells to TME is mediated by chemotaxis of CCL20/CCR6 [34], CCL22/CCR4 [35], CCL28/CCR10 [36], and CXCL12/CXCR4 [37,38]. Importantly, Treg cells abate tumor-specific T cell immunity involving CTLs, Th, natural killer (NK), and natural killer T cells, contributing to tumor growth and metastasis [33,35,39]. In addition, the cells can promote inflammation in the TME via expressing inflammatory cytokines [40]. By providing an escape route for cancers from the immune response, the expression of Treg cells is significantly correlated with worse overall survival (OS) in the majority of solid tumors. However, it is associated with better survival in several cancers, including colorectal, head and neck, or esophageal cancers with unclear mechanisms [41].
3.1.2. Natural Killer Cells
NK cells are well-known to play a role in antitumor immune responses. Their migration to inflamed tissues including tumor sites involves a series of chemokine receptors such as CCL3-5/CCR5 [42], CXCL10/CXCR3 [43], and CX3CL1/CX3CR1 [44]. Similar to CTLs, the cell-mediated cytotoxicity of NK cells also occurs via effector cytokines, cytotoxic molecules, and the Fas pathway [19,20,21,45]. Moreover, the elimination of tumors mediated by NK cells, subsequently, leads to tumor-specific T cell responses [45]. Especially, a high infiltration density of NK cells in a tumor nest is associated with better OS in esophageal cancer [46].
3.1.3. B Cells
B cells are central players in humoral immunity due to their antibody production capacity [47]. Chemokine axes such as CCL19, CCL21/CCR7, CCL20/CCR6, CXCL12/CXCR4, and CXCL13/CXCR5 (Table 1) correlate with B cell infiltration to tumor sites [15,48]. B cells exhibit antitumor functionality by directly killing tumor cells, producing specific antibodies for tumor antigens, acting as antigen-presenting cells (APCs) for T cell activation and memory T cell development, and facilitating CD4+ and CD8+ T cell immune responses [49,50,51,52,53]. However, B cells induce protumor effects by activating STAT3, promoting tumor angiogenesis and facilitating tumor progression [54]. Due to the dual roles of B cells, their high density is associated with good outcomes in non-small cell lung cancer (NSCLC) [55] but poor outcomes in ovarian cancer [56,57].
3.1.4. Dendritic Cells (DCs)
DCs have opposite effects in tumor response depending on their maturation stage. Immature DCs (iDCs) present antigens to T cells, and then induce immune tolerance including the generation of inducible Treg cells, T cell anergy and deletion [58]. iDCs are attracted to tumor tissue sites through CCL20, CXCL12, and CXCL14 chemotaxis [59,60,61,62]. Conversely, mature DCs (mDCs) assist the priming of CD4+ Th cells and CD8+ CTLs, the activation of B cells, and the initiation of adaptive immune responses [58]. CCL19 attracts mDCs and lymphocytes expressing CCR7 in T cell-rich areas, and thereby helping DCs meet tumor-associated antigen-specific T cells [63]. Due to their capacity to mediate T cell immunity, DCs can be used as adjuvants for cancer vaccination [58].
3.1.5. Neutrophils
Neutrophils also have a crucial regulatory role in tumor establishment and development [64]. Chemokines such as CCL2, CCL3, CXCL1, CXCL2, CXCL5, CXCL8, and CXCL12 promote neutrophil infiltration to tumors [64]. Importantly, neutrophils induce antitumor functions through direct cytotoxicity, antibody-dependent cellular cytotoxicity, and specific antigen presentation [65]. Nevertheless, neutrophils can induce genotoxicity and promote excessive angiogenesis and tumor proliferation [65]. Additionally, neutrophils can facilitate tumor metastasis by forming premetastatic niches and neutrophil extracellular traps (NETs) [14,64,65,66,67]. Intriguingly, since neutrophils have both pro- and antitumor effects, a higher density of neutrophils is associated with better response to 5-fluorouracil-based therapy in CRC patients [68], but worse clinical outcomes in the other types of human cancers [69].
3.1.6. Macrophages
Macrophages are attracted to tumor sites expressing chemotactic factors such as CCL2, CCL5, CCL7, CCL8, CXCL1, and CXCL12 (Table 1) [18,70]. Macrophages have been classified as classical M1 (antitumor macrophages) and alternative M2 (protumor macrophages) polarized subtypes. M1 macrophages can directly kill tumor cells and produce proinflammatory cytokines [71]. Contrarily, tumor-associated macrophages (TAMs) possess the properties of M2-polarized macrophages, produce immunosuppressive molecules to promote Treg cells, and suppress antitumor immunity [18,71,72,73]. Indeed, TAMs produce growth factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and prostaglandin to support angiogenesis and tumor growth [18]. TAMs establish a niche for cancer stem cells (CSCs) and support the epithelial–mesenchymal transition (EMT), which leads to cell migration and metastasis [73]. Collectively, macrophages exhibit either anti- or protumor functions based on their classification (M1 or M2).
3.1.7. Myeloid-Derived Suppressor Cells (MDSCs)
Tumor tissues contain MDSCs, which can suppress innate and adaptive antitumor immunity and contribute to tumor progression [74,75]. The infiltration of MDSCs into tumors is related to numerous chemokine and receptor axes such as CCL2, CCL7, CCL8/CCR2, CCL5/CCR5, CXCL1, CXCL2, CXCL5/CXCR2, and CXCL12/CXCR4 [18]. MDSCs that migrate to tumor sites increase STAT1 activity, leading to low levels of reactive oxygen species (ROS) and high levels of iNOS, NO, and arginase-1, which inhibit CD8+ T cell functions in a nonspecific manner [72]. Moreover, MDSCs can endow cancer cells with stem cell-like properties and are linked with cancer stemness [8]. MDSCs also support tumor angiogenesis by producing angiogenic factors such as VEGF, platelet-derived growth factor (PDGF), transforming growth factor beta, CXCL8, and matrix metalloproteinases (MMPs) such as MMP-2 and MMP-9 [18]. Data from one meta-analysis in solid tumors demonstrated that elevated levels of circulating MDSCs are negatively associated with the survival outcomes in most cancers [76].
Taken together, the chemokine system plays key roles in regulating the infiltration of immune cells into the TME, which leads to diverse functions in tumor immunity. While CD8+ CTLs, Th1, and NK cells induce antitumor responses, Th2, Treg cells, and MDSCs stimulate protumor functions, and B cells, DCs, neutrophils, and macrophages probably exhibit both anti- and protumor effects. Interestingly, however, one chemokine axis can attract different kinds of immune cells, which generate contrasting effects. The functional redundancies not only cause difficulties in the development of anticancer drugs that target the chemokine system but also lead to opposite prognoses. For example, the CCL5/CCR5 axis, which can recruit CD4+ and CD8+ T cells [77] and NK cells [42], can predict the improved efficiency of DC-immunotherapy in NSCLC [78]. Nevertheless, this pair also exhibits chemoattraction to TAMs and MDSCs [79,80], and has been reported to correlate with poor outcomes in BC patients [81].
3.2. Roles of Chemokines in Tumor Growth and Proliferation
Whereas normal cells strictly control the cellular homeostasis by regulating the synthesis and release of growth factors, tumor cells disrupt the regulatory mechanisms of the host for growth factor production and, then, sustain their growth and proliferative signals [82]. Numerous studies have demonstrated that the chemokine system is involved in tumor growth and proliferation through several mechanisms.
One of the mechanisms by which some chemokines such as CCL2 or CXCL8 promote tumor growth and proliferation involves acting as autocrine or paracrine growth factors [83,84,85]. Furthermore, chemokines including CCL2 and CCL5 contribute to tumor proliferation through the formation of an immunosuppressive TME by recruiting Treg cells or inflammatory monocytes and macrophages [86,87,88].
Phosphoinositide 3-kinase (PI3K)/AKT and extracellular signal-regulated protein kinases 1 and 2 (ERK 1/2) pathways are two key cellular signalling involved in tumor cell survival and proliferation [89,90]. Interestingly, these pathways are utilized by a series of chemokines and their receptors, such as CCL20/CCR6 [91], CCL25/CCR9 [92], CXCL1/CXCR2 [93], CXCL8/CXCR1-2 [94], CXCL12/CXCR4 [95], and CX3CL1/CX3CR1 [96], to inhibit apoptosis and promote tumor cell growth and proliferation. Intriguingly, both the PI3K/AKT and ERK 1/2 pathways induced by interactions between chemokines and their receptors can lead to nuclear factor kappa B (NF-κB) activation [97]. While the NF-κB pathway induces the upregulated expression of some chemokines such as CCL2, CCL5, CXCL5, CXCL8, CXCL10, CXCL12, and CX3CL1, it also participates in the antiapoptotic and proliferative effects of CCL5, CCL20, CXCL8, and CXCL12 in pancreatic cancer [98]. Importantly, chemokines can promote tumor cell survival by regulating the balance between NF-κB-associated pro- and antiapoptosis proteins. CCR5 and CX3CL1 were demonstrated to promote the expression of antiapoptotic and tumor-promoting proteins such as Bcl-xl, Bcl-2, and C-IAP1, as well as to reduce the expression of apoptotic proteins including cleaved caspase-3 and -9, PARP, and Bax via the NF-κB pathway [99,100].
In contrast, chemokines also inhibit tumor growth and proliferation. The term “cellular senescence” has been used to describe a state of stable and long-term proliferative arrest, despite maintained viability and metabolic activities [101]. Oncogene-induced senescence (OIS) is a highly stable antiproliferative response to oncogenic stress and acts as an effective barrier to tumor progression [102,103]. In the early stages of tumorigenesis, chemokines such as the CXCL1/CXCR2 axis can mediate OIS through NF-κB signalling to restrict tumor growth. However, in late stages of tumorigenesis, cellular senescence becomes a source of inflammation, recruiting MDSCs into the inflamed tumor, generating an immune suppressive microenvironment, and allowing cancer cell growth [103,104]. Moreover, CCL14 attenuates hepatocellular carcinoma (HCC) cell proliferation by inhibiting cell cycle progression and promoting apoptosis in vitro and suppresses HCC growth in vivo via the Wnt/β-catenin signalling pathway [105].
Taken together, the functions of chemokines and their receptors in inducing either pro- or antitumorigenic activities are highly complicated. On the one hand, chemokine systems can promote tumor growth and proliferation through the autocrine growth factor function, generation of immunosuppressive TME, and the PI3K/AKT and NF-κB signalling pathways. On the other hand, they can induce OIS or the Wnt/β-catenin signalling pathway to mitigate tumor development. Hence, future studies should be more focused on elucidating the underlying action mechanisms for these chemokine systems to decipher their distinct roles in tumor biology and to discover new targeted therapies for effective cancer treatment.
3.3. Roles of Chemokine System in Tumor Angiogenesis
Like normal tissues, tumors require sustenance from nutrients, oxygen, and the ability to excrete metabolic wastes and carbon dioxide, which are addressed by the process of angiogenesis, a tumor-associated neovasculature [82]. Many chemokine systems have been found to play important roles in tumor angiogenesis [106].
