Preclinical Targeted α- and β−-Radionuclide Therapy in HER2-Positive Brain Metastasis Using Camelid Single-Domain Antibodies
Abstract
1. Introduction
2. Results
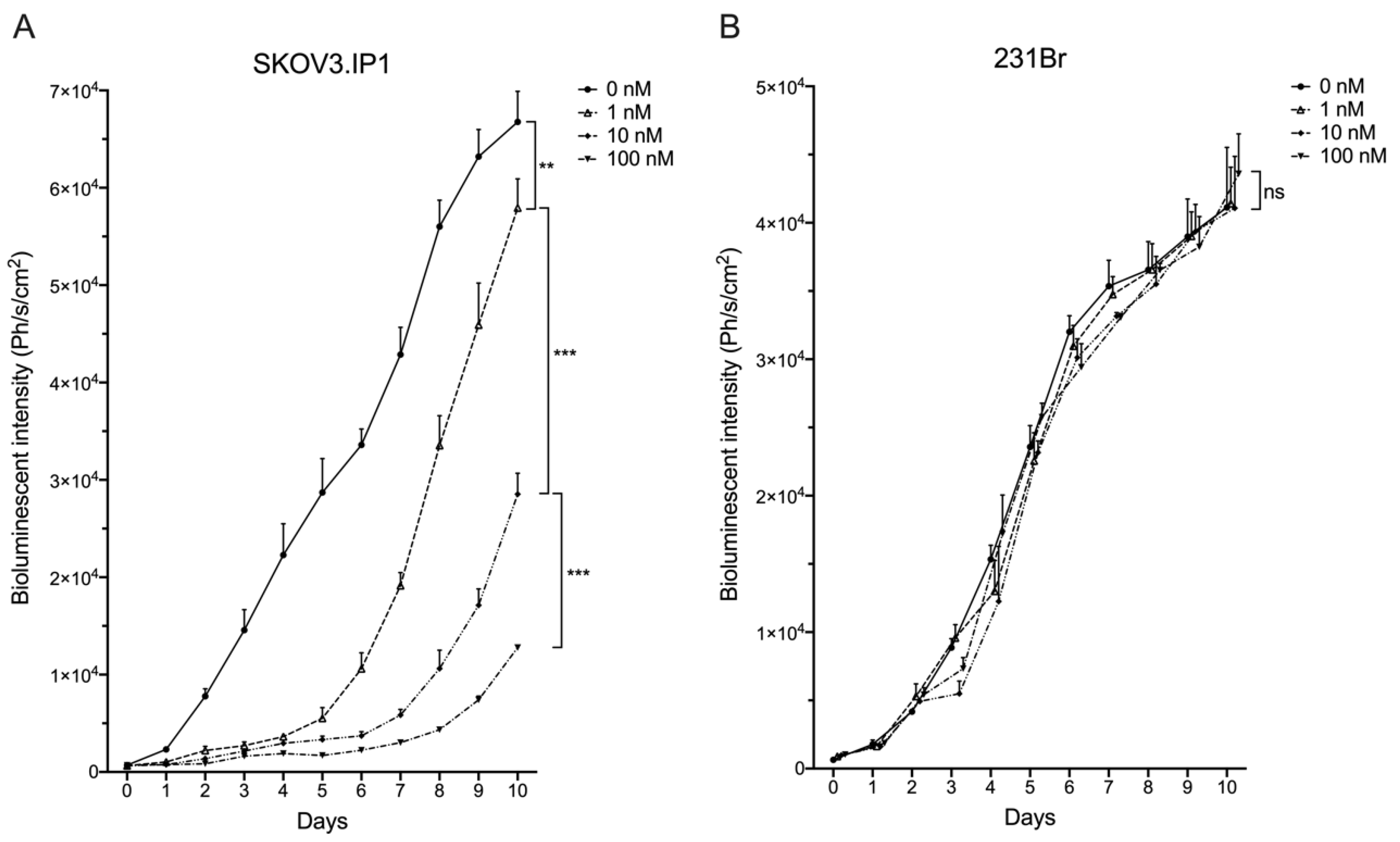
2.1. Different HER2pos Cell Lines React Differently In Vitro to Treatment with Trastuzumab
2.2. Intracranially Inoculated HER2pos Cells Show Aggressive Exponential Growth In Vivo
2.3. SdAbs Can Be Labeled Efficiently with Different Radionuclides
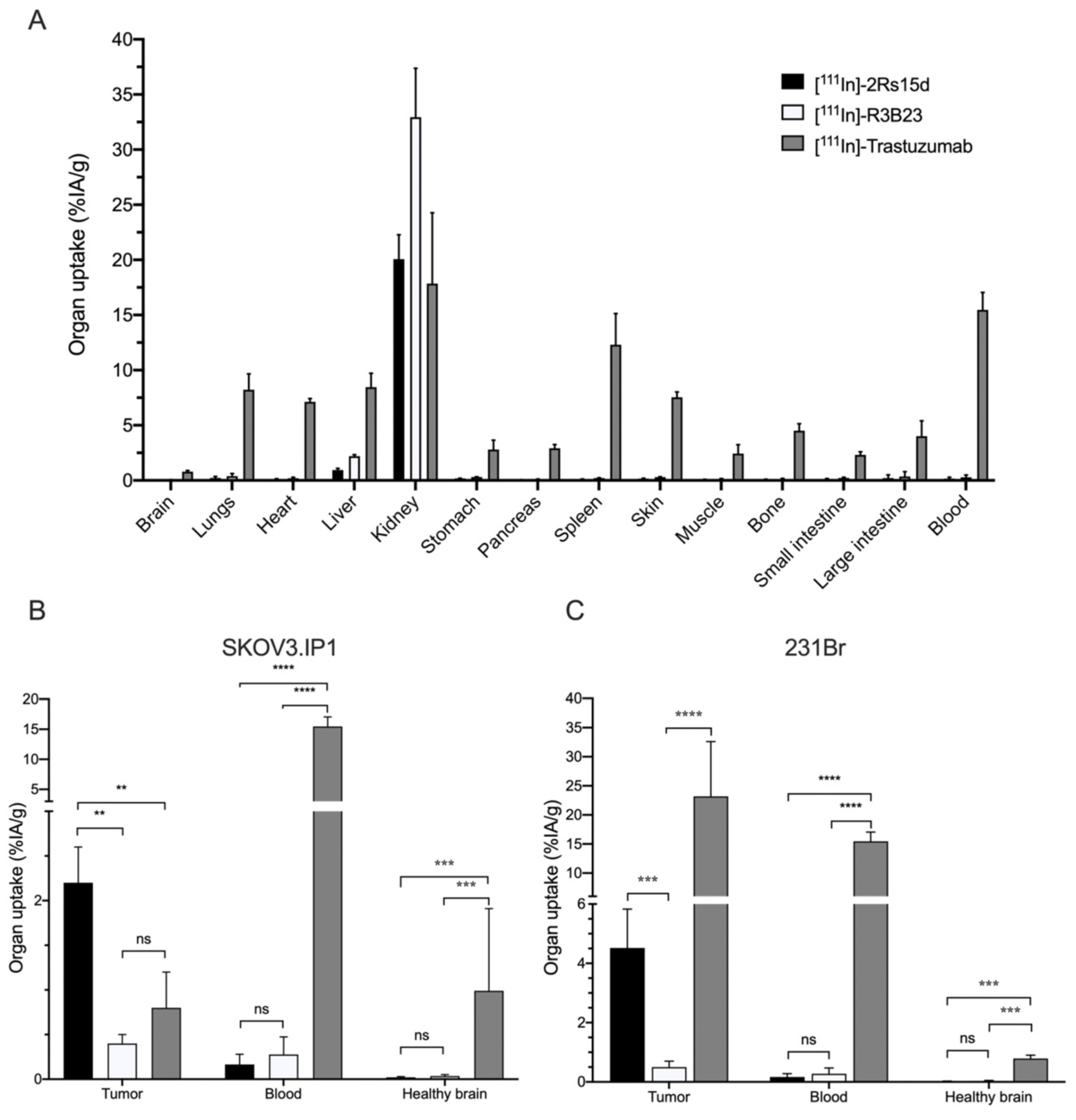
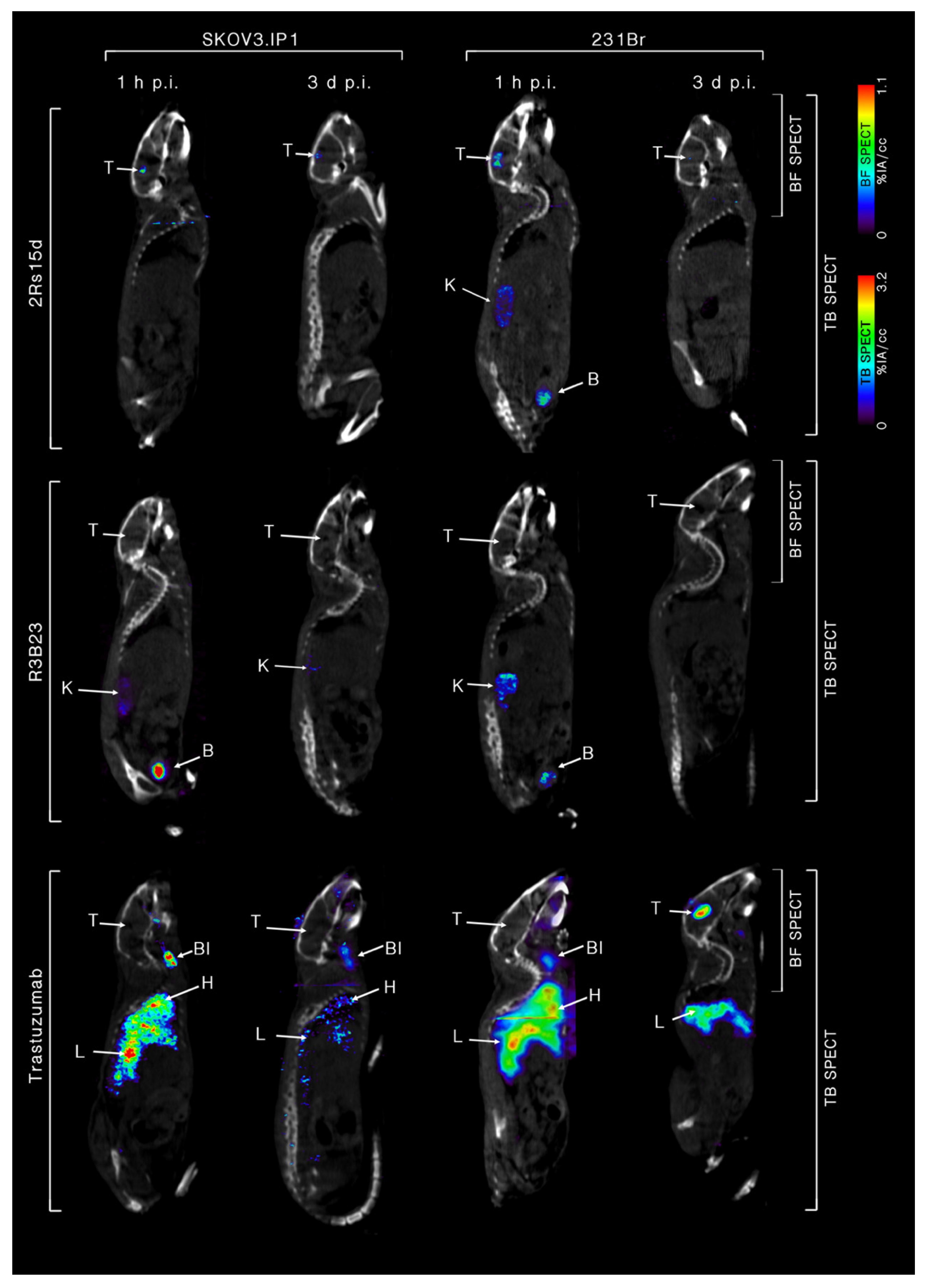
2.4. 111In-Labeled Anti-HER2 sdAb Shows Favorable In Vivo Biodistribution Compared to Trastuzumab
2.5. Dosimetry Calculations of A Single Dose [131I]-2Rs15d and [225Ac]-2Rs15d
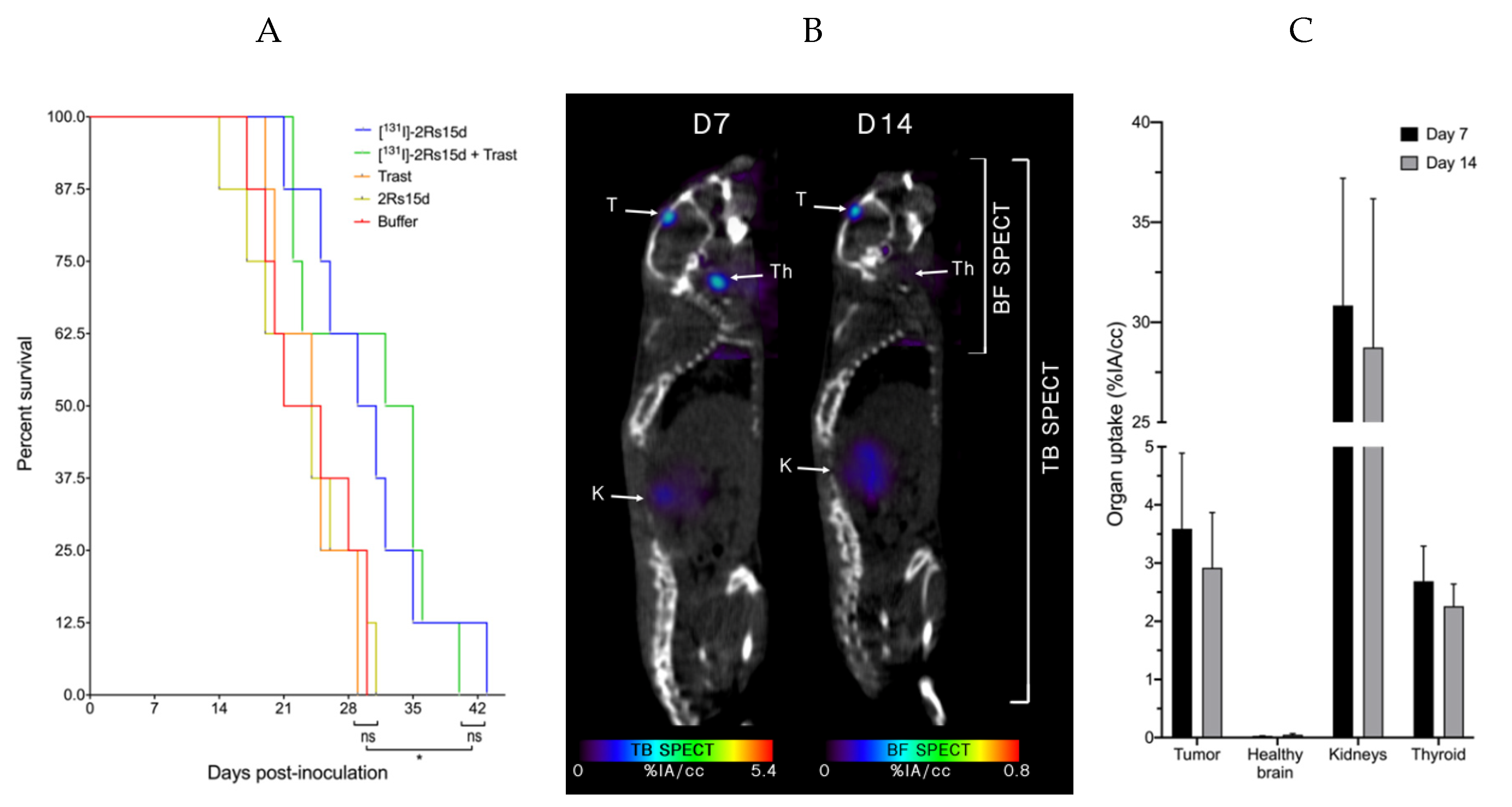
2.6. [131I]-2Rs15d Shows Theranostic Potential for HER2pos Brain Lesions
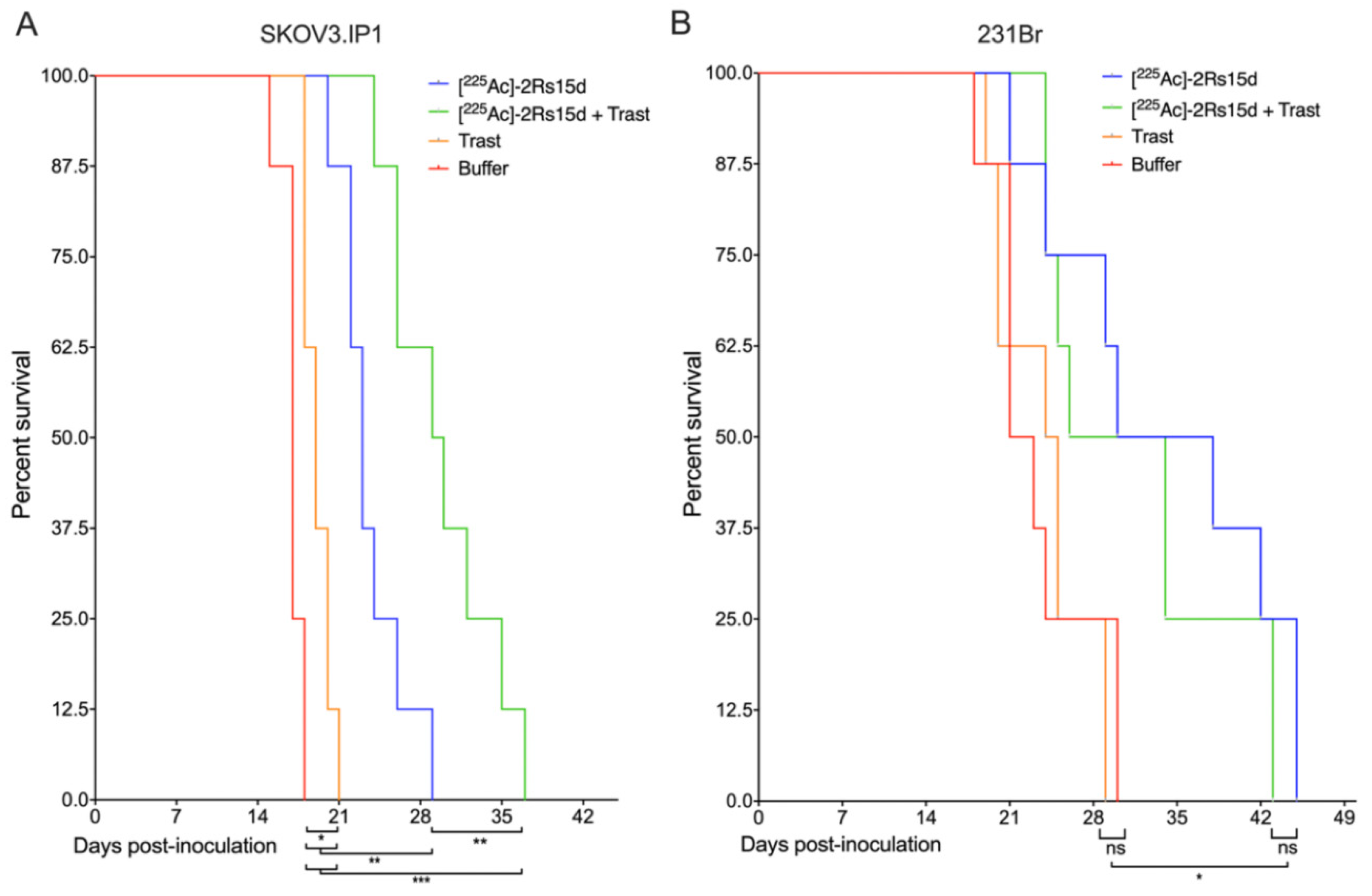
2.7. SdAb-Mediated Targeted Alpha Therapy Inhibits Cell Growth in Trastuzumab-Responsive and -Resistant Tumor Models
2.8. [225Ac]- and [131I]-sdAb TRNT Shows No Obvious In Vivo Toxicity
3. Discussion
4. Materials and Methods
4.1. General
4.2. Cell Culture Conditions
4.3. In Vitro Trastuzumab-Induced Growth Inhibition of HER2-Expressing Cells
4.4. Preparation of Radiolabeled Compounds
4.5. Tumor Inoculation and Follow-Up
4.6. In Vivo Tumor Targeting and Ex Vivo Biodistribution of 111In-Labeled Radioconjugates
4.7. Dosimetry Calculations of A Single Dose of [131I]- and [225Ac]-2Rs15d
4.8. Theranostic Application of [131I]-2Rs15d for Brain Lesions
4.9. Targeted Alpha Therapy of Brain Lesions
4.10. Toxicity of [225Ac]-2Rs15d and [131I]-2Rs15d
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arkosy, P.; Toth, J.; Beres, E.; Toth, D.; Szivos, L.; Nagy, J.; Klekner, A.; Virga, J. Prognosis and Treatment Outcomes of Patients Undergoing Resection of Brain Metastases from Breast Cancer. Anticancer Res. 2020, 40, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Winer, E.P. Brain metastases: The HER2 paradigm. Clin. Cancer Res. 2007, 13, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Rostami, R.; Mittal, S.; Rostami, P.; Tavassoli, F.; Jabbari, B. Brain metastasis in breast cancer: A comprehensive literature review. J. Neurooncol. 2016, 127, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, E.M.; Amonkar, M.M.; Shim, B. Incidence and prevalence of brain metastases among patients with advanced breast cancer in a United States managed-care population. J. Clin. Oncol. 2007, 25, 6624. [Google Scholar] [CrossRef]
- Stemmler, J.; Schmitt, M.; Willems, A.; Bernhard, H.; Harbeck, N.; Heinemann, V. Brain metastases in HER2-overexpressing metastatic breast cancer: Comparative analysis of trastuzumab levels in serum and cerebrospinal fluid. J. Clin. Oncol. 2006, 24, 1525. [Google Scholar] [CrossRef]
- Vogel, C.L.; Cobleigh, M.A.; Tripathy, D.; Gutheil, J.C.; Harris, L.N.; Fehrenbacher, L.; Slamon, D.J.; Murphy, M.; Novotny, W.F.; Burchmore, M.; et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 719–726. [Google Scholar] [CrossRef]
