The Emerging Role of MicroRNAs and Other Non-Coding RNAs in Cancer Cachexia
Abstract
1. Introduction
2. MicroRNAs in Muscle Wasting
3. MicroRNAs in Adipose Tissue Depletion
4. MicroRNAs in Cachexia-Homeostatic Control
5. Circulating MicroRNAs
6. Other Non-Coding RNAs in Cancer Cachexia
6.1. Long Non-Coding RNAs
6.1.1. LncRNAs in Muscle Wasting
6.1.2. LncRNAs in Adipose Tissue Depletion
6.2. Circular RNAs
CircRNAs in Adipose Tissue Depletion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fearon, K.C.H.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; Macdonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Baracos, V.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Prim. 2018, 4, 17105. [Google Scholar] [CrossRef] [PubMed]
- Pais, A.M.; Ferreira, R.; Gil Da Costa, R.M. Platinum-induced muscle wasting in cancer chemotherapy: Mechanisms and potential targets for therapeutic intervention. Life Sci. 2018, 208, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.P.; Santos, J.M.; E Silva, M.P.C.; Gil Da Costa, R.M.; Medeiros, R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachex Sarcopenia Muscle 2020. [Google Scholar] [CrossRef]
- Argilés, J.M.; Moore-Carrasco, R.; Fuster, G.; Busquets, S.; Lopez-Soriano, F.J. Cancer cachexia: The molecular mechanisms. Int. J. Biochem. Cell Biol. 2003, 35, 405–409. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Loumaye, A.; Thissen, J.-P. Biomarkers of cancer cachexia. Clin. Biochem. 2017, 50, 1281–1288. [Google Scholar] [CrossRef]
- Tisdale, M.J. Mechanisms of Cancer Cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef]
- Bruggeman, A.R.; Kamal, A.; Leblanc, T.W.; Ma, J.D.; Baracos, V.; Roeland, E. Cancer Cachexia: Beyond Weight Loss. J. Oncol. Pract. 2016, 12, 1163–1171. [Google Scholar] [CrossRef]
- Dev, R.; Wong, A.; Hui, D.; Bruera, E. The Evolving Approach to Management of Cancer Cachexia. Oncology 2017, 31, 23–32. [Google Scholar]
- Condrat, C.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Inui, A. Cancer anorexia-cachexia syndrome: Current issues in research and management. CA A Cancer J. Clin. 2002, 52, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.C.; Bachmann, J.; Prokopchuk, O.; Friess, H.; Martignoni, M.E. Molecular pathways leading to loss of skeletal muscle mass in cancer cachexia—Can findings from animal models be translated to humans? BMC Cancer 2016, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, J.L.; Lu, J.; Song, Y.; Kwak, K.S.; Jiao, Q.; Rosenfeld, R.; Chen, Q.; Boone, T.; Simonet, W.S.; et al. Reversal of Cancer Cachexia and Muscle Wasting by ActRIIB Antagonism Leads to Prolonged Survival. Cell 2010, 142, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-C.; Kulp, S.K.; Lai, I.