The Effect of Higher Level Computerized Clinical Decision Support Systems on Oncology Care: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Literature Search
2.3. Data Collection
2.4. Study Selection Criteria
2.5. Extraction and Assessment of Quality
3. Results
3.1. Eligible Studies
3.2. Study Characteristics
3.3. Impact of CDSS on Oncology Practice
3.3.1. Process Outcomes
- Use of CSF: Febrile neutropenia (FN) frequently complicates cancer chemotherapy; therefore CSF is often administered to reduce the risk and severity of FN. However, 30% of the patients that receive CSF have a low risk of FN, increasing costs and patient burden with no clinical benefit. Agiro et al. and Adeboyeje et al. showed that implementation of CDSSs significantly decreased the use of CSF as primary prevention for FN, which was interpreted as reduced overtreatment [26,27].
- Physician-prescribing behavior: Bouaud et al. showed that initial decisions in breast cancer management were modified in 31% of cases after implementation of a CDSS. Whatever the motivation for change, it was always directed towards an improvement in patient management [32].
- Frequency of pain assessment: Christ et al. showed that frequency of nursing pain assessments within 24 h after admission was significantly higher in the postimplementation group compared to the pre-implementation group (12.0 vs. 7.4 p < 0.001). Increased frequency of nursing pain assessment is associated with improved pain outcomes [28].
- Healthcare costs: Jackman et al. suggests that the use of a CDSS resulted in a significant reduction in total costs for stage IV non-small cell lung cancer patients one year after diagnosis, by approximately $17,000 (from $69,122 before to $52, 037) [33].
- Clinician’s workload: Verberne et al. calculated that on average a clinician needs 64 min per patient per year in the follow-up of colorectal cancer (CRC). Clinicians’ workload was significantly reduced to 23 min per patient per year after implementation of the CDSS, saving more than 40 min per patient per year during follow-up [31].
3.3.2. Guideline Adherence
3.3.3. Clinical Outcomes
3.4. Risk of Bias and Level of Evidence
4. Discussion
4.1. Summary of Evidence
4.2. Overall Completeness and Applicability of Evidence
4.3. Agreements and Disagreements with Literature
4.4. Strengths and Limitations
5. Future Research
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACM | Association of Computing Machinery |
| ASCO | American Society of Clinical Oncology |
| CDSS | Clinical Decision Support System |
| CEA | Carcino-Embryonic Antigen |
| CPG | Clinical Practice Guidelines |
| CRC | Colorectal Cancer |
| CSF | Colony Stimulating Factor |
| EHR | Electronic Health Record |
| EMR | Electronic Medical Record |
| EORTC | European Organisation for Research and Treatment of Cancer |
| ESMO | European Society for Medical Oncology |
| FN | Febrile Neutropenia |
| IEEE | Institute of Electrical and Electronics Engineers |
| MDT | Multidisciplinary Team |
| NCCN | National Comprehensive Cancer Network |
| PRISMA-P | Preferred Reporting Items for Systematic Reviews and Meta analyses Protocols |
| TRL | Technology Readiness Level |
| VAS | Visual Analogue Scale |
| WHO | World Health Organisation |
References
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current Challenges in Cancer Treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Lunenfeld, B.; Stratton, P. The clinical consequences of an ageing world and preventive strategies. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.J.; Martinez, C.H.; Mannino, D.M. Ageing and the epidemiology of multimorbidity. Eur. Respir. J. 2014, 44, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- LeVasseur, N.; Fergusson, D.; Clemons, M. Unnecessary variation in practice: How to improve cancer care through pragmatic trials. Curr. Oncol. 2018, 25, e263–e264. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.P.; Ganz, P.A.; Murphy, S.B.; Nass, S.J.; Ferrell, B.R.; Stovall, E. Patient-centered cancer treatment planning: Improving the quality of oncology care. Summary of an Institute of Medicine workshop. Oncologist 2011, 16, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Computer technology helps radiologists spot overlooked small breast cancers. Oncology (Williston Park) 2000, 14, 1450.
