WNK1 Kinase Stimulates Angiogenesis to Promote Tumor Growth and Metastasis
Abstract
1. Introduction
2. Results
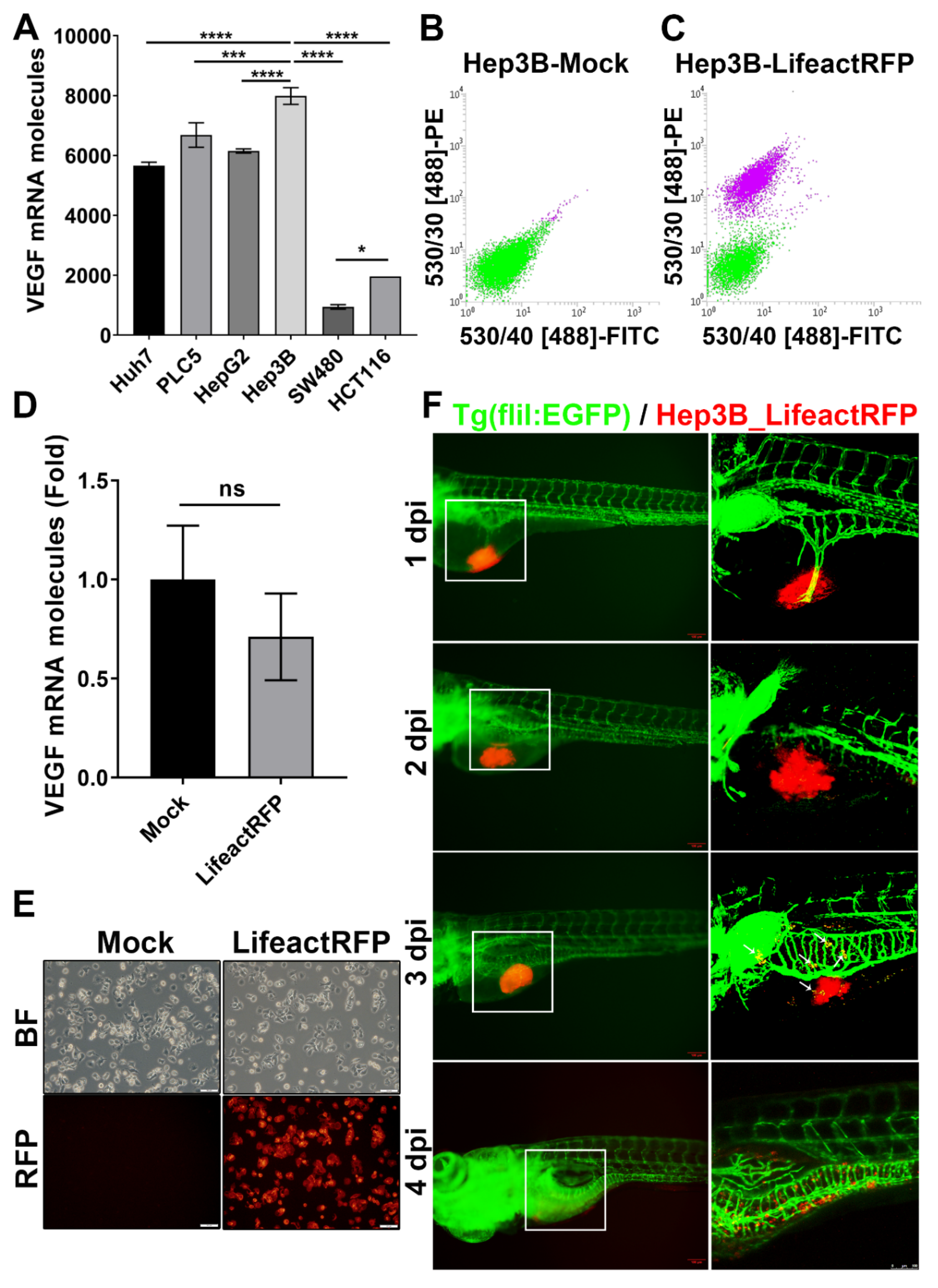
2.1. Identify a Hepatoma Cell Line (Hep3B) with High Angiogenic Activity and Establish Stable Hep3B_Lifeact-RFP Cells for Xenotransplantation Study
2.2. Xenotransplanted Hep3B_Lifeact-RFP Cells Induce Angiogenesis in Zebrafish Embryos
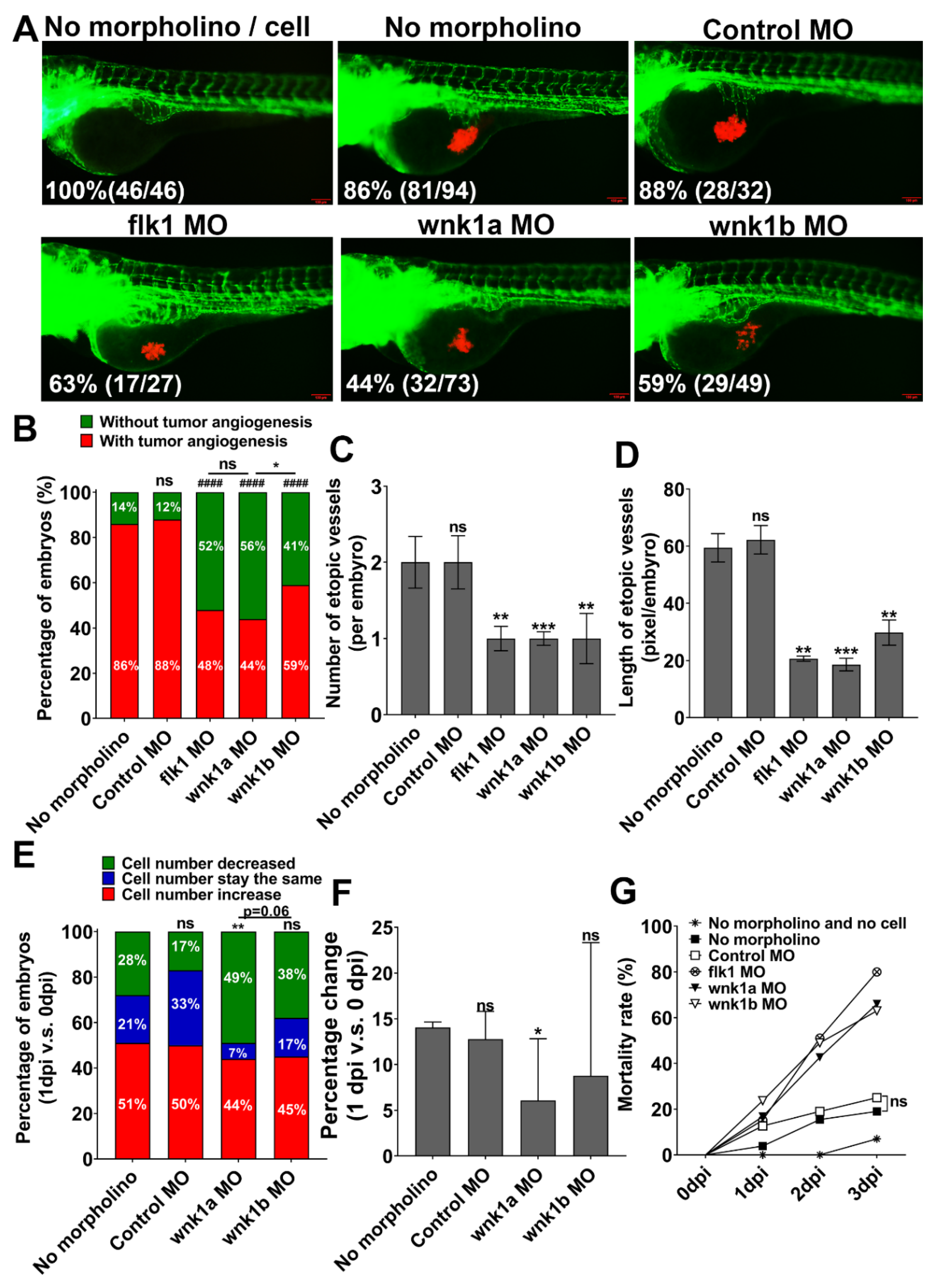
2.3. Knockdown wnk1 Reduces Tumor-Induced Angiogenesis and Prevents Tumor Cell Proliferation in Zebrafish Embryos
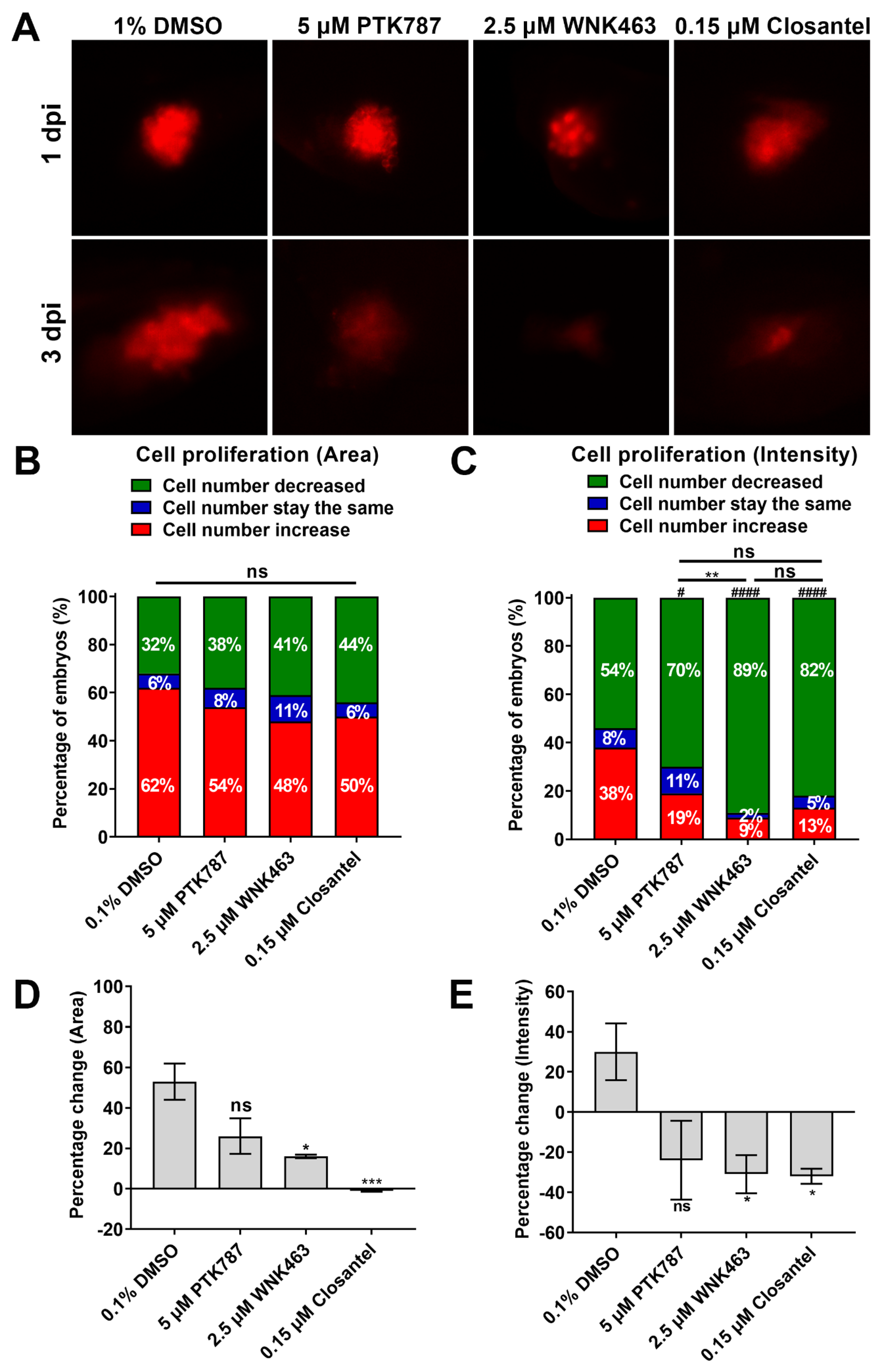
2.4. Inhibition of WNK Kinase Signaling Cascade by Small Molecule Inhibitors Is Anti-Angiogenic
2.5. WNK1 and OSR1/SPAK Inhibitors Exhibit Stronger Anti-Proliferation Activity in Xenotransplanted Tumor than VEGFR Inhibitor
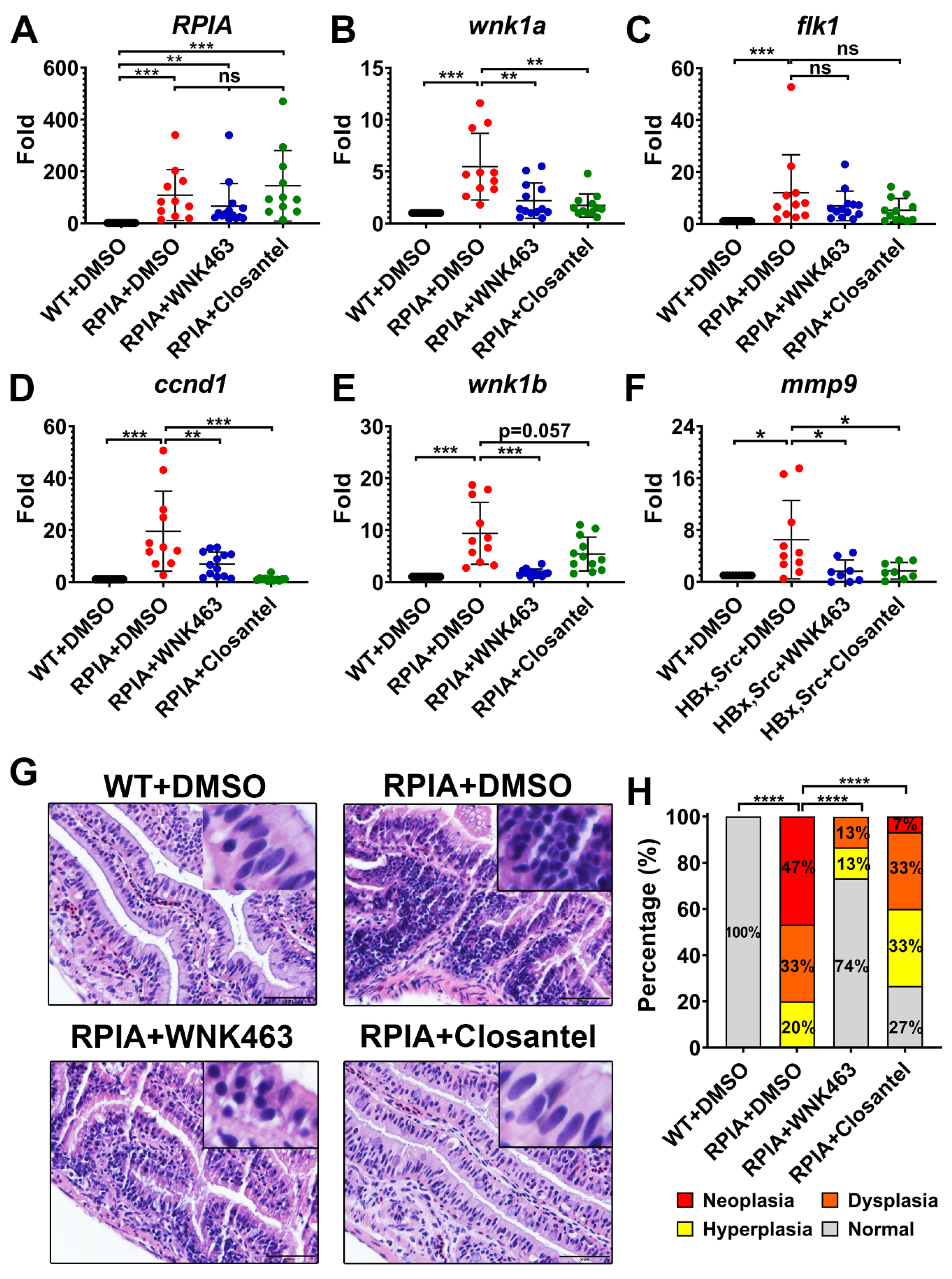
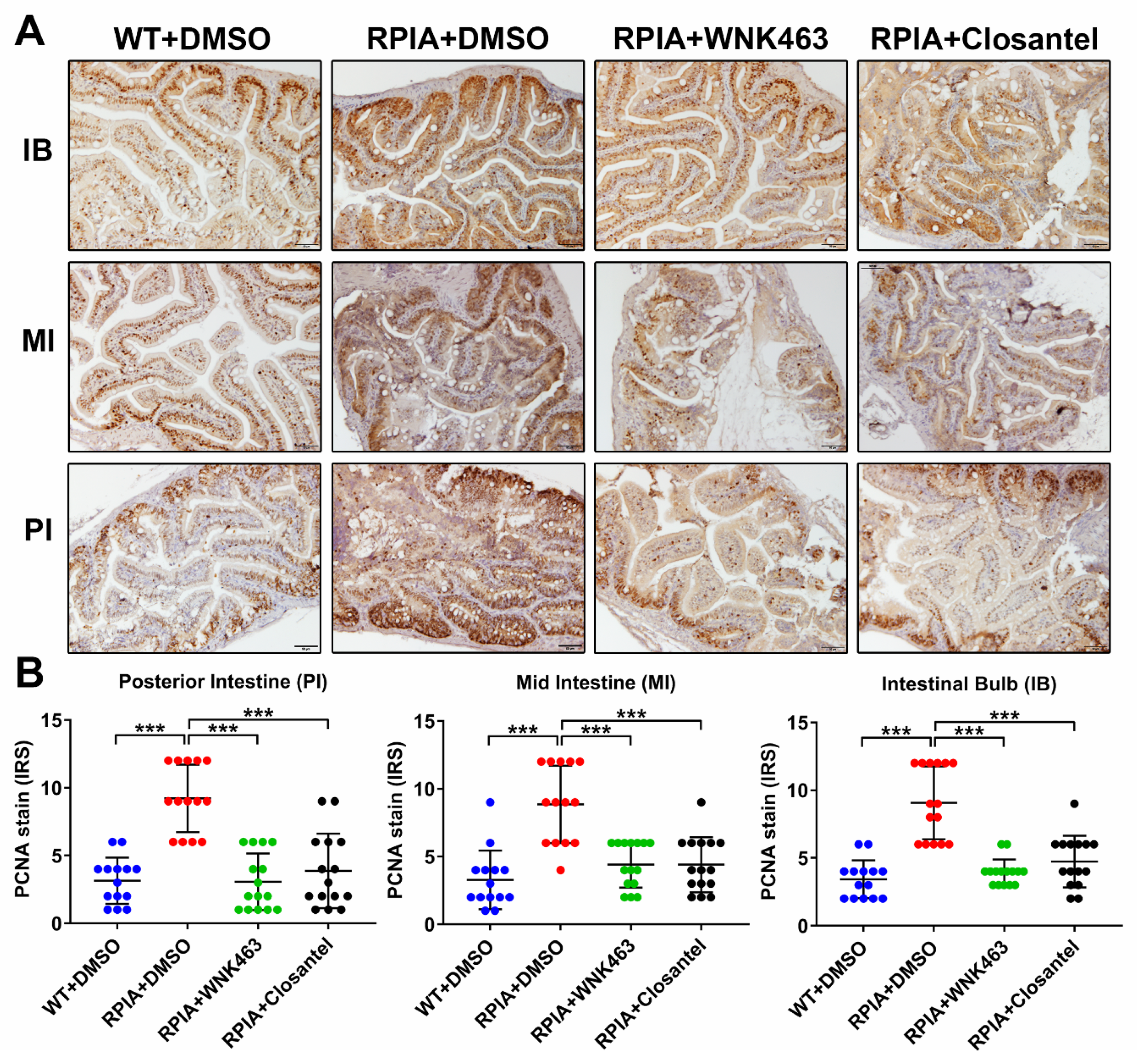
2.6. Oral Gavage of WNK463 and Closantel Reduce Tumorigenesis in Adult Zebrafish Colorectal Cancer Model
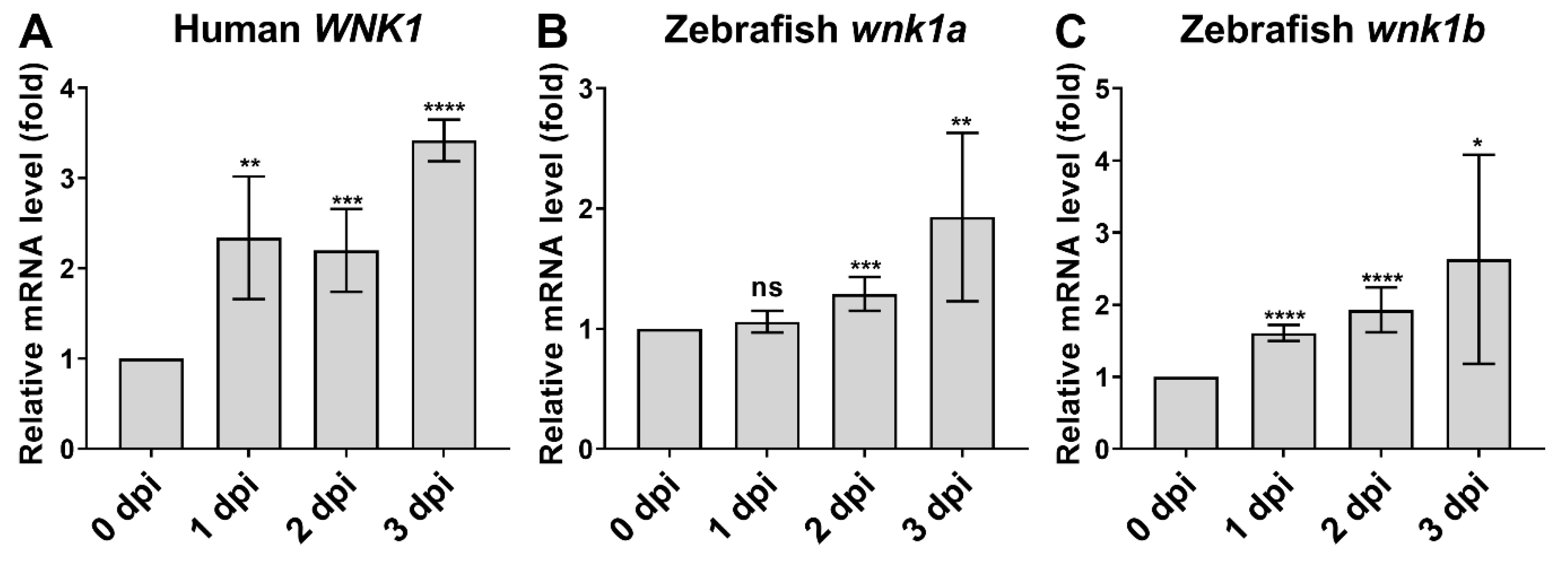
2.7. Oral Gavage of WNK1 Signaling Inhibitors Reduces Tumorigenesis in Adult Zebrafish Hepatocellular Carcinoma Model
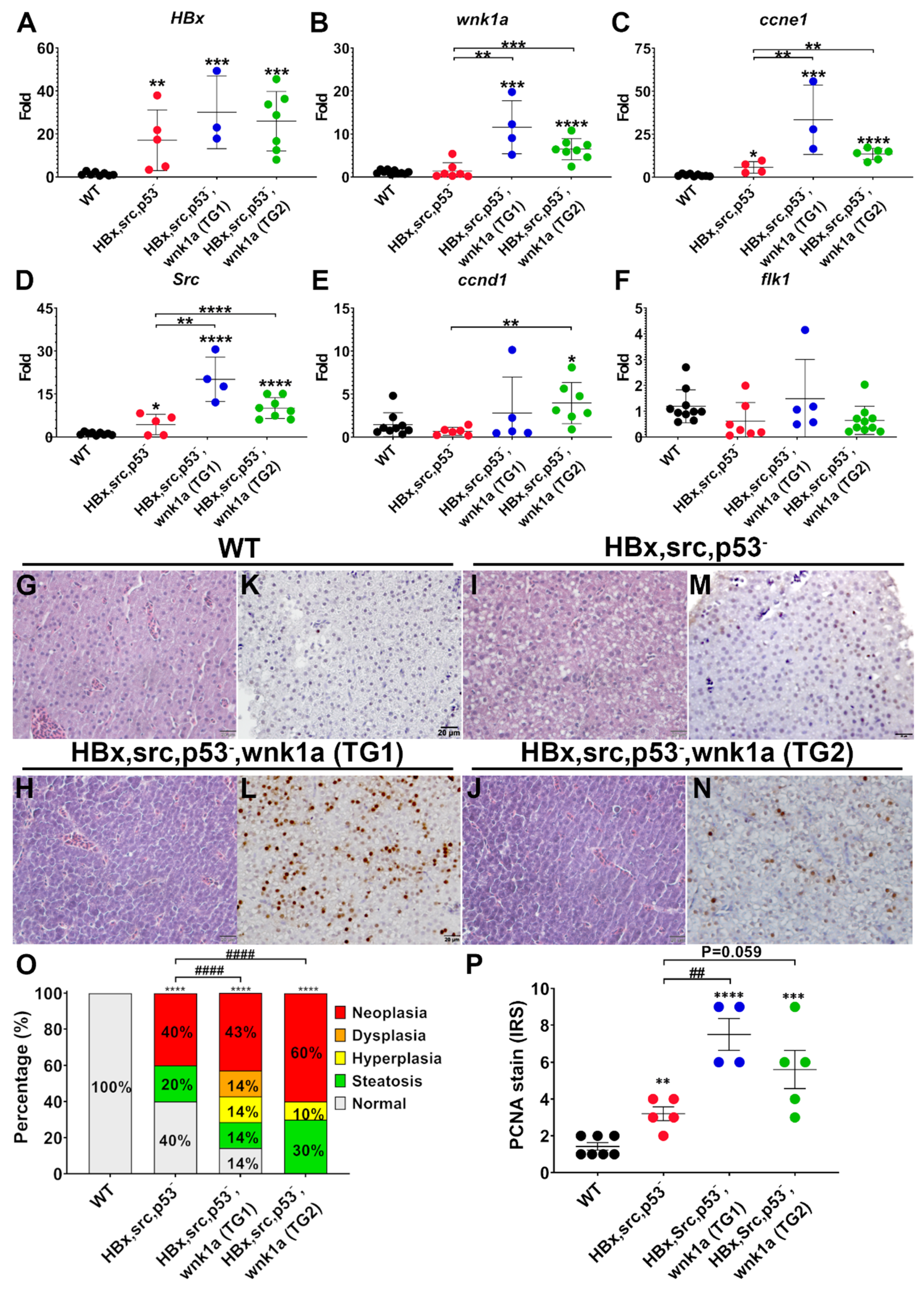
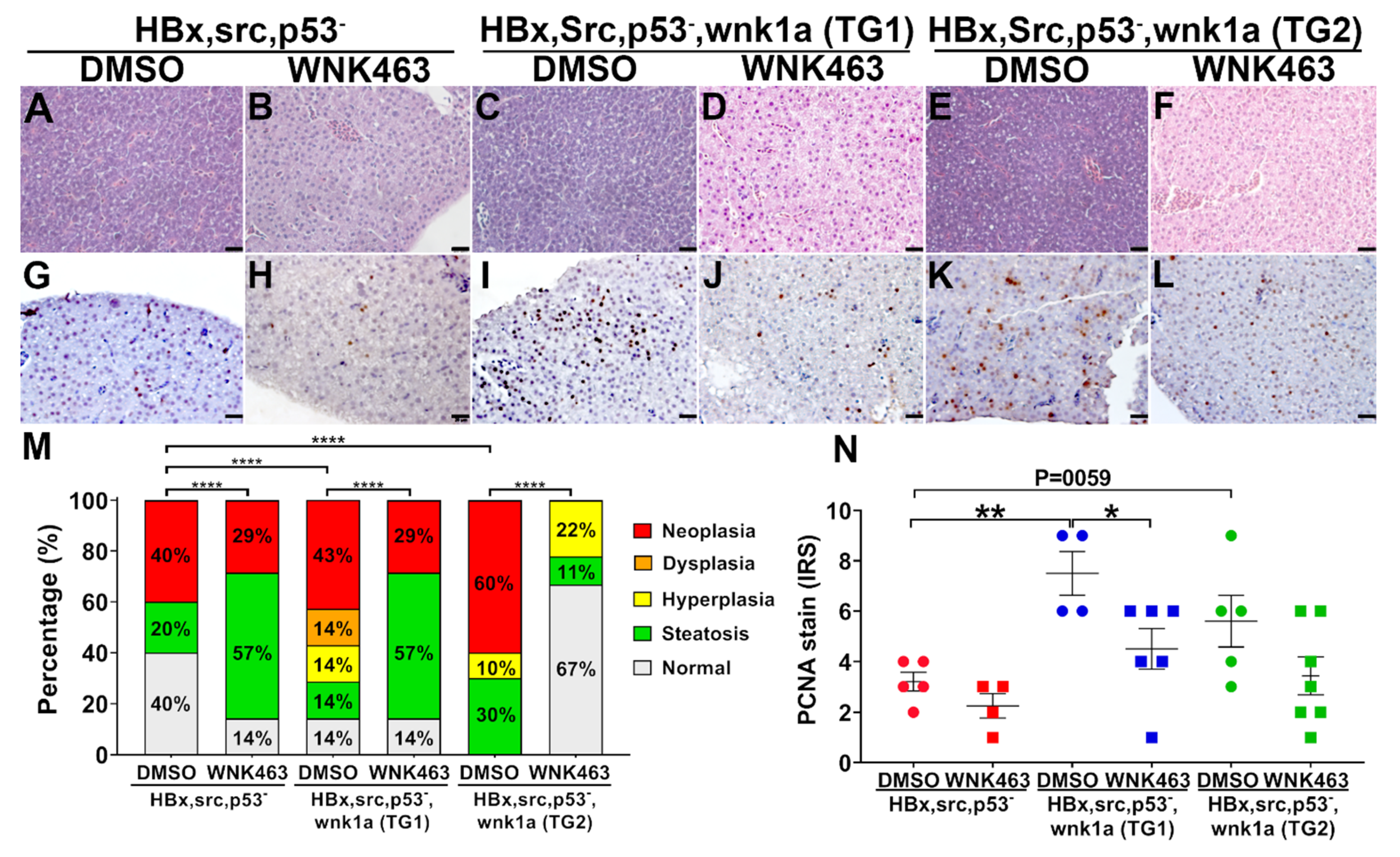
2.8. Endothelial-Specific Wnk1 Overexpression Enhances Hepatocarcinogenesis in HBx, src, (p53-) Transgenic Fish
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Zebrafish Maintenance and Transgenic Zebrafish Lines
4.3. Embryos Collection
4.4. Microinjection
4.5. Cell Culture
