Abstract
Deregulated cellular apoptosis is a hallmark of cancer and chemotherapy resistance. The B-cell lymphoma 2 (BCL-2) protein family members are sentinel molecules that regulate the mitochondrial apoptosis machinery and arbitrate cell fate through a delicate balance between pro- and anti-apoptotic factors. The recognition of the anti-apoptotic BCL2 gene as an oncogenic driver in hematological malignancies has directed attention toward unraveling the biological significance of each of the BCL-2 superfamily members in cancer progression and garnered interest in the targeting of apoptosis in cancer therapy. Accordingly, the approval of venetoclax (ABT-199), a small molecule BCL-2 inhibitor, in patients with chronic lymphocytic leukemia and acute myeloid leukemia has become the proverbial torchbearer for novel candidate drug approaches selectively targeting the BCL-2 superfamily. Despite the inspiring advances in this field, much remains to be learned regarding the optimal therapeutic context for BCL-2 targeting. Functional assays, such as through BH3 profiling, may facilitate prediction of treatment response, development of drug resistance and shed light on rational combinations of BCL-2 inhibitors with other branches of cancer therapy. This review summarizes the pathological roles of the BCL-2 family members in cancer, discusses the current landscape of their targeting in clinical practice, and highlights the potential for future therapeutic inroads in this important area.
1. Introduction—Apoptosis from the Chemotherapy Lens
Chemotherapy resistance in cancer has been attributed to multiple mechanisms, which often act in concert [1,2]. This repertoire includes altering drug transport through influx/efflux pumps such as ATP-binding cassette transporters [3] and P-glycoprotein overexpression [1]. Intracellularly, well-described downstream mechanisms include activation of key signaling pathways, drug-target alteration [4] and repair of drug-induced DNA damage [2]. Extrinsic to the cancer cell, cross-talk between tumor cells with the tumor microenvironment adds to chemo-resistance [5]. Tumor heterogeneity and the existence of cancer stem cells, may further limit treatment response.
Increasingly, dysregulation of drug-induced autophagy and apoptosis has been recognized as a key mechanism of carcinogenesis and chemotherapy resistance, whereby the surviving cancer cell continues to accumulate oncogenic mutations which further propagate tumor progression [6]. Targeting apoptosis therefore holds promise in overcoming resistance to cancer therapy. Recently, venetoclax (ABT-199) has successfully achieved USA Federal Drug Administration (FDA) approval for the treatment of patients with chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML), confirming that apoptosis-targeting strategies have finally come of age. In this article, we discuss the roles of the BCL-2 superfamily in carcinogenesis and treatment resistance, and review the successes and failures of strategies targeting the BCL-2 family members in cancer therapy.
2. The BCL-2 Superfamily and Its Role in Apoptosis
2.1. The BCL-2 Superfamily Controls the Intrinsic Apoptosis Pathway
Apoptosis is effected via the intrinsic and extrinsic pathways. The extrinsic, or death-receptor mediated pathway, is initiated when cell death receptors such as Fas, TNFR1, TRAIL-R1, TRAIL-R2, DR3 and DR6, interact with their ligands on the cell surface. Activation of Fas, TRAIL-R1 or TRAIL-R2 leads to the formation of a “death-inducing signaling complex” (DISC) and triggers a cascade of caspase activation culminating in apoptosis, while the activation of other receptors induces apoptosis by triggering different signaling pathways such as NF-κB [7,8]. Detailed discussion of the extrinsic pathway and its targeting is beyond the scope of this review. The intrinsic, or mitochondrial pathway, responds to intracellular apoptotic stimuli such as viral infection, oxidative stress, calcium flux and DNA damage caused by drug or radiation exposure [9,10]. When committed to apoptosis, mitochondria outer membrane permeabilization (MOMP) is the decisive event through which cytochrome c and the second mitochondria-derived activator of caspase (SMAC) are released into the cytoplasm, triggering apoptosome assembly and caspase 9 activation [7]. Downstream executioner caspases 3, 6 and 7 cause cellular dismantlement and cytoskeletal protein degradation, which lead to the classic morphological features of crenation, DNA condensation and, ultimately, cell death [11].
The BCL-2 family members are central regulatory players in the intrinsic mitochondrial apoptotic program, and their interplay controls cell fate [12] (Figure 1). More than 25 BCL-2 family members have been identified. Advances in structural resolution of these members have categorized them into three subfamilies—(1) the multidomain anti-apoptotic members (BCL-2, BCL-xL, MCL-1, BCL-w, BCL-B/Boo and BFL-1/A1), (2) the multidomain pro-apoptotic members (BAX, BAK), and (3) the BH3-only members (BAD, BID, NOXA, HRK, BMF, PUMA, BIM). Multi-domain members in (1) and (2) have four BCL-2 homology (BH) domains (BH1, BH2, BH3 and BH4) each, while the BH3-only members are comprised of only a single short BH3 domain [13,14,15]. The BH3 motif is composed of 9 to 15 amino acids and is uniquely conserved across all BCL-2 family members [16]. BH3 interactions are responsible for orchestrating the BCL-2 interactome via a BH3-into-hydrophobic groove mechanism [17,18], which allows the formation of homo- and heterodimers that control apoptotic function [19]. Minor alterations in the amino acid sequences of the binding grooves and BH3 domains control the specificity of these interactions.
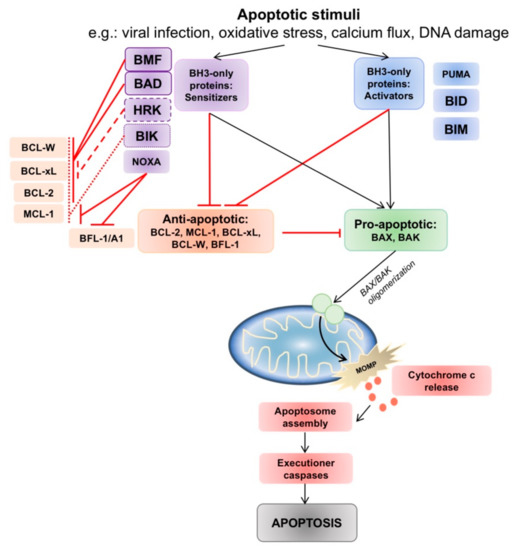
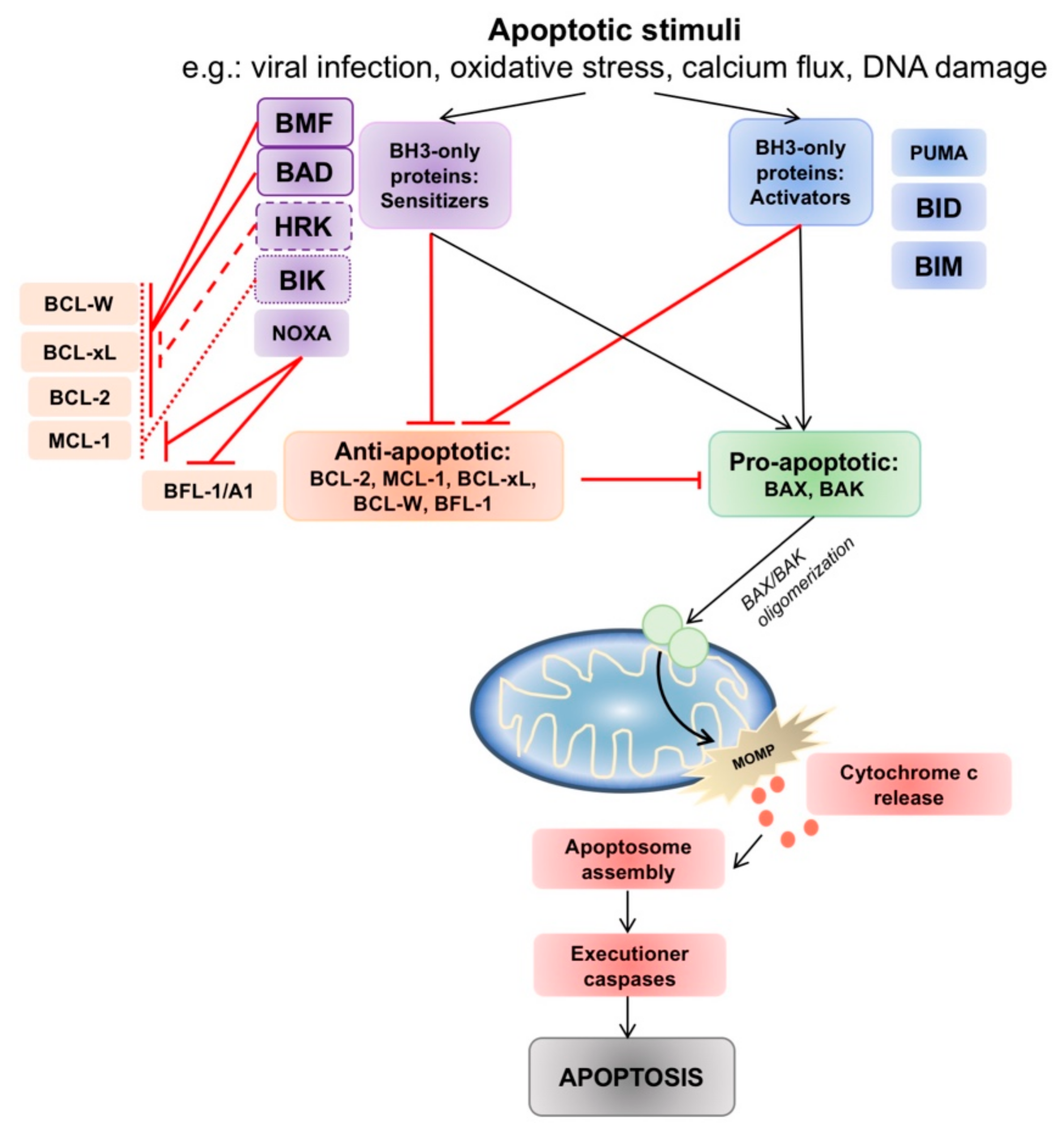
Figure 1.
The intrinsic apoptotic pathway and interactions between pro- and anti-apoptotic B-cell lymphoma 2 (BCL-2) family members. Intrinsic pathway apoptotic stimuli such as viral infection, oxidative stress, calcium flux and DNA damage lead to changes in the balance of pro- and anti-apoptotic BCL-2 family members. The anti-apoptotic proteins act to prevent BAX/BAK activation. Activator BH3-only proteins (PUMA, tBID, BIM) inhibit all anti-apoptotic members, whereas sensitizer BH3-only proteins interact and engage selective anti-apoptotic members, allowing BAX/BAK oligomerization and indirect activation. Oligomerization of BAX/BAK in the mitochondrial membrane commits the cell to mitochondria outer membrane permeabilization (MOMP), and triggers a downstream caspase cascade which ends in apoptosis.
The balance between pro- and anti-apoptotic family members determines if intrinsic apoptosis will proceed. When pro-apoptotic BAX or BAK are liberated, they are able to oligodimerize in the outer mitochondrial membrane, leading to the formation of a mitochondrial membrane pore which commits the cell to MOMP. The BH3-only proteins have complex roles as death sentinels that link apoptotic signals to the intrinsic pathway, and are divided between two roles– either as “direct activators” (tBID, BIM, PUMA) of BAX and BAK, exposing the BH3 domain of BAX and BAK to facilitate oligodimerization [20]; or as “inactivators” or “sensitizers” (BAD, BIK, BMF, HRK, NOXA) by binding with anti-apoptotic BCL-2 thus allowing BAX/BAK to be unrestrained to trigger MOMP [21] (Figure 1).
2.2. Regulation of BCL-2 Family Members
The importance of apoptosis in homeostasis requires that it is tightly regulated. The canonical roles of the BCL-2 subfamilies suggest that apoptosis may be triggered through inactivation of the anti-apoptotic multidomain subfamily proteins, or an increase in concentration of BH3-only proteins. Yet the BCL-2 puzzle has proven far more complex and often unpredictable, contributed by pleiotropic effects of multiple signaling controls, as well as post-transcriptional and post-translational modification processes, in modifying the affinity between BCL-2 family members [16]. For example, PI3K-Akt activation leads to phosphorylation and inactivation of BAD [22], leaving BCL-2 free to inhibit apoptosis, while increasing the expression of anti-apoptotic genes [11]. Similarly, activation of the extracellular signal regulated kinase (ERK) pathway results in increased transcription of the anti-apoptotic subfamily of BCL-2 members, and increased ubiquitination and subsequent degradation of pro-apoptotic members, leading to cell survival [23]. ERK-mediated phosphorylation of MCL-1 at T163 was further shown to stabilize MCL-1, leading to suppression of apoptosis in various hematological malignancy cell lines [24].