- O’Sullivan, D.; Fraccaro, P.; Carson, E.; Weller, P. Decision time for clinical decision support systems. Clin. Med. J. R. Coll. Physicians Lond. 2014, 14, 338–341. [Google Scholar] [CrossRef]
- Van de Velde, S.; Heselmans, A.; Delvaux, N.; Brandt, L.; Marco-Ruiz, L.; Spitaels, D.; Cloetens, H.; Kortteisto, T.; Roshanov, P.; Kunnamo, I.; et al. A systematic review of trials evaluating success factors of interventions with computerised clinical decision support. Implement. Sci. 2018, 13, 114. [Google Scholar] [CrossRef]
- Roshanov, P.S. Features of effective computerised clinical decision support systems: Meta-regression of 162 randomised trials. BMJ (Online) 2013, 346, f657. [Google Scholar] [CrossRef]
- Kawamoto, K.; Houlihan, C.A.; Balas, E.A.; Lobach, D.F. Improving clinical practice using clinical decision support systems: A systematic review of trials to identify features critical to success. BMJ 2005, 330, 765. [Google Scholar] [CrossRef]
- Pawloski, P.A.; Brooks, G.A.; Nielsen, M.E.; Olson-Bullis, B.A. A Systematic Review of Clinical Decision Support Systems for Clinical Oncology Practice. J. Natl. Compr. Cancer Netw. 2019, 17, 331–338. [Google Scholar] [CrossRef]
- Klarenbeek, S.E.; Weekenstroo, H.H.A.; Sedelaar, J.P.M.; Fütterer, J.J.; Prokop, M.; Tummers, M. Effect of computerized clinical decision support systems on cancer care: A systematic review and meta-analysis. In PROSPERO International Prospective Register of Systematic Reviews; 2019; Available online: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=124800 (accessed on 8 March 2019 ).
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic reviews and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Révész, D.; Engelhardt, E.G.; Tamminga, J.J.; Schramel, F.M.; Onwuteaka-Philipsen, B.D.; Van De Garde, E.M.W.; Steyerberg, E.W.; Jansma, E.P.; De Vet, H.C.; Coupé, V.M. Decision support systems for incurable non-small cell lung cancer: A systematic review. BMC Med. Inform. Decis. Mak. 2017, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Kazemi, A.; Moghaddasi, H.; Rafsanjani, K.A.; Bahoush, G. Specifications of Computerized Provider Order Entry and Clinical Decision Support Systems for Cancer Patients Undergoing Chemotherapy: A Systematic Review. Chemotherapy 2018, 63, 162–171. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.A.; Whelan, T.J.; Villasis-Keever, M.; Gafni, A.; Charles, C.; Roberts, R.; Schiff, S.; Cai, W. Are cancer-related decision aids effective? A Systematic review and meta-analysis. J. Clin. Oncol. 2009, 27, 974–985. [Google Scholar] [CrossRef]
- Ilic, D.; Jammal, W.; Chiarelli, P.; Gardiner, R.A.; Hughes, S.; Stefanovic, D.; Chambers, S.K. Assessing the effectiveness of decision AIDS for decision making in prostate cancer testing: A systematic review. Psycho Oncol. 2015, 24, 1303–1315. [Google Scholar] [CrossRef]
