Sexual Dimorphism in Cellular and Molecular Features in Human ACTH-Secreting Pituitary Adenomas
Abstract
1. Introduction
2. Results
2.1. ACTH Synthesis and Secretion Pattern
2.2. USP8 Sequencing
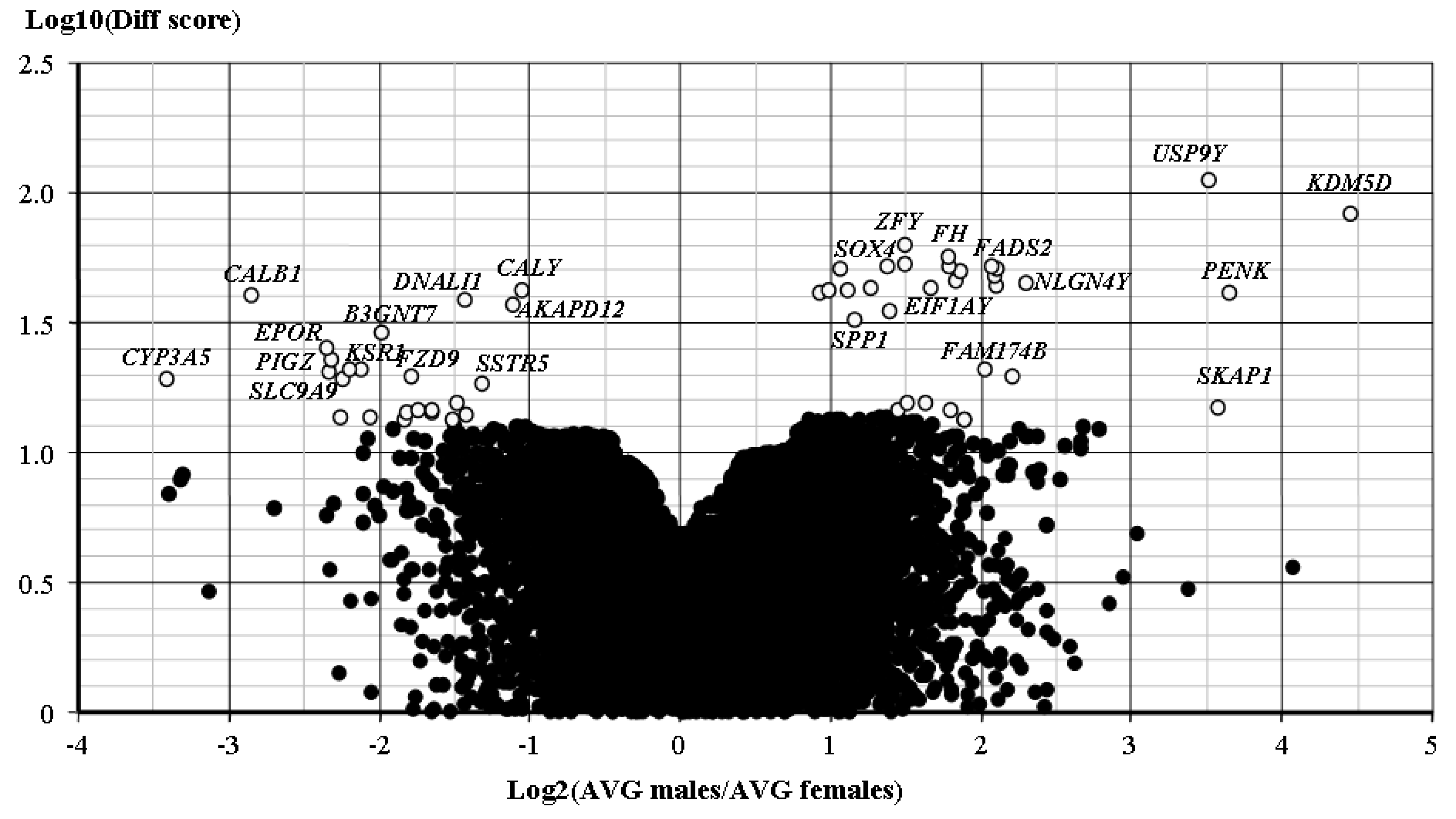
2.3. Gene Expression Pattern
3. Discussion
4. Materials and Methods
4.1. Specimens
4.2. Human Pituitary Adenoma Primary Culture
4.3. ACTH Assay
4.4. Microarray Analysis from Archival Specimens
4.5. Differential Gene Expression Analysis
4.6. Functional Annotation and Gene Ontology
4.7. USP8 Sequencing
4.8. Real-Time PCR
4.9. Ethics
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cushing, H. The basophil adenomas of the pituitary body and their clinical manifestations. Johns Hopkins Bull. 1932, 50, 137–195. [Google Scholar]
- Cavagnini, F.; Pecori Giraldi, F. Epidemiology and follow-up of patients with Cushing’s disease. Ann. Endocrinol. 2001, 62, 168–179. [Google Scholar]
- Pecori Giraldi, F.; Moro, M.; Cavagnini, F.; the Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypothalamic-Pituitary-Adrenal Axis. Gender-related differences in the presentation and course of Cushing’s disease. J. Clin. Endocrinol. Metab. 2003, 88, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.G.; Lad, S.P.; Harsh, G.R.; Laws, E.R., Jr.; Boakye, M. National trends, complications, and outcomes following transsphenoidal surgery for Cushing’s disease from 1993 to 2002. Neurosurg. Focus 2007, 23, E7–E12. [Google Scholar] [CrossRef]
- Castinetti, F.; Guignat, L.; Giraud, P.; Muller, M.; Kamenicky, P.; Drui, D.; Caron, P.; Luca, F.; Donadille, B.; Vantyghem, M.C.; et al. Ketoconazole in Cushing’s disease: Is it worth a try? J. Clin. Endocrinol. Metab. 2014, 99, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Zilio, M.; Barbot, M.; Ceccato, F.; Vamozzi, C.; Bilora, F.; Casonato, A.; Frigo, A.C.; Albiger, N.; Daidone, V.; Mazzai, L.; et al. Diagnosis and complications of Cushing’s disease: Gender-related differences. Clin. Endocrinol. 2014, 80, 403–410. [Google Scholar] [CrossRef]
- Chen, Y.; Mei, X.; Jian, F.; Ma, Q.; Chen, X.; Bian, L.; Sun, Q. Gender and magnetic resonance imaging classification-related differences in clinical and biochemical characteristics of Cushing’s disease: A single-centre study. Chin. Med. J. 2014, 127, 3948–3956. [Google Scholar]
- Huan, C.; Qu, Y.; Ren, Z. Gender differences in presentation and outcome of patients with Cushing’s disease in Han Chinese. Bio Med. Mater. Eng. 2014, 24, 3439–3446. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, X.; Zeng, M.; Zhuang, Y.; Zhou, Y.; Zhang, Z.; Yang, Y.; Wang, Y.; Ye, H.; Li, Y. Gender-specific differences in clinical profile and biochemical parameters in patients with Cushing’s disease: A single center experience. Int. J. Endocrinol. 2015, 2015, 949620. [Google Scholar] [CrossRef]
- Perez-Rivas, L.G.; Theodoropoulou, M.; Ferraù, F.; Nusser, C.; Kawaguchi, K.; Stratakis, C.A.; Rueda, F.F.; Wildemberg, L.E.; Assié, G.; Beschorner, R.; et al. The gene of the ubiquitin-specific protease 8 is frequently mutated in adenomas causing Cushing’s disease. J. Clin. Endocrinol. Metab. 2015, 100, E997–E1004. [Google Scholar] [CrossRef]
- Sbiera, S.; Perez-Rivas, L.G.; Taranets, L.; Weigand, I.; Flitsch, J.; Graf, E.; Monoranu, C.M.; Saeger, W.; Hagel, C.; Honegger, J.; et al. Driver mutations in USP8 wild type Cushing’s disease. Neuro Oncol. 2019, 21, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Sesta, A.; Cassarino, M.F.; Terreni, M.; Ambrogio, A.G.; Libera, L.; Bardelli, D.; Lasio, G.; Losa, M.; Pecori, G.F. Ubiquitin-specific protease 8 mutant corticotrope adenomas present unique secretory and molecular features and shed light on the role of ubiquitylation on ACTH processing. Neuroendocrinology 2020, 110, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Cassarino, M.F.; Ambrogio, A.G.; Cassarino, A.; Terreni, M.R.; Gentilini, D.; Sesta, A.; Cavagnini, F.; Losa, M.; Pecori Giraldi, F. Gene expression profiling in human corticotroph tumours reveals distinct, neuroendocrine profiles. J. Neuroendocrinol. 2018, 30, e12628. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.S.; Earp, H.S.; Harris, R.C.; Magnani, L.; Matthews, L.; Misior, A.M.; Orlic-Milacic, M.; Rothfels, K.; Shamovsky, V.; Stern, D.F.; et al. Estrogen-Dependent Gene Expression. Available online: https://reactome.org/content/detail/R-HSA-9018519 (accessed on 4 February 2020).
- Ross-Innes, C.S.; Stark, R.; Teschendorff, A.E.; Holmes, K.A.; Ali, H.R.; Dunning, M.J.; Brown, G.D.; Gojis, O.; Ellis, I.O.; Green, A.R.; et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 2012, 481, 389–393. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Clocchiatti, A.; Cora, E.; Zhang, Y.; Dotto, G.P. Sexual dimorphism and cancer. Nat. Rev. Cancer 2016, 16, 330–339. [Google Scholar] [CrossRef]
- Arnold, A.P.; Disteche, C.M. Sexual inequality in the cancer cell. Cancer Res. 2018, 78, 5504–5505. [Google Scholar] [CrossRef]