In the nucleus, genomic alterations such as chromosomal translocations and gene amplifications may increase BCL-2 levels. A notable example would be in CLL, where deletion of chromosome 13q in >50% of patients leads to silencing of the microRNAs miR-15 and miR-16, which are responsible for degrading BCL-2 RNA, resulting in BCL-2 overexpression [25]. Post-translational modifications moderate protein functions through ubiquitination, proteolysis, phosphorylation and proteasomal degradation [26]. Phosphorylation of BCL-2 at S70 [27] has been described to alter its anti-apoptotic ability [28] and confer resistance to taxane chemotherapy [29]. Specific BIM phosphorylation sites have the ability to affect its BCL-2 binding capability, resulting in an anti-apoptotic phenotype, while mutations at other phosphorylation sites (Ser-55, -65 and -73) tag BIM for proteasomal degradation, increasing therapy resistance [30]. Phosphorylation of BAX at specific residues (S184), mediated by Akt activation, has been suggested to switch BAX from pro- to anti-apoptotic in phenotype, by allowing it to sequester activator BH3 proteins [18].
2.3. Dysregulation of BCL-2 Family Members in Carcinogenesis and Treatment Resistance
Dysregulation of the anti-apoptotic members BCL-2, BCL-xL and MCL-1 have been widely described in carcinogenesis, cancer progression and chemotherapy resistance [31]. Cancer cells often upregulate anti-apoptotic BCL-2 proteins, thus tilting the ratio of anti- versus pro-apoptotic members to fall in favor of apoptosis evasion, even in the presence of stimuli from chemotherapeutic agents [6]. CLL is considered one of the classical hematological malignancies attributable to failure of apoptosis. Nearly all CLL patients have increased BCL-2 expression. Repression of BCL-2 at the post-transcriptional level allowed for the induction of apoptosis in CLL cell lines [25]. BCL-2 overexpression is a key event in follicular lymphoma (FL), driven by pathological chromosomal t(14; 18) translocation, whereby the BCL2 oncogene is pathogenically translocated to the immunoglobulin heavy chain (IGHV) gene locus, leading to its amplification. In diffuse large B-cell lymphoma (DLBCL), concomitant overexpression of BCL-2 and MYC is classified as a “double-hit” DLBCL, which is associated with a dismal prognosis, high risk for relapse, resistance to standard chemotherapy and justifies upfront escalation to more intensive treatment. These observations have fueled strategies therapeutically targeting the anti-apoptotic BCL-2 members in cancer treatment.
An interesting and somewhat non-canonical aspect of the functional biology of BCL-2 is ability to maintain a mild mitochondrial pro-oxidant milieu while preventing deleterious levels of reactive oxygen species (ROS) production triggered by oxidative stressors through the regulation of cytochrome c oxidase activity [32]. This mechanism appears to be the result of an interaction between BCL-2 and the subunit COX Va that shifts the ratio of COX Va to COX Vb subunits, thus modulating cytochrome c oxidase activity. The modulation of ROS production by BCL-2 expression is a critical component of its anti-apoptotic activity as cells subjected to oxidative stress inducers modulate their mitochondrial redox metabolism to buffer the excess ROS production, thereby promoting cell survival [33]. In addition, the pro-oxidant milieu generated through superoxide anion production by an increased expression of BCL-2 was shown to be linked to an interaction between BCL-2 and the small GTPase Rac1, a critical regulator of NADPH oxidase, responsible for superoxide production [34]. Interestingly, a mild to moderate increase in intracellular superoxide anion (pro-oxidant state) has also been shown to impact the phosphorylation status of BCL-2, specifically at S70 via the generation of peroxynitrite (a reaction product of superoxide and nitric oxide). This involves peroxynitrite mediated nitrative modification of the regulatory subunit B56δ of the protein phosphatase 2A (PP2A), which prevents holoenzyme assembly and results in the sustained S70 phosphorylation of BCL-2 to stabilize its anti-apoptotic activity [35]. These findings provide evidence for an intricate crosstalk between BCL-2 and cellular redox metabolism, thereby delineating a novel facet in the biology of this death regulatory protein with potential therapeutic implications.
MCL1 is one of the most highly amplified genes in human cancers [36]. In hematological malignancies, increased levels of MCL-1 have been described in multiple myeloma (MM) [37], DLBCL [38], AML, chronic myeloid leukemia (CML) and mantle cell lymphoma (MCL). Many chemotherapeutic agents affect apoptosis through the reduction of MCL-1 levels. In CLL cell lines, up-regulation of MCL-1 after co-culture with stroma was linked to fludarabine resistance [39]. Conversely, knock-down of MCL1 in mice models not only triggered apoptosis of transformed AML cells but also salvaged AML-afflicted mice from disease progression [40].
Finally, elevated BCL-xL expression has also been observed in MM [41] and non-Hodgkin’s lymphoma (NHL), and is implicated in their progression. In one study, transgenic mice with overexpression of BCL-xL readily developed lymphomas [42]. This is further supported by studies showing that interactions between pro-apoptotic BCL-xL and anti-apoptotic BIM control the apoptosis rate in MYC-related lymphoma [43].
Conversely, the loss of pro-apoptotic proteins appears to be relatively uncommon. Somatic inactivation of BAX (and BAK) has been reported in both solid and hematological cancers [44]. Deletion or silencing of NOXA, PUMA or BIM expression has been described in the pathogenesis of hematologic cancers and their response to chemotherapy [45,46]. Of note, BIM is deleted in 17% of MCL [47], while BAX mutations occur in 20% of hematologic cancers such as CLL, FL, MCL and NHL. In mouse fibroblast models, loss of both BAX and BAK led to resistance to chemotherapy-induced apoptosis [48]. Additionally, loss of BAX in colon cancer cells led to 5-fluorouracil resistance [49].
Indeed, the complex roles of the BCL-2 family members have created immense potential for targeting. Progressive and stepwise improvements in our mechanistic understanding of apoptosis have since allowed for the identification of entry points into this network, toward the promise of optimal therapeutic targeting in cancer. In the next section, we discuss the historical advancements in BCL-2 family targeting that have led to the success of venetoclax in modern day hematological malignancy treatment, and delve into upcoming novel strategies.
3. Targeting the BCL-2 Superfamily: A Summary of the Current Landscape
3.1. Antisense Oligonucleotides (ASO)
ASOs were the first approaches employed for BCL-2 inhibition. These are complementary strands that hybridize with and silence anti-apoptotic BCL-2 subfamily mRNA, leading to hydrolysis of the mRNA and promoting apoptosis [50,51]. Oblimersen is an 18-antisense oligonucleotide complementary to the first six codons of BCL-2 mRNA that was evaluated in a variety of hematological malignancies. Promising response rates were seen when combined with standard chemo-immunotherapy [52,53], and also allowed lower doses of chemotherapy to be administered. Reduced BCL-2 mRNA and protein levels were noted in AML patients who achieved a complete response (CR) with oblimersen, providing proof-of-principle of its mechanism of action [53]. Common toxicities included fever, fatigue, gastrointestinal side effects and night sweats. However, on several phase III studies, no survival advantage could be shown for oblimersen addition [52]. Despite this, several patients treated with oblimersen on study appeared to derive durable benefit from this drug [52]. Other ASOs under evaluation include SPC2996, PNT2258 and bispecific ASOs targeting BCL-2/BCL-xL.
3.2. BH3-Mimetics
The recognition of BH3-only proteins as natural inhibitors of BCL-2 proteins led to the development of BH3-mimetics. These small molecules are homologous to the BH3 domains of anti-apoptotic BH3-only proteins, and bind competitively to the hydrophobic groove of anti-apoptotic proteins, displacing BAX/BAK or pro-apoptotic BH3-only molecules, inducing apoptosis. Venetoclax (ABT-199, Abbvie Inc, North Chicago, IL, USA), which targets BCL-2, was the front-runner inhibitor in developmental pipelines for BH3-mimetics, and its FDA approval across four indications represents a major milestone in this field. To date, BH3-mimetics specifically inhibiting BCL-2, MCL-1 and BCL-xL, respectively are undergoing evaluation.
3.2.1. Gossypol and AT-101
Gossypol acts as a pan-BCL-2 family inhibitor [54], and has both BAX/BAK-dependent [55] and -independent [56] mechanisms of action. In preclinical studies, gossypol demonstrated promising activity through activation of the intrinsic apoptotic pathway in CML [57], NHL [58] and MM [59]. In-vivo studies in mouse models [60] showed significant slowing of tumor growth when gossypol was combined with CHOP chemotherapy, compared to either CHOP or gossypol alone [60]. However, gossypol has significant off-target side effects including dose-limiting thrombocytopenia, preventing it from advancing into clinical trials. The more potent, and orally-available enantiomer of gossypol, AT-101 progressed further in its development. However, despite promising preclinical data, early phase studies of AT-101 in combination with docetaxel for prostate cancer or non-small cell lung cancer (NSCLC) did not show improved outcomes [61,62] (Table 1). In CLL, AT-101 with rituximab showed only modest efficacy [63]. Gastrointestinal toxicities (such as nausea, vomiting and ileus), fatigue and neutropenia were the most common side effects noted [63]. Newer analogues of AT-101 include TW37 and TM-1206, which have improved affinity to BCL-2, MCL-1 and BCL-xL.

Table 1.
Early-generation BH3-mimetics inhibiting BCL-2. R/R: relapsed/refractory; CLL: chronic lymphocytic leukemia; HL: hodgkin’s lymphoma; MDS: myelodysplastic syndrome; ORR: objective response rate; FL: follicular lymphoma; FR: fludarabine plus rituximab; iwCLL: international workshop on CLL; MCL: mantle cell lymphoma; CR: complete response; PR: partial response; PFS: progression-free survival; RP2D: recommended phase 2 dose; ALL: acute lymphoblastic leukemia; SCLC: small cell lung cancer; NSCLC: non-small cell lung cancer; OS: overall survival.
3.2.2. Obatoclax
Obatoclax (GX15-070; Teva Pharmaceutical Industries Ltd, Parsippany, NJ, USA) is a relatively weak polypyrrole pan-BCL-2 family inhibitor that is able to bind to anti-apoptotic BCL-2, BCL-xL, BCL-w, BCL-B, BFL-1/A1 and also MCL-1 with sub-micromolar affinity, allowing BAK/BAX oligomerization and cell death [64]. Obatoclax is also purported to have BCL-2 independent mechanisms, via its effect on the Akt/mTOR signaling pathway [65], which increases the possibility of off-target toxicity. In various hematological malignancy cell lines, and in-vivo mouse models, obatoclax monotherapy showed anti-cancer activity [65,66]. However, raising obatoclax serum levels to clinically effective concentrations in mice models was associated with severe neurotoxicity. Accordingly, when obatoclax was tested in phase I/II trials for AML, CLL, acute lymphoblastic leukemia (ALL), myelodysplastic syndrome, MCL and classical Hodgkin lymphoma (HL) [67,68,69,70], only limited clinical activity was observed (Table 1). Common adverse events included mood disturbances and gastrointestinal side effects, while high grade toxicities appeared to be mainly hematological [68]. Development of obatoclax has been discontinued.
3.2.3. ABT-737
ABT-737 (Abbvie Inc, North Chicago, IL, USA) was the “first-in-class” small molecule inhibitor designed as a BH3 mimetic of BAD. ABT-737 was shown to bind with a much higher affinity (sub-nanomolar concentrations) and more selectively, compared to obatoclax, to BCL-2, BCL-xL and BCL-w [71]. Activity of ABT-737 was shown in MM, and AML cell lines (Table 1). Notably, in CML cell lines, ABT-737 plus imatinib reduced the development of BCL-2 driven imatinib-resistance [72]. The specific binding of ABT-737 to its intended targets resulted in an increase in MCL-1 expression and phosphorylation thus bypassing the effect of ABT-737 and leading to ABT-737-resistance in AML cells [73]. Compounding this, the unfavorable pharmacokinetic profile of ABT-737 further spurred the development of newer generations of BH3 mimetics.
3.2.4. Navitoclax
Navitoclax (ABT-263; Abbvie Inc, North Chicago, IL, USA) is a second-generation, orally bioavailable BH3-mimetic. Navitoclax binds preferentially to BCL-2, BCL-xL and BCL-w with nanomolar affinity, specifically disrupting BCL-2 and BCL-xL interactions with pro-death BH3 members. However, navitoclax still lacks the ability to antagonize MCL-1 and BFL-1/A1 dependent interactions. In-vivo xenograft models of small cell lung cancer (SCLC), ALL, NHL, MCL and MM showed promising tumor regression [74]. In a phase I study of 29 patients with relapsed or refractory CLL, navitoclax as a single-agent showed an overall response rate (ORR) of 35% in patients receiving a daily dose of at least 110mg, although no CR was observed [75]. Despite this, durable responses >12 months occurred, even in patients with the poor prognostic marker, deletion 17p. Furthermore, a higher ratio of BIM to MCL-1 and BIM to BCL-2 correlated with improved efficacy of navitoclax [75]. As expected, patients with lower pre-treatment MCL-1 levels had improved response to navitoclax. Prominent thrombocytopenia occurred early after treatment-initiation, this was often dose-limiting [75], and consistent with previous data showing that the homeostasis of mature platelets is dependent on BCL-xL. Currently, navitoclax is increasingly under investigation in solid tumors due to the inherent risks of severe thrombocytopenia in patients with hematological malignancies who are already myelosuppressed (Table 1).