- Souza, N.M.; Sebaldt, R.J.; Mackay, J.A.; Prorok, J.C.; Weise-Kelly, L.; Navarro, T.; Wilczynski, N.L.; Haynes, R.B. Computerized clinical decision support systems for primary preventive care: A decision-maker-researcher partnership systematic review of effects on process of care and patient outcomes. Implement. Sci. 2011, 6, 87. [Google Scholar] [CrossRef]
- Welch, B.M.; Kawamoto, K. Clinical decision support for genetically guided personalized medicine: A systematic review. J Am Med Inform. Assoc. 2013, 20, 388–400. [Google Scholar] [CrossRef]
- Zhong, W.; Smith, B.; Haghighi, K.; Mancuso, P. Systematic Review of Decision Aids for the Management of Men with Localized Prostate Cancer. Urology 2018, 114, 1–7. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Mankins, J.C. Technology readiness assessments: A retrospective. Acta Astronaut. 2009, 65, 1216–1223. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S.E. The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. 2011. Available online: www.handbook.cochrane.org (accessed on 15 December 2018).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Adeboyeje, G.; Agiro, A.; Malin, J.; Fisch, M.J.; DeVries, A. Reducing Overuse of Colony-Stimulating Factors in Patients With Lung Cancer Receiving Chemotherapy: Evidence From a Decision Support-Enabled Program. J. Oncol. Pract. 2017, 13, e337–e345. [Google Scholar] [CrossRef]
- Agiro, A.; DeVries, A.; Malin, J.; Fisch, M.J. Real-world impact of a decision support tool on colony-stimulating factor use and chemotherapy-induced febrile neutropenia among patients with breast cancer. J. Natl. Compr. Cancer Netw. 2018, 16, 162–169. [Google Scholar] [CrossRef]
- Christ, T.N.; Villadolid, J.J.; Choksi, A.; Malec, M.; Knoebel, R.W. Impact of a Clinical Decision Support Tool on Cancer Pain Management in Opioid-Tolerant Inpatients. Hosp. Pharm. 2018, 53, 256–262. [Google Scholar] [CrossRef]
- Rios, M.; Desandes, E.; Bresson, B.; Lesur, I.K.A.; Boisson, F.; Demange, V.; Bey, P. Clinical practice guidelines in cancerology: Comparative study of three decision support-systems for breast and prostate cancer in Lorraine french region. Bull. Cancer 2003, 90, 363–370. [Google Scholar]
- Séroussi, B.; Bouaud, J.; Gligorov, J.; Uzan, S. Supporting multidisciplinary staff meetings for guideline-based breast cancer management: A study with OncoDoc2. AMIA Annu. Symp. Proc. 2007, 2007, 656–660. [Google Scholar]
- Verberne, C.J.; Nijboer, C.H.; de Bock, G.H.; Grossmann, I.; Wiggers, T.; Havenga, K. Evaluation of the use of decision-support software in carcino-embryonic antigen (CEA)-based follow-up of patients with colorectal cancer. BMC Med. Inform. Decis. Mak. 2012, 12, 14. [Google Scholar] [CrossRef]