CXC chemokines, depending on the expression of the glutamic-leucine-arginine (ELR) motif at the N-terminal, can be classified into ELR+ chemokines with angiogenic effects and ELR− chemokines with angiostatic effects [107]. Angiogenic ELR+ CXC chemokines comprise CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8. Chemokines such as CXCL6 and CXCL8 can specifically bind and activate both CXCR1 and CXCR2 (Table 1), whereas other angiogenic ELR+ CXC chemokines mediate their angiogenic activity through CXCR2 [108,109]. Although CXCL12 is one of the ELR− CXC chemokines, it had been exceptionally implicated as a strong angiogenic chemokine [108]. All the angiogenic CXC chemokines contain a putative cis-element that recognizes NF-κB, leading to tumor-associated angiogenesis [109]. Furthermore, by working alone or interacting with other angiogenic factors such as VEGF, basic FGF (bFGF), and prostacyclin, the CXC axes such as CXCL8/CXCR2 and CXCL12/CXCR4 can act in either a direct, parallel, or serial manner to stimulate angiogenesis [109,110,111]. Interestingly, the pro-angiogenic effect of VEGF and CXCL8 was demonstrated to be further associated with the activation of neutrophils [112]. Furthermore, other chemokines such as CCL1, CCL2, CCL3, CCL11, CCL15, CCL16, CCL23, and CX3CL1 have also been implicated in tumor neovascularization by promoting migration and differentiation with or without the proliferation of endothelial cells and inducing new blood vessel formation [113,114,115,116,117,118,119,120]. CCL2 can recruit angiogenic factor-producing leukocytes such as macrophages into the TME to accelerate angiogenesis [121].
Except for CXCL12, other ELR− members of the CXC chemokine family including CXCL4, CXCL4L1, CXCL9, CXCL10, CXCL11, and CXCL14 are angiostatic [108,109]. CXCL4L1 is produced by the nonallelic variant gene of CXCL4 and differs from CXCL4 in only three amino acid residues but has more potent angiostatic effect than CXCL4 [109]. CXCR3 is a major receptor that has been identified for angiostatic CXC chemokines including CXCL4, CXCL9, CXCL10, and CXCL11. These chemokines are involved in the recruitment of Th1 cells expressing CXCR3, which acts as a receptor for the inhibition of angiogenesis [108,109,122]. Interestingly, the angiostatic capabilities of CXCL4 and CXCL10 also come from their suppression of bFGF and VEGF-induced angiogenesis and their inhibition of endothelial cell proliferation and chemotaxis [108,123,124,125]. CXCL14 has been shown to be a potent angiogenesis inhibitor but its receptor and underlying action mechanism remain unidentified [65,109]. Furthermore, CCL5 binding to CCR5 has also been demonstrated to mediate anti-angiogenic activity with an undefined mechanism [126].
In summary, chemokines stimulate or inhibit angiogenesis by the promotion or suppression of angiogenic factors such as VEGF and bFGF and the migration and proliferation of endothelial cells. Another way of chemokines to augment angiogenesis is through the recruitment of immune cells that support angiogenesis to the TME.
3.4. Roles of Chemokine System in Tumor Metastasis
Tumor metastasis is the movement of tumor cells from a primary site to progressively colonize distant organs and is a major contributor to the death of cancer patients [127]. After growing and proliferating at the primary tumor site, tumor cells migrate and invade the surrounding extracellular matrix (ECM), then proceed to enter the bloodstream or lymphatic system, becoming circulating tumor cells (CTCs). CTCs are disseminated along chemotactic gradients and induce extravasation at non-random and organ-specific sites, followed by tumor growth and proliferation at the new sites [14]. There are some organs in the body that are more susceptible to tumor metastasis such as the lung, brain, liver, lymph nodes, and bone marrow while others like the skin, kidneys, and pancreas are less prone [128]. While the mammalian body has a variety of active cellular highways, chemokines are considered to act as the “traffic directors” responsible for guiding cells that express appropriate receptors to specific locations. Metastatic cancer cells can “hijack” the chemokine receptor-regulated cell migration highway to initiate metastasis at distant sites [128].
At the new distant locations, cancer cells can exploit the chemokine system to establish immune system suppression and angiogenesis for the formation of a pre-metastatic niche, and to facilitate the proliferation of metastatic cancer cells [129]. For example, CCL2 stimulates metastatic seeding of BC cells by increasing the retention of metastasis-associated macrophages [130]. Furthermore, CXCR2 has a key role in metastatic progression, involving the migration of myeloid lineage cells such as neutrophils, macrophages, and MDSCs to both primary tumors and metastases [131]. Interestingly, in BC, the CCL5 secreted by lymphatic endothelial cells within the lungs and lymph nodes directs tumor dissemination into these tissues and promotes metastasis [132].
Cell migration is an integrated multistep process initiated by the process of membrane protrusion, which is driven by localized polymerization of actin filaments on the submembrane [133]. The binding of chemokines to their GPCRs activates a series of downstream signalling pathways that regulate integrin activation (adhesion) and actin cytoskeleton reorganization. This leads to actin polymerization and F-actin formation, followed by pseudopodia formation and, eventually, more efficient migration and invasion of tumor cells [134,135]. The CCL5/CCR5, CCL21/CCR7, and CXCL12/CXCR4 axes have been shown to promote cell migration through this mechanism [136,137].
The EMT is a phenotypic change from polarized epithelial cells to mesenchymal cells, resulting in the loss of cell-cell adhesion and cell polarity, increased migratory capacity and invasiveness, enhanced resistance to apoptosis, and substantial promotion of the production of ECM components [138,139]. Two of the most important properties that promote metastasis, namely invasiveness and stemness, converge during EMT [140]. Interestingly, various chemokines have been implicated to contribute to EMT progression in cancer cells. EMT can be induced by CXCL8 and its receptors through overexpression of the transcription factor Brachyury [141], CCL2 with the enhancement of Snail expression [142], the CXCL6/CXCR1/2 axis via the PI3K/AKT and Wnt/β-catenin pathways [143], and the CXCL1/LCN2 paracrine axis with the activation of Src signalling [144]. In addition, NF-κB is associated with the EMT induced by CCL5 and CCL18 [145,146]. In contrast, CCL28 treatment has been demonstrated to inhibit cell invasion and EMT in oral squamous cell carcinoma cells [147].
CSCs refer to undifferentiated and self-renewing tumor cells, which have the ability to initiate heterogeneous tumors and repopulate metastatic outgrowths [140]. Many studies have demonstrated a correlation between the chemokine system and CSC-like properties in cancer cells. The CXCL12/CXCR4 axis has been well-documented to have various roles in CSCs. The overexpression of CXCR4 or CXCL12γ, an isoform of CXCL12 found in CD44+/CD133+ prostate CSCs, suggests that one mechanism by which the CXCR4/CXCL12 axis promotes metastasis in prostate cancer is the maintenance of stemness in CSCs [148,149]. In addition, CXCR1 blockage reduced CSC properties in clear cell renal cell carcinoma [150], depleted the CSC population, and reduced systemic metastasis development in BC cells [151]. CCR5+ BC cells demonstrated several specific features of CSCs, including increased mammosphere formation, enhanced ability to initiate tumors, and metastatic capacity, as well as improved DNA repair activity [152].
Briefly, chemokines promote tumor metastasis through their hijacked cell migration highway, the establishment of a premetastatic niche, formation of pseudopodia, and induction of EMT and CSC properties. Therefore, chemokines that stimulate tumor metastasis can potentially serve as poor prognostic markers for cancer patients. Taken together, the chemokine system plays pivotal roles in regulating immune cell recruitment to the TME, tumor growth and proliferation, angiogenesis, and metastasis. The representative chemokines and chemokine receptors associated with their multifaceted roles in cancer progression are delineated in Figure 1.
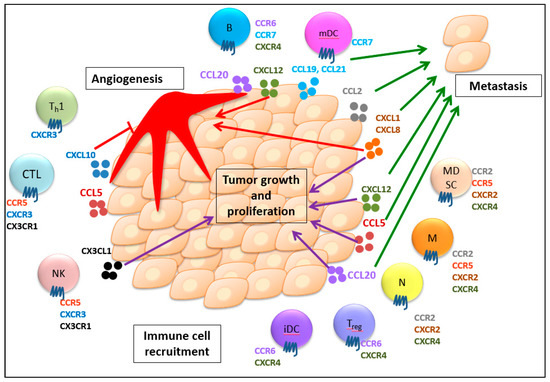
Figure 1.
Multifaceted roles of chemokines and their receptors in immune cell recruitment, tumor growth and proliferation, angiogenesis, and metastasis. Chemokines guide the trafficking of different immune cells expressing their respective receptors into the tumor microenvironment, which induces both anti- and protumor immunity. Additionally, the chemokine system generally stimulates tumor growth and proliferation. Chemokines can also regulate angiogenesis with their angiogenic or angiostatic functions. Furthermore, chemokines are involved in tumor migration to secondary sites to develop metastasis. CTL, CD8+ cytotoxic T lymphocyte; Th1, T helper cell; NK, natural killer cell; Treg, regulatory T cell; B, B cell; iDC, immature dendritic cells; mDC, mature dendritic cell; N, neutrophil, M, macrophage; MDSC, myeloid-derived suppressor cell. The purple arrows show the promotion of tumor growth and proliferation. The red arrows indicate the angiogenic effect. The red T line indicates the angiostatic effect. The green arrows indicate the promotion of metastasis. (For detailed information, please see the text.).
4. Role of Chemokine System in Cancer Prognosis
So far, this review has described the complicated and multifaceted roles of the chemokine system in cancer progression. These critical roles of the chemokine system could have value in predicting OS in cancer patients. We used the PubMed database as the primary source and Google as the secondary source and searched for relevant articles on the role of chemokine system in cancer prognosis, published up to October 2019. The following key words were employed in the search: chemokine/s, prognosis, prognostic, and cancer. There is increasing evidence from retrospective, prospective, prospective-retrospective studies, which are designed as retrospective analysis of archived tissues prospectively collected with follow-up data, and even meta-analysis studies to demonstrate the potential predictive value of the chemokine system for patients with different kinds of cancers. In this section, we will discuss the roles of chemokines as prognostic factors for cancer patients in correlation with their roles in disease progression.
4.1. CCL2
Interestingly, a hypothesis suggested that tumor cells produce CCL2 to amplify the recruitment of monocytes or macrophages, which might kill tumor cells by producing pro-inflammatory cytokines [153]. Even though CCL2 exhibits angiogenic effects in vivo, the activity is only observed at certain doses but not at higher doses of CCL2 [154]. Nevertheless, there is a series of results suggesting that the CCL2-dependent signalling pathway could promote the survival of tumor cells [155,156] and stimulate metastasis [157,158,159] and angiogenesis [121]. The dual roles of the CCL2/CCR2 axis in cancer development can lead to opposite results, as patients with higher CCL2 or CCR2 expression had significantly better OS in NSCLC [153] but shorter OS and progression-free survival in diffuse large B cell lymphoma (DLBCL) [160], although evidence about the roles of CCL2/CCR2 in DLBCL is limited. Further understandings are needed to clarify the value of CCL2/CCR2 as a prognostic factor in many different cancer types.
4.2. CCL5
The CCL5/CCR5 axis has been demonstrated to promote cancer cell migration through the recruitment and modulation of inflammatory cell activities, followed by the generation of an immunosuppressive environment including TAMs and MDSCs in BC [79,80,161]. Data from a study on BC patients showed that patients with higher serum levels of CCL5 had a greater probability of lymph node metastasis [81]. Similarly, in stage II BC patients, the positive tumor expression of CCL5 alone or when combined with the absence of estrogen receptor-α significantly increased the risk for disease progression [162]. Therefore, CCL5 can be considered a poor prognostic factor for BC, primarily in stage II patients.