- Cheng, F. Gender dimorphism creates divergent cancer susceptibilities. Trends Cancer 2016, 2, 325–326. [Google Scholar] [CrossRef]
- Gabriele, L.; Buoncervello, M.; Ascione, B.; Bellenghi, M.; Matarrese, P.; Care, A. The gender perspective in cancer research and therapy: Novel insights and on-going hypotheses. Ann. Ist. Super. Sanità 2016, 52, 213–222. [Google Scholar]
- Yager, J.D. Endogenous estrogens as carcinogens through metabolic activation. J. Natl. Cancer Inst. Monogr. 2000, 27, 67–73. [Google Scholar] [CrossRef]
- Shao, R. Progesterone receptor isoforms A and B: New insights into the mechanism of progesterone resistance for the treatment of endometrial carcinoma. Ecancermedicalscience 2013, 7, 381. [Google Scholar] [PubMed]
- Banerjee, P.P.; Banerjee, S.; Brown, T.R.; Zirkin, B.R. Androgen action in prostate function and disease. Am. J. Clin. Exp. Urol. 2018, 6, 62–77. [Google Scholar] [PubMed]
- Marzagalli, M.; Montagnani, M.M.; Casati, L.; Fontana, F.; Moretti, R.M.; Limonta, P. Estrogen receptor beta in melanoma: From molecular insights to potential clinical utility. Front. Endocrinol. 2016, 7, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, R.; Zhang, L.; Kebebew, E. Thyroid cancer gender disparity. Future Oncol. 2010, 6, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Buhl, R.; Hugo, H.H.; Mehdorn, H.M. Clinical and histological features of multiple meningiomas compared with solitary meningiomas. Neurol. Res. 2005, 27, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Garg, K.; Katiyar, V.; Tandon, V.; Agarwal, D.; Singh, M.; Chandra, S.P.; Suri, A.; Kale, S.S.; Mahapatra, A.K. The role of mifepristone in the management of meningiomas: A systematic review of literature. Neurol. India 2019, 67, 698–705. [Google Scholar]
- Lloyd, R.V. Estrogen-induced hyperplasia and neoplasia in the rat anterior pituitary gland. An immunohistochemical study. Am. J. Pathol. 1983, 113, 198–206. [Google Scholar]
- Sarkar, D.K. Genesis of prolactinomas: Studies using estrogen-treated animals. Front. Horm. Res. 2006, 35, 32–49. [Google Scholar]
- Cao, L.; Gao, H.; Gui, S.; Bai, G.; Lu, R.; Wang, F.; Zhang, Y. Effects of the estrogen receptor antagonist fulvestrant on F344 rat prolactinoma models. J. Neuro Oncol. 2014, 116, 523–531. [Google Scholar] [CrossRef]
- Caronti, B.; Palladini, G.; Calderaro, C.; Bevilacqua, M.C.; Petrangeli, E.; Esposito, V.; Tamburrano, G.; Gulino, A.; Jaffrain-Rea, M.L. Effects of gonadal steroids on the growth of human pituitary adenomas in Vitro. Tumor Biol. 1995, 16, 353–364. [Google Scholar] [CrossRef]
- Oomizu, S.; Honda, J.; Takeuchi, S.; Kakeya, T.; Masui, T.; Takahashi, S. Transforming growth factor-alpha stimulates proliferation of mammotrophs and corticotrophs in the mouse pituitary. J. Endocrinol. 2000, 165, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Song, Y.; Xu, H.; Zhou, K.; Zhang, W.; Chen, J.; Qin, M.; Yi, H.; Gustafsson, J.A.; Yang, H.; et al. In nonfunctional pituitary adenomas, estrogen receptors and slug contribute to development of invasiveness. J. Clin. Endocrinol. Metab. 2011, 96, E1237–E1245. [Google Scholar] [CrossRef] [PubMed]
- Estrella, J.S.; Ma, L.T.; Milton, D.R.; Yao, J.C.; Wang, H.; Rashid, A.; Broaddus, R.R. Expression of estrogen-induced genes and estrogen receptor beta in pancreatic neuroendocrine tumors: Implications for targeted therapy. Pancreas 2014, 43, 996–1002. [Google Scholar] [CrossRef]
- Manoranjan, B.; Salehi, F.; Scheithauer, B.W.; Rotondo, F.; Kovacs, K.; Cusimano, M.D. Estrogen receptors α and β immunohistochemical expression: Clinicopathological correlations in pituitary adenomas. Anticancer Res. 2010, 30, 2897–2904. [Google Scholar] [PubMed]
- Pereira-Lima, J.F.S.; Marroni, C.P.; Pizarro, C.B.; Barbosa Coutinho, L.M.; Ferreira, N.P.; Oliveira, M.C. Immunohistochemical detection of estrogen receptor alpha in pituitary adenomas and its correlation with cellular replication. Neuroendocrinology 2004, 79, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Burdman, J.A.; Paunl, M.; Heredia Sereno, G.M.; Bordon, A.E. Estrogen receptors in human pituitary tumors. Horm. Metab. Res. 2008, 40, 521–527. [Google Scholar] [CrossRef]
- Scheithauer, B.; Kovacs, K.; Zorludemir, S.; Lloyd, R.V.; Erdogan, S.; Slezak, J. Immunoexpression of androgen receptor in the nontumorous pituitary and in adenomas. Endocr. Pathol. 2008, 19, 27–33. [Google Scholar] [CrossRef]
- Ambrogio, A.G.; De Martin, M.; Ascoli, P.; Cavagnini, F.; Pecori Giraldi, F. Gender-dependent changes in haematological parameters in patients with Cushing’s disease before and after remission. Eur. J. Endocrinol. 2014, 170, 393–400. [Google Scholar] [CrossRef]
- Pecori Giraldi, F.; Toja, P.M.; Michailidis, G.; Metinidou, A.; De Martin, M.; Scacchi, M.; Stramba-Badiale, M.; Cavagnini, F. High prevalence of prolonged QT interval duration in male patients with Cushing’s disease. Exp. Clin. Endocrinol. Diabetes 2011, 119, 221–224. [Google Scholar] [CrossRef]