- Bouaud, J.; Séroussi, B.; Antoine, E.C.; Zelek, L.; Spielmann, M. A before-after study using OncoDoc, a guideline-based decision support-system on breast cancer management: Impact upon physician prescribing behaviour. Stud. Health Technol. Inform. 2001, 84 Pt 1, 420–424. [Google Scholar]
- Jackman, D.M.; Zhang, Y.; Dalby, C.; Nguyen, T.; Nagle, J.; Lydon, C.A.; Rabin, M.S.; McNiff, K.K.; Fraile, B.; Jacobson, J.O. Cost and Survival Analysis Before and After Implementation of Dana-Farber Clinical Pathways for Patients With Stage IV Non-Small-Cell Lung Cancer. J. Oncol. Pract. 2017, 13, e346–e352. [Google Scholar] [CrossRef]
- Bertsche, T.; Askoxylakis, V.; Habl, G.; Laidig, F.; Kaltschmidt, J.; Schmitt, S.P.; Ghaderi, H.; Zabel-du Bois, A.; Milker-Zabel, S.; Debus, J.; et al. Multidisciplinary pain management based on a computerized clinical decision support system in cancer pain patients. Pain 2009, 147, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Bouaud, J.; Blaszka-Jaulerry, B.; Zelek, L.; Spano, J.P.; Lefranc, J.P.; Cojean-Zelek, I.; Durieux, A.; Tournigand, C.; Rousseau, A.; Séroussi, B. Health information technology: Use it well, or don’t! Findings from the use of a decision support system for breast cancer management. AMIA Annu. Symp. Proc. 2014, 2014, 315–324. [Google Scholar] [PubMed]
- Bouaud, J.; Spano, J.P.; Lefranc, J.P.; Cojean-Zelek, I.; Blaszka-Jaulerry, B.; Zelek, L.; Durieux, A.; Tournigand, C.; Rousseau, A.; Vandenbussche, P.Y.; et al. Physicians’ Attitudes Towards the Advice of a Guideline-Based Decision Support System: A Case Study With OncoDoc2 in the Management of Breast Cancer Patients. Stud. Health Technol. Inform. 2015, 216, 264–269. [Google Scholar] [PubMed]
- Jia, P.; Zhang, L.; Chen, J.; Zhao, P.; Zhang, M. The Effects of Clinical Decision Support Systems on Medication Safety: An Overview. PLoS ONE 2016, 11, e0167683. [Google Scholar] [CrossRef] [PubMed]
- Bright, T.J.; Wong, A.; Dhurjati, R.; Bristow, E.; Bastian, L.; Coeytaux, R.R.; Samsa, G.; Hasselblad, V.; Williams, J.W.; Musty, M.D.; et al. Effect of clinical decision-support systems: A systematic review. Ann. Intern. Med. 2012, 157, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Damiani, G.; Pinnarelli, L.; Colosimo, S.C.; Almiento, R.; Sicuro, L.; Galasso, R.; Sommella, L.; Ricciardi, W. The effectiveness of computerized clinical guidelines in the process of care: A systematic review. BMC Health Serv. Res. 2010, 10, 2. [Google Scholar] [CrossRef]
- Chaudhry, B.; Wang, J.; Wu, S.; Maglione, M.; Mojica, W.; Roth, E.; Morton, S.C.; Shekelle, P.G. Systematic review: Impact of health information technology on quality, efficiency, and costs of medical care. Ann. Intern. Med. 2006, 144, 742–752. [Google Scholar] [CrossRef]