-L.; Hsu, E.-C.; He, W.A.; Frankhouser, D.E.; Yan, P.S.; Mo, X.; Bloomston, M.; Lesinski, G.B.; et al. Preclinical Investigation of the Novel Histone Deacetylase Inhibitor AR-42 in the Treatment of Cancer-Induced Cachexia. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef]
- Andreyev, H.; Norman, A.; Oates, J.; Cunningham, D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur. J. Cancer 1998, 34, 503–509. [Google Scholar] [CrossRef]
- Leandro-Merhi, V.A.; De Aquino, J.L.B.; De Camargo, J.G.T.; Frenhani, P.B.; Bernardi, J.L.D.; McLellan, K.C.P. Clinical and nutritional status of surgical patients with and without malignant diseases: Cross-sectional study. Arq. Gastroenterol. 2011, 48, 58–61. [Google Scholar] [CrossRef][Green Version]
- Ebner, N.; Von Haehling, S. Silver linings on the horizon: Highlights from the 10th Cachexia Conference. J. Cachexia Sarcopenia Muscle 2018, 9, 176–182. [Google Scholar] [CrossRef]
- Ebner, N.; Anker, S.D.; Von Haehling, S. Recent developments in the field of cachexia, sarcopenia, and muscle wasting: Highlights from the 11th Cachexia Conference. J. Cachex Sarcopenia Muscle 2019, 10, 218–225. [Google Scholar] [CrossRef]
- Lee, D.E.; Brown, J.L.; Rosa-Caldwell, M.E.; Blackwell, T.A.; Perry, R.A.; Brown, L.A.; Khatri, B.; Seo, D.; Bottje, W.G.; Washington, T.A.; et al. Cancer cachexia-induced muscle atrophy: Evidence for alterations in microRNAs important for muscle size. Physiol. Genom. 2017, 49, 253–260. [Google Scholar] [CrossRef]
- Narasimhan, A.; Ghosh, S.; Stretch, C.; Greiner, R.; Bathe, O.F.O.F.; Baracos, V.; Damaraju, S. Small RNAome profiling from human skeletal muscle: Novel miRNAs and their targets associated with cancer cachexia. J. Cachex Sarcopenia Muscle 2017, 8, 405–416. [Google Scholar] [CrossRef] [PubMed]
- He, W.A.; Calore, F.; Londhe, P.; Canella, A.; Guttridge, D.C.; Croce, C.M. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc. Natl. Acad. Sci. USA 2014, 111, 4525–4529. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Cabrera, A.; Fermoselle, C.; Salmela, I.; Yélamos, J.; Barreiro, E. MicroRNA expression and protein acetylation pattern in respiratory and limb muscles of Parp-1(−/−) and Parp-2(−/−) mice with lung cancer cachexia. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 2530–2543. [Google Scholar] [CrossRef] [PubMed]