- Kallioniemi, O.P.; Holli, K.; Visakorpi, T.; Koivula, T.; Helin, H.H.; Isola, J.J. Association of c-erbB-2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer. Int. J. Cancer. 1991, 49, 650–655. [Google Scholar] [CrossRef]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E., Jr.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef]
- Olson, E.M.; Abdel-Rasoul, M.; Maly, J.; Wu, C.S.; Lin, N.U.; Shapiro, C.L. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann. Oncol. 2013, 24, 1526–1533. [Google Scholar] [CrossRef]
- Lin, N.U.; Dieras, V.; Paul, D.; Lossignol, D.; Christodoulou, C.; Stemmler, H.J.; Roche, H.; Liu, M.C.; Greil, R.; Ciruelos, E.; et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin. Cancer Res. 2009, 15, 1452–1459. [Google Scholar] [CrossRef]
- Puttemans, J.; Lahoutte, T.; D’Huyvetter, M.; Devoogdt, N. Beyond the Barrier: Targeted Radionuclide Therapy in Brain Tumors and Metastases. Pharmaceutics 2019, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Y.; Xiao, Z.; Li, W.; Dimitrov, D.S.; Chen, W. Human Domain Antibodies to Conserved Epitopes on HER2 Potently Inhibit Growth of HER2-Overexpressing Human Breast Cancer Cells In Vitro. Antibodies (Basel) 2019, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Debie, P.; Lafont, C.; Defrise, M.; Hansen, I.; van Willigen, D.M.; van Leeuwen, F.W.B.; Gijsbers, R.; D’Huyvetter, M.; Devoogdt, N.; Lahoutte, T.; et al. Size and affinity kinetics of nanobodies influence targeting and penetration of solid tumours. J. Control. Release 2020, 317, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, M.; Xavier, C.; Everaert, H.; Vaneycken, I.; Fontaine, C.; Decoster, L.; Vanhoeij, M.; Caveliers, V.; Lahoutte, T. Phase II trial of HER2-PET/CT using 68Ga-anti-HER2 VHH1 for characterization of HER2 presence in brain metastases of breast cancer patients. Ann. Oncol. 2019, 30. [Google Scholar] [CrossRef]
- D’Huyvetter, M.; De Vos, J.; Xavier, C.; Pruszynski, M.; Sterckx, Y.G.J.; Massa, S.; Raes, G.; Caveliers, V.; Zalutsky, M.R.; Lahoutte, T.; et al. (131)I-labeled Anti-HER2 Camelid sdAb as a Theranostic Tool in Cancer Treatment. Clin. Cancer Res. 2017, 23, 6616–6628. [Google Scholar] [CrossRef]
- D’Huyvetter, M.; Vincke, C.; Xavier, C.; Aerts, A.; Impens, N.; Baatout, S.; De Raeve, H.; Muyldermans, S.; Caveliers, V.; Devoogdt, N.; et al. Targeted radionuclide therapy with A 177Lu-labeled anti-HER2 nanobody. Theranostics 2014, 4, 708–720. [Google Scholar] [CrossRef]
- Krasniqi, A.; D’Huyvetter, M.; Xavier, C.; Van der Jeught, K.; Muyldermans, S.; Van Der Heyden, J.; Lahoutte, T.; Tavernier, J.; Devoogdt, N. Theranostic Radiolabeled Anti-CD20 sdAb for Targeted Radionuclide Therapy of Non-Hodgkin Lymphoma. Mol. Cancer Ther. 2017, 16, 2828–2839. [Google Scholar] [CrossRef]