- Garg, A.X.; Adhikari, N.K.; McDonald, H.; Rosas-Arellano, M.P.; Devereaux, P.J.; Beyene, J.; Sam, J.; Haynes, R.B. Effects of Computerized Clinical Decision Support Systems on Practitioner Performance and Patient OutcomesA Systematic Review. JAMA 2005, 293, 1223–1238. [Google Scholar] [CrossRef]
- Jaspers, M.W.; Smeulers, M.; Vermeulen, H.; Peute, L.W. Effects of clinical decision-support systems on practitioner performance and patient outcomes: A synthesis of high-quality systematic review findings. J. Am. Med. Inform. Assoc. 2011, 18, 327–334. [Google Scholar] [CrossRef]
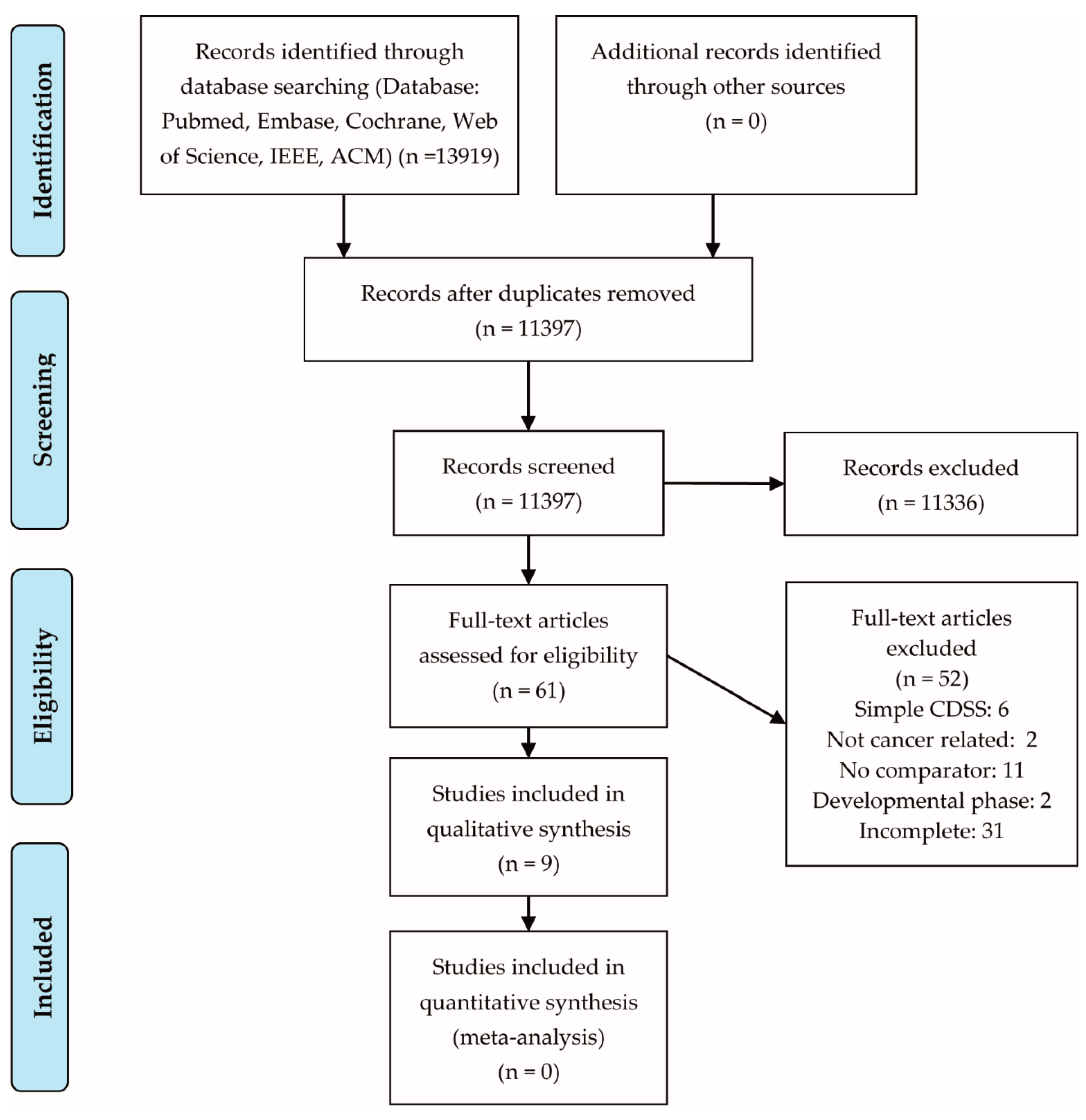
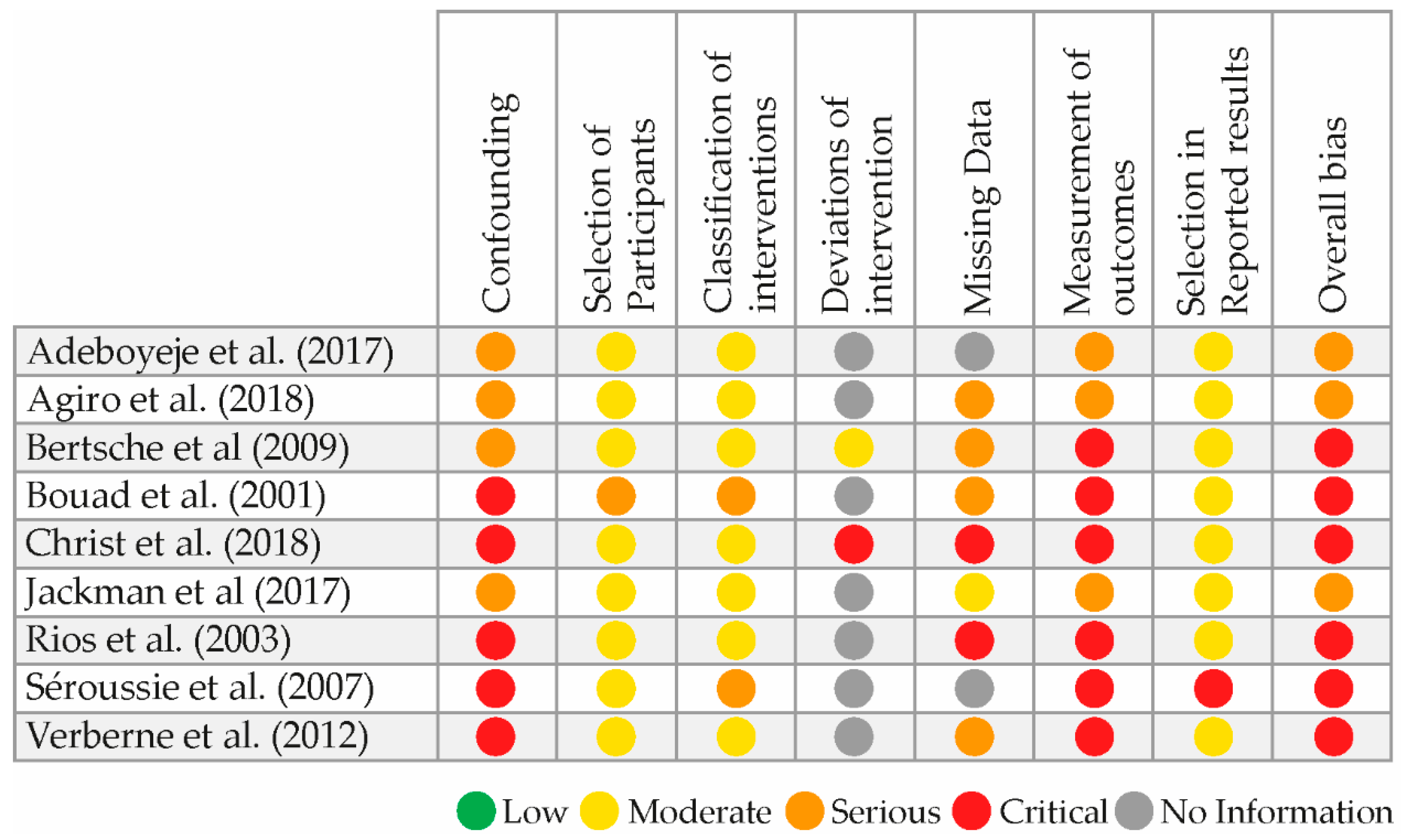
| Study | Study Design | Cancer | System Classification | Clinical Topic | Outcome Parameters | Total Sample Size | Control (N = Number of Participants) | Intervention (N = Number of Participants) | Risk of Bias | Quality of Evidence |
|---|---|---|---|---|---|---|---|---|---|---|
| Adeboyeje et al., 2017 [26] | Multicenter, before-after, cohort study | Lung | Decision support system | CSF support for chemotherapy | (1) % CSF use (2) % at high risk for febrile neutropenia | 1857 | National guideline (N = 707) | CDSS (N = 1150) | Serious | Low |
| Agiro et al., 2018 [27] | Multicenter, before-after, cohort study | Breast | Decision support system | CSF support for chemotherapy | (1) % CSF use (2) % at high risk for febrile neutropenia | 4001 | National guideline (N = 1991) | CDSS (N = 2010) | Serious | Low |
| Christ et al., 2018 [28] | Single center, before-after, cohort study | Hematologic malignancies and solid tumors | Decision support system | Pain management of opioid-tolerant oncology patients | (1) Attainment of analgesia (2) Frequency medication (3) Frequency pain assessment (4) Guideline adherence (5) Pain score | 62 | National guidelines (N = 30) | CDSS (N = 32) | Critical | Very low |
| Rios et al., 2003 [29] | Before-after, cohort study * | Breast and prostate | CPG system | Treatment planning | Guideline adherence | 907 | Standard of care (Breast N = 320, prostate N = 188) | CDSS (Breast N = 270, prostate N = 129) | Critical | Very low |
| Seroussi et al., 2007 [30] | Single center, before-after, cohort study | Breast | Decision support system | Treatment decisions by MDT | Guideline adherence | 316 | MDT (N = 139) | MDT supported by CDSS (N = 177) | Critical | Very low |
| Verberne et al., 2012 [31] | Single center, before-after, cohort study | Colorectal | Decision support system | Follow-up based on CEA testing | (1) Workload clinicians for follow-up (2) % of metastases found at follow-up (3) % curative metastasectomy | 245 | Standard of care (N = 61) | CDSS (N = 184) | Critical | Very low |
| Bouaud et al., 2001 [32] | Single center, before-after, cohort study | Breast | CPG system | Treatment decisions by MDT | (1) Treatment decision (2) Clinical trial inclusion rate (3) Compliance to CPG | 127 | MDT (N = 127) | MDT supported by CDSS (N = 127) | Critical | Very low |