- Van De Worp, W.R.; Schols, A.M.; Dingemans, A.C.; Kamp, C.M.O.D.; Degens, J.H.; Kelders, M.C.; Coort, S.; Woodruff, H.C.; Kratassiouk, G.; Harel-Bellan, A.; et al. Identification of microRNAs in skeletal muscle associated with lung cancer cachexia. J. Cachex Sarcopenia Muscle 2019, 11, 452–463. [Google Scholar] [CrossRef]
- Li, J.; Chan, M.C.; Yu, Y.; Bei, Y.; Chen, P.; Zhou, Q.; Cheng, L.; Chen, L.; Ziegler, O.; Rowe, G.C.; et al. miR-29b contributes to multiple types of muscle atrophy. Nat. Commun. 2017, 8, 15201. [Google Scholar] [CrossRef]
- Moraes, L.; Fernandez, G.J.; Vechetti-Junior, I.; Freire, P.P.; Souza, R.W.A.; Villacis, R.A.R.; Rogatto, S.R.; Reis, P.P.D.; Dal-Pai-Silva, M.; Carvalho, R.F. Integration of miRNA and mRNA expression profiles reveals microRNA-regulated networks during muscle wasting in cardiac cachexia. Sci. Rep. 2017, 7, 6998. [Google Scholar] [CrossRef]
- Zhang, A.; Li, M.; Wang, B.; Klein, J.D.; Price, S.R.; Wang, X. miRNA-23a/27a attenuates muscle atrophy and renal fibrosis through muscle-kidney crosstalk. J. Cachex Sarcopenia Muscle 2018, 9, 755–770. [Google Scholar] [CrossRef]
- Connolly, M.; Paul, R.; Garros, R.F.; Natanek, S.A.; Bloch, S.; Lee, J.; Lorenzo, J.P.; Patel, H.; Cooper, C.; Sayer, A.A.; et al. miR-424-5p reduces ribosomal RNA and protein synthesis in muscle wasting. J. Cachex Sarcopenia Muscle 2017, 9, 400–416. [Google Scholar] [CrossRef]
- Da’As, S.; Rizeq, B.; Nasrallah, G.K. Adipose tissue dysfunction in cancer cachexia. J. Cell. Physiol. 2018, 234, 13–22. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, S.; Xu, Z.; Yang, Q.; Li, B.; Zhu, S.; Wang, L.; Wu, J.; Yuan, J.; Wang, C.; et al. Tumour-originated exosomal miR-155 triggers cancer-associated cachexia to promote tumour progression. Mol. Cancer 2018, 17, 155. [Google Scholar] [CrossRef]
- Wan, Z.; Chen, X.; Gao, X.; Dong, Y.; Zhao, Y.; Wei, M.; Fan, W.; Yang, G.; Liu, L. Chronic myeloid leukemia-derived exosomes attenuate adipogenesis of adipose derived mesenchymal stem cells via transporting miR-92a-3p. J. Cell. Physiol. 2019, 234, 21274–21283. [Google Scholar] [CrossRef] [PubMed]
- Kulyté, A.; Lorente-Cebrián, S.; Gao, H.; Mejhert, N.; Agustsson, T.; Arner, P.; Ryden, M.; Dahlman, I. MicroRNA profiling links miR-378 to enhanced adipocyte lipolysis in human cancer cachexia. Am. J. Physiol. Metab. 2014, 306, E267–E274. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, A.J.; Scarlett, J.M.; Marks, D.L. Hypothalamic mechanisms in cachexia. Physiol. Behav. 2010, 100, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef] [PubMed]