- Shamim, W.; Yousufuddin, M.; Bakhai, A.; Coats, A.J.S.; Honour, J.W. Gender differences in the urinary excretion rates of cortisol and androgen metabolites. Ann. Clin. Biochem. 2000, 37, 770–774. [Google Scholar] [CrossRef]
- Pecori Giraldi, F.; Ambrogio, A.G. Variability in laboratory parameters used for management of Cushing’s syndrome. Endocrine 2015, 50, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, P.M.; Jones, A.F.; Ratcliffe, A.; Holder, G.; White, A.; Holder, R.; Ratcliffe, J.G.; London, D.R. Patterns of ACTH and cortisol pulsatility over twenty-four hours in normal males and females. Clin. Endocrinol. 1990, 32, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Jehle, S.; Walsh, J.E.; Freda, P.U.; Post, K.D. Selective use of bilateral inferior petrosal sinus sampling in patients with adrenocorticotropin-dependent Cushing’s syndrome prior to transsphenoidal surgery. J. Clin. Endocrinol. Metab. 2008, 93, 4624–4632. [Google Scholar] [CrossRef] [PubMed]
- Hammer, G.D.; Tyrrell, J.B.; Lamborn, K.R.; Applebury, C.B.; Hannegan, E.T.; Bell, S.; Rahl, R.; Lu, A.; Wilson, C.B. Transsphenoidal microsurgery for Cushing’s disease: Initial outcome and long-term results. J. Clin. Endocrinol. Metab. 2004, 89, 6348–6357. [Google Scholar] [CrossRef] [PubMed]
- Storr, H.L.; Isidori, A.M.; Monson, J.P.; Besser, G.M.; Grossman, A.B.; Savage, M.O. Prepubertal Cushing’s disease is more common in males, but there is no increase in severity at diagnosis. J. Clin. Endocrinol. Metab. 2004, 89, 3818–3820. [Google Scholar] [CrossRef] [PubMed]
- Libuit, L.G.; Karageorgiadis, A.S.; Sinaii, N.; Nguyen May, N.M.; Keil, M.F.; Lodish, M.B.; Stratakis, C.A. A gender-dependent analysis of Cushing’s disease in childhood: Pre- and postoperative follow-up. Clin. Endocrinol. 2015, 83, 72–77. [Google Scholar] [CrossRef]
- Bujko, M.; Kober, P.; Boresowicz, J.; Rusetska, N.; Paziewska, A.; Dabrowska, M.; Piascik, A.; Pekul, M.; Zielinski, G.; Kunicki, J.; et al. USP8 mutations in corticotroph adenomas determine a distinct gene expression profile irrespective of functional tumour status. Eur. J. Endocrinol. 2019, 181, 615–627. [Google Scholar] [CrossRef]
- Neou, M.; Villa, C.; Armignacco, R.; Jouinot, A.; Raffin-Sanson, M.L.; Septier, A.; Letourneur, F.; Diry, S.; Diedisheim, M.; Izac, B.; et al. Pangenomic classification of pituitary neuroendocrine tumors. Cancer Cell 2020, 37, 123–134. [Google Scholar] [CrossRef]
- Weigand, I.; Knobloch, L.; Flitsch, J.; Saeger, W.; Monoranu, C.M.; Höfner, K.; Herterich, S.; Rotermund, R.; Ronchi, C.L.; Buchfelder, M.; et al. Impact of USP8 gene mutations on protein deregulation in Cushing disease. J. Clin. Endocrinol. Metab. 2019, 104, 2535–2546. [Google Scholar] [CrossRef]
- Nieman, L.K.; Biller, B.M.K.; Findling, J.W.; Newell-Price, J.; Savage, M.O.; Stewart, P.M.; Montori, V.M. Diagnosis of Cushing’s syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2008, 93, 1526–1540. [Google Scholar] [CrossRef]
- Nieman, L.K.; Biller, B.M.; Findling, J.W.; Murad, M.H.; Newell-Price, J.; Savage, M.O.; Tabarin, A. Treatment of Cushing’s syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2015, 100, 2807–2831. [Google Scholar] [CrossRef] [PubMed]
- Cassarino, M.F.; Sesta, A.; Pagliardini, L.; Losa, M.; Lasio, G.; Cavagnini, F.; Pecori Giraldi, F. Proopiomelanocortin, glucocorticoid, and CRH receptor expression in human ACTH-secreting pituitary adenomas. Endocrine 2017, 55, 853–860. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Occhi, G.; Regazzo, D.; Albiger, N.M.; Ceccato, F.; Ferasin, S.; Scanarini, M.; Denaro, L.; Cosma, C.; Plebani, M.; Cassarino, M.F.; et al. Activation of the dopamine receptor type-2 (DRD2) promoter by 9-cis retinoic acid in a cellular model of Cushing’s disease mediates the inhibition of cell proliferation and ACTH secretion without a complete corticotroph-to-melanotroph transdifferentiation. Endocrinology 2014, 155, 3538–3549. [Google Scholar] [CrossRef] [PubMed]
- Hah, N.; Kraus, W.L. Hormone-regulated transcriptomes: Lessons learned from estrogen signaling pathways in breast cancer cells. Mol. Cell. Endocrinol. 2014, 382, 652–664. [Google Scholar] [CrossRef]
- Dorak, M.T.; Karpuzoglu, E. Gender differences in cancer susceptibility: An inadequately addessed issue. Front. Genet. 2012, 3, 268. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, L.; Chen, H.; Wang, Y.; Xu, Y.; Mao, H.; Li, J.; Mills, G.B.; Shu, Y.; Li, L.; et al. Comprehensive characterization of molecular differences in cancer between male and female patients. Cancer Cell 2016, 29, 711–722. [Google Scholar] [CrossRef]
- Staedtler, F.; Hartmann, N.; Letzkus, M.; Bongiovanni, S.; Scherer, A.; Marc, P.; Johnson, K.J.; Schumacher, M.M. Robust and tissue-independent gender-specific transcript biomarkers. Biomarkers 2013, 18, 436–445. [Google Scholar] [CrossRef]