4.3. CCL14
The functions of CCL14 in cancer progression have not been clearly identified. CCL14 promotes apoptosis, alleviates HCC cell proliferation and growth by inhibiting cell cycle progression through the Wnt/β-catenin signalling pathway, and contributes to longer OS in HCC patients [105]. Consistently, high expression of CCL14 genes effectively improved survival times in HCC [163]. In contrast, however, CCL14 was reported to promote bone marrow infiltration, proliferation, and the polarization of macrophages, which was considered to be associated with chemoresistance in MM [164]. More studies are needed to discover the mechanisms of action for this chemokine in cancer development and its prognostic functions in HCC and other cancer types.
4.4. CCL20
Studies have reported that the CCL20/CCR6 axis plays a key role in the tumor-chemokine network and promotes tumor progression in HCC and CRC. This axis has been shown to stimulate cell proliferation, probably via regulating the expression of p21, p27, and cyclin-D1 [91,165], induce EMT via PI3K/AKT and Wnt/β-catenin pathways [166], and eventually, promote metastasis [167,168]. The CCL20/CCR6 network facilitates Treg activity and induces immune suppression to mediate cancer cell elimination and metastasis [34]. With these protumor effects, CCL20 expression in HCC [169] and the co-expression of CCL20 and CXCL8 in CRC [167] were negatively associated with patient outcomes.
4.5. CCR7
CCR7 has been well-documented to comprehensively promote tumor development in many cancer types. CCR7 can weaken the host’s antitumor immunity by downregulating IFN-γ mediated inflammation in melanoma [170]. In addition, CCR7 has been demonstrated to inhibit apoptosis and stimulate proliferation by promoting G2/M phase progression through the ERK1/2 pathway in NSCLC [171]. CCR7 also induces tumor angiogenesis by promoting VEGF-C expression in prostate cancer [172]. Moreover, it enhances the proliferation and migration of endothelial cells and increases angiogenic capacity via the NF-κB/VEGF pathway in esophageal squamous carcinoma cells (ESCC) [173]. CCR7 has also been found to induce EMT in PDAC and lung cancer (LC) [174,175], promote MMP-2 and -9 expression in bladder cancer [176], and facilitate tumor cell dissemination, migration, and eventually metastasis formation [177]. With the strong protumor functions, the expression of CCR7 or CCL21 has been reported to be strongly correlated with poor survival [169,178]. Hence, CCR7 could become a predictive marker for a number of cancers, including colorectal liver metastasis.
4.6. CXCL1
CXCL1 has been reported to play an important role in CRC progression and metastasis by inducing glycolysis [179]. CXCL1 is also an ELR+ CXC chemokine that induces angiogenesis by binding to CXCR2 [109]. In addition, CXCL1 produced by TAMs recruits CXCR2+ MDSCs for the pre-metastatic niche to stimulate liver metastases in CRC [70]. Moreover, CXCL1 directly represses T cell infiltration and mitigates sensitivity to immunotherapy in pancreatic cancer [180]. Consistently, due to the tumor-promoting effects, high CXCL1 expression has been shown to be a risk factor for cancer prognosis, with poor OS, advanced tumor, node, and metastasis stage, and lymph node metastasis. This result strongly suggests the prognosis value of CXCL1 for various cancers including CRC, pancreatic cancer, and others [181]. It has also been suggested that CXCL1 mediates radioresistance by regulating the DNA damage response in a ROS-dependent manner in ESCC [182].
4.7. CXCL8
CXCL8 has been extensively investigated for its functions in promoting tumorigenesis. CXCL8 can stimulate proliferation and survival via autocrine activation in CRC [183], cervical cancer [184], and LC [185], or via the ERK1/2 pathway in NSCLC [94]. CXCL8 also promotes angiogenesis and cell migration, induces EMT in CRC [183,186], attracts and activates MDSCs to form NETs, and helps tumors evade the immune system [187]. Due to the above protumor effects, high expression of CXCL8 potentially serves as an unfavorable prognostic marker in numerous human cancers, including CRC [167,188], cervical cancer [189], and lung adenocarcinoma [190]. Consistently, CXCL8 has also been a strong predictor for poor outcome in various treatments including chemo-immunotherapy [191] in pancreatic cancer or aflibercept therapy in metastatic CRC [192].
4.8. CXCL10
CXCL10 has opposing effects on TME. On the one hand, local production of CXCL10 attracts CTLs into ESCC tissue and probably plays a positive role in patient survival [193]. On the other hand, an in vitro study showed that monocytes, orchestrated by CXCL10, promote the migration and invasion of tumor cells in B cell precursor acute lymphoblastic leukemia (ALL), and then stimulate metastasis [194]. Because of the above dual functions, elevated expression of CXCL10 has been associated with favorable outcomes in ESCC [195], but unfavorable prognosis in DLBCL [196].
4.9. CXCL12/CXCR4
CXCL12/CXCR4 is a well-investigated axis that is strongly involved in all stages of tumor progression in many kinds of cancer. CXCL12/CXCR4 plays an important role in the recruitment of Treg cells into the TME, and contributes to immune suppressive activities and tumor-related inflammation in HCC and malignant pleural mesothelioma [37,38]. As mentioned above, the CXCL12/CXCR4 pair promotes tumor cell growth and the proliferation of glioblastoma cells via the ERK1/2 and AKT pathways [95]. In addition, the axis is involved in tumor angiogenesis via VEGF-dependent mechanisms in BC [197] and glioblastoma [198]. This axis also promotes metastasis mediated by actin polymerization, pseudopodia formation [199], and EMT induction [200]. Moreover, CXCR4 plays a key role in the self-renewal of CSCs in drug-resistant NSCLC [201]. Due to the powerful protumor activities of CXCL12/CXCR4 axis, numerous studies have consistently shown that their expression is associated with poor prognosis for patients in esophageal cancer [202], acute myelogenous leukemia [203], BC [204,205], HCC [206], and NSCLC [207]. In addition, CXCR4 expression correlates with the degree of tumor infiltration and promotes a more aggressive phenotype in papillary thyroid carcinoma [208], and is also a negative prognostic marker for response to chemotherapy in NHL [209].
4.10. CX3CL1/CX3CR1
The CX3CL1/CX3CR1 axis has both tumor-promoting and suppressive effects in cancer progression, resulting in either favorable or unfavorable prognosis depending on the cancer types. Increased expression of CX3CL1 has been correlated with a better prognosis, with enhanced recruitment of CD8+ T and NK cells in gastric adenocarcinoma patients [210]. Furthermore, in HCC patients, CX3CL1/CX3CR1 regulates the cancer cell cycle and tumor progression via the autocrine or paracrine systems, which is associated with positive outcomes [211]. In contrast, CX3CL1/CX3CR1 has been proven to induce tumor growth, proliferation, and apoptosis resistance through activating the AKT/NF-κB [100] and JAK/STAT signalling pathways in PDAC [212]. Therefore, a study in PDAC patients showed evidence that high expression of the CX3CL1/CX3CR1 axis in tumor tissues led to a poor prognosis for OS [213].
4.11. XCL1
XCL1 attracts CD4+ and CD8+ T cells, neutrophils [214], and NK cells [215]. It probably augments antitumor responses, and therefore could be crucial in gene transfer immunotherapies in some cancers [214]. Interestingly, higher serum XCL1 levels at diagnosis and their progressive decline during chemotherapy were associated with higher survival in ALL [216]. This could be explained by the progressive decrease of the leukemic burden in the tumor’s response to cancer treatments. However, this result should be taken into consideration because patients with good predictive factors were also younger and had lower white blood cell counts [216]. The mechanism of XCL1 in cancer progression and its role in cancer prognosis should be examined further in future investigations.
In brief, many chemokines and chemokine receptors can act as prognostic markers for cancer patients. CCL2, CXCL10, and CX3CL1/CX3CR1 can serve as both favorable and unfavorable prognostic factors for cancers depending on cancer types. While CCL14 and XCL1 have shown only good prognostic value, other chemokines such as CCL5, CCL20/CCR6, CCR7, CXCL1, CXCL8, and CXCL12/CXCR4 have consistently played the role of poor prognostic markers in different kinds of cancer. The favorable and unfavorable roles of chemokines as prognostic factors in different types of cancers are summarized in Table 2. Interestingly, high levels of multiple chemokines were reported to be strongly associated with worse patient OS in several clinical trials [217,218,219]. In order to identify and validate multiple chemokines as promising and predictive tumor-based biomarkers for patient outcomes, further investigations in various clinical trials of anticancer treatments are necessary.

Table 2.
Roles of chemokine system as prognostic factors in cancers.
5. Conclusions
It is undeniable that during the past several decades, there have been enormous advances in our knowledge regarding the functions of the chemokine system in cancer. Accumulating evidence strongly supports the multifaceted roles of chemokines and their receptors in tumor progression. Importantly, chemokines act as chemoattractants to recruit both anti- and protumor immune cells into the TME. Furthermore, most chemokines function as promoting factors for tumor growth and proliferation, angiogenesis, and metastasis; however, some other chemokines have the opposite effects. The complicated effects of the chemokine system in cancer could be due to the promiscuity of chemokine and chemokine receptor interactions. One chemokine-receptor pair can serve as tumor suppressors in one type of cancer and as tumor promoters in other types of cancer. Therefore, some chemokines such as CCL2, CXCL10, and CX3CL1/CX3CR1 can be either favorable or unfavorable cancer prognostic factors depending on the cancer types. In contrast, CCL14 and XCL1 only serve as good prognostic factors for cancer patient outcomes. However, the chemokines and their receptors that particularly stimulate tumorigenesis, including CCR7, CXCL1, CXCL8, and CXCL12/CXCR4, could, consequently, act as poor prognostic markers for cancer patients.
Despite the substantial advances in our understanding of the complex nature of the chemokine system in tumor biology, knowledge about the multifaceted roles of chemokines and their prognostic value in different types of cancers, especially in response to diverse anticancer therapies, is still limited. Nonetheless, a considerable number of chemokine receptor inhibitors targeting different chemokine signalling pathways are currently being evaluated in many preclinical studies and clinical trials, with encouraging results when used in combination with chemotherapy or immune checkpoint therapy. For the validation of specific chemokines and their receptors as prognostic markers of specific cancer types, further extensive studies are essential to decipher their distinct roles and the action mechanisms involved in cancer progression.
Author Contributions
J.C. conceptualized the manuscript; H.T.T.D. wrote the initial draft and revised subsequent drafts; C.H.L. and J.C. reviewed and edited the manuscript prior to submission. All authors have read and agreed to the published version of the manuscript.