4.6. Establishment of Hep3B_Lifeact-RFP Stable Line
4.7. Morpholino Anti-Sense Oligonucleotide
4.8. Xenotransplantation of Hepatoma in Zebrafish
4.9. Resource of Compounds
4.10. Confocal Microscopy
4.11. Oral Gavage
4.12. Tissue preparation, Histological, and Immunochemical Analysis
4.13. RNA Isolation, Real Time Quantitative Polymerase Chain Reaction (QPCR)
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, B.E.; English, J.M.; Wilsbacher, J.L.; Stippec, S.; Goldsmith, E.J.; Cobb, M.H. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J. Biol. Chem. 2000, 275, 16795–16801. [Google Scholar] [CrossRef]
- Verissimo, F.; Jordan, P. WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene 2001, 20, 5562–5569. [Google Scholar] [CrossRef] [PubMed]
- Wilson, F.H.; Disse-Nicodeme, S.; Choate, K.A.; Ishikawa, K.; Nelson-Williams, C.; Desitter, I.; Gunel, M.; Milford, D.V.; Lipkin, G.W.; Achard, J.M.; et al. Human hypertension caused by mutations in WNK kinases. Science 2001, 293, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Vitari, A.C.; Deak, M.; Morrice, N.A.; Alessi, D.R. The WNK1 and WNK4 protein kinases that are mutated in Gordon’s hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem. J. 2005, 391, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Vitari, A.C.; Thastrup, J.; Rafiqi, F.H.; Deak, M.; Morrice, N.A.; Karlsson, H.K.; Alessi, D.R. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem. J. 2006, 397, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Choate, K.A.; Kahle, K.T.; Wilson, F.H.; Nelson-Williams, C.; Lifton, R.P. WNK1, a kinase mutated in inherited hypertension with hyperkalemia, localizes to diverse Cl- -transporting epithelia. Proc. Natl. Acad. Sci. USA 2003, 100, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Gamba, G. WNK lies upstream of kinases involved in regulation of ion transporters. Biochem. J. 2005, 391, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-L.; Zhu, X.; Wang, Z.; Subramanya, A.R.; Ellison, D.H. Mechanisms of WNK1 and WNK4 interaction in the regulation of thiazide-sensitive NaCl cotransport. J. Clin. Investig. 2005, 115, 1379–1387. [Google Scholar] [CrossRef]
- Dorwart, M.R.; Shcheynikov, N.; Wang, Y.; Stippec, S.; Muallem, S. SLC26A9 is a Cl(-) channel regulated by the WNK kinases. J. Physiol. 2007, 584, 333–345. [Google Scholar] [CrossRef]
- Wang, H.R.; Liu, Z.; Huang, C.L. Domains of WNK1 kinase in the regulation of ROMK1. American journal of physiology. Am. J. Physiol. Renal. Physiol. 2008, 295, F438–F445. [Google Scholar] [CrossRef]
- Gallolu Kankanamalage, S.; Karra, A.S.; Cobb, M.H. WNK pathways in cancer signaling networks. Cell Commun. Signal. 2018, 16, 72. [Google Scholar] [CrossRef] [PubMed]
- Mandai, S.; Mori, T.; Nomura, N.; Furusho, T.; Arai, Y.; Kikuchi, H.; Sasaki, E.; Sohara, E.; Rai, T.; Uchida, S. WNK1 regulates skeletal muscle cell hypertrophy by modulating the nuclear localization and transcriptional activity of FOXO4. Sci. Rep. 2018, 8, 9101. [Google Scholar] [CrossRef] [PubMed]