- Dekempeneer, Y.; Keyaerts, M.; Krasniqi, A.; Puttemans, J.; Muyldermans, S.; Lahoutte, T.; D’Huyvetter, M.; Devoogdt, N. Targeted alpha therapy using short-lived alpha-particles and the promise of nanobodies as targeting vehicle. Expert Opin. Biol. Ther. 2016, 16, 1035–1047. [Google Scholar] [CrossRef]
- Pruszynski, M.; D’Huyvetter, M.; Bruchertseifer, F.; Morgenstern, A.; Lahoutte, T. Evaluation of an Anti-HER2 Nanobody Labeled with (225)Ac for Targeted alpha-Particle Therapy of Cancer. Mol. Pharm. 2018, 15, 1457–1466. [Google Scholar] [CrossRef]
- Dekempeneer, Y.; Back, T.; Aneheim, E.; Jensen, H.; Puttemans, J.; Xavier, C.; Keyaerts, M.; Palm, S.; Albertsson, P.; Lahoutte, T.; et al. Labeling of Anti-HER2 Nanobodies with Astatine-211: Optimization and the Effect of Different Coupling Reagents on Their in Vivo Behavior. Mol. Pharm. 2019, 16, 3524–3533. [Google Scholar] [CrossRef]
- Vaneycken, I.; Devoogdt, N.; Van Gassen, N.; Vincke, C.; Xavier, C.; Wernery, U.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J. 2011, 25, 2433–2446. [Google Scholar] [CrossRef]
- Van Mechelen, M.; Van Herck, A.; Punie, K.; Nevelsteen, I.; Smeets, A.; Neven, P.; Weltens, C.; Han, S.; Vanderstichele, A.; Floris, G.; et al. Behavior of metastatic breast cancer according to subtype. Breast Cancer Res. Treat. 2020. [Google Scholar] [CrossRef]
- Gabos, Z.; Sinha, R.; Hanson, J.; Chauhan, N.; Hugh, J.; Mackey, J.R.; Abdulkarim, B. Prognostic Significance of Human Epidermal Growth Factor Receptor Positivity for the Development of Brain Metastasis After Newly Diagnosed Breast Cancer. J. Clin. Oncol. 2006, 24, 5658–5663. [Google Scholar] [CrossRef] [PubMed]
- Pestalozzi, B.C.; Holmes, E.; de Azambuja, E.; Metzger-Filho, O.; Hogge, L.; Scullion, M.; Lang, I.; Wardley, A.; Lichinitser, M.; Sanchez, R.I.; et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: A retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. 2013, 14, 244–248. [Google Scholar] [CrossRef]
- Dehdashti, F.; Wu, N.; Bose, R.; Naughton, M.J.; Ma, C.X.; Marquez-Nostra, B.V.; Diebolder, P.; Mpoy, C.; Rogers, B.E.; Lapi, S.E.; et al. Evaluation of [(89)Zr]trastuzumab-PET/CT in differentiating HER2-positive from HER2-negative breast cancer. Breast Cancer Res. Treat. 2018, 169, 523–530. [Google Scholar] [CrossRef]
- Baum, R.P.; Prasad, V.; Muller, D.; Schuchardt, C.; Orlova, A.; Wennborg, A.; Tolmachev, V.; Feldwisch, J. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J. Nucl. Med. 2010, 51, 892–897. [Google Scholar] [CrossRef]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schroder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Lyashchenko, S.K.; Riedl, C.; Ruan, S.; Zanzonico, P.B.; Lake, D.; Jhaveri, K.; Zeglis, B.; Lewis, J.S.; O’Donoghue, J.A. First-in-Human Human Epidermal Growth Factor Receptor 2-Targeted Imaging Using (89)Zr-Pertuzumab PET/CT: Dosimetry and Clinical Application in Patients with Breast Cancer. J. Nucl. Med. 2018, 59, 900–906. [Google Scholar] [CrossRef]