Funding
The National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT): NRF–2018R1A5A2023127.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.S.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef] [PubMed]
- Foxman, E.F.; Kunkel, E.J.; Butcher, E.C. Integrating conflicting chemotactic signals. The role of memory in leukocyte navigation. J. Cell Biol. 1999, 147, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Foxman, E.F.; Campbell, J.J.; Butcher, E.C. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J. Cell Biol. 1997, 139, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Cho, J. Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch. Pharmacal. Res. 2013, 36, 1039–1050. [Google Scholar] [CrossRef]
- Moser, B.; Loetscher, P. Lymphocyte traffic control by chemokines. Nat. Immunol. 2001, 2, 123–128. [Google Scholar] [CrossRef]
- Ansel, K.M.; Cyster, J.G. Chemokines in lymphopoiesis and lymphoid organ development. Curr. Opin. Immunol. 2001, 13, 172–179. [Google Scholar] [CrossRef]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559. [Google Scholar] [CrossRef]
- Fuji, S.; Utsunomiya, A.; Inoue, Y.; Miyagi, T.; Owatari, S.; Sawayama, Y.; Moriuchi, Y.; Choi, I.; Shindo, T.; Yoshida, S.-I. Outcomes of patients with relapsed aggressive adult T-cell leukemia-lymphoma: Clinical effectiveness of anti-CCR4 antibody and allogeneic hematopoietic stem cell transplantation. Haematologica 2018, 103, e211–e214. [Google Scholar] [CrossRef]
- Micallef, I.N.; Stiff, P.J.; Nademanee, A.P.; Maziarz, R.T.; Horwitz, M.E.; Stadtmauer, E.A.; Kaufman, J.L.; McCarty, J.M.; Vargo, R.; Cheverton, P.D. Plerixafor plus granulocyte colony-stimulating factor for patients with non-Hodgkin lymphoma and multiple myeloma: Long-term follow-up report. Biol. Blood Marrow Transpl. 2018, 24, 1187–1195. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. The chemokine superfamily revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef] [PubMed]
- David, B.A.; Kubes, P. Exploring the complex role of chemokines and chemoattractants in vivo on leukocyte dynamics. Immunol. Rev. 2019, 289, 9–30. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzi, E.; Angioni, R.; Molon, B.; Calì, B. Chemokines and chemokine receptors: Orchestrating tumor metastasization. Int. J. Mol. Sci. 2019, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Vilgelm, A.E.; Richmond, A. Chemokines Modulate Immune Surveillance in Tumorigenesis, Metastasis, and Response to Immunotherapy. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 2004, 4, 540. [Google Scholar] [CrossRef]
- Ruffell, B.; DeNardo, D.G.; Affara, N.I.; Coussens, L.M. Lymphocytes in cancer development: Polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. 2010, 21, 3–10. [Google Scholar] [CrossRef]
- Mukaida, N.; Sasaki, S.-I.; Baba, T. Chemokines in cancer development and progression and their potential as targeting molecules for cancer treatment. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef]
- Moretta, A. Molecular mechanisms in cell-mediated cytotoxicity. Cell 1997, 90, 13–18. [Google Scholar] [CrossRef][Green Version]
- Kagi, D.; Vignaux, F.; Ledermann, B.; Burki, K.; Depraetere, V.; Nagata, S.; Hengartner, H.; Golstein, P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 1994, 265, 528–530. [Google Scholar] [CrossRef]
- Russell, J.H.; Ley, T.J. Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 2002, 20, 323–370. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.-L.; Li, L.; Guo, Y.-W.; Yu, P.; Yin, X.-J.; Wang, S.; Liu, C.-P. CD8+ cytotoxic and FoxP3+ regulatory T lymphocytes serve as prognostic factors in breast cancer. Am. J. Transl. Res. 2019, 11, 5039. [Google Scholar] [PubMed]
- Nagarsheth, N.; Peng, D.; Kryczek, I.; Wu, K.; Li, W.; Zhao, E.; Zhao, L.; Wei, S.; Frankel, T.; Vatan, L.; et al. PRC2 Epigenetically Silences Th1-Type Chemokines to Suppress Effector T-Cell Trafficking in Colon Cancer. Cancer Res. 2016, 76, 275–282. [Google Scholar] [CrossRef]
- Peng, D.; Kryczek, I.; Nagarsheth, N.; Zhao, L.; Wei, S.; Wang, W.; Sun, Y.; Zhao, E.; Vatan, L.; Szeliga, W.; et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015, 527, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Bonecchi, R.; Bianchi, G.; Bordignon, P.P.; D’Ambrosio, D.; Lang, R.; Borsatti, A.; Sozzani, S.; Allavena, P.; Gray, P.A.; Mantovani, A.; et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998, 187, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Andalib, A.; Doulabi, H.; Maracy, M.R.; Rezaei, A.; Hasheminia, S.J. CCR3, CCR4, CCR5, and CXCR3 expression in peripheral blood CD4+ lymphocytes in gastric cancer patients. Adv. Biomed. Res. 2013, 2, 31. [Google Scholar] [CrossRef]
- Nizri, E.; Greenman-Maaravi, N.; Bar-David, S.; Ben-Yehuda, A.; Weiner, G.; Lahat, G.; Klausner, J. Analysis of histological and immunological parameters of metastatic lymph nodes from colon cancer patients reveals that T-helper 1 type immune response is associated with improved overall survival. Medicine 2016, 95, e5340. [Google Scholar] [CrossRef]
- Nizri, E.; Sternbach, N.; Bar-David, S.; Ben-Yehuda, A.; Gerstenhaber, F.; Ofir, T.; Wolf, I.; Weiner, G.; Lahat, G.; Klausner, J. T-Helper 1 immune response in metastatic lymph nodes of pancreatic ductal adenocarcinoma: A marker for prolonged survival. Ann. Surg. Oncol. 2018, 25, 475–481. [Google Scholar] [CrossRef]
- Wynn, T.A. Type 2 cytokines: Mechanisms and therapeutic strategies. Nat. Rev. Immunol. 2015, 15, 271–282. [Google Scholar] [CrossRef]
- Islam, S.A.; Chang, D.S.; Colvin, R.A.; Byrne, M.H.; McCully, M.L.; Moser, B.; Lira, S.A.; Charo, I.F.; Luster, A.D. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ T H 2 cells. Nat. Immunol. 2011, 12, 167. [Google Scholar] [CrossRef]
- Kim, C.H.; Nagata, K.; Butcher, E.C. Dendritic cells support sequential reprogramming of chemoattractant receptor profiles during naive to effector T cell differentiation. J. Immunol. 2003, 171, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Piro, G.; Simionato, F.; Carbone, C.; Frizziero, M.; Malleo, G.; Zanini, S.; Casolino, R.; Santoro, R.; Mina, M.M.; Zecchetto, C. A circulating TH2 cytokines profile predicts survival in patients with resectable pancreatic adenocarcinoma. Oncoimmunology 2017, 6, e1322242. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Sakaguchi, S. Regulatory T cells in tumor immunity. Int. J. Cancer 2010, 127, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Li, W.M.; Liu, H.R. CCL 20-CCR 6 Cytokine Network Facilitate Treg Activity in Advanced Grades and Metastatic Variants of Hepatocellular Carcinoma. Scand. J. Immunol. 2016, 83, 33–37. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942. [Google Scholar] [CrossRef]
- Facciabene, A.; Peng, X.; Hagemann, I.S.; Balint, K.; Barchetti, A.; Wang, L.-P.; Gimotty, P.A.; Gilks, C.B.; Lal, P.; Zhang, L. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T reg cells. Nature 2011, 475, 226. [Google Scholar] [CrossRef]
- Shimtzu, Y.; Dobashi, K.; Imai, H.; Sunaga, N.; Ono, A.; Sano, T.; Hikino, T.; Shimizu, K.; Tanaka, S.; Ishizuka, T. CXCR4+ FOXP3+ CD25+ lymphocytes accumulate in CXCL12-expressing malignant pleural mesothelioma. Int. J. Immunopathol. Pharmacol. 2009, 22, 43–51. [Google Scholar] [CrossRef]
- Shen, X.; Li, N.; Li, H.; Zhang, T.; Wang, F.; Li, Q. Increased prevalence of regulatory T cells in the tumor microenvironment and its correlation with TNM stage of hepatocellular carcinoma. J. Cancer Res. Clin. 2010, 136, 1745–1754. [Google Scholar] [CrossRef]
- Zhao, E.; Wang, L.; Dai, J.; Kryczek, I.; Wei, S.; Vatan, L.; Altuwaijri, S.; Sparwasser, T.; Wang, G.; Keller, E.T. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology 2012, 1, 152–161. [Google Scholar] [CrossRef]
- Kryczek, I.; Wang, L.; Wu, K.; Li, W.; Zhao, E.; Cui, T.; Wei, S.; Liu, Y.; Wang, Y.; Vatan, L. Inflammatory regulatory T cells in the microenvironments of ulcerative colitis and colon carcinoma. Oncoimmunology 2016, 5, e1105430. [Google Scholar] [CrossRef]
- Shang, B.; Liu, Y.; Jiang, S.-j.; Liu, Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lou, Y.; Lizée, G.; Qin, H.; Liu, S.; Rabinovich, B.; Kim, G.J.; Wang, Y.-H.; Ye, Y.; Sikora, A.G. Plasmacytoid dendritic cells induce NK cell–dependent, tumor antigen–specific T cell cross-priming and tumor regression in mice. J. Clin. Investig. 2008, 118, 1165–1175. [Google Scholar] [CrossRef]
- Wendel, M.; Galani, I.E.; Suri-Payer, E.; Cerwenka, A. Natural killer cell accumulation in tumors is dependent on IFN-γ and CXCR3 ligands. Cancer Res. 2008, 68, 8437–8445. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, E.; Combadière, B.; Bonduelle, O.; Iga, M.; Gao, J.-L.; Maho, M.; Boissonnas, A.; Murphy, P.M.; Debré, P.; Combadière, C. Fractalkine mediates natural killer-dependent antitumor responses in vivo. Cancer Res. 2003, 63, 7468–7474. [Google Scholar] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chen, L.; Li, J.; Zheng, X.; Shi, L.; Wu, C.; Jiang, J. Prognostic value of tumor infiltrating NK cells and macrophages in stage II+ III esophageal cancer patients. Oncotarget 2016, 7, 74904. [Google Scholar] [CrossRef]
- Largeot, A.; Pagano, G.; Gonder, S.; Moussay, E.; Paggetti, J. The B-side of cancer immunity: The underrated tune. Cells 2019, 8, 449. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef]
- DiLillo, D.J.; Yanaba, K.; Tedder, T.F. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: Therapeutic B cell depletion enhances B16 melanoma growth in mice. J. Immunol. 2010, 184, 4006–4016. [Google Scholar] [CrossRef]
- Crawford, A.; MacLeod, M.; Schumacher, T.; Corlett, L.; Gray, D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J. Immunol. 2006, 176, 3498–3506. [Google Scholar] [CrossRef]