3.2.5. Venetoclax: A Selective BCL-2 Inhibitor
Hydrogen bonds between venetoclax and Asp103 on BCL-2 result in the increased selectivity of venetoclax for BCL-2 compared to previous compounds [85]. Venetoclax is an orally-available, extremely potent and selective BCL-2 only inhibitor, and is platelet-sparing [85]. Due to its improved therapeutic window, this drug emerged as the front-runner BH3-mimetic, particularly in malignancies which are BCL-2 dependent.
As described earlier, the central role of the BCL2 super family in CLL has made this disease a key substrate for studying and developing BCL-2-targeted therapy. In a phase I dose-escalation study of 116 relapsed/refractory CLL and NHL patients treated with venetoclax, ORR of 79% and CR rate of 20% was seen in patients with CLL. This was particularly impressive as the target-population had included heavily-pretreated CLL patients and 90% of patients harbored at least 2 poor prognostic markers, such as chromosome 17p-deletion, 11q deletion, fludarabine-resistance, bulky lymphadenopathy and lack of mutation in IGHV. This potent and rapid cell kill was further confirmed by the unexpectedly high rate of tumor lysis (TLS) in 18% of patients leading to fatalities. Amending the dose schedule to feature a risk-mitigating ramp-up dose, together with monitoring and adequate TLS prophylaxis, helped to prevent this feared side effect. Despite rampant expression of BCL-2 in healthy tissues, other adverse events (AEs) were manageable, such as diarrhea, nausea and neutropenia. Treatment with venetoclax in the dose-escalation cohort resulted in an estimated 2-year overall survival (OS) rate of 84% [86] (Table 2).

Table 2.
Key venetoclax trials including upcoming novel combinations. MRD: minimal residual disease; TLS: tumor-lysis syndrome; CRi: complete remission with incomplete marrow recovery; SLL: small lymphocytic lymphoma; AML: acute myeloid leukemia; IDH2: isocitrate dehydrogenase 2; NHL: non-hodgkin’s lymphoma; DLBCL: diffuse large B-cell lymphoma; WM: waldenstrom macroglobulinemia; MZL: marginal zone lymphoma; MM: multiple myeloma; FCR: fludarabine/cyclophosphamide/rituximab; BR: bendamustine/rituximab.
These impressive results led to a pivotal phase II study of 107 patients with relapsed/refractory deletion-17p CLL treated with venetoclax. ORR with venetoclax was 79%, including CR 8%, and responses were seen regardless of the presence of poor-prognostic markers [86] (Table 2). Specifically in CLL patients treated with venetoclax after progressing on the B cell receptor inhibitors (BCRis) ibrutinib or idelalisib, the phase II M14-032 study reported ORR 67% and time-to-response of 2.5 months. Even in a small exploratory subgroup of 28 patients who had previously received more than 1 previous BCRi, encouraging activity was noted [87] (Table 2).
Venetoclax therapy is made even more convincing by its ability to result in unprecedented phenomenon of undetectable minimal residual disease (uMRD), which is defined when there is <1 CLL cell per 10,000 lymphocytes in marrow or peripheral blood. Low or uMRD has been shown to correlate with improvements in OS [88]. In a pooled analysis of 2 phase II studies of relapsed/refractory CLL patients treated with venetoclax, the PFS rate was 92.8% in patients achieving uMRD at 24 months on treatment [89,90]. The first approval for venetoclax in patients with CLL came in 2016, where patients with 17p deletion were approved to receive venetoclax in the subsequent-line setting. This was later extended to patients with CLL or small lymphocytic lymphoma (SLL) in June 2018, regardless of 17p deletion, in the subsequent-line setting.
The combination of rituximab, an anti-CD20 antibody, to venetoclax has also shown to be highly effective and able to achieve high uMRD rates in relapsed/refractory CLL. Preclinical data showed that this combination was able to counteract micro-environmental signals that were contributing to venetoclax resistance in CLL [91]. The phase III MURANO study [92] compared venetoclax-rituximab for 6 cycles followed by a 2 year-maintenance treatment, to 6 cycles of bendamustine-rituximab, and showed remarkable improvements in 2-year progression-free survival of 84.9% versus 36.3% [92], as well as 3-year uMRD rate (62% versus 13%) [93]. Another phase III study recently reported results comparing venetoclax-obinutuzumab versus chlorambucil-obinutuzumab in previously untreated CLL patients. Venetoclax-obinutuzumab was associated with significantly improved PFS at 24 months (24-month PFS rate 88.2% versus 64.1%), and this benefit was extended to patients with poor prognostic factors [94]. These impressive results relating to uMRD, together with pooled analysis data suggesting that venetoclax should be sequenced earlier in treatment paradigms, ultimately led to the FDA indication being expanded to all adult patients with CLL or SLL in May 2019.
Venetoclax monotherapy is modestly active in relapsed/refractory AML. Of note, patients harboring IDH1/2 mutations appeared to perform better with venetoclax therapy, with CR rate of 33% [95,96]. Further phase Ib studies have also combined venetoclax with hypomethylating agents based on preclinical models demonstrating synergy [97]. When combined with low dose cytarabine, decitabine or azacytidine in untreated elderly patients, CR/CRi rates ranged between 54–68% across studies with a median time to response of 1.2–1.4 months, with tolerable toxicity [98,99] (Table 2). This led to a further FDA breakthrough status in November 2018 for venetoclax in combination with hypomethylating drugs for newly-diagnosed elderly AML patients ineligible for intensive chemotherapy.
Venetoclax has also shown promising activity in relapsed/refractory MCL. In a phase I trial of 106 patients with relapsed/refractory NHL, patients with MCL had particularly high response rates (ORR of 75%, CR 21% [100]. Venetoclax plus ibrutinib was evaluated on a phase II study, which recruited a majority of relapsed/refractory MCL patients, again showing high response rates of ORR 71%, CR 63% [101], and this is being explored further on a phase III study (Table 2). Venetoclax monotherapy appears to be less active in other NHL, in particular relapsed/refractory DLBCL, where only modest response rates of around 18% were noted [100]. Similarly, in relapsed/refractory MM, ORR for venetoclax monotherapy was 21% [102]. In cell lines, the t(11; 14) (q13;q32) translocation was shown to increase BCL-2:MCL-1 ratio and lead to lower BCL-xL levels, and patients harboring this translocation may benefit the most [103].
As alluded to, venetoclax has heralded the way for the development of other BCL-2 inhibitors. Newer BCL-2 inhibitors in the pipeline include S55746 (Servier, Suresnes, France) which has dual BCL-2/ BCL-xL inhibiting capabilities [104,105] (Table 2). It is likely that the role for BCL-2 inhibitors is likely to expand in cancer therapy, and further results are awaited.
3.2.6. BCL-xL—Selective BH3-Mimetics
BCL-xL dependency has been described across tumor types, aggregating mainly in solid tumors [106]. This makes selective BCL-xL inhibition an attractive target, especially in the treatment of venetoclax- resistant cancers. As described earlier, BCL-xL expression in AML, MM and some solid tumor models, is associated with chemotherapy and venetoclax resistance [107,108]. WEHI-539 (The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) was the first selective BCL-xL inhibitor published. When bound to BCL-xL, WEHI-539 induced BAK-mediated cell death in SCLC cell lines. When used in osteosarcoma cells that overexpressed BCL-xL, WEHI-539 was able to potentiate the effect of low-dose doxorubicin [109]. However, further development of this compound has been halted due to in-vivo toxicity. On-target toxicities of such inhibitors include thrombocytopenia, which occurs rapidly and reversibly, similar to what was observed with navitoclax. Further BCL-xL selective inhibitors under pre-clinical evaluation include A-1155463 and A-1331852 (Abbvie Inc., North Chicago, IL, USA) (Table 3).

Table 3.
Other BCL2 family inhibitors under investigation. SCLC: small cell lung cancer; NSCLC: non-small cell lung cancer; R/R: relapsed/refractory; MM: multiple myeloma; AML: acute myeloid leukemia; NHL: non-hodgkin’s lymphoma; DLBLC: diffuse large B cell lymphoma; ORR: objective response rate; PFS: progression-free survival.
3.2.7. MCL-1 Antagonists
In healthy tissues, MCL-1 regulates neural and cardiac cell survival. In cancer, not only has the MCL-1 protein been shown to regulate cell survival in myeloid and lymphoid cancers including MM, AML and NHL [36,110], but MCL1 amplifications have been found in >10% of solid tumor cancer types [106]. In triple-negative breast cancer, MCL1 amplification correlates with poor prognosis [111].
Drug development in MCL-1 inhibition is ongoing with several candidate compounds in early phase testing (Table 3). A-1210477 (Abbvie Inc., North Chicago, IL, USA) was the first inhibitor able to disrupt MCL-1-NOXA and MCL-1-BIM2A interactions selectively [107]. When used in MM and NSCLC cell lines that showed MCL-1 dependency, A-1210477 triggered MOMP and apoptosis [107]. A more potent MCL-1 inhibitor, S63845 (Servier, Suresnes, France) [112], is also undergoing evaluation, and has been shown to have sub-molar affinity to the MCL-1 BH3 binding groove with BCL-2/ BCL-xL binding. In vitro, S63485 induced BAX/BAK-mediated apoptosis in solid tumors, as well as elicited intriguing synergism with tyrosine kinase inhibitors(TKIs) [112]. Several other MCL-1 inhibitors [AZD5991(AstraZeneca), AMG-176, AMG-397(Amgen), S64315/MIK665(Novartis)] are currently undergoing phase I clinical trials in a variety of hematological malignancies (Table 3). Despite these advancements, the concern for the development of side effects of MCL-1-targeting agents on cardiac and neurological systems may pose challenges to clinical development of these agents, and further results are awaited.
3.3. Targeting the BH4 Domain
Similar to BH3, the BH4 domain is conserved amongst the members of the BCL-2 superfamily. Aside from its crucial role in the anti-apoptotic activity of BCL-2, the BH4 domain also is required for other non-canonical functions of the BCL-2 superfamily, such as in calcium homeostasis at the ER [113]. Notably, losing the BH4 domain greatly diminishes the anti-apoptotic function of BCL-2 [114]. Targeting BH4 is therefore emerging as a novel strategy in cancer therapy (Table 3).
3.4. Interference Technology
Interference technologies at the DNA and RNA level utilize a nucleic acid-based approach to block transcription and translation of BCL2 respectively. Silencing BCL2 by utilizing RNA interference (RNAi) technology is still in its infancy. Early data regarding the efficacy of this approach have been generated using ALL cell lines and xenografts [115]. PNT2258 (ProNAi Therapeutics Inc., Vancouver, Canada) a first-in-class DNAi drug that consists of a 24-base sequence complementary to regions of DNA that are upstream from sites of gene transcription, thus preventing BCL-2 transcription [116]. In pre-clinical studies, PNT2258 was active in BCL-2 driven xenografts, including in NHL, prostate cancer and melanoma [117]. Differential activity was seen in different NHL cell lines according to their levels of BCL-2 overexpression. Initial phase I studies of PNT2258 confirmed a safe toxicity profile with tolerable lympho- and thrombocytopenia [118]. Initial interesting responses, especially in DLBCL patients, were noted on a phase I study of PNT2258 in relapsed/refractory NHL. However, these results were not corroborated in a phase II study of relapsed/refractory DLBCL, and the development of PNT2258 was subsequently discontinued [119] (Table 3).
5. Expanding Clinical Contexts for BCL-2 Targeting
5.1. Promising Combination Strategies in Hematological Malignancies
Despite the gains we have made through venetoclax in specific clinical contexts, rational combinations of BCL2-targeting therapy with chemotherapeutics and other targeted therapy hold promise to advance treatment paradigms. Venetoclax is currently being combined with different branches of cancer therapy in different hematological and solid malignancies, chosen based on known pathways that are aberrant in specific tumor types (Table 2). Aside from combination with chemotherapy, other important combinations under investigation include those with proteasome inhibitors (bortezomib, carfilzomib), PI3K inhibitors (idelalisib), BTK inhibitors (ibrutinib), CDK inhibitors (dinaciclib, palbociclib), MEK inhibitors (cobimetinib), MDM2 inhibitors (idasanutlin) and other novel agents (Table 2). Co-targeting of different BCL-2 family members to overcome resistance, such as concurrent BCL-2 and MCL-1 targeting or BCL-2/ BCL-xL targeting is also under study (Table 2).
The rationale to combine BCL-2 targeted drugs with chemotherapy is based on the understanding of mitochondrial priming. Treatment with BH3-mimetics is expected to raise the mitochondrial priming state, thereby allowing them to act as “chemosensitizers” for synergism with cytotoxic chemotherapy [143] (Table 2). Furthermore, this approach holds benefit not just in enhancing cell kill, but also may reduce treatment doses, thus reducing toxic side effects.