- Boskovitz, A.; McLendon, R.E.; Okamura, T.; Sampson, J.H.; Bigner, D.D.; Zalutsky, M.R. Treatment of HER2-positive breast carcinomatous meningitis with intrathecal administration of alpha-particle-emitting (211)At-labeled trastuzumab. Nucl. Med. Biol. 2009, 36, 659–669. [Google Scholar] [CrossRef]
- Jannetti, S.A.; Carlucci, G.; Carney, B.; Kossatz, S.; Shenker, L.; Carter, L.M.; Salinas, B.; Brand, C.; Sadique, A.; Donabedian, P.L.; et al. PARP-1-Targeted Radiotherapy in Mouse Models of Glioblastoma. J. Nucl. Med. 2018, 59, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Vaidyanathan, G.; Koumarianou, E.; Kang, C.M.; Zalutsky, M.R. Astatine-211 labeled anti-HER2 5F7 single domain antibody fragment conjugates: Radiolabeling and preliminary evaluation. Nucl. Med. Biol. 2018, 56, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.K.; Shaller, C.; Garmestani, K.; Plascjak, P.S.; Hodge, K.M.; Yuan, Q.A.; Marks, J.D.; Waldmann, T.A.; Brechbiel, M.W.; Adams, G.P. Effective treatment of established human breast tumor xenografts in immunodeficient mice with a single dose of the alpha-emitting radioisotope astatine-211 conjugated to anti-HER2/neu diabodies. Clin. Cancer Res. 2008, 14, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Cedrowska, E.; Pruszynski, M.; Gaweda, W.; Zuk, M.; Krysinski, P.; Bruchertseifer, F.; Morgenstern, A.; Karageorgou, M.A.; Bouziotis, P.; Bilewicz, A. Trastuzumab Conjugated Superparamagnetic Iron Oxide Nanoparticles Labeled with (225)Ac as a Perspective Tool for Combined alpha-Radioimmunotherapy and Magnetic Hyperthermia of HER2-Positive Breast Cancer. Molecules 2020, 25, 1025. [Google Scholar] [CrossRef]
- Keyaerts, M.; Vos, J.D.; Duhoux, F.P.; Caveliers, V.; Fontaine, C.; Vanhoeij, M.; D’Huyvetter, M.; Everaert, H.; Ghykiere, P.; Devoogdt, N.; et al. Phase I results of CAM-H2: Safety profile and tumor targeting in patients. J. Clin. Oncol. 2018, 36, e13017. [Google Scholar] [CrossRef]
- Betzer, O.; Shilo, M.; Opochinsky, R.; Barnoy, E.; Motiei, M.; Okun, E.; Yadid, G.; Popovtzer, R. The effect of nanoparticle size on the ability to cross the blood-brain barrier: An in vivo study. Nanomedicine (Lond.) 2017, 12, 1533–1546. [Google Scholar] [CrossRef]
- Blanchette, M.; Tremblay, L.; Lepage, M.; Fortin, D. Impact of drug size on brain tumor and brain parenchyma delivery after a blood-brain barrier disruption. J. Cereb. Blood Flow Metab. 2014, 34, 820–826. [Google Scholar] [CrossRef]
- Mendes, D.; Alves, C.; Afonso, N.; Cardoso, F.; Passos-Coelho, J.L.; Costa, L.; Andrade, S.; Batel-Marques, F. The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer--a systematic review. Breast Cancer Res. 2015, 17, 140. [Google Scholar] [CrossRef]
- Barok, M.; Joensuu, H.; Isola, J. Trastuzumab emtansine: Mechanisms of action and drug resistance. Breast Cancer Res. 2014, 16, 209. [Google Scholar] [CrossRef]
- David, M.A.; Jones, D.R.; Tayebi, M. Potential candidate camelid antibodies for the treatment of protein-misfolding diseases. J. Neuroimmunol. 2014, 272, 76–85. [Google Scholar] [CrossRef]