- Bouaziz, J.-D.; Yanaba, K.; Venturi, G.M.; Wang, Y.; Tisch, R.M.; Poe, J.C.; Tedder, T.F. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc. Natl. Acad. Sci. USA 2007, 104, 20878–20883. [Google Scholar] [CrossRef] [PubMed]
- Germain, C.; Gnjatic, S.; Dieu-Nosjean, M.-C. Tertiary lymphoid structure-associated B cells are key players in anti-tumor immunity. Front. Immunol. 2015, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-Y.; Gao, Q.; Wang, Z.-C.; Zhou, J.; Wang, X.-Y.; Min, Z.-H.; Shi, Y.-H.; Shi, G.-M.; Ding, Z.-B.; Ke, A.-W. Margin-infiltrating CD20+ B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin. Cancer Res. 2013, 19, 5994–6005. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lee, H.; Pal, S.; Jove, V.; Deng, J.; Zhang, W.; Hoon, D.S.; Wakabayashi, M.; Forman, S.; Yu, H. B cells promote tumor progression via STAT3 regulated-angiogenesis. PLoS ONE 2013, 8, e64159. [Google Scholar] [CrossRef] [PubMed]
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; Lepelley, A.; Becht, E.; Katsahian, S.; Bizouard, G. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am. J. Respir. Crit. Care Med. 2014, 189, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, S.; Berntsson, J.; Nodin, B.; Micke, P.; Jirström, K. Prognostic impact of tumour-associated B cells and plasma cells in epithelial ovarian cancer. J. Ovarian Res. 2016, 9, 21. [Google Scholar] [CrossRef]
- Yang, C.; Lee, H.; Jove, V.; Deng, J.; Zhang, W.; Liu, X.; Forman, S.; Dellinger, T.H.; Wakabayashi, M.; Yu, H. Prognostic significance of B-cells and pSTAT3 in patients with ovarian cancer. PLoS ONE 2013, 8, e54029. [Google Scholar] [CrossRef]
- Banchereau, J.; Palucka, A.K. Dendritic cells as therapeutic vaccines against cancer. Nat. Rev. Immunol. 2005, 5, 296. [Google Scholar] [CrossRef]
- Bell, D.; Chomarat, P.; Broyles, D.; Netto, G.; Harb, G.M.; Lebecque, S.; Valladeau, J.; Davoust, J.; Palucka, K.A.; Banchereau, J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J. Exp. Med. 1999, 190, 1417–1426. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 2012, 12, 265. [Google Scholar] [CrossRef]
- Shurin, G.V.; Ferris, R.; Tourkova, I.L.; Perez, L.; Lokshin, A.; Balkir, L.; Collins, B.; Chatta, G.S.; Shurin, M.R. Loss of new chemokine CXCL14 in tumor tissue is associated with low infiltration by dendritic cells (DC), while restoration of human CXCL14 expression in tumor cells causes attraction of DC both in vitro and in vivo. J. Immunol. 2005, 174, 5490–5498. [Google Scholar] [CrossRef] [PubMed]
- Shellenberger, T.D.; Wang, M.; Gujrati, M.; Jayakumar, A.; Strieter, R.M.; Burdick, M.D.; Ioannides, C.G.; Efferson, C.L.; El-Naggar, A.K.; Roberts, D. BRAK/CXCL14 is a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells. Cancer Res. 2004, 64, 8262–8270. [Google Scholar] [CrossRef] [PubMed]
- Dieu, M.-C.; Vanbervliet, B.; Vicari, A.; Bridon, J.-M.; Oldham, E.; Aït-Yahia, S.; Brière, F.; Zlotnik, A.; Lebecque, S.; Caux, C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 1998, 188, 373–386. [Google Scholar] [CrossRef] [PubMed]
- SenGupta, S.; Subramanian, B.C.; Parent, C.A. Getting TANned: How the tumor microenvironment drives neutrophil recruitment. J. Leukoc. Biol 2019, 105, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Fridlender, Z.G.; Granot, Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 2015, 8, 125–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, Y.; Han, Y.; Zhang, Q.; Jiang, Z.; Zhang, X.; Huang, B.; Xu, X.; Zheng, J.; Cao, X. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell 2016, 30, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Seubert, B.; Grünwald, B.; Kobuch, J.; Cui, H.; Schelter, F.; Schaten, S.; Siveke, J.T.; Lim, N.H.; Nagase, H.; Simonavicius, N. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology 2015, 61, 238–248. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Bianchi, P.; Grizzi, F.; Di Caro, G.; Basso, G.; Ponzetta, A.; Bonavita, E.; Barbagallo, M.; Tartari, S.; Polentarutti, N. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int. J. Cancer 2016, 139, 446–456. [Google Scholar] [CrossRef]
- Donskov, F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin. Cancer Biol. 2013, 23, 200–207. [Google Scholar] [CrossRef]
- Wang, D.; Sun, H.; Wei, J.; Cen, B.; DuBois, R.N. CXCL1 is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res. 2017, 77, 3655–3665. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Schaar, B.; Tallapragada, S.; Dorigo, O. Tumor associated macrophages in gynecologic cancers. Gynecol. Oncol. 2018, 149, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Serafini, P.; De Santo, C.; Marigo, I.; Cingarlini, S.; Dolcetti, L.; Gallina, G.; Zanovello, P.; Bronte, V. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol. Immunother. 2004, 53, 64–72. [Google Scholar] [CrossRef]
- Sinha, P.; Clements, V.K.; Bunt, S.K.; Albelda, S.M.; Ostrand-Rosenberg, S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 2007, 179, 977–983. [Google Scholar] [CrossRef]
- Wang, P.-F.; Song, S.-Y.; Wang, T.-J.; Ji, W.-J.; Li, S.-W.; Liu, N.; Yan, C.-X. Prognostic role of pretreatment circulating MDSCs in patients with solid malignancies: A meta-analysis of 40 studies. Oncoimmunology 2018, 7, e1494113. [Google Scholar] [CrossRef]
- González-Martín, A.; Gómez, L.; Lustgarten, J.; Mira, E.; Mañes, S. Maximal T cell–mediated antitumor responses rely upon CCR5 expression in both CD4+ and CD8+ T cells. Cancer Res. 2011, 71, 5455–5466. [Google Scholar] [CrossRef]
- Skachkova, O.; Khranovska, N.; Gorbach, O.; Svergun, N.; Sydor, R.; Nikulina, V. Immunological markers of anti-tumor dendritic cells vaccine efficiency in patients with non-small cell lung cancer. Exp. Oncol. 2013, 35, 109–113. [Google Scholar]
- Cheng, R.; Billet, S.; Liu, C.; Haldar, S.; Choudhury, D.; Tripathi, M.; Hav, M.; Merchant, A.; Hu, T.; Huang, H. Periodontal inflammation recruits distant metastatic breast cancer cells by increasing myeloid-derived suppressor cells. Oncogene 2019, 1–14. [Google Scholar] [CrossRef]
- Azenshtein, E.; Luboshits, G.; Shina, S.; Neumark, E.; Shahbazian, D.; Weil, M.; Wigler, N.; Keydar, I.; Ben-Baruch, A. The CC chemokine RANTES in breast carcinoma progression: Regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002, 62, 1093–1102. [Google Scholar]
- Wan, H.; Du, Z.; Long, Q.; Lü, Q.; Li, H. Criteria derived from serum markers can precisely evaluate axillary status in breast cancer patients. J. Surg. Res. 2017, 208, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cai, Z.; Galson, D.L.; Xiao, G.; Liu, Y.; George, D.E.; Melhem, M.F.; Yao, Z.; Zhang, J. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate 2006, 66, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Kamohara, H.; Takahashi, M.; Ishiko, T.; Ogawa, M.; Baba, H. Induction of interleukin-8 (CXCL-8) by tumor necrosis factor-α and leukemia inhibitory factor in pancreatic carcinoma cells: Impact of CXCL-8 as an autocrine growth factor. Int. J. Oncol. 2007, 31, 627–632. [Google Scholar] [CrossRef]
- Masood, R.; Cai, J.; Tulpule, A.; Zheng, T.; Hamilton, A.; Sharma, S.; Espina, B.M.; Smith, D.L.; Gill, P.S. Interleukin 8 is an autocrine growth factor and a surrogate marker for Kaposi’s sarcoma. Clin. Cancer Res. 2001, 7, 2693–2702. [Google Scholar]
- Kalbasi, A.; Komar, C.; Tooker, G.M.; Liu, M.; Lee, J.W.; Gladney, W.L.; Ben-Josef, E.; Beatty, G.L. Tumor-derived CCL2 mediates resistance to radiotherapy in pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2017, 23, 137–148. [Google Scholar] [CrossRef]
- Tan, M.C.; Goedegebuure, P.S.; Belt, B.A.; Flaherty, B.; Sankpal, N.; Gillanders, W.E.; Eberlein, T.J.; Hsieh, C.-S.; Linehan, D.C. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J. Immunol. 2009, 182, 1746–1755. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, L.; Pan, K.; Zhang, Z.; Zhou, M.; Cao, G. Integrated analysis of mRNA and miRNA expression profiles in pancreatic ductal adenocarcinoma. Oncol Rep. 2017, 37, 2779–2786. [Google Scholar] [CrossRef]
- Mebratu, Y.; Tesfaigzi, Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle 2009, 8, 1168–1175. [Google Scholar] [CrossRef]
- Jason, S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar]
- Brand, S.; Olszak, T.; Beigel, F.; Diebold, J.; Otte, J.M.; Eichhorst, S.T.; Göke, B.; Dambacher, J. Cell differentiation dependent expressed CCR6 mediates ERK-1/2, SAPK/JNK, and Akt signaling resulting in proliferation and migration of colorectal cancer cells. J. Cell. Biochem. 2006, 97, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Edwards, R.; Tucci, S.; Bu, P.; Milsom, J.; Lee, S.; Edelmann, W.; Gümüs, Z.H.; Shen, X.; Lipkin, S. Chemokine 25–induced signaling suppresses colon cancer invasion and metastasis. J. Clin. Investig. 2012, 122, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Bolitho, C.; Hahn, M.A.; Baxter, R.C.; Marsh, D.J. The chemokine CXCL1 induces proliferation in epithelial ovarian cancer cells by transactivation of the epidermal growth factor receptor. Endocr. Relat. Cancer 2010, 17, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Luppi, F.; Longo, A.; De Boer, W.; Rabe, K.; Hiemstra, P. Interleukin-8 stimulates cell proliferation in non-small cell lung cancer through epidermal growth factor receptor transactivation. Lung Cancer 2007, 56, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Barbero, S.; Bonavia, R.; Bajetto, A.; Porcile, C.; Pirani, P.; Ravetti, J.L.; Zona, G.L.; Spaziante, R.; Florio, T.; Schettini, G. Stromal cell-derived factor 1α stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Res. 2003, 63, 1969–1974. [Google Scholar] [PubMed]
- Gaudin, F.; Nasreddine, S.; Donnadieu, A.-C.; Emilie, D.; Combadiere, C.; Prevot, S.; Machelon, V.; Balabanian, K. Identification of the chemokine CX3CL1 as a new regulator of malignant cell proliferation in epithelial ovarian cancer. PLoS ONE 2011, 6, e21546. [Google Scholar] [CrossRef]