Venetoclax in combination with BTK inhibitors in CLL and MCL treatment is actively being explored. Samples taken from CLL patients receiving ibrutinib were analyzed in-vitro with the addition of venetoclax, and proved synergism of this combination [144]. Ibrutinib appears to downregulate MCL-1 and BCL-xL, potentiating venetoclax’s effect [144]. Adding venetoclax to obinutuzumab and ibrutinib in combination is being evaluated on a phase Ib study (NCT02427451), and a phase III study (GLOW/CLL3011) is studying ibrutinib plus venetoclax versus obinutuzumab plus chlorambucil (Table 2).
BAK, BAX and other pro-apoptotic members are degraded by ubiquitination and the proteasomal pathway. Therefore, proteasomal inhibition allows for their stabilization and accumulation in mitochondria, increasing the pro- to anti-apoptotic protein ratio [145]. In relapsed/refractory MM treatment, venetoclax, bortezomib and dexamethasone combination therapy initially showed a high ORR. Patients achieving PR or better had higher levels of BCL-2 [129]. However, on the phase III BELLINI study randomizing patients with relapsed/refractory MM to bortezomib combined with venetoclax or matched placebo, although the study showed improved PFS, ORR and uMRD for the venetoclax-containing arm, 13 treatment-emergent deaths occurred in the venetoclax-containing arm. Most deaths were attributable to infection, and this risk strengthened the urge toward a biomarker-driven approach. Authors suggested that this combination could be most relevant in patients with t(11; 14), where a trend towards improved OS was also noted, limiting exposure of toxicity to a smaller group of patients [131,146]. An additional phase II study investigating venetoclax with carfilzomib and dexamethasone (NCT02899052) is underway, interim results describe no new safety signals [130].
Strategies inhibiting important cyclin dependent kinases (CDKs) are also promising. CDK9 is a key component of positive transcription elongation factor (pTEFb) which is a transcriptional regulator complex. Inhibition of CDK9 blocks transcription resulting in MCL-1 repression [147]. CDK9 inhibition also down-regulates miRNAs that in turn negatively regulates pro-apoptotic BCL-2 family members, leading to a net activation of pro-apoptotic members. Voruciclib, a CDK1, 4, 6 and 9 inhibitor, synergized with venetoclax in DLBCL models to induce tumor remission [148]. In MM, several pre-clinical studies have similarly described how CDK inhibition down-regulates MCL-1 in cell lines. In AML, inhibition of CDK9 was demonstrated to transcriptionally silence MCL1, and thus overcome MCL-1 dependent drug resistance [149]. In venetoclax-resistant AML cell lines and mouse xenografts, voruciclib combined with venetoclax were synergistic in triggering BIM-dependent apoptosis [150]. Several early phase clinical trials investigating combinations of venetoclax with CDK inhibitors are ongoing (Table 2).
Promising results are also emerging from the combination of PI3K inhibitors (PI3Ki) with venetoclax and other therapies, particularly in CLL. Recent data released from a phase I/II study of umbralisib (a PI3Ki), ublituximab (a CD-20 antibody) and venetoclax in relapsed/refractory CLL included 27 patients, starting with umbralisib-ublituximab debulking to reduce the risk for tumor lysis syndrome, followed by umbralisib-venetoclax starting from the fourth cycle onwards. In 13 patients treated for >7 cycles of triple combination treatment, the ORR was 100% after cycle 7, and in 9 patients who received 12 or more cycles of treatment, 100% of patients attained uMRD. At short follow up of 6.4 months, none of the 27 patients had experienced disease progression [128] (Table 2).
Finally, novel therapies are being combined with BCL-2 inhibition. MDM2 inhibition has been shown to promote MCL-1 degradation in preclinical AML models [151]. Early results from a phase Ib study combining idasanutlin with venetoclax in relapsed/refractory AML have shown a response rate of 35.9%, with manageable toxicity [152]. Further studies combining venetoclax with novel therapies such as gemtuzumab ozogamicin, enasidenib and liposomal cytarabine and daunorubicin are ongoing (Table 2).
5.2. Targeting BCL2 Pathways in Solid Tumor Therapy
Currently, results from targeting the BCL-2 superfamily in solid tumors, using venetoclax or navitoclax have been disappointing [153]. On a wide study of multiple solid tumor cell lines, MCL-1 mRNA was the anti-apoptotic BCL-2 member with the highest levels in glioma, lung, renal, prostate, ovarian and breast cancer lines. In comparison, BCL-2 and BFL-1/A1 mRNA levels were highest in leukemia/lymphoma and melanoma cell lines [154]. This may explain why therapeutic success with venetoclax monotherapy has been thus far limited to hematological malignancies. Strategies targeting MCL-1 in solid tumors, or combinations including MCL-1 could achieve more success [154]. In cervical cancer cell lines, resistance to venetoclax, the BCL-xL selective inhibitor A1331852 or the MCL-1 inhibitor A-1210477 was noted when these agents were used individually. However, combining MCL-1 and BCL-xL inhibitors, or MCL-1 and BCL-2 inhibitors led to inhibition of proliferation in the same cell lines [155].
In other solid tumors, BCL-2 pathway targeting could sensitize to standard therapy, possibly related to its effects on mitochondrial priming. In hormone receptor (HR)-positive breast cancer xenografts, the BH3 mimetics venetoclax and ABT-737 potentiated tumor responses to tamoxifen. Further synergy was seen when the BH3 mimetics were combined with PI3K/mTOR inhibitors, which are already approved therapy in HR-positive advanced breast cancer, in addition to tamoxifen [156]. Currently, a randomized phase II study is comparing fulvestrant versus fulvestrant plus venetoclax in advanced HR-positive breast cancer (NCT03584009), and a phase Ib study of combination letrozole, palbociclib and venetoclax in metastatic ER-positive breast cancer is planned (NCT03900884).
BH3 mimetics were also shown to potentiate chemotherapy efficacy in basal-like HR-negative breast cancer xenografts. Immunocompromised mouse xenografts were treated with either ABT-737, docetaxel or both [157]. As expected, treatment with ABT-737 alone was ineffective, but treatment with combination therapy led to significant improvements in tumor response and OS in-vivo in breast cancer xenografts which overexpressed BCL-2. This finding correlated with a marked increase in apoptosis and BIM-BCL-2 dissociation, and suggests a role for BH3 mimetics to sensitize breast cancers to docetaxel chemotherapy. These results further corroborate with in-vitro experiments showing that endogenous BCL-2 phosphorylation occurs with spindle poison treatment which then leads to increased endogenous BCL-2/BIM binding. The addition of BCL-2 inhibitors was able to disrupt mitotic BCL-2/BIM binding in-vitro, enhancing paclitaxel cytotoxicity [29].
New strategies are exploiting the signaling pathways that induce dependency on BCL-2-like proteins [158]. Oncogenic addiction of a cell to RAS, HER2 or EGFR inhibits apoptosis by downregulating BH3-only activator proteins through the MAPK/ERK pathway [158], however, this may also trigger a second oncogenic signal through MYC which promotes BIM expression. Overall, this may lead to increased BCL-2-like protein dependency and increase sensitivity of oncogene addicted cells to apoptosis induced by BH3 mimetics. Two studies have reported on the upregulation of BCL-2-like members in EGFR-TKI-resistant NSCLC which harbor oncogenic EGFR mutations [159,160]. In one study, erlotinib-resistant EGFR mutant lung cancer cells showed increased MCL-1 expression, and were sensitive to EGFR TKIs when combined with navitoclax [161].
In melanoma, low BCL-xL expression was shown to bias the anti-apoptotic pool towards MCL-1. The combination of MCL-1 inhibition using AZD5991 with MEK1/2 inhibitors (MEKi) was noted to induce synthetic lethality by BAX/BAK-dependent cell death in-vivo [162]. AZD5991 was also shown to delay the development of acquired BRAFi/MEKi resistance, and enhanced the efficacy of ERKi in previously-resistant models [162]. Similar observations were made in patient-derived xenograft models of high-grade serous ovarian cancer which were resistant to the MEKi, cobimetinib. Proteomic interrogation showed that cobimetinib upregulated BIM, increasing mitochondrial ‘priming’, and sensitized models to synergistic targeting with the dual BCL-2/XL inhibitor navitoclax [163]. A phase Ib study combining navitoclax with trametinib in RAS-mutant advanced solid tumors is underway (Table 1).
6. Future Directions and Challenges
The recognition of the BCL-2 protein superfamily in regulating intrinsic apoptosis has brought attention to its targeting in overcoming treatment-related resistance in cancer therapy. Progressive refinement in the development of selective BCL-2 inhibitors has led to the successful approval of venetoclax, and significant improvement in clinical outcomes of CLL and AML therapy, while minimizing off-target toxicities. This success has catalyzed the progressive development of other BH3 mimetics, which is likely to change practice in the coming decade. Thus far, the limited success seen in other hematological malignancies and solid tumors only serves to underscore the following challenges we face in harnessing the benefit of BCL-2 inhibitors more broadly.
Firstly, though active development of BCL-xL and MCL-1 inhibitors is ongoing, it is uncertain if these inhibitors will maintain sufficient safety profile for widespread use [164]. Glaringly, no selective BFL-1/A1 inhibitors have been developed, although the ML214 probe may be useful to evaluate potential interaction sites for BFL-1/A1 inhibition [165]. Successful efforts targeting the pro-apoptotic family members are also notably missing from this space; however, apoptotic modulators such as BAM7 which are able to engage the BAX trigger site toward functional oligomerization are under investigation [166].
The selectivity of venetoclax has undeniable benefit in allowing off-target toxicity to be minimized. However, this selectivity itself promotes resistance and compensatory upregulation of non-target anti-apoptotic members such as MCL-1, which may necessitate combination or sequential targeting approaches. At the juncture, it remains to be seen if multiple BH3 mimetics can be successfully used in combination due to overlapping toxicity, and clinical trials evaluating the safety of these combinations are underway.
Thirdly, increasing data is emerging regarding the regulatory role that the mitochondrial membrane itself exerts on the BCL-2 superfamily. Membrane insertion and BAX oligomerization are the rate limiting steps for intrinsic apoptosis to proceed. Changes in the mechanical properties of the mitochondrial membrane may regulate BCL-2 proteins, or the membrane itself may have direct effects in modulating BCL-2 family member function [19]. One study has reported increased resistance for BAX-BCL-xL complexes when membrane inserted, and it is proposed that the inhibition of BAX oligomerization by BCL-2 proteins in the context of cellular membranes may be an effective means to allow the cell to avoid BAX activation [167]. Examining BCL-2 family member interactions in the presence of membranes appears imperative to forward our efforts.
The invention of BH3 profiling technology has made it plausible that a means of examining functional BCL-2 protein dependency and its dynamism during cancer development and progression is now available. Its predictive benefit should be consistently evaluated on prospective clinical trials. Additionally, BH3 profiling was developed in and has immense potential in the current era of BH3 mimetics. However, it is not clear if this technology will also help predict benefit to other anti-BCL-2 therapies such as interference strategies [168].
Finally, the BCL-2 family members have numerous non-canonical functions such as cross-talk with metabolic pathways, cellular redox status, involvement in ER calcium homeostasis and autophagy. These pleiotropic effects mean that targeting BCL-2 and BCL-2-like proteins may have a multitude of effects on cancer cell fate, and these consequences on anti-cancer therapy remain under investigation. Recent data have also shown that the BCL-2 family members have an unexpected immunological role, which is provocative for development. In melanoma cells with strong BCL-2 expression, the addition of ABT-737 to co-culture with expanded peripheral blood cytotoxic T-lymphocytes amplified tumor cell kill. As postulated, the addition of BCL-2 inhibitors may sensitize the target tumor cells to perforin/granzyme-B mediated cell kill [169]. More recently, Brokatzky and colleagues have described that activation of BAX/BAK induces mitochondrial DNA release [170], which can go on to trigger the innate immune system through the cGAS-STING signaling pathway. This may have the potential to increase the immunogenicity of immunologically “cold” tumors. In this way, the mitochondrial apoptosis pathway may serve as a modulator of anti-tumor immunity, therefore paving the way for novel combinations of drugs targeting the BCL-2 family together with immune-checkpoint blockade.
7. Conclusions
Despite the exciting advances made in our understanding of the BCL-2 proteins’ role in controlling cell fate and treatment resistance, these observations indicate that the benefit of BCL-2 targeting therapy is not yet fully exploited. The promise of personalized biomarker technology and rational combinations of BCL-2 inhibitors with other branches of cancer therapy are imminent, and will certainly add to our therapeutic arsenal to improve outcomes in a wider group of patients.
Author Contributions
Conceptualization, N.Y.L.N., G.B. and S.P.; data curation, N.N, G.B.; writing—original draft preparation, N.Y.L.N.; writing—review and editing, N.Y.L.N., C.C., J.L., G.B., A.L.W., B.C.G., S.P.; visualization, N.Y.L.N., G.B., S.P.; supervision, S.P.; project administration, N.Y.L.N. All authors have read and agreed to the published version of the manuscript.