- Nabuurs, R.J.A.; Rutgers, K.S.; Welling, M.M.; Metaxas, A.; de Backer, M.E.; Rotman, M.; Bacskai, B.J.; van Buchem, M.A.; van der Maarel, S.M.; van der Weerd, L. In vivo detection of amyloid-β deposits using heavy chain antibody fragments in a transgenic mouse model for Alzheimer’s disease. PLoS ONE 2012, 7, e38284. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Vandesquille, M.; Koukouli, F.; Dudeffant, C.; Youssef, I.; Lenormand, P.; Ganneau, C.; Maskos, U.; Czech, C.; Grueninger, F.; et al. Camelid single-domain antibodies: A versatile tool for in vivo imaging of extracellular and intracellular brain targets. J. Control. Release 2016, 243, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Bhatt, M.; Butler, D.; De Genst, E.; Dobson, C.M.; Messer, A.; Kordower, J.H. Proteasome-targeted nanobodies alleviate pathology and functional decline in an alpha-synuclein-based Parkinson’s disease model. NPJ Parkinsons Dis. 2018, 4, 25. [Google Scholar] [CrossRef]
- Iljina, M.; Hong, L.; Horrocks, M.H.; Ludtmann, M.H.; Choi, M.L.; Hughes, C.D.; Ruggeri, F.S.; Guilliams, T.; Buell, A.K.; Lee, J.E.; et al. Nanobodies raised against monomeric a-synuclein inhibit fibril formation and destabilize toxic oligomeric species. BMC Biol. 2017, 15, 57. [Google Scholar] [CrossRef]
- Zhou, Z.; Vaidyanathan, G.; McDougald, D.; Kang, C.M.; Balyasnikova, I.; Devoogdt, N.; Ta, A.N.; McNaughton, B.R.; Zalutsky, M.R. Fluorine-18 Labeling of the HER2-Targeting Single-Domain Antibody 2Rs15d Using a Residualizing Label and Preclinical Evaluation. Mol. Imaging Biol. 2017, 19, 867–877. [Google Scholar] [CrossRef]
- Palmieri, D.; Bronder, J.L.; Herring, J.M.; Yoneda, T.; Weil, R.J.; Stark, A.M.; Kurek, R.; Vega-Valle, E.; Feigenbaum, L.; Halverson, D.; et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007, 67, 4190–4198. [Google Scholar] [CrossRef]
- Terrell-Hall, T.B.; Nounou, M.I.; El-Amrawy, F.; Griffith, J.I.G.; Lockman, P.R. Trastuzumab distribution in an in-vivo and in-vitro model of brain metastases of breast cancer. Oncotarget 2017, 8, 83734–83744. [Google Scholar] [CrossRef]
- Santoro, L.; Mora-Ramirez, E.; Trauchessec, D.; Chouaf, S.; Eustache, P.; Pouget, J.-P.; Kotzki, P.-O.; Bardiès, M.; Deshayes, E. Implementation of patient dosimetry in the clinical practice after targeted radiotherapy using [(177)Lu-[DOTA0, Tyr3]-octreotate. EJNMMI Res. 2018, 8, 103. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. ²¹³Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: A first-in-human experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted alpha-Therapy of Metastatic Castration-Resistant Prostate Cancer with (225)Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef]
- Krolicki, L.; Bruchertseifer, F.; Morgenstern, A.; Kunikowska, J.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Apostolidis, C.; Rola, R.; et al. Safety and Therapeutic Efficacy of 225Ac-DOTA-Substance P for Therapy of Brain Tumors. JMIRS 2019, 50, S22. [Google Scholar] [CrossRef]