- Nowak, M.; Markowska, A.; Nussdorfer, G.G.; Tortorella, C.; Malendowicz, L.K. Evidence that endogenous vasoactive intestinal peptide (VIP) is involved in the regulation of rat pituitary-adrenocortical function: In Vivo studies with a VIP antagonist. Neuropeptides 1994, 27, 297–303. [Google Scholar] [CrossRef]
- Ma, J.; Malladi, S.; Beck, A.H. Systematic analysis of sex-linked molecular alterations and therapies in cancer. Sci. Rep. 2016, 6, 19119. [Google Scholar] [CrossRef]
- Colaco, S.; Modi, D. Genetics of the human Y chromosome and its association with male infertility. Reprod. Biol. Endocrinol. 2018, 16, 14–38. [Google Scholar] [CrossRef]
- Fan, H.; Dong, G.; Zhao, G.; Liu, F.; Yao, G.; Zhu, Y.; Hou, Y. Gender differences of B cell signature in healthy subjects underlie disparities in incidence and course of SLE related to estrogen. J. Immunol. Res. 2014, 2014, 814598. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, M.D.; Espinosa, A.B.; Maillo, A.; Rebelo, O.; Vera, J.F.; Sayagues, J.M.; Merino, M.; Diaz, P.; Sousa, P.; Orfao, A. Patient gender is associated with distinct patterns of chromosomal abnormalities and sex chromosome-linked gene-expression profiles in meningiomas. Oncologist 2007, 12, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.S. SOX4: The unappreciated oncogene. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Schmit, K.; Michiels, C. TMEM proteins in cancer: A review. Front. Pharmacol. 2018, 9, 1345–1358. [Google Scholar] [CrossRef]
- Schmidt, C.; Sciacovelli, M.; Frezza, C. Fumarate hydratase in cancer: A multifaceted tumour suppressor. Semin. Cell Dev. Biol. 2019, 98, 15–25. [Google Scholar] [CrossRef]
- Song, G.; Xu, J.; He, L.; Sun, X.; Xiong, R.; Luo, Y.; Hu, X.; Zhang, R.; Yue, Q.; Liu, K.; et al. Systematic profiling identifies PDLIM2 as a novel prognostic predictor for oesophageal squamous cell carcinoma (ESCC). J. Cell. Mol. Med. 2019, 23, 5751–5761. [Google Scholar] [CrossRef] [PubMed]
- Hamann, M.; Grill, S.; Struck, J.; Bergmann, A.; Hartmann, O.; Polcher, M.; Kiechle, M. Detection of early breast cancer beyond mammographic screening: A promising biomarker panel. Biomark. Med. 2019, 13, 1107–1117. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Jou, Y.C.; Tsai, H.T.; Shiau, A.L.; Wu, C.L.; Tzai, T.S. Prothymosin-alpha enhances phosphatase and tensin homolog expression and binds with tripartite motif-containing protein 21 to regulate Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2 signaling in human bladder cancer. Cancer Sci. 2019, 110, 1208–1219. [Google Scholar] [CrossRef]
- Wierzbicka-Tutka, I.; Sokolowski, G.; Baldys-Waligorska, A.; Adamek, D.; Radwanska, E.; Golkowski, F. Prothymosin-alpha and Ki-67 expression in pituitary adenomas. Postepy Hig. Med. Dosw. 2016, 70, 1117–1123. [Google Scholar] [CrossRef]
- Quiroz, Y.; Lopez, M.; Mavropoulos, A.; Motte, P.; Martial, J.A.; Hammerschmidt, M.; Muller, M. The HMG-box transcription factor Sox4b is required for pituitary expression of gata2a and specification of thyrotrope and gonadotrope cells in zebrafish. Mol. Endocrinol. 2012, 26, 1014–1027. [Google Scholar] [CrossRef]
- Ma, Y.; Qi, X.; Du, J.; Song, S.; Feng, D.; Qi, J.; Zhu, Z.; Zhang, X.; Xiao, H.; Han, Z.; et al. Identification of candidate genes for human pituitary development by EST analysis. BMC Genom. 2009, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, Y.; Sheng, M.; Bu, D.; Huang, F.; Liu, X.; Zhou, C.; Dai, C.; Sun, B.; Zhu, J.; et al. Phenotype-genotype association analysis of ACTH-secreting pituitary adenoma and its molecular link to patient osteoporosis. Int. J. Mol. Sci. 2016, 17, 1654. [Google Scholar] [CrossRef] [PubMed]
- Ehrchen, J.; Heuer, H.; Sigmund, R.; Schafer, M.K.; Bauer, K. Expression and regulation of osteopontin and connective tissue growth factor transcripts in rat anterior pituitary. J. Endocrinol. 2001, 169, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Yagisawa, T.; Ito, F.; Osaka, Y.; Amano, H.; Kobayashi, C.; Toma, H. The influence of sex hormones on renal osteopontin expression and urinary constituents in experimental urolithiasis. J. Urol. 2001, 166, 1078–1082. [Google Scholar] [CrossRef]
- Latoche, J.D.; Ufelle, A.C.; Fazzi, F.; Ganguly, K.; Leikauf, G.D.; Fattman, C.L. Secreted phosphoprotein 1 and sex-specific differences in silica-induced pulmonary fibrosis in mice. Environ. Health Perspect. 2016, 124, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.; Godino-Gimeno, A.; Cerda-Reverter, J.M. Evolution of proopiomelanocortin. Vitam. Horm. 2019, 111, 1–16. [Google Scholar] [PubMed]
- Inturrisi, C.E.; Branch, A.D.; Robertson, H.D.; Howells, R.D.; Franklin, S.O.; Shapiro, J.R.; Calvano, S.E.; Yoburn, B.C. Glucocorticoid regulation of enkephalins in cultured rat adrenal medulla. Mol. Endocrinol. 1988, 2, 633–640. [Google Scholar] [CrossRef]