Funding
N.Y.L.N. is supported by the National Medical Research Council, Singapore (MOH-FLWSHP19may-0006), A.L.W. is supported by the National Medical Research Council, Singapore (NMRC/TA17nov003). B.C.G. is supported by the National Medical Research Council, Singapore (NMRC/CSA-SI/0006/2016). S.P. is supported by the National Medical Research Council, Singapore (NMRC CIRG/1433/2015 and OFIRG/0041/2017).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pan, S.T.; Li, Z.L.; He, Z.X.; Qiu, J.X.; Zhou, S.F. Molecular mechanisms for tumour resistance to chemotherapy. Clin. Exp. Pharmacol. Physiol. 2016, 43, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Pietrocola, F.; Levine, B.; Kroemer, G. Metabolic control of autophagy. Cell 2014, 159, 1263–1276. [Google Scholar] [CrossRef]
- Shukla, S.; Chen, Z.S.; Ambudkar, S.V. Tyrosine kinase inhibitors as modulators of ABC transporter-mediated drug resistance. Drug Resist. Updates 2012, 15, 70–80. [Google Scholar] [CrossRef]
- Gul, O.; Basaga, H.; Kutuk, O. Apoptotic blocks and chemotherapy resistance: Strategies to identify Bcl-2 protein signatures. Brief. Funct. Genom. Proteom. 2008, 7, 27–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramos, P.; Bentires-Alj, M. Mechanism-based cancer therapy: Resistance to therapy, therapy for resistance. Oncogene 2015, 34, 3617–3626. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Tumor resistance to apoptosis. Int. J. Cancer 2009, 124, 511–515. [Google Scholar] [CrossRef]
- Green, D.R.; Walczak, H. Apoptosis therapy: Driving cancers down the road to ruin. Nat. Med. 2013, 19, 131–133. [Google Scholar] [CrossRef]
- Green, D.R.; Llambi, F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Saelens, X.; Festjens, N.; Vande Walle, L.; van Gurp, M.; van Loo, G.; Vandenabeele, P. Toxic proteins released from mitochondria in cell death. Oncogene 2004, 23, 2861–2874. [Google Scholar] [CrossRef]
- Wu, H.; Medeiros, L.J.; Young, K.H. Apoptosis signaling and BCL-2 pathways provide opportunities for novel targeted therapeutic strategies in hematologic malignances. Blood Rev. 2018, 32, 8–28. [Google Scholar] [CrossRef]
- Du Toit, A. Cell death: Balance through a bivalent regulator. Nat. Rev. Mol. Cell Biol. 2013, 14, 546. [Google Scholar] [CrossRef]
- Tessoulin, B.; Papin, A.; Gomez-Bougie, P.; Bellanger, C.; Amiot, M.; Pellat-Deceunynck, C.; Chiron, D. BCL2-Family Dysregulation in B-Cell Malignancies: From Gene Expression Regulation to a Targeted Therapy Biomarker. Front. Oncol. 2018, 8, 645. [Google Scholar] [CrossRef]
- Ni Chonghaile, T.; Letai, A. Mimicking the BH3 domain to kill cancer cells. Oncogene 2008, 27, S149–S157. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, K.; Kashkar, H. Targeting the mitochondrial apoptotic pathway: A preferred approach in hematologic malignancies? Cell Death Dis. 2014, 5, e1098. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Placzek, W.J. Post-Transcriptional Regulation of Anti-Apoptotic BCL2 Family Members. Int. J. Mol. Sci. 2018, 19, 308. [Google Scholar] [CrossRef]
- Chen, H.C.; Kanai, M.; Inoue-Yamauchi, A.; Tu, H.C.; Huang, Y.; Ren, D.; Kim, H.; Takeda, S.; Reyna, D.E.; Chan, P.M.; et al. An interconnected hierarchical model of cell death regulation by the BCL-2 family. Nat. Cell Biol. 2015, 17, 1270–1281. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef]
- Flores-Romero, H.; Garcia-Saez, A.J. The Incomplete Puzzle of the BCL2 Proteins. Cells 2019, 8, 1176. [Google Scholar] [CrossRef]
- Dewson, G.; Kratina, T.; Sim, H.W.; Puthalakath, H.; Adams, J.M.; Colman, P.M.; Kluck, R.M. To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3: Groove interactions. Mol. Cell 2008, 30, 369–380. [Google Scholar] [CrossRef]
- Gillies, L.A.; Kuwana, T. Apoptosis regulation at the mitochondrial outer membrane. J. Cell. Biochem. 2014, 115, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Little, A.S.; Balmanno, K.; Sale, M.J.; Smith, P.D.; Cook, S.J. Tumour cell responses to MEK1/2 inhibitors: Acquired resistance and pathway remodelling. Biochem. Soc. Trans. 2012, 40, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Nifoussi, S.K.; Vrana, J.A.; Domina, A.M.; De Biasio, A.; Gui, J.; Gregory, M.A.; Hann, S.R.; Craig, R.W. Thr 163 phosphorylation causes Mcl-1 stabilization when degradation is independent of the adjacent GSK3-targeted phosphodegron, promoting drug resistance in cancer. PLoS ONE 2012, 7, e47060. [Google Scholar] [CrossRef]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef] [PubMed]
- Vucic, D.; Dixit, V.M.; Wertz, I.E. Ubiquitylation in apoptosis: A post-translational modification at the edge of life and death. Nat. Rev. Mol. Cell Biol. 2011, 12, 439–452. [Google Scholar] [CrossRef]
- Ito, T.; Deng, X.; Carr, B.; May, W.S. Bcl-2 phosphorylation required for anti-apoptosis function. J. Biol. Chem. 1997, 272, 11671–11673. [Google Scholar] [CrossRef]
- Deng, X.; Ruvolo, P.; Carr, B.; May, W.S., Jr. Survival function of ERK1/2 as IL-3-activated, staurosporine-resistant Bcl2 kinases. Proc. Natl. Acad. Sci. USA 2000, 97, 1578–1583. [Google Scholar] [CrossRef]
- Dai, H.; Ding, H.; Meng, X.W.; Lee, S.H.; Schneider, P.A.; Kaufmann, S.H. Contribution of Bcl-2 phosphorylation to Bak binding and drug resistance. Cancer Res. 2013, 73, 6998–7008. [Google Scholar] [CrossRef]
- Ng, K.P.; Hillmer, A.M.; Chuah, C.T.; Juan, W.C.; Ko, T.K.; Teo, A.S.; Ariyaratne, P.N.; Takahashi, N.; Sawada, K.; Fei, Y.; et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat. Med. 2012, 18, 521–528. [Google Scholar] [CrossRef]
- Lessene, G.; Czabotar, P.E.; Colman, P.M. BCL-2 family antagonists for cancer therapy. Nat. Rev. Drug Discov. 2008, 7, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.X.; Pervaiz, S. Involvement of cytochrome c oxidase subunits Va and Vb in the regulation of cancer cell metabolism by Bcl-2. Cell Death Differ. 2010, 17, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Low, I.C.; Chen, Z.X.; Pervaiz, S. Bcl-2 modulates resveratrol-induced ROS production by regulating mitochondrial respiration in tumor cells. Antioxid. Redox Signal. 2010, 13, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Velaithan, R.; Kang, J.; Hirpara, J.L.; Loh, T.; Goh, B.C.; Le Bras, M.; Brenner, C.; Clement, M.V.; Pervaiz, S. The small GTPase Rac1 is a novel binding partner of Bcl-2 and stabilizes its antiapoptotic activity. Blood 2011, 117, 6214–6226. [Google Scholar] [CrossRef] [PubMed]
- Low, I.C.; Loh, T.; Huang, Y.; Virshup, D.M.; Pervaiz, S. Ser70 phosphorylation of Bcl-2 by selective tyrosine nitration of PP2A-B56delta stabilizes its antiapoptotic activity. Blood 2014, 124, 2223–2234. [Google Scholar] [CrossRef] [PubMed]
- Kozopas, K.M.; Yang, T.; Buchan, H.L.; Zhou, P.; Craig, R.W. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc. Natl. Acad. Sci. USA 1993, 90, 3516–3520. [Google Scholar] [CrossRef]
- Zhang, B.; Gojo, I.; Fenton, R.G. Myeloid cell factor-1 is a critical survival factor for multiple myeloma. Blood 2002, 99, 1885–1893. [Google Scholar] [CrossRef]
- Wenzel, S.S.; Grau, M.; Mavis, C.; Hailfinger, S.; Wolf, A.; Madle, H.; Deeb, G.; Dorken, B.; Thome, M.; Lenz, P.; et al. MCL1 is deregulated in subgroups of diffuse large B-cell lymphoma. Leukemia 2013, 27, 1381–1390. [Google Scholar] [CrossRef]
- Pepper, C.; Lin, T.T.; Pratt, G.; Hewamana, S.; Brennan, P.; Hiller, L.; Hills, R.; Ward, R.; Starczynski, J.; Austen, B.; et al. Mcl-1 expression has in vitro and in vivo significance in chronic lymphocytic leukemia and is associated with other poor prognostic markers. Blood 2008, 112, 3807–3817. [Google Scholar] [CrossRef]
- Glaser, S.P.; Lee, E.F.; Trounson, E.; Bouillet, P.; Wei, A.; Fairlie, W.D.; Izon, D.J.; Zuber, J.; Rappaport, A.R.; Herold, M.J.; et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 2012, 26, 120–125. [Google Scholar] [CrossRef]
- Chauhan, D.; Velankar, M.; Brahmandam, M.; Hideshima, T.; Podar, K.; Richardson, P.; Schlossman, R.; Ghobrial, I.; Raje, N.; Munshi, N.; et al. A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene 2007, 26, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.N.; Grabow, S.; Delbridge, A.R.; Strasser, A.; Adams, J.M. Endogenous Bcl-xL is essential for Myc-driven lymphomagenesis in mice. Blood 2011, 118, 6380–6386. [Google Scholar] [CrossRef] [PubMed]
- Delbridge, A.R.; Strasser, A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015, 22, 1071–1080. [Google Scholar] [CrossRef]
- Schuyer, M.; van der Burg, M.E.; Henzen-Logmans, S.C.; Fieret, J.H.; Klijn, J.G.; Look, M.P.; Foekens, J.A.; Stoter, G.; Berns, E.M. Reduced expression of BAX is associated with poor prognosis in patients with epithelial ovarian cancer: A multifactorial analysis of TP53, p21, BAX and BCL-2. Br. J. Cancer 2001, 85, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Pinon, J.D.; Labi, V.; Egle, A.; Villunger, A. Bim and Bmf in tissue homeostasis and malignant disease. Oncogene 2008, 27, S41–S52. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.Z.; Ziffra, J.; Stennett, L.; Bodner, B.; Bonish, B.K.; Chaturvedi, V.; Bennett, F.; Pollock, P.M.; Trent, J.M.; Hendrix, M.J.; et al. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res. 2005, 65, 6282–6293. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, H.; Karnan, S.; Suzuki, R.; Matsuo, K.; Zhang, X.; Ota, A.; Morishima, Y.; Nakamura, S.; Seto, M. Genome-wide array-based CGH for mantle cell lymphoma: Identification of homozygous deletions of the proapoptotic gene BIM. Oncogene 2005, 24, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.C.; Zong, W.X.; Cheng, E.H.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef]
- Violette, S.; Poulain, L.; Dussaulx, E.; Pepin, D.; Faussat, A.M.; Chambaz, J.; Lacorte, J.M.; Staedel, C.; Lesuffleur, T. Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-X(L) in addition to Bax and p53 status. Int. J. Cancer 2002, 98, 498–504. [Google Scholar] [CrossRef]
- Scarfo, L.; Ghia, P. Reprogramming cell death: BCL2 family inhibition in hematological malignancies. Immunol. Lett. 2013, 155, 36–39. [Google Scholar] [CrossRef]