- Brito, H.O.; Radulski, D.; Wilhelms, D.B.; Stojakovic, A.; Brito, L.M.O.; Gil Da Costa, R.M.; Trindade, E.; Engblom, D.; Franco, C.R.C.; Zampronio, A.R. Immune-mediated febrile response in female rats: Role of central hypothalamic mediators. Sci. Rep. 2020, 10, 4073. [Google Scholar] [CrossRef] [PubMed]
- Mercader, J.M.; González, J.R.; Lozano, J.J.; Bak, M.; Kauppinen, S.; Sumoy, L.; Dierssen, M.; Fernández-Aranda, F.; Visa, J.; Gratacòs, M.; et al. Aberrant brain microRNA target and miRISC gene expression in the anx/anx anorexia mouse model. Gene 2012, 497, 181–190. [Google Scholar] [CrossRef]
- Ghai, V.; Lee, I.; Wang, K. Chapter 13—Circulating miRNAs as Tumor Biomarkers. In Oncogenomics; Dammacco, F., Silvestris, F., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 191–206. [Google Scholar] [CrossRef]
- Reid, G.; Kirschner, M.B.; Van Zandwijk, N. Circulating microRNAs: Association with disease and potential use as biomarkers. Crit. Rev. Oncol. 2011, 80, 193–208. [Google Scholar] [CrossRef]
- Powrózek, T.; Mlak, R.; Brzozowska, A.; Mazurek, M.; Gołębiowski, P.; Małecka-Massalska, T. miRNA-130a Significantly Improves Accuracy of SGA Nutritional Assessment Tool in Prediction of Malnutrition and Cachexia in Radiotherapy-Treated Head and Neck Cancer Patients. Cancers 2018, 10, 294. [Google Scholar] [CrossRef]
- Okugawa, Y.; Toiyama, Y.; Hur, K.; Yamamoto, A.; Yin, C.; Ide, S.; Kitajima, T.; Fujikawa, H.; Yasuda, H.; Koike, Y.; et al. Circulating miR-203 derived from metastatic tissues promotes myopenia in colorectal cancer patients. J. Cachex Sarcopenia Muscle 2019, 10, 536–548. [Google Scholar] [CrossRef]
- Paul, R.; Lee, J.; Donaldson, A.V.; Connolly, M.; Sharif, M.; Natanek, S.A.; Rosendahl, U.; Polkey, M.I.; Griffiths, M.; Kemp, P.R. miR-422a suppresses SMAD4 protein expression and promotes resistance to muscle loss. J. Cachex Sarcopenia Muscle 2017, 9, 119–128. [Google Scholar] [CrossRef]
- Yao, R.; Wang, Y.; Chen, L.-L. Cellular functions of long noncoding RNAs. Nature 2019, 21, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-K.; Li, J.; Guan, D.; Liang, C.; Zhuo, Z.; Liu, J.; Lu, A.; Ge, Z.; Zhang, B.-T. A newly identified lncRNA MAR1 acts as a miR-487b sponge to promote skeletal muscle differentiation and regeneration. J. Cachex Sarcopenia Muscle 2018, 9, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, M.D.; Hatzigeorgiou, A.G. Analyzing MiRNA–LncRNA Interactions. Ther. Antibodies 2016, 1402, 271–286. [Google Scholar] [CrossRef]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2015, 17, 47–62. [Google Scholar] [CrossRef]