- Richmond, A. NF-κB, chemokine gene transcription and tumour growth. Nat. Rev. Immunol. 2002, 2, 664. [Google Scholar] [CrossRef]
- Geismann, C.; Schäfer, H.; Gundlach, J.-P.; Hauser, C.; Egberts, J.-H.; Schneider, G.; Arlt, A. NF-κB Dependent Chemokine Signaling in Pancreatic Cancer. Cancers 2019, 11, 1445. [Google Scholar] [CrossRef]
- Song, J.K.; Park, M.H.; Choi, D.-Y.; Yoo, H.S.; Han, S.B.; Hong, J.T. Deficiency of CC chemokine receptor 5 suppresses tumor development via inactivation of NF-κB and upregulation of IL-1Ra in melanoma model. PLoS ONE 2012, 7, e33747. [Google Scholar]
- Wang, H.; Cai, J.; Du, S.; Guo, Z.; Xin, B.; Wang, J.; Wei, W.; Shen, X. Fractalkine/CX3CR1 induces apoptosis resistance and proliferation through the activation of the AKT/NF-κB cascade in pancreatic cancer cells. Cell Biochem. Funct. 2017, 35, 315–326. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef] [PubMed]
- Vizioli, M.G.; Santos, J.; Pilotti, S.; Mazzoni, M.; Anania, M.C.; Miranda, C.; Pagliardini, S.; Pierotti, M.A.; Gil, J.; Greco, A. Oncogenic RAS-induced senescence in human primary thyrocytes: Molecular effectors and inflammatory secretome involved. Oncotarget 2014, 5, 8270. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Myeloid-derived suppressor cells (MDSC): An important partner in cellular/tissue senescence. Biogerontology 2018, 19, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Lesina, M.; Wörmann, S.M.; Morton, J.; Diakopoulos, K.N.; Korneeva, O.; Wimmer, M.; Einwächter, H.; Sperveslage, J.; Demir, I.E.; Kehl, T. RelA regulates CXCL1/CXCR2-dependent oncogene-induced senescence in murine Kras-driven pancreatic carcinogenesis. J. Clin. Investig. 2016, 126, 2919–2932. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Xu, W.; Wei, C.; Huang, J.; Xu, J.; Zhang, Y.; Zhao, Y.; Chen, J.; Dong, S.; Liu, B. CCL14 serves as a novel prognostic factor and tumor suppressor of HCC by modulating cell cycle and promoting apoptosis. Cell Death Dis. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dimberg, A. Chemokines in angiogenesis. In The Chemokine System in Experimental and Clinical Hematology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 59–80. [Google Scholar]
- Bonecchi, R.; Mollica Poeta, V.; Capucetti, A.; Massara, M. Chemokines and chemokine receptors: New targets for cancer immunotherapy. Front. Immunol. 2019, 10, 379. [Google Scholar]
- Chow, M.T.; Luster, A.D. Chemokines in cancer. Cancer Immunol. Res. 2014, 2, 1125–1131. [Google Scholar] [CrossRef]
- Strieter, R.M.; Burdick, M.D.; Mestas, J.; Gomperts, B.; Keane, M.P.; Belperio, J.A. Cancer CXC chemokine networks and tumour angiogenesis. Eur. J. Cancer 2006, 42, 768–778. [Google Scholar] [CrossRef]
- Martin, D.; Galisteo, R.; Gutkind, J.S. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFκB through the CBM (Carma3/Bcl10/Malt1) complex. J. Biol. Chem. 2009, 284, 6038–6042. [Google Scholar] [CrossRef]
- Salcedo, R.; Oppenheim, J.J. Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation 2003, 10, 359–370. [Google Scholar] [CrossRef]
- Schruefer, R.; Lutze, N.; Schymeinsky, J.; Walzog, B. Human neutrophils promote angiogenesis by a paracrine feedforward mechanism involving endothelial interleukin-8. Am. J. Physiol Heart Circ. Physiol. 2005, 288, H1186–H1192. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Son, K.-N.; Kim, C.W.; Ko, J.; Na, D.S.; Kwon, B.S.; Gho, Y.S.; Kim, J. Human CC chemokine CCL23, a ligand for CCR1, induces endothelial cell migration and promotes angiogenesis. Cytokine 2005, 30, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, R.; Young, H.A.; Ponce, M.L.; Ward, J.M.; Kleinman, H.K.; Murphy, W.J.; Oppenheim, J.J. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J. Immunol. 2001, 166, 7571–7578. [Google Scholar] [CrossRef] [PubMed]
- Strasly, M.; Doronzo, G.; Capello, P.; Valdembri, D.; Arese, M.; Mitola, S.; Moore, P.; Alessandri, G.; Giovarelli, M.; Bussolino, F. CCL16 activates an angiogenic program in vascular endothelial cells. Blood 2004, 103, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Yuzhalin, A.E.; Gordon-Weeks, A.N.; Muschel, R.J. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 2016, 7, 28697. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, G.; Spinetti, G.; Ribatti, D.; Camarda, G.; Morbidelli, L.; Ziche, M.; Santoni, A.; Capogrossi, M.C.; Napolitano, M. I-309 binds to and activates endothelial cell functions and acts as an angiogenic molecule in vivo. Blood 2000, 96, 4039–4045. [Google Scholar] [CrossRef]
- Ryschich, E.; Lizdenis, P.; Ittrich, C.; Benner, A.; Stahl, S.; Hamann, A.; Schmidt, J.; Knolle, P.; Arnold, B.; Hämmerling, G.J. Molecular fingerprinting and autocrine growth regulation of endothelial cells in a murine model of hepatocellular carcinoma. Cancer Res. 2006, 66, 198–211. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, C.W.; Son, K.-N.; Han, K.Y.; Lee, K.H.; Kleinman, H.K.; Ko, J.; Na, D.S.; Kwon, B.S.; Gho, Y.S. Angiogenic activity of human CC chemokine CCL15 in vitro and in vivo. FEBS Lett. 2004, 570, 47–51. [Google Scholar] [CrossRef]
- You, J.-J.; Yang, C.-H.; Huang, J.-S.; Chen, M.-S.; Yang, C.-M. Fractalkine, a CX3C chemokine, as a mediator of ocular angiogenesis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5290–5298. [Google Scholar] [CrossRef]
- Ueno, T.; Toi, M.; Saji, H.; Muta, M.; Bando, H.; Kuroi, K.; Koike, M.; Inadera, H.; Matsushima, K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin. Cancer Res. 2000, 6, 3282–3289. [Google Scholar]
- Metzemaekers, M.; Vanheule, V.; Janssens, R.; Struyf, S.; Proost, P. Overview of the mechanisms that may contribute to the non-redundant activities of interferon-inducible CXC chemokine receptor 3 ligands. Front. Immunol. 2018, 8, 1970. [Google Scholar] [CrossRef] [PubMed]
- Perollet, C.; Han, Z.C.; Savona, C.; Caen, J.P.; Bikfalvi, A. Platelet factor 4 modulates fibroblast growth factor 2 (FGF-2) activity and inhibits FGF-2 dimerization. Blood 1998, 91, 3289–3299. [Google Scholar] [CrossRef] [PubMed]
- Luster, A.D.; Greenberg, S.M.; Leder, P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J. Exp. Med. 1995, 182, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Campanella, G.S.; Colvin, R.A.; Luster, A.D. CXCL10 can inhibit endothelial cell proliferation independently of CXCR3. PLoS ONE 2010, 5, e12700. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, L.S.; Coelho, A.M.; Russo, R.C.; Guabiraba, R.; Souza, A.L.; Bruno-Lima, G., Jr.; Proudfoot, A.E.; Andrade, S.P.; Teixeira, M.M. Role of the chemokines CCL3/MIP-1α and CCL5/RANTES in sponge-induced inflammatory angiogenesis in mice. Microvasc. Res. 2009, 78, 148–154. [Google Scholar] [CrossRef]
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201. [Google Scholar] [CrossRef]
- Zlotnik, A.; Burkhardt, A.M.; Homey, B. Homeostatic chemokine receptors and organ-specific metastasis. Nat. Rev. Immunol. 2011, 11, 597. [Google Scholar] [CrossRef]
- Rezaeeyan, H.; Shirzad, R.; McKee, T.D.; Saki, N. Role of chemokines in metastatic niche: New insights along with a diagnostic and prognostic approach. APMIS 2018, 126, 359–370. [Google Scholar] [CrossRef]
- Kitamura, T.; Qian, B.-Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef]
- Steele, C.W.; Karim, S.A.; Leach, J.D.; Bailey, P.; Upstill-Goddard, R.; Rishi, L.; Foth, M.; Bryson, S.; McDaid, K.; Wilson, Z. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell 2016, 29, 832–845. [Google Scholar] [CrossRef]
- Lee, E.; Fertig, E.J.; Jin, K.; Sukumar, S.; Pandey, N.B.; Popel, A.S. Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nat. Commun. 2014, 5, 4715. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Condeelis, J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Cordero, J.J.; Hodgson, L.; Condeelis, J. Directed cell invasion and migration during metastasis. Curr. Opin. Cell Biol. 2012, 24, 277–283. [Google Scholar] [CrossRef]
- Singh, S.K.; Mishra, M.K.; Eltoum, I.-E.A.; Bae, S.; Lillard, J.W.; Singh, R. CCR5/CCL5 axis interaction promotes migratory and invasiveness of pancreatic cancer cells. Sci. Rep. 2018, 8, 1323. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Celià-Terrassa, T.; Jolly, M.K. Cancer Stem Cells and Epithelial-to-Mesenchymal Transition in Cancer Metastasis. Cold Spring Harb. Perspect. Med. 2019, a036905. [Google Scholar] [CrossRef]
- Fernando, R.I.; Castillo, M.D.; Litzinger, M.; Hamilton, D.H.; Palena, C. IL-8 signaling plays a critical role in the epithelial–mesenchymal transition of human carcinoma cells. Cancer Res. 2011, 71, 5296–5306. [Google Scholar] [CrossRef]
- Ling, Z.; Yang, X.; Chen, X.; Xia, J.; Cheng, B.; Tao, X. CCL2 promotes cell migration by inducing epithelial-mesenchymal transition in oral squamous cell carcinoma. J. Oral. Pathol. Med. 2019. [Google Scholar] [CrossRef]
- Liu, G.; An, L.; Zhang, H.; Du, P.; Sheng, Y. Activation of CXCL6/CXCR1/2 Axis Promotes the Growth and Metastasis of Osteosarcoma Cells in vitro and in vivo. Front. Pharm. 2019, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Dong, B.; Xu, F.; Xu, Y.; Pan, J.; Song, J.; Zhang, J.; Huang, Y.; Xue, W. CXCL1-LCN2 paracrine axis promotes progression of prostate cancer via the Src activation and epithelial-mesenchymal transition. Cell Commun. Signal. 2019, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Wu, F.; Huang, S.; Feng, L.; Bai, J.; Gu, S.; Zhao, X. Effects of CCL5 on the biological behavior of breast cancer and the mechanisms of its interaction with tumor-associated macrophages. Oncol. Rep. 2019, 42, 2499–2511. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, J.; Zhu, G.; Li, G.; Tan, H.; Chen, C.; Pi, L.; She, L.; Chen, X.; Wei, M. CCL18 promotes the metastasis of squamous cell carcinoma of the head and neck through MTDH-NF-κB signalling pathway. J. Cell. Mol. Med. 2019, 23, 2689–2701. [Google Scholar] [CrossRef]