| Jackman et al., 2017 [33] | Single center, before-after, cohort study | Non-small cell lung | Clinical pathway | Treatment for stage IV | (1) Costs (2) Overall survival | 370 | Standard of care (N = 160) | CDSS (N = 210) | Serious | Low |
| Bertsche et al., 2009 [34] | Single center, before-after, cohort study | All types | Decision support system | Treatment of tumor-induced-pain | (1) % deviation from guideline (2) Pain score | 100 | Standard of care (N = 50) | CDSS (N = 50) | Critical | Very low |
| Study | Installation | System Features | Key Outcomes Associated with CDSS | Results (Control vs. Intervention) |
|---|---|---|---|---|
| Adeboyeje et al., 2017 [26] | Web based | CPG 1–4 based, calculates febrile neutropenia risk and recommends CSF use | Percentage CSF use based on febrile neutropenia risk assessment | 48.4% vs. 35.6%, p = 0.001, 95% CI: −14.7 to −2.7 |
| Agiro et al., 2018 [27] | Web based | CPG 3,4 based, calculates febrile neutropenia risk and recommends CSF use | Percentage CSF use based on febrile neutropenia risk assessment | 74.9% vs. 68.5%, p = 0.006, 95% CI: −6.0 to −4.7 |
| Christ et al., 2018 [28] | Integrated in EMR | CPG 3 based, identifies patients who require pain assessment, displays patient-specific information and the most recent and maximum pain score |
|
|
| Verberne et al., 2012 [31] | Intranet-based | Assigns patients to one of three follow-up intervals based on CEA change, writes appropriate follow-up letter |
|
|
| Bouaud et al., 2001 [32] | Not mentioned | Hypertextual navigation in CPG * structured decision tree flowchart |
|
|
| Jackman et al., 2017 [33] | Web based | Algorithms define the best fitting care pathway for patients at each point in care | Costs of care for 1 year after time of diagnosis in US dollar:
|
|
| Study | Installation | System Features | Key Outcomes Associated with CDSS | Results (Control vs. Intervention) |
|---|---|---|---|---|
| Christ et al., 2018 [28] | Integrated in EMR | CPG 1 based, identifies patients who require pain assessment, displays patient-specific information and the most recent and maximum pain score | Percentage of guideline-adherent pain regimens | 40.0% vs. 46.9%, p = 0.97 |
| Rios et al., 2003 [29] | Not mentioned | CPG 2 based, organizes patient data and generates patient specific recommendations | Percentage of guideline-adherent treatment decisions
|
|
| Seroussi et al., 2007 [30] | Not mentioned | CPG 3 based, contextualizes both guideline medical knowledge and patient information and generates patient specific recommendations | Percentage of guideline-adherent treatment decisions | 79.2% vs. 93.4%, p < 10−5 |
| Bouaud et al., 2001 [32] | Not mentioned | Hyper textual navigation in CPG * structured decision tree flowchart | Percentage of guideline-adherent treatment decisions | 61.42% vs. 85.03%, p < 10−4 |