- Mercer, T.R.; E Dinger, M.; Mattick, J. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Shen, L.; Han, J.; Wang, H.; Meng, Q.; Chen, L.; Liu, Y.; Feng, Y.; Wu, G. Cachexia-related long noncoding RNA, CAAlnc1, suppresses adipogenesis by blocking the binding of HuR to adipogenic transcription factor mRNAs. Int. J. Cancer 2019, 145, 1809–1821. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, T.; Wang, B.; Li, L.; Ye, D.; Yu, S. Identification and functional analysis of a potential key lncRNA involved in fat loss of cancer cachexia. J. Cell. Biochem. 2017, 119, 1679–1688. [Google Scholar] [CrossRef]
- Gong, C.; Li, Z.; Ramanujan, K.; Clay, I.; Zhang, Y.; Lemire-Brachat, S.; Glass, D.J. A Long Non-coding RNA, LncMyoD, Regulates Skeletal Muscle Differentiation by Blocking IMP2-Mediated mRNA Translation. Dev. Cell 2015, 34, 181–191. [Google Scholar] [CrossRef]
- Li, Z.; Cai, B.; Abdalla, B.A.; Zhu, X.; Zheng, M.; Han, P.; Nie, Q.; Zhang, X. LncIRS1 controls muscle atrophy via sponging miR-15 family to activate IGF1-PI3K/AKT pathway. J. Cachex Sarcopenia Muscle 2019, 10, 391–410. [Google Scholar] [CrossRef]
- Dong, X.; Park, S.; Lin, X.; Copps, K.; Yi, X.; White, M.F. Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth. J. Clin. Investig. 2005, 116, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Mammucari, C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights from genetic models. Skelet. Muscle 2011, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Si, M.; Liu, X.; Choi, J.M.; Wang, Y.; Thomas, S.S.; Peng, H.; Hu, Z. Long-noncoding RNA Atrolnc-1 promotes muscle wasting in mice with chronic kidney disease. J. Cachex Sarcopenia Muscle 2018, 9, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Yang, Z.; Jia, R.; Ge, S. The novel roles of circRNAs in human cancer. Mol. Cancer 2019, 18, 6. [Google Scholar] [CrossRef]
- Schneider, T.; Bindereif, A. Circular RNAs: Coding or noncoding? Cell Res. 2017, 27, 724–725. [Google Scholar] [CrossRef]
- Patop, I.L.; Wüst, S.; Kadener, S. Past, present, and future of circ RNA s. EMBO J. 2019, 38, e100836. [Google Scholar] [CrossRef]
- Lee, E.C.S.; Elhassan, S.A.M.; Lim, G.P.L.; Kok, W.H.; Tan, S.W.; Leong, E.N.; Tan, S.H.; Chan, E.W.L.; Bhattamisra, S.K.; Rajendran, R.; et al. The roles of circular RNAs in human development and diseases. Biomed. Pharmacother. 2019, 111, 198–208. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Zhu, L.; Bai, M.; Liu, Y.; Zhan, Y.; Deng, T.; Yang, H.; Sun, W.; Wang, X.; Zhu, K.; et al. Exosomal circRNA derived from gastric tumor promotes white adipose browning by targeting the miR-133/PRDM16 pathway. Int. J. Cancer 2019, 144, 2501–2515. [Google Scholar] [CrossRef]