- Sathekge, M.M.; Bruchertseifer, F.; Lawal, I.O.; Vorster, M.; Knoesen, O.; Lengana, T.; Boshomane, T.G.; Mokoala, K.K.; Morgenstern, A. Treatment of brain metastases of castration-resistant prostate cancer with 225Ac-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1756–1757. [Google Scholar] [CrossRef] [PubMed]
- Bruchertseifer, F.; Morgenstern, A.; Giesel, F.; Apostolidis, C.; Haberkorn, U.; Kratochwil, C. Targeted Alpha Therapy of mCRPC with 225Actinium-PSMA617: Dosimetry, toxicity and duration of tumor-control. J. Nucl. Med. 2018, 59, 530. [Google Scholar]
- Baselga, J.; Albanell, J. Mechanism of action of anti-HER2 monoclonal antibodies. Ann. Oncol. 2001, 12, S35–S41. [Google Scholar] [CrossRef] [PubMed]
- Sattiraju, A.; Xiong, X.; Pandya, D.N.; Wadas, T.J.; Xuan, A.; Sun, Y.; Jung, Y.; Sai, K.K.S.; Dorsey, J.F.; Li, K.C.; et al. Alpha Particle Enhanced Blood Brain/Tumor Barrier Permeabilization in Glioblastomas Using Integrin Alpha-v Beta-3-Targeted Liposomes. Mol. Cancer Ther. 2017, 16, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Sattiraju, A.; Xiong, X.; Pandya, D.; Sun, Y.; Jung, Y.; Wadas, T.; Li, K.; Mintz, A. Permeabilizing the blood-brain-barrier with α-particle therapy. J. Nucl. Med. 2015, 56, 1230. [Google Scholar]
- Lemaire, M.; D’Huyvetter, M.; Lahoutte, T.; Van Valckenborgh, E.; Menu, E.; De Bruyne, E.; Kronenberger, P.; Wernery, U.; Muyldermans, S.; Devoogdt, N.; et al. Imaging and radioimmunotherapy of multiple myeloma with anti-idiotypic Nanobodies. Leukemia 2014, 28, 444–447. [Google Scholar] [CrossRef] [PubMed]
- De Vlieghere, E.; Carlier, C.; Ceelen, W.; Bracke, M.; De Wever, O. Data on in vivo selection of SK-OV-3 Luc ovarian cancer cells and intraperitoneal tumor formation with low inoculation numbers. Data Brief. 2016, 6, 542–549. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Breckpot, K.; Dullaers, M.; Bonehill, A.; van Meirvenne, S.; Heirman, C.; de Greef, C.; van der Bruggen, P.; Thielemans, K. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J. Gene Med. 2003, 5, 654–667. [Google Scholar] [CrossRef]
- Goyvaerts, C.; Dingemans, J.; De Groeve, K.; Heirman, C.; Van Gulck, E.; Vanham, G.; De Baetselier, P.; Thielemans, K.; Raes, G.; Breckpot, K. Targeting of human antigen-presenting cell subsets. J. Virol. 2013, 87, 11304–11308. [Google Scholar] [CrossRef]
- Pruszynski, M.; Koumarianou, E.; Vaidyanathan, G.; Revets, H.; Devoogdt, N.; Lahoutte, T.; Lyerly, H.K.; Zalutsky, M.R. Improved tumor targeting of anti-HER2 nanobody through N-succinimidyl 4-guanidinomethyl-3-iodobenzoate radiolabeling. J. Nucl. Med. 2014, 55, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, M.; Verschueren, J.; Bos, T.J.; Tchouate-Gainkam, L.O.; Peleman, C.; Breckpot, K.; Vanhove, C.; Caveliers, V.; Bossuyt, A.; Lahoutte, T. Dynamic bioluminescence imaging for quantitative tumour burden assessment using IV or IP administration of D: -luciferin: Effect on intensity, time kinetics and repeatability of photon emission. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Loening, A.M.; Gambhir, S.S. AMIDE: A free software tool for multimodality medical image analysis. Mol. Imaging 2003, 2, 131–137. [Google Scholar] [CrossRef] [PubMed]