- Durig, J.; Duhrsen, U.; Klein-Hitpass, L.; Worm, J.; Hansen, J.B.; Orum, H.; Wissenbach, M. The novel antisense Bcl-2 inhibitor SPC2996 causes rapid leukemic cell clearance and immune activation in chronic lymphocytic leukemia. Leukemia 2011, 25, 638–647. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’Brien, S.; Moore, J.O.; Boyd, T.E.; Larratt, L.M.; Skotnicki, A.; Koziner, B.; Chanan-Khan, A.A.; Seymour, J.F.; Bociek, R.G.; Pavletic, S.; et al. Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J. Clin. Oncol. 2007, 25, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, G.; Stock, W.; Dai, G.; Klisovic, R.B.; Liu, S.; Klisovic, M.I.; Blum, W.; Kefauver, C.; Sher, D.A.; Green, M.; et al. Phase I study of oblimersen sodium, an antisense to Bcl-2, in untreated older patients with acute myeloid leukemia: Pharmacokinetics, pharmacodynamics, and clinical activity. J. Clin. Oncol. 2005, 23, 3404–3411. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.; Jin, C.; Satterthwait, A.C.; Reed, J.C. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006, 13, 1419–1421. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Aggarwal, S.; Wierda, W.; Gandhi, V. Bax and Bak are required for apogossypolone, a BH3-mimetic, induced apoptosis in chronic lymphocytic leukemia cells. Leuk. Lymphoma 2013, 54, 1097–1100. [Google Scholar] [CrossRef][Green Version]
- Lei, X.; Chen, Y.; Du, G.; Yu, W.; Wang, X.; Qu, H.; Xia, B.; He, H.; Mao, J.; Zong, W.; et al. Gossypol induces Bax/Bak-independent activation of apoptosis and cytochrome c release via a conformational change in Bcl-2. FASEB J. 2006, 20, 2147–2149. [Google Scholar] [CrossRef]
- Meng, Y.; Li, Y.; Li, J.; Li, H.; Fu, J.; Liu, Y.; Liu, H.; Chen, X. (-)Gossypol and its combination with imatinib induce apoptosis in human chronic myeloid leukemic cells. Leuk. Lymphoma 2007, 48, 2204–2212. [Google Scholar] [CrossRef]
- Masood, A.; Sher, T.; Paulus, A.; Miller, K.C.; Chitta, K.S.; Chanan-Khan, A. Targeted treatment for chronic lymphocytic leukemia. OncoTargets Ther. 2011, 4, 169–183. [Google Scholar] [CrossRef]
- Kline, M.P.; Rajkumar, S.V.; Timm, M.M.; Kimlinger, T.K.; Haug, J.L.; Lust, J.A.; Greipp, P.R.; Kumar, S. R-(-)-gossypol (AT-101) activates programmed cell death in multiple myeloma cells. Exp. Hematol. 2008, 36, 568–576. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Wang, S.; Aboukameel, A.; Chen, B.; Wu, X.; Chen, J.; Al-Katib, A. Preclinical studies of a nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-X(L) [(-)-gossypol] against diffuse large cell lymphoma. Mol. Cancer Ther. 2005, 4, 13–21. [Google Scholar]
- Liu, G.; Kelly, W.K.; Wilding, G.; Leopold, L.; Brill, K.; Somer, B. An open-label, multicenter, phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin. Cancer Res. 2009, 15, 3172–3176. [Google Scholar] [CrossRef] [PubMed]
- Sonpavde, G.; Matveev, V.; Burke, J.M.; Caton, J.R.; Fleming, M.T.; Hutson, T.E.; Galsky, M.D.; Berry, W.R.; Karlov, P.; Holmlund, J.T.; et al. Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule Bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. Ann. Oncol. 2012, 23, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.E.; Loria, O.J.; Aguillon, R.A.; James, D.; Llanos, C.A.; Rassenti, L.; Wood, B.A.; Homlund, J.T.; Kipps, T.J. A Phase II, Open Label Study of AT-101 in Combination with Rituximab in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia. Evaluation of Two Dose Regimens. Blood 2007, 110, 3119. [Google Scholar] [CrossRef]
- Nguyen, M.; Marcellus, R.C.; Roulston, A.; Watson, M.; Serfass, L.; Murthy Madiraju, S.R.; Goulet, D.; Viallet, J.; Belec, L.; Billot, X.; et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc. Natl. Acad. Sci. USA 2007, 104, 19512–19517. [Google Scholar] [CrossRef]
- Konopleva, M.; Watt, J.; Contractor, R.; Tsao, T.; Harris, D.; Estrov, Z.; Bornmann, W.; Kantarjian, H.; Viallet, J.; Samudio, I.; et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax). Cancer Res. 2008, 68, 3413–3420. [Google Scholar] [CrossRef]
- Trudel, S.; Li, Z.H.; Rauw, J.; Tiedemann, R.E.; Wen, X.Y.; Stewart, A.K. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood 2007, 109, 5430–5438. [Google Scholar] [CrossRef]
- Schimmer, A.D.; Raza, A.; Carter, T.H.; Claxton, D.; Erba, H.; DeAngelo, D.J.; Tallman, M.S.; Goard, C.; Borthakur, G. A multicenter phase I/II study of obatoclax mesylate administered as a 3- or 24-hour infusion in older patients with previously untreated acute myeloid leukemia. PLoS ONE 2014, 9, e108694. [Google Scholar] [CrossRef]
- Arellano, M.L.; Borthakur, G.; Berger, M.; Luer, J.; Raza, A. A phase II, multicenter, open-label study of obatoclax mesylate in patients with previously untreated myelodysplastic syndromes with anemia or thrombocytopenia. Clin. Lymphoma Myeloma Leuk. 2014, 14, 534–539. [Google Scholar] [CrossRef]
- Urtishak, K.A.; Edwards, A.Y.; Wang, L.S.; Hudome, A.; Robinson, B.W.; Barrett, J.S.; Cao, K.; Cory, L.; Moore, J.S.; Bantly, A.D.; et al. Potent obatoclax cytotoxicity and activation of triple death mode killing across infant acute lymphoblastic leukemia. Blood 2013, 121, 2689–2703. [Google Scholar] [CrossRef]
- Goy, A.; Hernandez-Ilzaliturri, F.J.; Kahl, B.; Ford, P.; Protomastro, E.; Berger, M. A phase I/II study of the pan Bcl-2 inhibitor obatoclax mesylate plus bortezomib for relapsed or refractory mantle cell lymphoma. Leuk. Lymphoma 2014, 55, 2761–2768. [Google Scholar] [CrossRef]
- Oltersdorf, T.; Elmore, S.W.; Shoemaker, A.R.; Armstrong, R.C.; Augeri, D.J.; Belli, B.A.; Bruncko, M.; Deckwerth, T.L.; Dinges, J.; Hajduk, P.J.; et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005, 435, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.Z.; Mak, P.Y.; Mu, H.; Zhou, H.; Mak, D.H.; Schober, W.; Leverson, J.D.; Zhang, B.; Bhatia, R.; Huang, X.; et al. Combined targeting of BCL-2 and BCR-ABL tyrosine kinase eradicates chronic myeloid leukemia stem cells. Sci. Transl. Med. 2016, 8, 355ra117. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; Choudhary, G.S.; Al-Harbi, S.; Almasan, A. Mcl-1 Phosphorylation defines ABT-737 resistance that can be overcome by increased NOXA expression in leukemic B cells. Cancer Res. 2012, 72, 3069–3079. [Google Scholar] [CrossRef] [PubMed]
- Ackler, S.; Xiao, Y.; Mitten, M.J.; Foster, K.; Oleksijew, A.; Refici, M.; Schlessinger, S.; Wang, B.; Chemburkar, S.R.; Bauch, J.; et al. ABT-263 and rapamycin act cooperatively to kill lymphoma cells in vitro and in vivo. Mol. Cancer Ther. 2008, 7, 3265–3274. [Google Scholar] [CrossRef]
- Roberts, A.W.; Seymour, J.F.; Brown, J.R.; Wierda, W.G.; Kipps, T.J.; Khaw, S.L.; Carney, D.A.; He, S.Z.; Huang, D.C.; Xiong, H.; et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: Results of a phase I study of navitoclax in patients with relapsed or refractory disease. J. Clin. Oncol. 2012, 30, 488–496. [Google Scholar] [CrossRef]
- Van Delft, M.F.; Wei, A.H.; Mason, K.D.; Vandenberg, C.J.; Chen, L.; Czabotar, P.E.; Willis, S.N.; Scott, C.L.; Day, C.L.; Cory, S.; et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell 2006, 10, 389–399. [Google Scholar] [CrossRef]
- Vogler, M.; Dinsdale, D.; Sun, X.M.; Young, K.W.; Butterworth, M.; Nicotera, P.; Dyer, M.J.; Cohen, G.M. A novel paradigm for rapid ABT-737-induced apoptosis involving outer mitochondrial membrane rupture in primary leukemia and lymphoma cells. Cell Death Differ. 2008, 15, 820–830. [Google Scholar] [CrossRef]
- Oki, Y.; Copeland, A.; Hagemeister, F.; Fayad, L.E.; Fanale, M.; Romaguera, J.; Younes, A. Experience with obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist in patients with relapsed or refractory classical Hodgkin lymphoma. Blood 2012, 119, 2171–2172. [Google Scholar] [CrossRef]
- O’Brien, S.M.; Claxton, D.F.; Crump, M.; Faderl, S.; Kipps, T.; Keating, M.J.; Viallet, J.; Cheson, B.D. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood 2009, 113, 299–305. [Google Scholar] [CrossRef]
- Goy, A.; Berger, M.; Ford, P.; Feldman, T.; Mato, A.; Bejot, C.; Fung, H.C. Sequential single-agent obatoclax mesylate (GX15-070MS) followed by combination with rituximab in patients with previously untreated follicular lymphoma. Leuk. Lymphoma 2014, 55, 2932–2934. [Google Scholar] [CrossRef]
- Brown, J.R.; Tesar, B.; Yu, L.; Werner, L.; Takebe, N.; Mikler, E.; Reynolds, H.M.; Thompson, C.; Fisher, D.C.; Neuberg, D.; et al. Obatoclax in combination with fludarabine and rituximab is well-tolerated and shows promising clinical activity in relapsed chronic lymphocytic leukemia. Leuk. Lymphoma 2015, 56, 3336–3342. [Google Scholar] [CrossRef] [PubMed]
- Hantel, A.; Wynne, J.; Lacayo, N.; Khaw, S.L.; Rubnitz, J.; Mullighan, C.; Schmidt, M.; Zhou, Y.; Ross, J.A.; Rosenwinkel, L.; et al. Safety and Efficacy of the BCL Inhibitors Venetoclax and Navitoclax in Combination with Chemotherapy in Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma. Clin. Lymphoma Myeloma Leuk. 2018, 18, S184–S185. [Google Scholar] [CrossRef]
- Baggstrom, M.Q.; Qi, Y.; Koczywas, M.; Argiris, A.; Johnson, E.A.; Millward, M.J.; Murphy, S.C.; Erlichman, C.; Rudin, C.M.; Govindan, R.; et al. A phase II study of AT-101 (Gossypol) in chemotherapy-sensitive recurrent extensive-stage small cell lung cancer. J. Thorac. Oncol. 2011, 6, 1757–1760. [Google Scholar] [CrossRef] [PubMed]
- Ready, N.; Karaseva, N.A.; Orlov, S.V.; Luft, A.V.; Popovych, O.; Holmlund, J.T.; Wood, B.A.; Leopold, L. Double-blind, placebo-controlled, randomized phase 2 study of the proapoptotic agent AT-101 plus docetaxel, in second-line non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.W.; Stilgenbauer, S.; Seymour, J.F.; Huang, D.C.S. Venetoclax in Patients with Previously Treated Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2017, 23, 4527–4533. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Wierda, W.G.; Choi, M.Y.; Davids, M.S.; Cheson, B.D.; Furman, R.R.; Lamanna, N.; Barr, P.M.; Eradat, H.A.; Halwani, A.S.; et al. Venetoclax activity in CLL patients who have relapsed after or are refractory to ibrutinib or idelalisib. J. Clin. Oncol. 2016, 34, 7519. [Google Scholar] [CrossRef]
- Bottcher, S.; Ritgen, M.; Fischer, K.; Stilgenbauer, S.; Busch, R.M.; Fingerle-Rowson, G.; Fink, A.M.; Buhler, A.; Zenz, T.; Wenger, M.K.; et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: A multivariate analysis from the randomized GCLLSG CLL8 trial. J. Clin. Oncol. 2012, 30, 980–988. [Google Scholar] [CrossRef]
- Thompson, P.A.; Wierda, W.G. Eliminating minimal residual disease as a therapeutic end point: Working toward cure for patients with CLL. Blood 2016, 127, 279–286. [Google Scholar] [CrossRef]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Hillmen, P.; Seymour, J.F.; Coutre, S.; Jurczak, W.; Mulligan, S.P.; Schuh, A.; Assouline, S.; et al. Venetoclax for Patients With Chronic Lymphocytic Leukemia With 17p Deletion: Results From the Full Population of a Phase II Pivotal Trial. J. Clin. Oncol. 2018, 36, 1973–1980. [Google Scholar] [CrossRef]