- Corchero, J.; Fuentes, J.A.; Manzanares, J. Gender differences in proenkephalin gene expression response to delta9-tetrahydrocannabinol in the hypothalamus of the rat. J. Psychopharmacol. 2002, 16, 283–289. [Google Scholar] [CrossRef]
- Watts, A.G. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: A complexity beyond negative feedback. Front. Neuroendocrinol. 2005, 26, 109–130. [Google Scholar] [CrossRef]
- Wu, X.; Wu, T.; Li, K.; Li, Y.; Hu, T.T.; Wang, W.F.; Qiang, S.J.; Xue, S.B.; Liu, W.W. The mechanism and influence of AKAP12 in different cancers. Biomed. Environ. Sci. 2018, 31, 927–932. [Google Scholar]
- Kondapalli, K.C.; Llongueras, J.P.; Capilla-Gonzalez, V.; Prasad, H.; Hack, A.; Smith, C.; Guerrero-Cazares, H.; Quinones-Hinojosa, A.; Rao, R. A leak pathway for luminal protons in endosomes drives oncogenic signalling in glioblastoma. Nat. Commun. 2015, 6, 6289. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, H.; Cooper, O.; Ben-Shlomo, A.; Mamelak, A.N.; Ren, S.G.; Bruyette, D.; Melmed, S. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J. Clin. Investig. 2011, 121, 4712–4721. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Liu, X.; Kameda, H.; Tone, Y.; Fukuoka, H.; Tone, M.; Melmed, S. EGFR induces E2F1-mediated corticotroph tumorigenesis. J. Endocr. Soc. 2017, 20, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity. Int. J. Oncol. 2017, 51, 1357–1369. [Google Scholar] [CrossRef]
- Dey, S.; Scullen, T.; Noguchi, C.T. Erythropoietin negatively regulates ACTH secretion. Brain Res. 2015, 1608, 14–20. [Google Scholar] [CrossRef]
- Ibanez-Costa, A.; Rivero-Cortes, E.; Vazquez-Borrego, M.C.; Gahete, M.D.; Jimenez-Reina, L.; Venegas-Moreno, E.; de la, R.A.; Arraez, M.A.; Gonzalez-Molero, I.; Schmid, H.A.; et al. Octreotide and pasireotide (dis) similarly inhibit pituitary tumor cells In Vitro. J. Endocrinol. 2016, 231, 135–145. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, C.; Pereira, A.M.; Feelders, R.A.; Romijn, J.A.; Roelfsema, F.; Sprij-Mooij, D.; van Aken, M.O.; van der Lely, A.J.; De Herder, W.W.; Lamberts, S.W.J.; et al. Coexpression of dopamine and somatostatin receptor subtypes in corticotroph adenomas. J. Clin. Endocrinol. Metab. 2009, 94, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Newell-Price, J.; Pivonello, R.; Tabarin, A.; Fleseriu, M.; Witek, P.; Gadelha, M.R.; Petersenn, S.; Tauchmanova, L.; Ravichandran, S.; Gupta, P.; et al. Use of late-night salivary cortisol to monitor response to medical treatment in Cushing’s disease. Eur. J. Endocrinol. 2019, 182, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Abel, J.L.; Rissman, E.F. Location, location, location: Genetic regulation of neural sex differences. Rev. Endocr. Metab. Disord. 2012, 13, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Reyes, R.; Martinez, S.; Gonzalez, M.; Tramu, G.; Bello, A.R. Origin of adenohypophysial lobes and cells from Rathke’s pouch in Swiss albino mice. Proliferation and expression of Pitx 2 and Calbindin D28K in corticotropic and somatotropic cell differentiation. Anat. Histol. Embryol. 2008, 37, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Amano, O.; Yamakuni, T.; Takahashi, Y.; Kondo, H. Localization of spot 35-calbindin (rat cerebellar calbindin) in the anterior pituitary of the rat: Developmental and sexual differences. Arch. Histol. Cytol. 1990, 53, 585–591. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lolodi, O.; Wang, Y.M.; Wright, W.C.; Chen, T. Differential regulation of CYP3A4 and CYP3A5 and its implication in drug discovery. Curr. Drug Metab. 2017, 18, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. World Health Organization: WHO Classification of Tumours of Endocrine Organs, 4th ed.; IARC Press: Lyon, France, 2017. [Google Scholar]
- Pecori Giraldi, F. Recent challenges in the diagnosis of Cushing’s syndrome. Horm. Res. 2009, 71, 123–127. [Google Scholar] [CrossRef]
- Invitti, C.; Pecori Giraldi, F.; Dubini, A.; Moroni, P.; Losa, M.; Piccoletti, R.; Cavagnini, F. Galanin is released by adrenocorticotropin-secreting pituitary adenomas in Vivo and in Vitro. J. Clin. Endocrinol. Metab. 1999, 84, 1351–1356. [Google Scholar] [CrossRef]
- Pecori Giraldi, F.; Marini, E.; Torchiana, E.; Mortini, P.; Dubini, A.; Cavagnini, F. Corticotrophin-releasing activity of desmopressin in Cushing’s disease. Lack of correlation between In Vivo and In Vitro responsiveness. J. Endocrinol. 2003, 177, 373–379. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pecori Giraldi, F.; Pagliardini, L.; Cassarino, M.F.; Losa, M.; Lasio, G.; Cavagnini, F. Responses to CRH and dexamethasone in a large series of human ACTH-secreting pituitary adenomas In Vitro reveal manifold corticotroph tumoural phenotypes. J. Neuroendocrinol. 2011, 23, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Cassarino, M.F.; Sesta, A.; Pagliardini, L.; Losa, M.; Lasio, G.; Cavagnini, F.; Pecori Giraldi, F. AZA-Deoxycytidine stimulates proopiomelanocortin gene expression and ACTH secretion in human pituitary ACTH-secreting tumors. Pituitary 2014, 17, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Li, W. Volcano plots in analyzing differential expressions with mRNA microarrays. J. Bioinform. Comput. Biol. 2012, 10, 121003. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Losa, M.; Mortini, P.; Pagnano, A.; Detomas, M.; Cassarino, M.F.; Pecori Giraldi, F. Clinical characteristics and surgical outcome in USP8-mutated human adrenocorticotropic hormone-secreting pituitary adenomas. Endocrine 2019, 63, 240–246. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Female Patients | Male Patients |