- Park, J.; Zhang, X.; Lee, S.K.; Song, N.-Y.; Son, S.H.; Kim, K.R.; Shim, J.H.; Park, K.-K.; Chung, W.-Y. CCL28-induced RARβ expression inhibits oral squamous cell carcinoma bone invasion. J. Clin. Investig. 2019. [Google Scholar] [CrossRef]
- Dubrovska, A.; Elliott, J.; Salamone, R.J.; Telegeev, G.D.; Stakhovsky, A.E.; Schepotin, I.B.; Yan, F.; Wang, Y.; Bouchez, L.C.; Kularatne, S.A. CXCR4 expression in prostate cancer progenitor cells. PLoS ONE 2012, 7, e31226. [Google Scholar] [CrossRef]
- Jung, Y.; Cackowski, F.C.; Yumoto, K.; Decker, A.M.; Wang, J.; Kim, J.K.; Lee, E.; Wang, Y.; Chung, J.-S.; Gursky, A.M. CXCL12γ Promotes Metastatic Castration-Resistant Prostate Cancer by Inducing Cancer Stem Cell and Neuroendocrine Phenotypes. Cancer Res. 2018, 78, 2026–2039. [Google Scholar] [CrossRef]
- Corrò, C.; Healy, M.E.; Engler, S.; Bodenmiller, B.; Li, Z.; Schraml, P.; Weber, A.; Frew, I.J.; Rechsteiner, M.; Moch, H. IL-8 and CXCR1 expression is associated with cancer stem cell-like properties of clear cell renal cancer. J. Pathol. 2019. [Google Scholar] [CrossRef]
- Ginestier, C.; Liu, S.; Diebel, M.E.; Korkaya, H.; Luo, M.; Brown, M.; Wicinski, J.; Cabaud, O.; Charafe-Jauffret, E.; Birnbaum, D. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J. Clin. Investig. 2010, 120, 485–497. [Google Scholar] [CrossRef]
- Jiao, X.; Velasco-Velázquez, M.A.; Wang, M.; Li, Z.; Rui, H.; Peck, A.R.; Korkola, J.E.; Chen, X.; Xu, S.; DuHadaway, J.B. CCR5 governs DNA damage repair and breast cancer stem cell expansion. Cancer Res. 2018, 78, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-w.; Qin, X.; Qin, C.Y.; Yin, Y.-l.; Chen, Y.; Zhu, H.-l. Expression of monocyte chemoattractant protein-1 and CC chemokine receptor 2 in non-small cell lung cancer and its significance. Cancer Immunol. Immunother. 2013, 62, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, R.; Ponce, M.L.; Young, H.A.; Wasserman, K.; Ward, J.M.; Kleinman, H.K.; Oppenheim, J.J.; Murphy, W.J. Human endothelial cells express CCR2 and respond to MCP-1: Direct role of MCP-1 in angiogenesis and tumor progression. Blood 2000, 96, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Brummer, G.; Acevedo, D.S.; Hu, Q.; Portsche, M.; Fang, W.B.; Yao, M.; Zinda, B.; Myers, M.; Alvarez, N.; Fields, P. Chemokine signaling facilitates early-stage breast cancer survival and invasion through fibroblast-dependent mechanisms. Mol. Cancer Res. 2018, 16, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Fang, W.; Smart, C.; Hu, Q.; Huang, S.; Alvarez, N.; Fields, P.; Cheng, N. CCR2 Chemokine Receptors Enhance Growth and Cell-Cycle Progression of Breast Cancer Cells through SRC and PKC Activation. Mol. Cancer Res. 2019, 17, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lei, Z.; Zhao, J.; Gong, W.; Liu, J.; Chen, Z.; Liu, Y.; Li, D.; Yuan, Y.; Zhang, G.-M. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007, 252, 86–92. [Google Scholar] [CrossRef]
- Yang, J.; Lv, X.; Chen, J.; Xie, C.; Xia, W.; Jiang, C.; Zeng, T.; Ye, Y.; Ke, L.; Yu, Y. CCL2-CCR2 axis promotes metastasis of nasopharyngeal carcinoma by activating ERK1/2-MMP2/9 pathway. Oncotarget 2016, 7, 15632. [Google Scholar] [CrossRef]
- Yasui, H.; Kajiyama, H.; Tamauchi, S.; Suzuki, S.; Peng, Y.; Yoshikawa, N.; Sugiyama, M.; Nakamura, K.; Kikkawa, F. CCL2 secreted from cancer-associated mesothelial cells promotes peritoneal metastasis of ovarian cancer cells through the P38-MAPK pathway. Clin. Exp. Metastasis 2019, 1–14. [Google Scholar] [CrossRef]
- Li, Y.-L.; Shi, Z.-H.; Wang, X.; Gu, K.-S.; Zhai, Z.-m. Prognostic significance of monocyte chemoattractant protein-1 and CC chemokine receptor 2 in diffuse large B cell lymphoma. Ann. Hematol. 2019, 98, 413–422. [Google Scholar] [CrossRef]
- Soria, G.; Lebel-Haziv, Y.; Ehrlich, M.; Meshel, T.; Suez, A.; Avezov, E.; Rozenberg, P.; Ben-Baruch, A. Mechanisms regulating the secretion of the promalignancy chemokine CCL5 by breast tumor cells: CCL5’s 40s loop and intracellular glycosaminoglycans. Neoplasia 2012, 14, 1–19. [Google Scholar] [CrossRef]
- Yaal-Hahoshen, N.; Shina, S.; Leider-Trejo, L.; Barnea, I.; Shabtai, E.L.; Azenshtein, E.; Greenberg, I.; Keydar, I.; Ben-Baruch, A. The chemokine CCL5 as a potential prognostic factor predicting disease progression in stage II breast cancer patients. Clin. Cancer Res. 2006, 12, 4474–4480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wan, J.-X.; Ke, Z.-P.; Wang, F.; Chai, H.-X.; Liu, J.-Q. TMEM88, CCL14 and CLEC3B as prognostic biomarkers for prognosis and palindromia of human hepatocellular carcinoma. Tumor Biol 2017, 39, 1010428317708900. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Y.; Li, T.; Wang, Q.; Qian, J.; Lu, Y.; Zhang, M.; Bi, E.; Yang, M.; Reu, F. Chemokines CCL2, 3, 14 stimulate macrophage bone marrow homing, proliferation, and polarization in multiple myeloma. Oncotarget 2015, 6, 24218. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lv, H.; Jia, X.; Liu, G.; Li, T.; Xu, Z.; Li, J. CC chemokine receptor 6 expression predicts poor prognosis in hepatocellular carcinoma. J. Surg. Oncol. 2014, 110, 151–155. [Google Scholar] [CrossRef]
- Hou, K.-Z.; Fu, Z.-Q.; Gong, H. Chemokine ligand 20 enhances progression of hepatocellular carcinoma via epithelial-mesenchymal transition. World J. Gastroenterol. 2015, 21, 475. [Google Scholar] [CrossRef]
- Cheng, X.-S.; Li, Y.-F.; Tan, J.; Sun, B.; Xiao, Y.-C.; Fang, X.-B.; Zhang, X.-F.; Li, Q.; Dong, J.-H.; Li, M. CCL20 and CXCL8 synergize to promote progression and poor survival outcome in patients with colorectal cancer by collaborative induction of the epithelial–mesenchymal transition. Cancer Lett. 2014, 348, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Rubie, C.; Oliveira, V.; Kempf, K.; Wagner, M.; Tilton, B.; Rau, B.; Kruse, B.; König, J.; Schilling, M. Involvement of chemokine receptor CCR6 in colorectal cancer metastasis. Tumor Biol. 2006, 27. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Shu, G.; Liu, H.; Zhang, Q.; Ma, Z.; Ren, C.; Guo, H.; Shi, J.; Liu, J.; Zhang, C. The Diagnostic Value of Chemokine/Chemokine Receptor Pairs in Hepatocellular Carcinoma and Colorectal Liver Metastasis. J. Histochem. Cytochem. 2019, 67, 299–308. [Google Scholar] [CrossRef]
- Takekoshi, T.; Fang, L.; Paragh, G.; Hwang, S.T. CCR7-expressing B16 melanoma cells downregulate interferon-γ-mediated inflammation and increase lymphangiogenesis in the tumor microenvironment. Oncogenesis 2012, 1, e9. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, L.; Qiu, X.; Liu, Z.; Li, H.; Li, Z.; Luo, W.; Wang, E. CCL21/CCR7 prevents apoptosis via the ERK pathway in human non-small cell lung cancer cells. PLoS ONE 2012, 7, e33262. [Google Scholar] [CrossRef] [PubMed]
- Chi, B.-J.; Du, C.-L.; Fu, Y.-F.; Zhang, Y.-N.; Wang, R.W. Silencing of CCR7 inhibits the growth, invasion and migration of prostate cancer cells induced by VEGFC. Int. J. Clin. Exp. Pathol. 2015, 8, 12533. [Google Scholar] [PubMed]
- Cai, Q.-Y.; Liang, G.-Y.; Zheng, Y.-F.; Tan, Q.-Y.; Wang, R.-W.; Li, K. CCR7 enhances the angiogenic capacity of esophageal squamous carcinoma cells in vitro via activation of the NF-κB/VEGF signaling pathway. Am. J. Transl. Res. 2017, 9, 3282. [Google Scholar] [PubMed]
- Li, K.; Xu, B.; Xu, G.; Liu, R. CCR7 regulates Twist to induce the epithelial-mesenchymal transition in pancreatic ductal adenocarcinoma. Tumor Biol. 2016, 37, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Chen, L.; Yin, R.; Qu, Y.; Bao, Y.; Xiao, Q.; Zhang, Z.; Shen, Y.; Li, C.; Xu, Y. Chemokine (C-C motif) ligand 21/C-C chemokine receptor type 7 triggers migration and invasion of human lung cancer cells by epithelial-mesenchymal transition via the extracellular signal-regulated kinase signaling pathway. Mol. Med. Rep. 2017, 15, 4100–4108. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.; Zhou, M.; Wang, L.; Qi, L.; Zhou, K.; Liu, L.-F.; Chen, Z.; Zu, X.-B. CCL21/CCR7 enhances the proliferation, migration, and invasion of human bladder cancer T24 cells. PLoS ONE 2015, 10, e0119506. [Google Scholar] [CrossRef] [PubMed]
- Legler, D.F.; Uetz-von Allmen, E.; Hauser, M.A. CCR7: Roles in cancer cell dissemination, migration and metastasis formation. Int. J. Biochem. Cell Biol. 2014, 54, 78–82. [Google Scholar] [CrossRef]
- Zu, G.; Luo, B.; Yang, Y.; Tan, Y.; Tang, T.; Zhang, Y.; Chen, X.; Sun, D. Meta-analysis of the prognostic value of CC chemokine receptor type 7 in patients with solid tumors. Cancer Manag. Res. 2019, 11, 1881. [Google Scholar] [CrossRef]
- Zhuo, C.; Wu, X.; Li, J.; Hu, D.; Jian, J.; Chen, C.; Zheng, X.; Yang, C. Chemokine (CXC motif) ligand 1 is associated with tumor progression and poor prognosis in patients with colorectal cancer. Biosci. Rep. 2018, 38, BSR20180580. [Google Scholar] [CrossRef]
- Li, J.; Byrne, K.T.; Yan, F.; Yamazoe, T.; Chen, Z.; Baslan, T.; Richman, L.P.; Lin, J.H.; Sun, Y.H.; Rech, A.J. Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity 2018, 49, 178–193. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Jiang, Y.; Luo, Y.; Zhang, H.; Zhan, Y. Prognostic and clinicopathological significance of CXCL1 in cancers: A systematic review and meta-analysis. Cancer Biol. 2019, 20, 1380–1388. [Google Scholar] [CrossRef]
- Zhang, H.; Yue, J.; Jiang, Z.; Zhou, R.; Xie, R.; Xu, Y.; Wu, S. CAF-secreted CXCL1 conferred radioresistance by regulating DNA damage response in a ROS-dependent manner in esophageal squamous cell carcinoma. Cell Death Dis. 2017, 8, e2790. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Manegold, P.C.; Hong, Y.K.; Zhang, W.; Pohl, A.; Lurje, G.; Winder, T.; Yang, D.; LaBonte, M.J.; Wilson, P.M. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int. J. Cancer 2011, 128, 2038–2049. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-B.; Xie, F.; Chang, K.-K.; Li, M.-Q.; Meng, Y.-H.; Wang, X.-H.; Li, H.; Li, D.-J.; Yu, J.-J. Hypoxia promotes the proliferation of cervical carcinoma cells through stimulating the secretion of IL-8. Int. J. Clin. Exp. Pathol. 2014, 7, 575. [Google Scholar] [PubMed]