- Rodan, A.R.; Jenny, A. WNK Kinases in Development and Disease. Curr. Top. Dev. Biol. 2017, 123, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wu, T.; Xu, K.; Huang, I.K.; Cleaver, O.; Huang, C.L. Endothelial-specific expression of WNK1 kinase is essential for angiogenesis and heart development in mice. Am. J. Pathol. 2009, 175, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yoon, J.; Yang, S.S.; Lin, S.H.; Huang, C.L. WNK1 protein kinase regulates embryonic cardiovascular development through the OSR1 signaling cascade. J. Biol. Chem. 2013, 288, 8566–8574. [Google Scholar] [CrossRef]
- Lai, J.G.; Tsai, S.M.; Tu, H.C.; Chen, W.C.; Kou, F.J.; Lu, J.W.; Wang, H.D.; Huang, C.L.; Yuh, C.H. Zebrafish WNK lysine deficient protein kinase 1 (wnk1) affects angiogenesis associated with VEGF signaling. PLoS ONE 2014, 9, e106129. [Google Scholar] [CrossRef]
- Xu, B.E.; Stippec, S.; Lenertz, L.; Lee, B.H.; Zhang, W.; Lee, Y.K.; Cobb, M.H. WNK1 activates ERK5 by an MEKK2/3-dependent mechanism. J. Biol. Chem. 2004, 279, 7826–7831. [Google Scholar] [CrossRef]
- Serysheva, E.; Berhane, H.; Grumolato, L.; Demir, K.; Balmer, S.; Bodak, M.; Boutros, M.; Aaronson, S.; Mlodzik, M.; Jenny, A. Wnk kinases are positive regulators of canonical Wnt/beta-catenin signalling. EMBO Rep. 2013, 14, 718–725. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Kimmel, C.B. Genetics and early development of zebrafish. Trends Genet. 1989, 5, 283–288. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Park, H.M.; Rigel, D.F.; DiPetrillo, K.; Whalen, E.J.; Anisowicz, A.; Beil, M.; Berstler, J.; Brocklehurst, C.E.; Burdick, D.A.; et al. Small-molecule WNK inhibition regulates cardiovascular and renal function. Nat. Chem. Biol. 2016, 12, 896–898. [Google Scholar] [CrossRef] [PubMed]
- AlAmri, M.A.; Kadri, H.; Alderwick, L.J.; Simpkins, N.S.; Mehellou, Y. Rafoxanide and Closantel Inhibit SPAK and OSR1 Kinases by Binding to a Highly Conserved Allosteric Site on Their C-terminal Domains. Chem. Med. Chem. 2017, 12, 639–645. [Google Scholar] [CrossRef]
- Chou, Y.T.; Jiang, J.K.; Yang, M.H.; Lu, J.W.; Lin, H.K.; Wang, H.D.; Yuh, C.H. Identification of a noncanonical function for ribose-5-phosphate isomerase A promotes colorectal cancer formation by stabilizing and activating beta-catenin via a novel C-terminal domain. PLoS Biol. 2018, 16, e2003714. [Google Scholar] [CrossRef]
- Yang, W.Y.; Rao, P.S.; Luo, Y.C.; Lin, H.K.; Huang, S.H.; Yang, J.M.; Yuh, C.H. Omics-based Investigation of Diet-induced Obesity Synergized with HBx, Src, and p53 Mutation Accelerating Hepatocarcinogenesis in Zebrafish Model. Cancers (Basel) 2019, 11, 1899. [Google Scholar] [CrossRef]
- Moniz, S.; Jordan, P. Emerging roles for WNK kinases in cancer. Cell. Mol. Life Sci. 2010, 67, 1265–1276. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, K.H.; Eom, M.; Kim, M.; Park, E.Y.; Jeong, Y.; Park, K.S.; Cha, S.K. WNK1 promotes renal tumor progression by activating TRPC6-NFAT pathway. FASEB J. 2019, 33, 8588–8599. [Google Scholar] [CrossRef]