|---|---|---|
| ACTH 4 h baseline (ng/100,000 cells) | 8.26 ± 1.76 | 3.26 ± 1.69 * |
| ACTH 4 h DEX (ng/100,000 cells) | 7.18 ± 2.03 | 0.87 ± 0.39 * |
| ACTH 4 h CRH (ng/100,000 cells) | 30.98 ± 8.37 | 12.79 ± 6.63 * |
| ACTH 24 h baseline (ng/100,000 cells) | 25.35 ± 5.88 | 7.94 ± 3.61 * |
| ACTH 24 h DEX (ng/100,000 cells) | 16.69 ± 5.02 | 1.49 ± 0.44 * |
| ACTH 24 h CRH (ng/100,000 cells) | 63.49 ± 14.61 | 50.42 ± 29.76 |
| ACTH 4 h % change with CRH | 395.09 ± 71.11 | 323.59 ± 98.76 |
| ACTH 24 h % change with DEX | 116.18 ± 24.18 | 114.76 ± 18.59 |
| POMC baseline (relative to RPLP0) | 98.2 ± 29.41 | 74.69 ± 62.93 |
| POMC 24 h CRH (ratio vs. baseline) | 1.65 ± 0.16 | 3.44 ± 1.38 |
| POMC 24 h DEX (ratio vs. baseline) | 0.83 ± 0.08 | 0.67 ± 0.27 |
| Category | Term | Count | p Value |
|---|---|---|---|
| Annotation Cluster 1 | Enrichment Score: 2.5871443516464256 | ||
| UP_KEYWORDS | Transit peptide | 32 | 0.0001 |
| UP_SEQ_FEATURE | transit peptide:Mitochondrion | 30 | 0.0002 |
| GOTERM_CC_DIRECT | GO:0005743~mitochondrial inner membrane | 26 | 0.0021 |
| GOTERM_CC_DIRECT | GO:0005759~mitochondrial matrix | 20 | 0.0054 |
| UP_KEYWORDS | Mitochondrion | 45 | 0.0165 |
| GOTERM_CC_DIRECT | GO:0005739~mitochondrion | 51 | 0.0670 |
| Annotation Cluster 2 | Enrichment Score: 1.5413963866565796 | ||
| UP_KEYWORDS | Iron-sulfur | 7 | 0.0067 |
| UP_KEYWORDS | 4Fe-4S | 5 | 0.0196 |
| GOTERM_MF_DIRECT | GO:0051539~4 iron, 4 sulfur cluster binding | 5 | 0.0406 |
| UP_KEYWORDS | Iron | 14 | 0.1282 |
| Annotation Cluster 3 | Enrichment Score: 1.535426320231244 | ||
| UP_KEYWORDS | Iron-sulfur | 7 | 0.0067 |
| GOTERM_MF_DIRECT | GO:0051537~2 iron, 2 sulfur cluster binding | 4 | 0.0412 |
| UP_KEYWORDS | 2Fe-2S | 3 | 0.0899 |
| Annotation Cluster 4 | Enrichment Score: 1.384474121480598 | ||
| GOTERM_CC_DIRECT | GO:0005913~cell-cell adherens junction | 18 | 0.0202 |
| GOTERM_MF_DIRECT | GO:0098641~cadherin binding involved in cell-cell adhesion | 16 | 0.0374 |
| GOTERM_BP_DIRECT | GO:0098609~cell-cell adhesion | 14 | 0.0931 |
| Annotation Cluster 5 | Enrichment Score: 1.340383397277057 | ||
| GOTERM_BP_DIRECT | GO:0006362~transcription elongation from RNA polymerase I promoter | 5 | 0.0144 |
| GOTERM_BP_DIRECT | GO:0045815~positive regulation of gene expression, epigenetic | 6 | 0.0469 |
| GOTERM_BP_DIRECT | GO:0006363~termination of RNA polymerase I transcription | 4 | 0.0745 |
| GOTERM_BP_DIRECT | GO:0006361~transcription initiation from RNA polymerase I promoter | 4 | 0.0864 |
| Category | Term | Count | p Value |
|---|---|---|---|
| Annotation Cluster 1 | Enrichment Score: 4.211755780679299 | ||
| GOTERM_MF_DIRECT | GO:0098641~cadherin binding involved in cell-cell adhesion | 29 | 0.0000 |
| GOTERM_BP_DIRECT | GO:0098609~cell-cell adhesion | 27 | 0.0001 |
| GOTERM_CC_DIRECT | GO:0005913~cell-cell adherens junction | 29 | 0.0001 |
| Annotation Cluster 2 | Enrichment Score: 3.9643562287208445 | ||
| GOTERM_BP_DIRECT | GO:0000398~mRNA splicing, via spliceosome | 28 | 0.0000 |
| UP_KEYWORDS | mRNA splicing | 27 | 0.0000 |
| UP_KEYWORDS | Spliceosome | 17 | 0.0000 |
| UP_KEYWORDS | mRNA processing | 30 | 0.0000 |
| GOTERM_CC_DIRECT | GO:0071013~catalytic step 2 spliceosome | 14 | 0.0001 |
| GOTERM_BP_DIRECT | GO:0008380~RNA splicing | 18 | 0.0006 |
| GOTERM_CC_DIRECT | GO:0005681~spliceosomal complex | 11 | 0.0045 |
| KEGG_PATHWAY | hsa03040:Spliceosome | 11 | 0.0847 |
| Annotation Cluster 3 | Enrichment Score: 3.2395038273067827 | ||
| UP_KEYWORDS | Mitochondrion | 77 | 0.0000 |
| UP_SEQ_FEATURE | transit peptide: Mitochondrion | 34 | 0.0011 |
| UP_KEYWORDS | Transit peptide | 34 | 0.0034 |
| GOTERM_CC_DIRECT | GO:0005759~mitochondrial matrix | 19 | 0.1097 |
| Annotation Cluster 4 | Enrichment Score: 2.823640223068603 | ||
| UP_KEYWORDS | Ribonucleoprotein | 30 | 0.0000 |
| UP_KEYWORDS | Ribosomal protein | 20 | 0.0001 |
| GOTERM_BP_DIRECT | GO:0006412~translation | 24 | 0.0004 |
| GOTERM_MF_DIRECT | GO:0003735~structural constituent of ribosome | 22 | 0.0004 |
| GOTERM_CC_DIRECT | GO:0005840~ribosome | 18 | 0.0004 |
| GOTERM_BP_DIRECT | GO:0000184~nuclear-transcribed mRNA catabolic process, nonsense-mediated decay | 15 | 0.0004 |
| KEGG_PATHWAY | hsa03010:Ribosome | 16 | 0.0014 |
| GOTERM_BP_DIRECT | GO:0006614~SRP-dependent cotranslational protein targeting to membrane | 12 | 0.0018 |