- Thijssen, R.; Slinger, E.; Weller, K.; Geest, C.R.; Beaumont, T.; van Oers, M.H.; Kater, A.P.; Eldering, E. Resistance to ABT-199 induced by microenvironmental signals in chronic lymphocytic leukemia can be counteracted by CD20 antibodies or kinase inhibitors. Haematologica 2015, 100, e302–e306. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’Rozario, J.; Assouline, S.; Owen, C.; Gerecitano, J.; Robak, T.; De la Serna, J.; et al. Venetoclax-Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2018, 378, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Kater, A.P.; Seymour, J.F.; Hillmen, P.; Eichhorst, B.; Langerak, A.W.; Owen, C.; Verdugo, M.; Wu, J.; Punnoose, E.A.; Jiang, Y.; et al. Fixed Duration of Venetoclax-Rituximab in Relapsed/Refractory Chronic Lymphocytic Leukemia Eradicates Minimal Residual Disease and Prolongs Survival: Post-Treatment Follow-Up of the MURANO Phase III Study. J. Clin. Oncol. 2019, 37, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2019, 380, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.M.; Thomas, D.; Corces-Zimmerman, M.R.; Xavy, S.; Rastogi, S.; Hong, W.J.; Zhao, F.; Medeiros, B.C.; Tyvoll, D.A.; Majeti, R. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat. Med. 2015, 21, 178–184. [Google Scholar] [CrossRef]
- Konopleva, M.; Pollyea, D.A.; Potluri, J.; Chyla, B.; Hogdal, L.; Busman, T.; McKeegan, E.; Salem, A.H.; Zhu, M.; Ricker, J.L.; et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016, 6, 1106–1117. [Google Scholar] [CrossRef]
- Bogenberger, J.M.; Kornblau, S.M.; Pierceall, W.E.; Lena, R.; Chow, D.; Shi, C.X.; Mantei, J.; Ahmann, G.; Gonzales, I.M.; Choudhary, A.; et al. BCL-2 family proteins as 5-Azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia 2014, 28, 1657–1665. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef]
- Wei, A.H.; Strickland, S.A., Jr.; Hou, J.Z.; Fiedler, W.; Lin, T.L.; Walter, R.B.; Enjeti, A.; Tiong, I.S.; Savona, M.; Lee, S.; et al. Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study. J. Clin. Oncol. 2019, 37, 1277–1284. [Google Scholar] [CrossRef]
- Davids, M.S.; Roberts, A.W.; Seymour, J.F.; Pagel, J.M.; Kahl, B.S.; Wierda, W.G.; Puvvada, S.; Kipps, T.J.; Anderson, M.A.; Salem, A.H.; et al. Phase I First-in-Human Study of Venetoclax in Patients With Relapsed or Refractory Non-Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 826–833. [Google Scholar] [CrossRef]
- Tam, C.S.; Anderson, M.A.; Pott, C.; Agarwal, R.; Handunnetti, S.; Hicks, R.J.; Burbury, K.; Turner, G.; Di Iulio, J.; Bressel, M.; et al. Ibrutinib plus Venetoclax for the Treatment of Mantle-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kaufman, J.L.; Gasparetto, C.; Mikhael, J.; Vij, R.; Pegourie, B.; Benboubker, L.; Facon, T.; Amiot, M.; Moreau, P.; et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 2017, 130, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Matulis, S.M.; Gupta, V.A.; Nooka, A.K.; Hollen, H.V.; Kaufman, J.L.; Lonial, S.; Boise, L.H. Dexamethasone treatment promotes Bcl-2 dependence in multiple myeloma resulting in sensitivity to venetoclax. Leukemia 2016, 30, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Nemati, F.; de Montrion, C.; Lang, G.; Kraus-Berthier, L.; Carita, G.; Sastre-Garau, X.; Berniard, A.; Vallerand, D.; Geneste, O.; de Plater, L.; et al. Targeting Bcl-2/Bcl-XL induces antitumor activity in uveal melanoma patient-derived xenografts. PLoS ONE 2014, 9, e80836. [Google Scholar] [CrossRef]
- Loriot, Y.; Mordant, P.; Dugue, D.; Geneste, O.; Gombos, A.; Opolon, P.; Guegan, J.; Perfettini, J.L.; Pierre, A.; Berthier, L.K.; et al. Radiosensitization by a novel Bcl-2 and Bcl-XL inhibitor S44563 in small-cell lung cancer. Cell Death Dis. 2014, 5, e1423. [Google Scholar] [CrossRef] [PubMed]
- Beroukhim, R.; Mermel, C.H.; Porter, D.; Wei, G.; Raychaudhuri, S.; Donovan, J.; Barretina, J.; Boehm, J.S.; Dobson, J.; Urashima, M.; et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010, 463, 899–905. [Google Scholar] [CrossRef]
- Leverson, J.D.; Phillips, D.C.; Mitten, M.J.; Boghaert, E.R.; Diaz, D.; Tahir, S.K.; Belmont, L.D.; Nimmer, P.; Xiao, Y.; Ma, X.M.; et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci. Transl. Med. 2015, 7, 279ra240. [Google Scholar] [CrossRef]
- Punnoose, E.A.; Leverson, J.D.; Peale, F.; Boghaert, E.R.; Belmont, L.D.; Tan, N.; Young, A.; Mitten, M.; Ingalla, E.; Darbonne, W.C.; et al. Expression Profile of BCL-2, BCL-XL, and MCL-1 Predicts Pharmacological Response to the BCL-2 Selective Antagonist Venetoclax in Multiple Myeloma Models. Mol. Cancer Ther. 2016, 15, 1132–1144. [Google Scholar] [CrossRef]
- Baranski, Z.; de Jong, Y.; Ilkova, T.; Peterse, E.F.; Cleton-Jansen, A.M.; van de Water, B.; Hogendoorn, P.C.; Bovee, J.V.; Danen, E.H. Pharmacological inhibition of Bcl-xL sensitizes osteosarcoma to doxorubicin. Oncotarget 2015, 6, 36113–36125. [Google Scholar] [CrossRef]
- Legartova, S.; Krejci, J.; Harnicarova, A.; Hajek, R.; Kozubek, S.; Bartova, E. Nuclear topography of the 1q21 genomic region and Mcl-1 protein levels associated with pathophysiology of multiple myeloma. Neoplasma 2009, 56, 404–413. [Google Scholar] [CrossRef][Green Version]
- Goodwin, C.M.; Rossanese, O.W.; Olejniczak, E.T.; Fesik, S.W. Myeloid cell leukemia-1 is an important apoptotic survival factor in triple-negative breast cancer. Cell Death Differ. 2015, 22, 2098–2106. [Google Scholar] [CrossRef] [PubMed]
- Kotschy, A.; Szlavik, Z.; Murray, J.; Davidson, J.; Maragno, A.L.; Le Toumelin-Braizat, G.; Chanrion, M.; Kelly, G.L.; Gong, J.N.; Moujalled, D.M.; et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 2016, 538, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Distelhorst, C.W. Bcl-2 protein family members: Versatile regulators of calcium signaling in cell survival and apoptosis. Annu. Rev. Physiol. 2008, 70, 73–91. [Google Scholar] [CrossRef]
- Huang, D.C.; Adams, J.M.; Cory, S. The conserved N-terminal BH4 domain of Bcl-2 homologues is essential for inhibition of apoptosis and interaction with CED-4. EMBO J. 1998, 17, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Scherr, M.; Elder, A.; Battmer, K.; Barzan, D.; Bomken, S.; Ricke-Hoch, M.; Schroder, A.; Venturini, L.; Blair, H.J.; Vormoor, J.; et al. Differential expression of miR-17~92 identifies BCL2 as a therapeutic target in BCR-ABL-positive B-lineage acute lymphoblastic leukemia. Leukemia 2014, 28, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, A.S.; Kandouz, M.; Liddane, A.; Sabbagh, H.; Hou, Y.; Li, C.; Al-Katib, A. PNT2258, a novel deoxyribonucleic acid inhibitor, induces cell cycle arrest and apoptosis via a distinct mechanism of action: A new class of drug for non-Hodgkin’s lymphoma. Oncotarget 2016, 7, 42374–42384. [Google Scholar] [CrossRef] [PubMed]
- Rodrigueza, W.V.; Whitehead, C.; Mohammad, R.; McGovern, J.P.; Wick, M.J.; Rasco, D.; Tolcher, A.W.; Bisgaier, C.L. Abstract 2764: Effect of PNT2258 combinations with docetaxel, dacarbazine, or vemurafenib on the A375 melanoma xenograft. Cancer Res. 2012, 72, 2764. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Rodrigueza, W.V.; Rasco, D.W.; Patnaik, A.; Papadopoulos, K.P.; Amaya, A.; Moore, T.D.; Gaylor, S.K.; Bisgaier, C.L.; Sooch, M.P.; et al. A phase 1 study of the BCL2-targeted deoxyribonucleic acid inhibitor (DNAi) PNT2258 in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2014, 73, 363–371. [Google Scholar] [CrossRef]
- Harb, W.A.; Lakhani, N.; Logsdon, A.; Steigelman, M.; Smith-Green, H.; Gaylor, S.; Rodrigueza, W.; Woolliscroft, M.; Sooch, M.; Messmann, R.A.; et al. The BCL2 Targeted Deoxyribonucleic Acid Inhibitor (DNAi) PNT2258 Is Active in Patients with Relapsed or Refractory Non-Hodgkin’s Lymphoma. Blood 2014, 124, 1716. [Google Scholar] [CrossRef]
- Valentin, R.; Grabow, S.; Davids, M.S. The rise of apoptosis: Targeting apoptosis in hematologic malignancies. Blood 2018, 132, 1248–1264. [Google Scholar] [CrossRef]
- Brocco, F.; ter Burg, H.; Fernandes, S.; Tam, C.S.; Forconi, F.; Guerra, R.M.; Bird, G.; Walensky, L.D.; Brown, J.R.; Kater, A.P.; et al. Dissecting the Role of Individual Bcl-2 Members in Response and Resistance to Ibrutinib or Venetoclax in CLL. Blood 2017, 130, 262. [Google Scholar] [CrossRef]
- Choudhary, G.S.; Al-Harbi, S.; Mazumder, S.; Hill, B.T.; Smith, M.R.; Bodo, J.; Hsi, E.D.; Almasan, A. MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis. 2015, 6, e1593. [Google Scholar] [CrossRef] [PubMed]
- Yecies, D.; Carlson, N.E.; Deng, J.; Letai, A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood 2010, 115, 3304–3313. [Google Scholar] [CrossRef] [PubMed]
- Jayappa, K.D.; Portell, C.A.; Gordon, V.L.; Capaldo, B.J.; Bekiranov, S.; Axelrod, M.J.; Brett, L.K.; Wulfkuhle, J.D.; Gallagher, R.I.; Petricoin, E.F.; et al. Microenvironmental agonists generate de novo phenotypic resistance to combined ibrutinib plus venetoclax in CLL and MCL. Blood Adv. 2017, 1, 933–946. [Google Scholar] [CrossRef]
- Seymour, J.F.; Ma, S.; Brander, D.M.; Choi, M.Y.; Barrientos, J.; Davids, M.S.; Anderson, M.A.; Beaven, A.W.; Rosen, S.T.; Tam, C.S.; et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: A phase 1b study. Lancet Oncol. 2017, 18, 230–240. [Google Scholar] [CrossRef]
- Flinn, I.; Brunvand, M.; Dyer, M.J.; Hillman, P.; Jones, J.; Lymp, J.; Elhamy, M.; Vosganian, G.; Huang, J.; Kipps, T.J. Preliminary Results of a Phase 1b Study (GP28331) Combining GDC-0199 (ABT-199) and Obinutuzumab in Patients with Relapsed/Refractory or Previously Untreated Chronic Lymphocytic Leukemia. Blood 2014, 124, 4687. [Google Scholar] [CrossRef]
- Rogers, K.A.; Huang, Y.; Ruppert, A.S.; Awan, F.T.; Heerema, N.A.; Hoffman, C.; Lozanski, G.; Maddocks, K.J.; Moran, M.E.; Reid, M.A.; et al. Phase 1b study of obinutuzumab, ibrutinib, and venetoclax in relapsed and refractory chronic lymphocytic leukemia. Blood 2018, 132, 1568–1572. [Google Scholar] [CrossRef]
- Barr, P.M.; Hill, B.T.; Ma, S.; Baran, A.M.; Bui, A.; Meacham, P.J.; Morrison, A.; Liesveld, J.L.; Mulford, D.A.; Sportelli, P.; et al. A Phase 1/2 Study of Umbralisib Ublituximab and Venetoclax in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL). Blood 2019, 134, 360. [Google Scholar] [CrossRef]
- Moreau, P.; Chanan-Khan, A.; Roberts, A.W.; Agarwal, A.B.; Facon, T.; Kumar, S.; Touzeau, C.; Punnoose, E.A.; Cordero, J.; Munasinghe, W.; et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood 2017, 130, 2392–2400. [Google Scholar] [CrossRef]
- Costa, L.J.; Stadtmauer, E.A.; Morgan, G.J.; Monohan, G.P.; Kovacsovics, T.; Burwick, N.; Jakubowiak, A.J.; Mobasher, M.; Freise, K.; Ross, J.A.; et al. Phase 2 study of venetoclax plus carfilzomib and dexamethasone in patients with relapsed/refractory multiple myeloma. J. Clin. Oncol. 2018, 36, 8004. [Google Scholar] [CrossRef]
- Moreau, P.; Harrison, S.; Cavo, M.; De La Rubia, J.; Popat, R.; Gasparetto, C.; Hungria, V.T.M.; Salwender, H.; Suzuki, K.; Kim, I.; et al. Updated Analysis of Bellini, a Phase 3 Study of Venetoclax or Placebo in Combination with Bortezomib and Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2019, 134, 1888. [Google Scholar] [CrossRef]