| Ex Vivo Biodistribution | Dosimetry | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ | 1 h p.i. | 4 h p.i. | 12 h p.i. | 24 h p.i. | 48 h p.i. | 72 h p.i. | Absorbed | ||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Dose | |||||||
| Brain | 0.09 | ± | 0.03 | 0.05 | ± | 0.03 | 0.03 | ± | 0.01 | 0.01 | ± | 0.00 | 0.00 | ± | 0.00 | 0.00 | ± | 0.00 | 0.04 |
| Lungs | 0.73 | ± | 0.25 | 0.36 | ± | 0.12 | 0.13 | ± | 0.02 | 0.08 | ± | 0.02 | 0.03 | ± | 0.01 | 0.02 | ± | 0.00 | 0.27 |
| Heart | 0.53 | ± | 0.11 | 0.29 | ± | 0.12 | 0.16 | ± | 0.02 | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | 0.00 | ± | 0.00 | 0.18 |
| Liver | 1.55 | ± | 0.23 | 0.64 | ± | 0.16 | 0.19 | ± | 0.07 | 0.08 | ± | 0.01 | 0.06 | ± | 0.01 | 0.01 | ± | 0.00 | 0.43 |
| Kidneys | 62.63 | ± | 9.54 | 19.20 | ± | 3.57 | 6.59 | ± | 2.46 | 1.88 | ± | 0.52 | 0.84 | ± | 0.23 | 0.44 | ± | 0.02 | 12.50 |
| Spleen | 0.33 | ± | 0.10 | 0.19 | ± | 0.10 | 0.05 | ± | 0.02 | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | 0.00 | ± | 0.00 | 0.10 |
| Muscle | 0.54 | ± | 0.09 | 0.29 | ± | 0.05 | 0.10 | ± | 0.03 | 0.05 | ± | 0.01 | 0.03 | ± | 0.01 | 0.01 | ± | 0.00 | 0.21 |
| Bone | 0.46 | ± | 0.08 | 0.20 | ± | 0.09 | 0.11 | ± | 0.04 | 0.05 | ± | 0.01 | 0.02 | ± | 0.01 | 0.01 | ± | 0.00 | 0.18 |
| Small intestines | 0.50 | ± | 0.14 | 0.42 | ± | 0.04 | 0.09 | ± | 0.02 | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | 0.00 | ± | 0.00 | 0.17 |
| Large intestines | 0.44 | ± | 0.19 | 0.23 | ± | 0.10 | 0.18 | ± | 0.06 | 0.02 | ± | 0.01 | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | 0.18 |
| Blood | 0.86 | ± | 0.14 | 0.31 | ± | 0.08 | 0.05 | ± | 0.01 | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | 0.00 | ± | 0.00 | 0.16 |
| Thyroid | 2.01 | ± | 0.93 | 1.97 | ± | 0.27 | 1.56 | ± | 0.77 | 0.91 | ± | 0.00 | 0.76 | ± | 0.25 | 0.52 | ± | 0.02 | 3.99 |
| Tumor | 4.51 | ± | 2.55 | 4.81 | ± | 2.49 | 3.54 | ± | 2.01 | 1.89 | ± | 1.90 | 0.62 | ± | 0.32 | 0.29 | ± | 0.01 | 4.90 |
| Ex Vivo Biodistribution | Dosimetry | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ | 1 h p.i. | 4 h p.i. | 12 h p.i. | 24 h p.i. | 48 h p.i. | 72 h p.i. | Absorbed | ||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Dose | |||||||
| Brain | 0.07 | ± | 0.03 | 0.06 | ± | 0.02 | 0.03 | ± | 0.01 | 0.01 | ± | 0.00 | 0.00 | ± | 0.00 | 0.00 | ± | 0.00 | 0.02 |
| Lungs | 0.69 | ± | 0.22 | 0.34 | ± | 0.09 | 0.11 | ± | 0.02 | 0.04 | ± | 0.02 | 0.01 | ± | 0.01 | 0.01 | ± | 0.00 | 0.08 |