- Zhu, Y.M.; Webster, S.; Flower, D.; Woll, P. Interleukin-8/CXCL8 is a growth factor for human lung cancer cells. Br. J. Cancer 2004, 91, 1970. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Yang, Z.; Cheng, X.; Xiao, Y.; Yu, K.; Cai, X.; Xia, C.; Li, Y. CXCL8 induces epithelial-mesenchymal transition in colon cancer cells via the PI3K/Akt/NF-κB signaling pathway. Oncol. Rep. 2017, 37, 2095–2100. [Google Scholar] [CrossRef]
- Gonzalez-Aparicio, M.; Alfaro, C. Influence of Interleukin-8 and Neutrophil Extracellular Trap (NET) Formation in the Tumor Microenvironment: Is There a Pathogenic Role? J. Immunol. Res. 2019, 2019. [Google Scholar] [CrossRef]
- Di Caro, G.; Carvello, M.; Pesce, S.; Erreni, M.; Marchesi, F.; Todoric, J.; Sacchi, M.; Montorsi, M.; Allavena, P.; Spinelli, A. Circulating inflammatory mediators as potential prognostic markers of human colorectal cancer. PLoS ONE 2016, 11, e0148186. [Google Scholar]
- Yan, R.; Shuai, H.; Luo, X.; Wang, X.; Guan, B. The clinical and prognostic value of CXCL8 in cervical carcinoma patients: Immunohistochemical analysis. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef]
- Sunaga, N.; Kaira, K.; Tomizawa, Y.; Shimizu, K.; Imai, H.; Takahashi, G.; Kakegawa, S.; Ohtaki, Y.; Nagashima, T.; Kasahara, N. Clinicopathological and prognostic significance of interleukin-8 expression and its relationship to KRAS mutation in lung adenocarcinoma. Br. J. Cancer 2014, 110, 2047. [Google Scholar] [CrossRef]
- Tsukinaga, S.; Kajihara, M.; Takakura, K.; Ito, Z.; Kanai, T.; Saito, K.; Takami, S.; Kobayashi, H.; Matsumoto, Y.; Odahara, S. Prognostic significance of plasma interleukin-6/-8 in pancreatic cancer patients receiving chemoimmunotherapy. World J. Gastroenterol. 2015, 21, 11168. [Google Scholar] [CrossRef]
- Lambrechts, D.; Thienpont, B.; Thuillier, V.; Sagaert, X.; Moisse, M.; Peuteman, G.; Pericay, C.; Folprecht, G.; Zalcberg, J.; Zilocchi, C. Evaluation of efficacy and safety markers in a phase II study of metastatic colorectal cancer treated with aflibercept in the first-line setting. Br. J. Cancer 2015, 113, 1027. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, F.; Ping, Y.; Wang, L.; Chen, X.; Wang, D.; Cao, L.; Zhao, S.; Li, B.; Kalinski, P. Local production of the chemokines CCL5 and CXCL10 attracts CD8+ T lymphocytes into esophageal squamous cell carcinoma. Oncotarget 2015, 6, 24978. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Chittezhath, M.; André, V.; Zhao, H.; Poidinger, M.; Biondi, A.; D’Amico, G.; Biswas, S.K. Protumoral role of monocytes in human B-cell precursor acute lymphoblastic leukemia: Involvement of the chemokine CXCL10. Blood 2012, 119, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Motoyama, S.; Nanjo, H.; Wakita, A.; Yoshino, K.; Sasaki, T.; Nagaki, Y.; Liu, J.; Imai, K.; Saito, H. CXCL10 expression status is prognostic in patients with advanced thoracic esophageal squamous cell carcinoma. Ann. Surg. Oncol. 2016, 23, 936–942. [Google Scholar] [CrossRef]
- Ansell, S.M.; Maurer, M.J.; Ziesmer, S.C.; Slager, S.L.; Habermann, T.M.; Link, B.K.; Witzig, T.E.; Macon, W.R.; Dogan, A.; Cerhan, J.R. Elevated pretreatment serum levels of interferon-inducible protein-10 (CXCL10) predict disease relapse and prognosis in diffuse large B-cell lymphoma patients. Am. J. Hematol. 2012, 87, 865–869. [Google Scholar] [CrossRef]
- Liang, Z.; Brooks, J.; Willard, M.; Liang, K.; Yoon, Y.; Kang, S.; Shim, H. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem. Biophys. Res. Commun. 2007, 359, 716–722. [Google Scholar] [CrossRef]
- Zagzag, D.; Lukyanov, Y.; Lan, L.; Ali, M.A.; Esencay, M.; Mendez, O.; Yee, H.; Voura, E.B.; Newcomb, E.W. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: Implications for angiogenesis and glioma cell invasion. Lab. Investig. 2006, 86, 1221. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, G.; Hao, M.; Zheng, J.; Zhou, X.; Zhang, J.; Taichman, R.S.; Pienta, K.J.; Wang, J. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010, 29, 709–722. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Chang, Y.; Xu, Q.; Wu, Z.; Ma, Q.; Wang, Z. The SDF-1/CXCR4 axis induces epithelial–mesenchymal transition in hepatocellular carcinoma. Mol. Cell Biochem. 2014, 392, 77–84. [Google Scholar] [CrossRef]
- Jung, M.; Rho, J.; Kim, Y.; Jung, J.; Jin, Y.; Ko, Y.-G.; Lee, J.; Lee, S.J.; Lee, J.; Park, M. Upregulation of CXCR4 is functionally crucial for maintenance of stemness in drug-resistant non-small cell lung cancer cells. Oncogene 2013, 32, 209–221. [Google Scholar] [CrossRef]
- Uchi, Y.; Takeuchi, H.; Matsuda, S.; Saikawa, Y.; Kawakubo, H.; Wada, N.; Takahashi, T.; Nakamura, R.; Fukuda, K.; Omori, T. CXCL12 expression promotes esophageal squamous cell carcinoma proliferation and worsens the prognosis. BMC Cancer 2016, 16, 514. [Google Scholar] [CrossRef] [PubMed]
- Spoo, A.C.; Lübbert, M.; Wierda, W.G.; Burger, J.A. CXCR4 is a prognostic marker in acute myelogenous leukemia. Blood 2007, 109, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.-p.; Shen, H.; Liu, L.-x.; Shu, Y.-q. The impact of chemokine receptor CXCR4 on breast cancer prognosis: A meta-analysis. Cancer Epidemiol. 2013, 37, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Xia, W.; Conforti, R.; Wei, Y.; Boulet, T.; Tomasic, G.; Spielmann, M.; Zoubir, M.; Berrada, N.; Arriagada, R. CXCR4 expression in early breast cancer and risk of distant recurrence. Oncol. 2009, 14, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Miao, L.; Zhao, Y.; Xiao, Y.-Y.; Xu, Q. A meta-analysis for CXC chemokine receptor type 4 as a prognostic marker and potential drug target in hepatocellular carcinoma. Drug Des. Dev. Ther. 2015, 9, 3625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, J.; Han, Y.; Jiang, J. A meta-analysis for CXCR4 as a prognostic marker and potential drug target in non-small cell lung cancer. Drug Des. Dev. Ther. 2015, 9, 3267. [Google Scholar]
- Torregrossa, L.; Giannini, R.; Borrelli, N.; Sensi, E.; Melillo, R.M.; Leocata, P.; Materazzi, G.; Miccoli, P.; Santoro, M.; Basolo, F. CXCR4 expression correlates with the degree of tumor infiltration and BRAF status in papillary thyroid carcinomas. Mod. Pathol. 2012, 25, 46–55. [Google Scholar] [CrossRef]
- Mazur, G.; Butrym, A.; Kryczek, I.; Dlubek, D.; Jaskula, E.; Lange, A.; Kuliczkowski, K.; Jelen, M. Decreased expression of CXCR4 chemokine receptor in bone marrow after chemotherapy in patients with non-Hodgkin lymphomas is a good prognostic factor. PLoS ONE 2014, 9, e98194. [Google Scholar] [CrossRef]
- Hyakudomi, M.; Matsubara, T.; Hyakudomi, R.; Yamamoto, T.; Kinugasa, S.; Yamanoi, A.; Maruyama, R.; Tanaka, T. Increased expression of fractalkine is correlated with a better prognosis and an increased number of both CD8+ T cells and natural killer cells in gastric adenocarcinoma. Ann. Surg. Oncol. 2008, 15, 1775–1782. [Google Scholar] [CrossRef]
- Matsubara, T.; Ono, T.; Yamanoi, A.; Tachibana, M.; Nagasue, N. Fractalkine-CX3CR1 axis regulates tumor cell cycle and deteriorates prognosis after radical resection for hepatocellular carcinoma. J. Surg. Oncol. 2007, 95, 241–249. [Google Scholar] [CrossRef]
- Huang, L.; Ma, B.; Ma, J.; Wang, F. Fractalkine/CX3CR1 axis modulated the development of pancreatic ductal adenocarcinoma via JAK/STAT signaling pathway. Biochem. Biophys. Res. Commun. 2017, 493, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y.; Chen, J.; Ma, H.; Shao, Z.; Chen, H.; Jin, G. High expression of CX3CL1/CX3CR1 axis predicts a poor prognosis of pancreatic ductal adenocarcinoma. J. Gastrointest. Surg. 2012, 16, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Cairns, C.M.; Gordon, J.R.; Li, F.; Baca-Estrada, M.E.; Moyana, T.; Xiang, J. Lymphotactin expression by engineered myeloma cells drives tumor regression: Mediation by CD4+ and CD8+ T cells and neutrophils expressing XCR1 receptor. J. Immunol. 2001, 167, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, J.A.; Saylor, V.; Figueroa, D.; Mizoue, L.; Xu, Y.; Menon, S.; Abrams, J.; Handel, T.; Zlotnik, A. Lymphotactin is produced by NK cells and attracts both NK cells and T cells in vivo. J. Immunol. 1997, 158, 1533–1540. [Google Scholar] [PubMed]
- Gutiérrez-Aguirre, C.H.; Flores-Jiménez, J.A.; Alatorre-Ricardo, J.; Cantú-Rodríguez, O.G.; Rosas-Taraco, A.; Salazar-Riojas, R.; Jaime-Pérez, J.C.; Sánchez-Cárdenas, M.; López-Silva, L.; Martínez-Castilla, A.M. The prognostic significance of serum XCL1 concentration in patients with acute lymphoblastic leukemia: A pilot study. Ann. Hematol. 2017, 96, 2015–2024. [Google Scholar] [CrossRef]
- Jamal, R.; Lapointe, R.; Cocolakis, E.; Thebault, P.; Kazemi, S.; Friedmann, J.E.; Dionne, J.; Cailhier, J.F.; Belanger, K.; Ayoub, J.P.; et al. Peripheral and local predictive immune signatures identified in a phase II trial of ipilimumab with carboplatin/paclitaxel in unresectable stage III or stage IV melanoma. J. Immunother. Cancer 2017, 5, 83. [Google Scholar] [CrossRef]
- Noonan, A.M.; Farren, M.R.; Geyer, S.M.; Huang, Y.; Tahiri, S.; Ahn, D.; Mikhail, S.; Ciombor, K.K.; Pant, S.; Aparo, S.; et al. Randomized Phase 2 Trial of the Oncolytic Virus Pelareorep (Reolysin) in Upfront Treatment of Metastatic Pancreatic Adenocarcinoma. Mol. Ther. 2016, 24, 1150–1158. [Google Scholar] [CrossRef]
- Ciombor, K.K.; Feng, Y.; Benson, A.B., III; Su, Y.; Horton, L.; Short, S.P.; Kauh, J.S.; Staley, C.; Mulcahy, M.; Powell, M.; et al. Phase II trial of bortezomib plus doxorubicin in hepatocellular carcinoma (E6202): A trial of the Eastern Cooperative Oncology Group. Investig. New Drugs 2014, 32, 1017–1027. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).