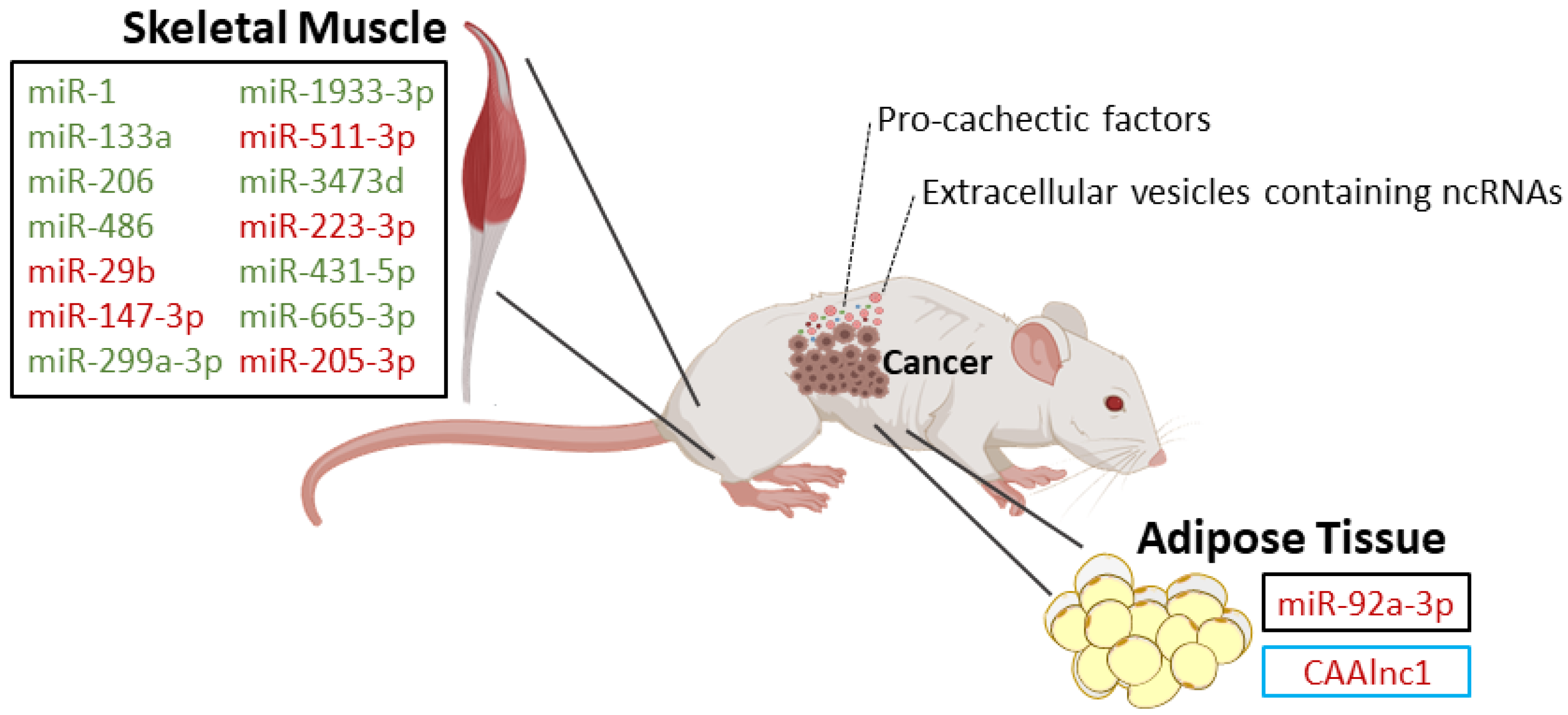
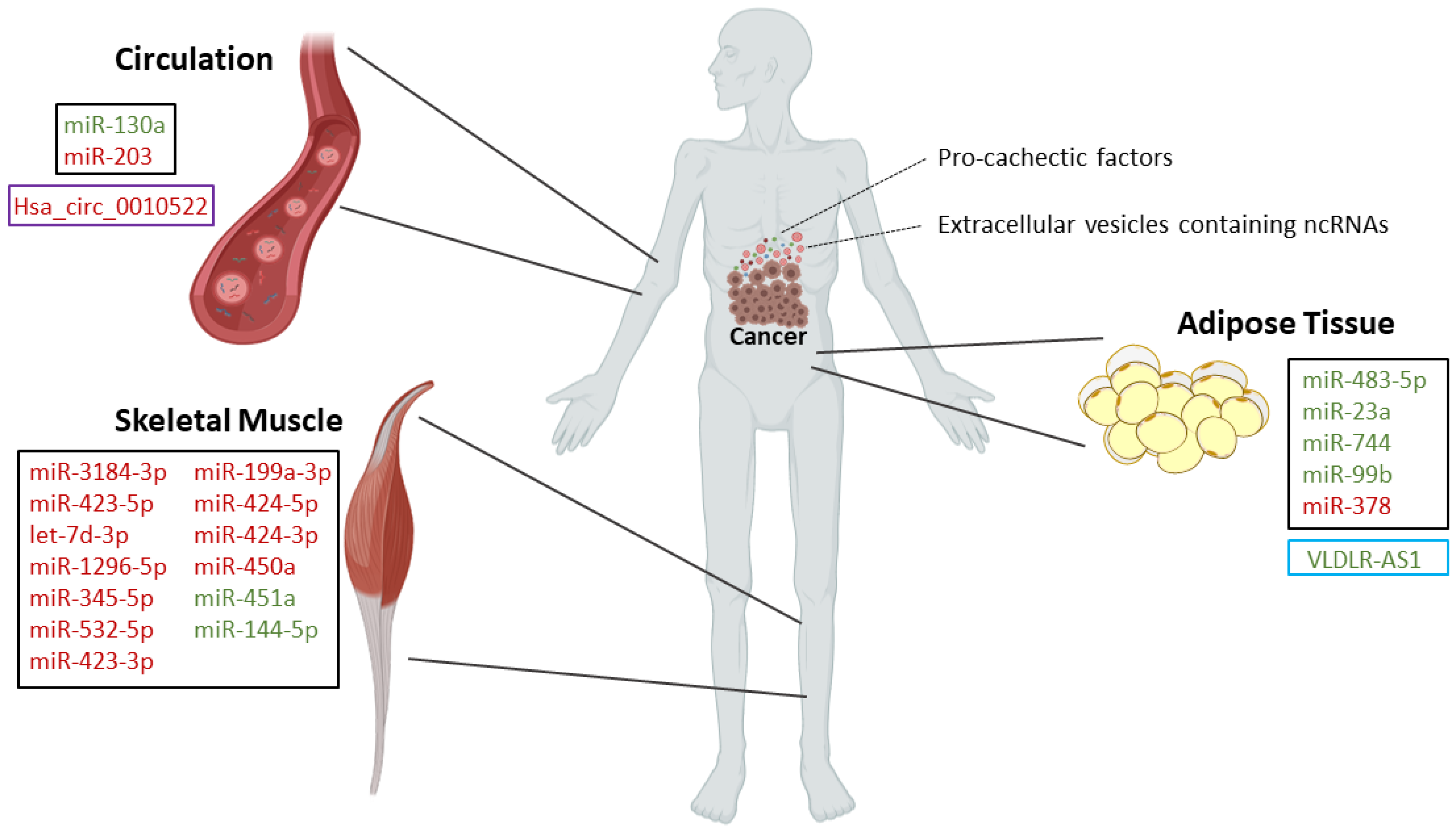
| Type of Study | MicroRNA | Expression | Targets | Biological Significance |
|---|---|---|---|---|
| In vitro: myoblasts from TLR7−/−and TLR7+/+ mice [22] | miR-21 [22] | Overexpressed in microvesicles secreted by lung and pancreatic cancer cell lines [22] | - | TLR7+/+ myoblast cell death [22] |
| In vivo: wild-type, Parp-1−/− and Parp-2−/− mice with and without lung cancer and cachexia [23] | miR-1 [23] | Downregulated in both diaphragm and gastrocnemius in all cachectic models [23] | - | These miRs are involved in biological process such as myoblast proliferation, hypertrophy, cell differentiation, and innervation [23]. Differential results for the diaphragm and gastrocnemius point out the site specificity of signaling pathways controlled by miRs involved in cancer cachexia [23]. |
| miR-133a [23] | Downregulated in diaphragm of all cachectic models and in gastrocnemius of Parp-2−/− and wild-type cachectic mice [23] | |||
| miR-206 [23] | Downregulated in diaphragm of all cachectic models and in gastrocnemius of wild-type cachectic mice [23] | |||
| miR-486 [23] | Downregulated in diaphragm and gastrocnemius of Parp-2−/− and wild-type cachectic micev [23] | |||
| In vivo: tibialis anterior muscle from mice that developed cachexia associated with Lewis lung carcinoma [20] | miR-147-3p [20] | Upregulated [20] | - | Altered cell-to-cell signaling, cell development, cell growth, and inflammatory response [20] |
| miR-299a-3p [20] | Downregulated [20] | |||
| miR-1933-3p [20] | Downregulated [20] | |||
| miR-511-3p [20] | Upregulated [20] | |||
| miR-3473d [20] | Downregulated [20] | |||
| miR-223-3p [20] | Upregulated [20] | |||
| miR-431-5p [20] | Downregulated [20] | |||
| miR-665-3p [20] | Downregulated [20] | |||
| miR-205-3p [20] | Upregulated [20] | |||
| In vivo: rectus abdominis from pancreatic and colorectal cancer patients [21] | miR-3184-3p [21] | Upregulated [21] | - | Roles in adipogenesis, myogenesis, signal transduction pathways, inflammation, and innate immune response [21] |
| miR-423-5p [21] | Upregulated [21] | |||
| let-7d-3p [21] | Upregulated [21] | |||
| miR-1296-5p [21] | Upregulated [21] | |||
| miR-345-5p [21] | Upregulated [21] | |||
| miR-532-5p [21] | Upregulated [21] | |||
| miR-423-3p [21] | Upregulated [21] | |||
| miR-199a-3p [21] | Upregulated [21] | |||