| Heart | 0.49 | ± | 0.10 | 0.36 | ± | 0.09 | 0.16 | ± | 0.02 | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | 0.00 | ± | 0.00 | 0.07 |
| Liver | 1.15 | ± | 0.20 | 0.71 | ± | 0.12 | 0.13 | ± | 0.06 | 0.02 | ± | 0.01 | 0.04 | ± | 0.01 | 0.01 | ± | 0.00 | 0.12 |
| Kidneys | 32.03 | ± | 8.49 | 16.30 | ± | 2.78 | 5.79 | ± | 2.09 | 2.65 | ± | 0.44 | 0.53 | ± | 0.25 | 0.23 | ± | 0.02 | 3.49 |
| Spleen | 0.36 | ± | 0.09 | 0.13 | ± | 0.08 | 0.05 | ± | 0.02 | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | 0.00 | ± | 0.00 | 0.03 |
| Muscle | 0.24 | ± | 0.08 | 0.21 | ± | 0.04 | 0.12 | ± | 0.03 | 0.04 | ± | 0.01 | 0.01 | ± | 0.01 | 0.01 | ± | 0.00 | 0.06 |
| Bone | 0.38 | ± | 0.07 | 0.22 | ± | 0.07 | 0.17 | ± | 0.03 | 0.03 | ± | 0.01 | 0.02 | ± | 0.01 | 0.01 | ± | 0.00 | 0.07 |
| Small intestines | 0.57 | ± | 0.12 | 0.34 | ± | 0.03 | 0.04 | ± | 0.02 | 0.02 | ± | 0.00 | 0.01 | ± | 0.00 | 0.00 | ± | 0.00 | 0.05 |
| Large intestines | 0.49 | ± | 0.17 | 0.23 | ± | 0.08 | 0.15 | ± | 0.05 | 0.04 | ± | 0.01 | 0.01 | ± | 0.00 | 0.01 | ± | 0.00 | 0.07 |
| Blood | 0.73 | ± | 0.12 | 0.25 | ± | 0.06 | 0.10 | ± | 0.01 | 0.02 | ± | 0.00 | 0.01 | ± | 0.00 | 0.00 | ± | 0.00 | 0.06 |
| Thyroid | 0.00 | ± | 0.83 | 0.00 | ± | 0.21 | 0.00 | ± | 0.65 | 0.00 | ± | 0.00 | 0.00 | ± | 0.28 | 0.00 | ± | 0.03 | 0.00 |
| Tumor | 3.81 | ± | 1.13 | 4.01 | ± | 1.08 | 3.54 | ± | 1.08 | 1.39 | ± | 0.89 | 0.62 | ± | 0.15 | 0.44 | ± | 0.01 | 1.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puttemans, J.; Dekempeneer, Y.; Eersels, J.L.; Hanssens, H.; Debie, P.; Keyaerts, M.; Windhorst, A.D.; van der Aa, F.; Lecocq, Q.; Breckpot, K.; et al. Preclinical Targeted α- and β−-Radionuclide Therapy in HER2-Positive Brain Metastasis Using Camelid Single-Domain Antibodies. Cancers 2020, 12, 1017. https://doi.org/10.3390/cancers12041017
Puttemans J, Dekempeneer Y, Eersels JL, Hanssens H, Debie P, Keyaerts M, Windhorst AD, van der Aa F, Lecocq Q, Breckpot K, et al. Preclinical Targeted α- and β−-Radionuclide Therapy in HER2-Positive Brain Metastasis Using Camelid Single-Domain Antibodies. Cancers. 2020; 12(4):1017. https://doi.org/10.3390/cancers12041017
Chicago/Turabian StylePuttemans, Janik, Yana Dekempeneer, Jos L. Eersels, Heleen Hanssens, Pieterjan Debie, Marleen Keyaerts, Albert D. Windhorst, Frank van der Aa, Quentin Lecocq, Karine Breckpot, and et al. 2020. "Preclinical Targeted α- and β−-Radionuclide Therapy in HER2-Positive Brain Metastasis Using Camelid Single-Domain Antibodies" Cancers 12, no. 4: 1017. https://doi.org/10.3390/cancers12041017
APA StylePuttemans, J., Dekempeneer, Y., Eersels, J. L., Hanssens, H., Debie, P., Keyaerts, M., Windhorst, A. D., van der Aa, F., Lecocq, Q., Breckpot, K., Morgenstern, A., Bruchertseifer, F., Lahoutte, T., Devoogdt, N., & D’Huyvetter, M. (2020). Preclinical Targeted α- and β−-Radionuclide Therapy in HER2-Positive Brain Metastasis Using Camelid Single-Domain Antibodies. Cancers, 12(4), 1017. https://doi.org/10.3390/cancers12041017