| GOTERM_BP_DIRECT | GO:0019083~viral transcription | 12 | 0.0068 |
| GOTERM_BP_DIRECT | GO:0006364~rRNA processing | 18 | 0.0080 |
| GOTERM_BP_DIRECT | GO:0006413~translational initiation | 12 | 0.0275 |
| GOTERM_CC_DIRECT | GO:0022625~cytosolic large ribosomal subunit | 7 | 0.0557 |
| GOTERM_CC_DIRECT | GO:0022627~cytosolic small ribosomal subunit | 4 | 0.3048 |
| Annotation Cluster 5 | Enrichment Score: 2.56685352683656 | ||
| UP_KEYWORDS | Nucleotide-binding | 96 | 0.0003 |
| UP_KEYWORDS | ATP-binding | 77 | 0.0005 |
| UP_SEQ_FEATURE | nucleotide phosphate-binding region:ATP | 55 | 0.0064 |
| GOTERM_MF_DIRECT | GO:0005524~ATP binding | 82 | 0.0072 |
| UP_KEYWORDS | Kinase | 39 | 0.0257 |
| Annotation Cluster 6 | Enrichment Score: 1.9383888797605597 | ||
| INTERPRO | IPR000089:Biotin/lipoyl attachment | 4 | 0.0062 |
| INTERPRO | IPR011053:Single hybrid motif | 4 | 0.0082 |
| UP_KEYWORDS | Lipoyl | 3 | 0.0128 |
| INTERPRO | IPR003016:2-oxo acid dehydrogenase, lipoyl-binding site | 3 | 0.0147 |
| GOTERM_BP_DIRECT | GO:0046487~glyoxylate metabolic process | 5 | 0.0213 |
| Annotation Cluster 7 | Enrichment Score: 1.818526737361256 | ||
| GOTERM_MF_DIRECT | GO:0019888~protein phosphatase regulator activity | 7 | 0.0010 |
| GOTERM_MF_DIRECT | GO:0008601~protein phosphatase type 2A regulator activity | 6 | 0.0014 |
| GOTERM_MF_DIRECT | GO:0051721~protein phosphatase 2A binding | 5 | 0.0240 |
| GOTERM_CC_DIRECT | GO:0000159~protein phosphatase type 2A complex | 4 | 0.0445 |
| GOTERM_BP_DIRECT | GO:0050790~regulation of catalytic activity | 4 | 0.5201 |
| Annotation Cluster 8 | Enrichment Score: 1.8075356513460852 | ||
| KEGG_PATHWAY | hsa04728:Dopaminergic synapse | 16 | 0.0007 |
| KEGG_PATHWAY | hsa04261:Adrenergic signaling in cardiomyocytes | 12 | 0.0521 |
| KEGG_PATHWAY | hsa04071:Sphingolipid signaling pathway | 10 | 0.0998 |
| Annotation Cluster 9 | Enrichment Score: 1.4944394402931314 | ||
| INTERPRO | IPR025995:RNA binding activity-knot of a chromodomain | 3 | 0.0215 |
| INTERPRO | IPR016197:Chromo domain-like | 5 | 0.0374 |
| GOTERM_BP_DIRECT | GO:0016575~histone deacetylation | 6 | 0.0410 |
| Annotation Cluster 10 | Enrichment Score: 1.3698761413023455 | ||
| GOTERM_MF_DIRECT | GO:0044183~protein binding involved in protein folding | 4 | 0.0183 |
| INTERPRO | IPR027413:GroEL-like equatorial domain | 4 | 0.0201 |
| GOTERM_BP_DIRECT | GO:1904874~positive regulation of telomerase RNA localization to Cajal body | 4 | 0.0223 |
| INTERPRO | IPR027409:GroEL-like apical domain | 4 | 0.0241 |
| INTERPRO | IPR002423:Chaperonin Cpn60/TCP-1 | 4 | 0.0241 |
| GOTERM_BP_DIRECT | GO:1904871~positive regulation of protein localization to Cajal body | 3 | 0.0408 |
| INTERPRO | IPR002194:Chaperonin TCP-1, conserved site | 3 | 0.0476 |
| GOTERM_CC_DIRECT | GO:0005832~chaperonin-containing T-complex | 3 | 0.0483 |
| INTERPRO | IPR027410:TCP-1-like chaperonin intermediate domain | 3 | 0.0806 |
| INTERPRO | IPR017998:Chaperone tailless complex polypeptide 1 (TCP-1) | 3 | 0.0806 |
| GOTERM_MF_DIRECT | GO:0051082~unfolded protein binding | 9 | 0.0861 |
| GOTERM_BP_DIRECT | GO:0032212~positive regulation of telomere maintenance via telomerase | 4 | 0.1459 |
| Annotation Cluster 11 | Enrichment Score: 1.3267539609067143 | ||
| GOTERM_BP_DIRECT | GO:0042752~regulation of circadian rhythm | 7 | 0.0153 |
| UP_KEYWORDS | Biological rhythms | 11 | 0.0184 |
| GOTERM_BP_DIRECT | GO:0032922~circadian regulation of gene expression | 6 | 0.0870 |
| GOTERM_BP_DIRECT | GO:0043153~entrainment of circadian clock by photoperiod | 3 | 0.2007 |
| Annotation Cluster 12 | Enrichment Score: 1.3244168484975751 | ||
| UP_KEYWORDS | Glycogen metabolism | 5 | 0.0148 |
| UP_KEYWORDS | Carbohydrate metabolism | 8 | 0.0611 |
| GOTERM_BP_DIRECT | GO:0005977~glycogen metabolic process | 4 | 0.1174 |
| Annotation Cluster 13 | Enrichment Score: 1.322070667802913 | ||
| UP_SEQ_FEATURE | domain:Leucine-zipper | 11 | 0.0118 |
| SMART | SM00338:BRLZ | 6 | 0.0390 |
| INTERPRO | IPR004827:Basic-leucine zipper domain | 6 | 0.0636 |
| UP_SEQ_FEATURE | DNA-binding region:Basic motif | 10 | 0.1765 |
| Annotation Cluster 14 | Enrichment Score: 1.302497203152933 | ||
| UP_SEQ_FEATURE | repeat:WD 5 | 17 | 0.0212 |
| UP_SEQ_FEATURE | repeat:WD 7 | 12 | 0.0315 |
| INTERPRO | IPR020472:G-protein beta WD-40 repeat | 9 | 0.0324 |
| UP_SEQ_FEATURE | repeat:WD 4 | 17 | 0.0380 |
| SMART | SM00320:WD40 | 17 | 0.0384 |
| INTERPRO | IPR017986:WD40-repeat-containing domain | 20 | 0.0388 |
| UP_SEQ_FEATURE | repeat:WD 6 | 14 | 0.0398 |
| UP_SEQ_FEATURE | repeat:WD 3 | 17 | 0.0566 |
| UP_KEYWORDS | WD repeat | 17 | 0.0616 |
| UP_SEQ_FEATURE | repeat:WD 1 | 17 | 0.0714 |
| UP_SEQ_FEATURE | repeat:WD 2 | 17 | 0.0714 |
| INTERPRO | IPR001680:WD40 repeat | 17 | 0.0717 |
| INTERPRO | IPR015943:WD40/YVTN repeat-like-containing domain | 20 | 0.0725 |
| INTERPRO | IPR019775:WD40 repeat, conserved site | 11 | 0.1285 |