- Lu, J.W.; Yang, W.Y.; Tsai, S.M.; Lin, Y.M.; Chang, P.H.; Chen, J.R.; Wang, H.D.; Wu, J.L.; Jin, S.L.; Yuh, C.H. Liver-specific expressions of HBx and src in the p53 mutant trigger hepatocarcinogenesis in zebrafish. PLoS ONE 2013, 8, e76951. [Google Scholar] [CrossRef]
- Dbouk, H.A.; Weil, L.M.; Perera, G.K.; Dellinger, M.T.; Pearson, G.; Brekken, R.A.; Cobb, M.H. Actions of the protein kinase WNK1 on endothelial cells are differentially mediated by its substrate kinases OSR1 and SPAK. Proc. Natl. Acad. Sci. USA 2014, 111, 15999–16004. [Google Scholar] [CrossRef]
- Abdalla, A.M.E.; Xiao, L.; Ullah, M.W.; Yu, M.; Ouyang, C.; Yang, G. Current Challenges of Cancer Anti-angiogenic Therapy and the Promise of Nanotherapeutics. Theranostics 2018, 8, 533–548. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.A.; Ellison, D.H. The WNKs: Atypical protein kinases with pleiotropic actions. Physiol. Rev. 2011, 91, 177–219. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Gao, L.; Xia, T.; Gu, Y.; Yu, R.K. Expression of the mouse WNK1 gene in correlation with ganglioside GD3 and functional analysis of the mouse WNK1 promoter. Gene 2005, 344, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.J.; Yang, S.S.; Hua, K.F.; Tsai, Y.L.; Lin, S.H.; Ka, S.M. SPAK plays a pathogenic role in IgA nephropathy through the activation of NF-kappaB/MAPKs signaling pathway. Free Radic. Biol. Med. 2016, 99, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb. Perspect. Med. 2012, 2, a006502. [Google Scholar] [CrossRef] [PubMed]