| Bertsche et al., 2009 [34] | Integrated in hospital drug information system. | Algorithms based on CPG 4 generate pain specific recommendations | Percentages of deviations from guidelines
| Percentages of deviations
|
| Study | Installation | System Features | Key Outcomes Associated with CDSS | Results (Control vs. Intervention) |
|---|---|---|---|---|
| Adeboyeje et al., 2017 [26] | Web based | CPG 1–4 based, calculates febrile neutropenia risk and recommends CSF use | Percentage of patients at high risk for febrile neutropenia | 2.8% vs. 4.3%, p = 0.927, 95% CI: −0.35 to 0.10 |
| Agiro et al., 2018 [27] | Web based | CPG 3,4 based, calculates febrile neutropenia risk and recommends CSF use | Percentage of patients at high risk for febrile neutropenia | 5% vs. 5.5%, p = 0.778, 95% CI: −0.2 to 0.3 |
| Christ et al., 2018 [28] | Integrated in EMR | CPG 3 based, identifies patients who require pain assessment, displays patient-specific information and the most recent and maximum pain score |
|
|
| Verberne et al., 2012 [31] | Intranet-based | Assigns patients to one of three follow-up intervals based on CEA change, writes appropriate follow-up letter |
|
|
| Jackman et al., 2017 [33] | Web based | Algorithms define the best fitting care pathway for patients at each point in care |
|
|
| Bertsche et al., 2009 [34] | Integrated in hospital drug information system | CPG5 based algorithms generate pain specific recommendations | Pain intensity score (NVAS) on day 5 after admission
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klarenbeek, S.E.; Weekenstroo, H.H.A.; Sedelaar, J.P.M.; Fütterer, J.J.; Prokop, M.; Tummers, M. The Effect of Higher Level Computerized Clinical Decision Support Systems on Oncology Care: A Systematic Review. Cancers 2020, 12, 1032. https://doi.org/10.3390/cancers12041032
Klarenbeek SE, Weekenstroo HHA, Sedelaar JPM, Fütterer JJ, Prokop M, Tummers M. The Effect of Higher Level Computerized Clinical Decision Support Systems on Oncology Care: A Systematic Review. Cancers. 2020; 12(4):1032. https://doi.org/10.3390/cancers12041032
Chicago/Turabian StyleKlarenbeek, Sosse E., Harm H.A. Weekenstroo, J.P. Michiel Sedelaar, Jurgen J. Fütterer, Mathias Prokop, and Marcia Tummers. 2020. "The Effect of Higher Level Computerized Clinical Decision Support Systems on Oncology Care: A Systematic Review" Cancers 12, no. 4: 1032. https://doi.org/10.3390/cancers12041032
APA StyleKlarenbeek, S. E., Weekenstroo, H. H. A., Sedelaar, J. P. M., Fütterer, J. J., Prokop, M., & Tummers, M. (2020). The Effect of Higher Level Computerized Clinical Decision Support Systems on Oncology Care: A Systematic Review. Cancers, 12(4), 1032. https://doi.org/10.3390/cancers12041032