| Genes Up-Regulated in Adenomas from Male Patients | |||
| SYMBOL | DiffScore | Chr | DEFINITION |
| B3GALT1 | 45,498 | 2 | beta 1,3-galactosyltransferase 1 |
| C3 | 40,959 | 19 | complement component 3 |
| EIF1AY | 21,706 | Y | eukaryotic translation initiation factor 1A, Y-linked |
| FADS2 | 52,155 | 11 | fatty acid desaturase 2 |
| FAM174B | 19,587 | 15 | family with sequence similarity 174, member B |
| FGF5 | 47,607 | 4 | fibroblast growth factor 5 |
| FH | 15,527 | 1 | fumarate hydratase |
| HAPLN3 | 42,829 | 15 | hyaluronan and proteoglycan link protein 3 |
| ISG20 | 13,330 | 15 | interferon stimulated exonuclease gene 20 kDa |
| JAM2 | 41,419 | 21 | junctional adhesion molecule 2 |
| KDM5D | 83,236 | Y | lysine demethylase 5D (former JARID1D) |
| MAP4K2 | 14,458 | 11 | mitogen-activated protein kinase kinase kinase kinase 2 |
| MIR612 | 49,436 | microRNA 612 | |
| NETO2 | 42,006 | 16 | neuropilin (NRP) and tolloid (TLL)-like 2 |
| NLGN4Y | 43,635 | Y | neuroligin 4, Y-linked |
| NXPH2 | 51,966 | 2 | neurexophilin 2 |
| PDLIM2 | 41,894 | 8 | PDZ and LIM domain 2 (mystique) |
| PENK | 40,750 | 8 | proenkephalin |
| PTMA | 13,638 | 2 | prothymosin alpha |
| SKAP1 | 14,899 | 17 | src kinase associated phosphoprotein 1 |
| SOX4 | 51,205 | 6 | SRY (sex determining region Y)-box 4 |
| SPP1 | 43,227 | 4 | secreted phosphoprotein 1 (osteopontin) |
| THAP12 | 14,458 | 11 | THAP domain containing 12 (former PRKRIR) |
| TMEM97 | 15,527 | 17 | transmembrane protein 97 (former MAC30) |
| TMSB4Y | 50,840 | Y | thymosin beta 4, Y-linked |
| TTTY14 | 51,522 | Y | testis-specific transcript, Y-linked 14 |
| TXLNGY | 44,773 | Y | taxilin gamma pseudogene, Y-linked |
| USP9Y | 38,904 | Y | ubiquitin specific peptidase 9, Y-linked |
| WFS1 | 20,982 | 4 | Wolfram syndrome 1 (wolframin) |
| ZFY | 62,438 | Y | zinc finger protein, Y-linked |
| ZNF256 | 13,638 | 19 | zinc finger protein 256 |
| Genes up-regulated in adenomas from female patients | |||
| SYMBOL | DiffScore | Chr | DEFINITION |
| AKAP12 | −35,303 | 6 | A kinase anchor protein 12 (gravin) |
| ANKRD24 | −13,814 | 19 | ankyrin repeat domain 24 |
| ATAD2 | −20,693 | 8 | ATPase family, AAA domain containing 2 |
| B3GNT7 | −13,766 | 2 | eta-1,3-N-acetylglucosaminyltransferase 7 |
| C19orf18 | −19,587 | 19 | chromosome 19 open reading frame 18 |
| CALB1 | −39,948 | 8 | calbindin 1, 28 kDa |
| CALY | −42,171 | 10 | calcyon neuron specific vesicular protein (former DRD1IP) |
| COL4A3 | −13,394 | 2 | collagen, type IV, alpha 3 (Goodpasture antigen) |
| CYP3A5 | −19,323 | 7 | cytochrome P450, family 3, subfamily A, member 5 |
| DAPL1 | −19,323 | 2 | death associated protein-like 1 |
| DNALI1 | −38,678 | 1 | dynein, axonemal, light intermediate chain 1 |
| DNM1P46 | −20,693 | 15 | dynamin 1 pseudogene 46 |
| DPCD | −14,466 | 8 | deleted in mouse model of primary ciliary dyskinesia |
| DPF1 | −14,458 | 19 | double PHD fingers 1 |
| EPOR | −20,388 | 19 | erythropoietin receptor |
| FOXD4 | −14,149 | 9 | forkhead box D4 |
| FZD9 | −18,458 | 7 | frizzled class receptor 9 |
| KSR1 | −27,506 | 17 | kinase suppressor of ras 1 |
| NIM1K | −14,149 | 5 | NIM1 serine/threonine-protein kinase |
| PIGZ | −25,072 | 3 | phosphatidylinositol glycan anchor biosynthesis, class Z |
| RAB11FIP1 | −13,097 | 8 | RAB11 family interacting protein 1 |
| SLC9A9 | −22,712 | 3 | solute carrier family member 9 |
| SSTR5 | −15,488 | 16 | somatostatin receptor 5 |
| Parameter | Female Patients (n = 122) | Male Patients (n = 31) |
|---|---|---|
| age (years) | 38.9 ± 1.27 | 44.0 ± 2.89 * |
| microadenoma (% entire series) | 56.3% | 50% |
| invasiveness (% entire series) | 9.8% | 12.5% |
| surgical remission (% entire series) | 69.2% | 67.0% |
| recurrence (% remission series) | 4.1% | 3.2% |
| ACTH staining (% cells) | 87.2 ± 1.36 | 87.1 ± 2.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecori Giraldi, F.; Cassarino, M.F.; Sesta, A.; Terreni, M.; Lasio, G.; Losa, M. Sexual Dimorphism in Cellular and Molecular Features in Human ACTH-Secreting Pituitary Adenomas. Cancers 2020, 12, 669. https://doi.org/10.3390/cancers12030669
Pecori Giraldi F, Cassarino MF, Sesta A, Terreni M, Lasio G, Losa M. Sexual Dimorphism in Cellular and Molecular Features in Human ACTH-Secreting Pituitary Adenomas. Cancers. 2020; 12(3):669. https://doi.org/10.3390/cancers12030669
Chicago/Turabian StylePecori Giraldi, Francesca, Maria Francesca Cassarino, Antonella Sesta, Mariarosa Terreni, Giovanni Lasio, and Marco Losa. 2020. "Sexual Dimorphism in Cellular and Molecular Features in Human ACTH-Secreting Pituitary Adenomas" Cancers 12, no. 3: 669. https://doi.org/10.3390/cancers12030669
APA StylePecori Giraldi, F., Cassarino, M. F., Sesta, A., Terreni, M., Lasio, G., & Losa, M. (2020). Sexual Dimorphism in Cellular and Molecular Features in Human ACTH-Secreting Pituitary Adenomas. Cancers, 12(3), 669. https://doi.org/10.3390/cancers12030669