- Tao, Z.F.; Hasvold, L.; Wang, L.; Wang, X.; Petros, A.M.; Park, C.H.; Boghaert, E.R.; Catron, N.D.; Chen, J.; Colman, P.M.; et al. Discovery of a Potent and Selective BCL-XL Inhibitor with in Vivo Activity. ACS Med. Chem. Lett. 2014, 5, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Tron, A.E.; Belmonte, M.A.; Adam, A.; Aquila, B.M.; Boise, L.H.; Chiarparin, E.; Cidado, J.; Embrey, K.J.; Gangl, E.; Gibbons, F.D.; et al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat. Commun. 2018, 9, 5341. [Google Scholar] [CrossRef] [PubMed]
- Maragno, A.L.; Mistry, P.; Kotschy, A.; Szlavik, Z.; Murray, J.; Davidson, J.; Toumelin-Braizat, G.L.; Chanrion, M.; Bruno, A.; Claperon, A.; et al. Abstract 4482: S64315 (MIK665) is a potent and selective Mcl1 inhibitor with strong antitumor activity across a diverse range of hematologic tumor models. Cancer Res. 2019, 79, 4482. [Google Scholar] [CrossRef]
- Halilovic, E.; Chanrion, M.; Mistry, P.; Wartmann, M.; Qiu, S.; Sanghavi, S.; Chen, Y.; Lysiak, G.; Maragno, A.L.; Pfaar, U.; et al. Abstract 4477: MIK665/S64315, a novel Mcl-1 inhibitor, in combination with Bcl-2 inhibitors exhibits strong synergistic antitumor activity in a range of hematologic malignancies. Cancer Res. 2019, 79, 4477. [Google Scholar] [CrossRef]
- Deng, J.; Park, D.; Wang, M.; Nooka, A.; Deng, Q.; Matulis, S.; Kaufman, J.; Lonial, S.; Boise, L.H.; Galipeau, J.; et al. BCL2-BH4 antagonist BDA-366 suppresses human myeloma growth. Oncotarget 2016, 7, 27753–27763. [Google Scholar] [CrossRef]
- Gupta, D.; Kumar, M.; Tyagi, P.; Kapoor, S.; Tyagi, A.; Barman, T.K.; Kharbanda, S.; Kufe, D.; Singh, H. Concomitant Delivery of Paclitaxel and NuBCP-9 peptide for synergistic enhancement of cancer therapy. Nanomedicine 2018, 14, 1301–1313. [Google Scholar] [CrossRef]
- Certo, M.; Del Gaizo Moore, V.; Nishino, M.; Wei, G.; Korsmeyer, S.; Armstrong, S.A.; Letai, A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 2006, 9, 351–365. [Google Scholar] [CrossRef]
- Touzeau, C.; Ryan, J.; Guerriero, J.; Moreau, P.; Chonghaile, T.N.; Le Gouill, S.; Richardson, P.; Anderson, K.; Amiot, M.; Letai, A. BH3 profiling identifies heterogeneous dependency on Bcl-2 family members in multiple myeloma and predicts sensitivity to BH3 mimetics. Leukemia 2016, 30, 761–764. [Google Scholar] [CrossRef]
- Alford, S.E.; Kothari, A.; Loeff, F.C.; Eichhorn, J.M.; Sakurikar, N.; Goselink, H.M.; Saylors, R.L.; Jedema, I.; Falkenburg, J.H.; Chambers, T.C. BH3 Inhibitor Sensitivity and Bcl-2 Dependence in Primary Acute Lymphoblastic Leukemia Cells. Cancer Res. 2015, 75, 1366–1375. [Google Scholar] [CrossRef]
- Anderson, M.A.; Deng, J.; Seymour, J.F.; Tam, C.; Kim, S.Y.; Fein, J.; Yu, L.; Brown, J.R.; Westerman, D.; Si, E.G.; et al. The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism. Blood 2016, 127, 3215–3224. [Google Scholar] [CrossRef] [PubMed]
- Bhola, P.D.; Mar, B.G.; Lindsley, R.C.; Ryan, J.A.; Hogdal, L.J.; Vo, T.T.; DeAngelo, D.J.; Galinsky, I.; Ebert, B.L.; Letai, A. Functionally identifiable apoptosis-insensitive subpopulations determine chemoresistance in acute myeloid leukemia. J. Clin. Investig. 2016, 126, 3827–3836. [Google Scholar] [CrossRef] [PubMed]
- Strasser, A.; Vaux, D.L. Viewing BCL2 and cell death control from an evolutionary perspective. Cell Death Differ. 2018, 25, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Gomez, F.; Lamothe, B.; Woyach, J.A.; Wierda, W.G.; Keating, M.J.; Balakrishnan, K.; Gandhi, V. Pharmacological and Protein Profiling Suggests Venetoclax (ABT-199) as Optimal Partner with Ibrutinib in Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2015, 21, 3705–3715. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dou, Q.P. Bax degradation by the ubiquitin/proteasome-dependent pathway: Involvement in tumor survival and progression. Proc. Natl. Acad. Sci. USA 2000, 97, 3850–3855. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Cavo, M.; De La Rubia, J.; Popat, R.; Gasparetto, C.; Hungria, V.T.M.; Salwender, H.; Suzuki, K.; Kim, I.; Moreau, P.; et al. T(11;14) and High BCL2 Expression Are Predictive Biomarkers of Response to Venetoclax in Combination with Bortezomib and Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma: Biomarker Analyses from the Phase 3 Bellini Study. Blood 2019, 134, 142. [Google Scholar] [CrossRef]
- Wang, S.; Fischer, P.M. Cyclin-dependent kinase 9: A key transcriptional regulator and potential drug target in oncology, virology and cardiology. Trends Pharmacol. Sci. 2008, 29, 302–313. [Google Scholar] [CrossRef]
- Dey, J.; Deckwerth, T.L.; Kerwin, W.S.; Casalini, J.R.; Merrell, A.J.; Grenley, M.O.; Burns, C.; Ditzler, S.H.; Dixon, C.P.; Beirne, E.; et al. Voruciclib, a clinical stage oral CDK9 inhibitor, represses MCL-1 and sensitizes high-risk Diffuse Large B-cell Lymphoma to BCL2 inhibition. Sci. Rep. 2017, 7, 18007. [Google Scholar] [CrossRef]
- Yeh, Y.Y.; Chen, R.; Hessler, J.; Mahoney, E.; Lehman, A.M.; Heerema, N.A.; Grever, M.R.; Plunkett, W.; Byrd, J.C.; Johnson, A.J. Up-regulation of CDK9 kinase activity and Mcl-1 stability contributes to the acquired resistance to cyclin-dependent kinase inhibitors in leukemia. Oncotarget 2015, 6, 2667–2679. [Google Scholar] [CrossRef]
- Luedtke, D.A.; Su, Y.; Edwards, H.; Polin, L.; Kushner, J.; Dzinic, S.H.; Lin, H.; Taub, J.W.; Ge, Y. Voruciclib, an Oral, Selective CDK9 Inhibitor, Enhances Cell Death Induced By the Bcl-2 Selective Inhibitor Venetoclax in Acute Myeloid Leukemia. Blood 2018, 132, 1361. [Google Scholar] [CrossRef]
- Lehmann, C.; Friess, T.; Birzele, F.; Kiialainen, A.; Dangl, M. Superior anti-tumor activity of the MDM2 antagonist idasanutlin and the Bcl-2 inhibitor venetoclax in p53 wild-type acute myeloid leukemia models. J. Hematol. Oncol. 2016, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.G.; Pollyea, D.A.; Garcia, J.S.; Jonas, B.A.; Yee, K.W.L.; Fenaux, P.; Assouline, S.; Vey, N.; Olin, R.; Roboz, G.J.; et al. Safety, Efficacy, Pharmacokinetic (PK) and Biomarker Analyses of BCL2 Inhibitor Venetoclax (Ven) Plus MDM2 Inhibitor Idasanutlin (idasa) in Patients (pts) with Relapsed or Refractory (R/R) AML: A Phase Ib, Non-Randomized, Open-Label Study. Blood 2018, 132, 767. [Google Scholar] [CrossRef]
- Rudin, C.M.; Hann, C.L.; Garon, E.B.; Ribeiro de Oliveira, M.; Bonomi, P.D.; Camidge, D.R.; Chu, Q.; Giaccone, G.; Khaira, D.; Ramalingam, S.S.; et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin. Cancer Res. 2012, 18, 3163–3169. [Google Scholar] [CrossRef] [PubMed]
- Placzek, W.J.; Wei, J.; Kitada, S.; Zhai, D.; Reed, J.C.; Pellecchia, M. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis. 2010, 1, e40. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.Y.; Lin, L.T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 2015, 35, S78–S103. [Google Scholar] [CrossRef]
- Vaillant, F.; Merino, D.; Lee, L.; Breslin, K.; Pal, B.; Ritchie, M.E.; Smyth, G.K.; Christie, M.; Phillipson, L.J.; Burns, C.J.; et al. Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell 2013, 24, 120–129. [Google Scholar] [CrossRef]
- Oakes, S.R.; Vaillant, F.; Lim, E.; Lee, L.; Breslin, K.; Feleppa, F.; Deb, S.; Ritchie, M.E.; Takano, E.; Ward, T.; et al. Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc. Natl. Acad. Sci. USA 2012, 109, 2766–2771. [Google Scholar] [CrossRef]
- Juin, P.; Geneste, O.; Gautier, F.; Depil, S.; Campone, M. Decoding and unlocking the BCL-2 dependency of cancer cells. Nat. Rev. Cancer 2013, 13, 455–465. [Google Scholar] [CrossRef]
- Rice, S.J.; Liu, X.; Wang, H.G.; Belani, C.P. EGFR mutations and AKT phosphorylation are markers for sensitivity to combined MCL-1 and BCL-2/xL inhibition in non-small cell lung cancer. PLoS ONE 2019, 14, e0217657. [Google Scholar] [CrossRef]
- Cheong, H.T.; Xu, F.; Choy, C.T.; Hui, C.W.C.; Mok, T.S.K.; Wong, C.H. Upregulation of Bcl2 in NSCLC with acquired resistance to EGFR-TKI. Oncol. Lett. 2018, 15, 901–907. [Google Scholar] [CrossRef]
- Hata, A.N.; Niederst, M.J.; Archibald, H.L.; Gomez-Caraballo, M.; Siddiqui, F.M.; Mulvey, H.E.; Maruvka, Y.E.; Ji, F.; Bhang, H.E.; Krishnamurthy Radhakrishna, V.; et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat. Med. 2016, 22, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Sale, M.J.; Minihane, E.; Monks, N.R.; Gilley, R.; Richards, F.M.; Schifferli, K.P.; Andersen, C.L.; Davies, E.J.; Vicente, M.A.; Ozono, E.; et al. Targeting melanoma’s MCL1 bias unleashes the apoptotic potential of BRAF and ERK1/2 pathway inhibitors. Nat. Commun. 2019, 10, 5167. [Google Scholar] [CrossRef] [PubMed]
- Lavarone, E.; Barbieri, C.M.; Pasini, D. Dissecting the role of H3K27 acetylation and methylation in PRC2 mediated control of cellular identity. Nat. Commun. 2019, 10, 1679. [Google Scholar] [CrossRef] [PubMed]
- Vogler, M.; Walter, H.S.; Dyer, M.J.S. Targeting anti-apoptotic BCL2 family proteins in haematological malignancies—From pathogenesis to treatment. Br. J. Haematol. 2017, 178, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Bittker, J.A.; Weiwer, M.; Wei, G.; Germain, A.; Brown, E.; Dandapani, S.; Munoz, B.; Palmer, M.; Golub, T.; Schreiber, S.L. Discovery of Inhibitors of Anti-Apoptotic Protein A1. In Probe Reports from the NIH Molecular Libraries Program; National Center for Biotechnology Information: Bethesda, MD, USA, 2010. [Google Scholar]
- Gavathiotis, E.; Reyna, D.E.; Bellairs, J.A.; Leshchiner, E.S.; Walensky, L.D. Direct and selective small-molecule activation of proapoptotic BAX. Nat. Chem. Biol. 2012, 8, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Unsay, J.D.; Cosentino, K.; Sporbeck, K.; Garcia-Saez, A.J. Pro-apoptotic cBid and Bax exhibit distinct membrane remodeling activities: An AFM study. Biochim. Biophys. Acta Biomembr. 2017, 1859, 17–27. [Google Scholar] [CrossRef]
- Ebrahim, A.S.; Sabbagh, H.; Liddane, A.; Raufi, A.; Kandouz, M.; Al-Katib, A. Hematologic malignancies: Newer strategies to counter the BCL-2 protein. J. Cancer Res. Clin. Oncol. 2016, 142, 2013–2022. [Google Scholar] [CrossRef]
- Lickliter, J.D.; Cox, J.; McCarron, J.; Martinez, N.R.; Schmidt, C.W.; Lin, H.; Nieda, M.; Nicol, A.J. Small-molecule Bcl-2 inhibitors sensitise tumour cells to immune-mediated destruction. Br. J. Cancer 2007, 96, 600–608. [Google Scholar] [CrossRef]
- Brokatzky, D.; Dorflinger, B.; Haimovici, A.; Weber, A.; Kirschnek, S.; Vier, J.; Metz, A.; Henschel, J.; Steinfeldt, T.; Gentle, I.E.; et al. A non-death function of the mitochondrial apoptosis apparatus in immunity. EMBO J. 2019, 38. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).