- Masckauchan, T.N.; Shawber, C.J.; Funahashi, Y.; Li, C.M.; Kitajewski, J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis 2005, 8, 43–51. [Google Scholar] [CrossRef]
- Lin, H.S.; Huang, Y.L.; Wang, Y.S.; Hsiao, E.; Hsu, T.A.; Shiao, H.Y.; Jiaang, W.T.; Sampurna, B.P.; Lin, K.H.; Wu, M.S.; et al. Identification of Novel Anti-Liver Cancer Small Molecules with Better Therapeutic Index than Sorafenib via Zebrafish Drug Screening Platform. Cancers (Basel) 2019, 11, 739. [Google Scholar] [CrossRef]
- Muller, P.Y.; Milton, M.N. The determination and interpretation of the therapeutic index in drug development. Nat. Rev. Drug Discov. 2012, 11, 751–761. [Google Scholar] [CrossRef]

| Gene Name | Primer Name | Sequence (5′ to 3′) |
|---|---|---|
| VEGFA | Q-hVEGFA-F | GGGCAGAATCATCACGAAGT |
| Q-hVEGFA-R | TGGTGATGTTGGACTCCTCA | |
| WNK1 | Q-hWNK1-F | GAGCGTCATCTGTGACTCCA |
| Q-hWNK1-R | CGGTCTTTGCTGGTACTGCT | |
| EGFP Standard Curve | Q-EGFP-N1-F | ACGTAAACGGCCACAAGTTC |
| Q-EGFP-N1-R | AAGTCGTGCTGCTTCATGTG | |
| pEGFP-N1-F | CCTGATTCTGTGGATAACCGTAT | |
| pEGFP-N1-R | TGCATTCTAGTTGTGGTTTGTCC | |
| RPIA | Q-RPIA-F | CATGCTGTGCAGCGAATAGC |
| Q-RPIA-R | TGGCGGGCCTGGAAGGAAGT | |
| ccnd1 | Q-ccnd1-F | TTGCCTCTCATCCCAGAACCT |
| Q-ccnd1-R | CCTGACACGATCGCAGACAGT | |
| wnk1a | Q-wnk1a-F | TCGAGATAGGACGTGGCTCT |
| Q-wnk1a-R | TCAAGGATGATTCCCAGGAG | |
| wnk1b | Q-wnk1b-F | CCGGGTCAGCTGTGCCCAAG |
| Q-wnk1b-R | TGGCCCAGGGGTTGTGAGGT | |