| In vivo: quadriceps (vastus lateralis) muscle from non-small cell lung cancer patients [24] | miR-424-5p [24] | Upregulated [24] | - | Roles in interleukin 6, TGF-β, TNF-α, insulin, and PI3K-Akt signaling pathways [24] |
| miR-424-3p [24] | Upregulated [24] | |||
| miR-450a [24] | Upregulated [24] | |||
| miR-451a [24] | Downregulated [24] | |||
| miR-144-5p [24] | Downregulated [24] | |||
| In vivo/In vitro: gastrocnemius from numerous muscle atrophy models, including mice inoculated with mouse colon cancer C26 cells/C2C12 cells [25] | miR-29b [25] | Upregulated [25] | Igf-1 and Pi3k (p85) [25] | To drive skeletal muscle atrophy [25] |
| Type of Study | MicroRNA | Expression | Targets | Biological Significance |
|---|---|---|---|---|
| In vitro: 3T3-L1 cell line [30] | miR-155 [30] | Upregulated in exosomes from breast cancer cells (4T1 cell line) [30] | PPARG [30] | Promotes brown differentiation and remodels adipocyte metabolism [30] |
| In vivo/In vitro: mice injected with K562 cells-derived exosomes/adipose-derived mesenchymal stem cells obtained from patients [31] | miR-92a-3p [31] | Upregulated in exosomes from chronic myeloid leukemia cells (K562 cells) [31] | Cebpα [31] | Loss of body fat in mice and suppression of the adipogenic ability of adipose-derived mesenchymal stem cells [31] |
| In vivo/In vitro: Abdominal subcutaneous adipose tissue from cachectic patients with gastrointestinal cancers/primary human adipocytes [32] | miR-483-5p [32] | Downregulated [32] | - | MiR-378 enhances adipocyte lipolysis [32] |
| miR-23a [32] | Downregulated [32] | - | ||
| miR-744 [32] | Downregulated [32] | - | ||
| miR-99b [32] | Downregulated [32] | - | ||
| miR-378 [32] | Upregulated [32] | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.M.O.; Peixoto da Silva, S.; Gil da Costa, R.M.; Medeiros, R. The Emerging Role of MicroRNAs and Other Non-Coding RNAs in Cancer Cachexia. Cancers 2020, 12, 1004. https://doi.org/10.3390/cancers12041004
Santos JMO, Peixoto da Silva S, Gil da Costa RM, Medeiros R. The Emerging Role of MicroRNAs and Other Non-Coding RNAs in Cancer Cachexia. Cancers. 2020; 12(4):1004. https://doi.org/10.3390/cancers12041004
Chicago/Turabian StyleSantos, Joana M. O., Sara Peixoto da Silva, Rui M. Gil da Costa, and Rui Medeiros. 2020. "The Emerging Role of MicroRNAs and Other Non-Coding RNAs in Cancer Cachexia" Cancers 12, no. 4: 1004. https://doi.org/10.3390/cancers12041004
APA StyleSantos, J. M. O., Peixoto da Silva, S., Gil da Costa, R. M., & Medeiros, R. (2020). The Emerging Role of MicroRNAs and Other Non-Coding RNAs in Cancer Cachexia. Cancers, 12(4), 1004. https://doi.org/10.3390/cancers12041004