| mmp9 | Q-mmp9-F | GGTGATTGACGACGCCTTTG |
| Q-mmp9-R | GAAATGGGCGTCTCCCTGAA | |
| flk-1 | Q-flk1-F | TCTTCACTCTTCACGTGCTTTTTAG |
| Q-flk1-R | GAAGGTGTGTATCTCCATCAGGAA | |
| hpse | Q-hpse-F | GCTCTGGTTTGGAGCTCATC |
| Q-hpse-R | GAAATCCCGACCAAGTTGAA | |
| chrebp1 | Q-chrebp-F | GGAGATGGACTCGCTCTTTG |
| Q-chrebp-R | GCAGAGGCTCAAAAGTGTCC | |
| actin | Q-actin-F | CTCCATCATGAAGTGCGACGT |
| Q-actin-R | CAGACGGAGTATTTGCGCTCA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sie, Z.-L.; Li, R.-Y.; Sampurna, B.P.; Hsu, P.-J.; Liu, S.-C.; Wang, H.-D.; Huang, C.-L.; Yuh, C.-H. WNK1 Kinase Stimulates Angiogenesis to Promote Tumor Growth and Metastasis. Cancers 2020, 12, 575. https://doi.org/10.3390/cancers12030575
Sie Z-L, Li R-Y, Sampurna BP, Hsu P-J, Liu S-C, Wang H-D, Huang C-L, Yuh C-H. WNK1 Kinase Stimulates Angiogenesis to Promote Tumor Growth and Metastasis. Cancers. 2020; 12(3):575. https://doi.org/10.3390/cancers12030575
Chicago/Turabian StyleSie, Zong-Lin, Ruei-Yang Li, Bonifasius Putera Sampurna, Po-Jui Hsu, Shu-Chen Liu, Horng-Dar Wang, Chou-Long Huang, and Chiou-Hwa Yuh. 2020. "WNK1 Kinase Stimulates Angiogenesis to Promote Tumor Growth and Metastasis" Cancers 12, no. 3: 575. https://doi.org/10.3390/cancers12030575
APA StyleSie, Z.-L., Li, R.-Y., Sampurna, B. P., Hsu, P.-J., Liu, S.-C., Wang, H.-D., Huang, C.-L., & Yuh, C.-H. (2020). WNK1 Kinase Stimulates Angiogenesis to Promote Tumor Growth and Metastasis. Cancers, 12(3), 575. https://doi.org/10.3390/cancers12030575





